Antidiabetic Effect of Urolithin A in Cultured L6 Myotubes and Type 2 Diabetic Model KK-Ay/Ta Mice with Glucose Intolerance
Abstract
1. Introduction
2. Materials and Methods
2.1. Determination of Glucose Uptake in Cultured L6 Myotubes
2.2. Extraction of Total Protein from Cultured L6 Myotubes
2.3. Extraction of Total Protein from Plasma Membrane in Cultured L6 Myotubes
2.4. Expression Analysis of Target Protein (Western Blotting)
2.5. Intraperitoneal Glucose Tolerance Test in KK-Ay/Ta Mice
2.6. Statistical Analysis
3. Results
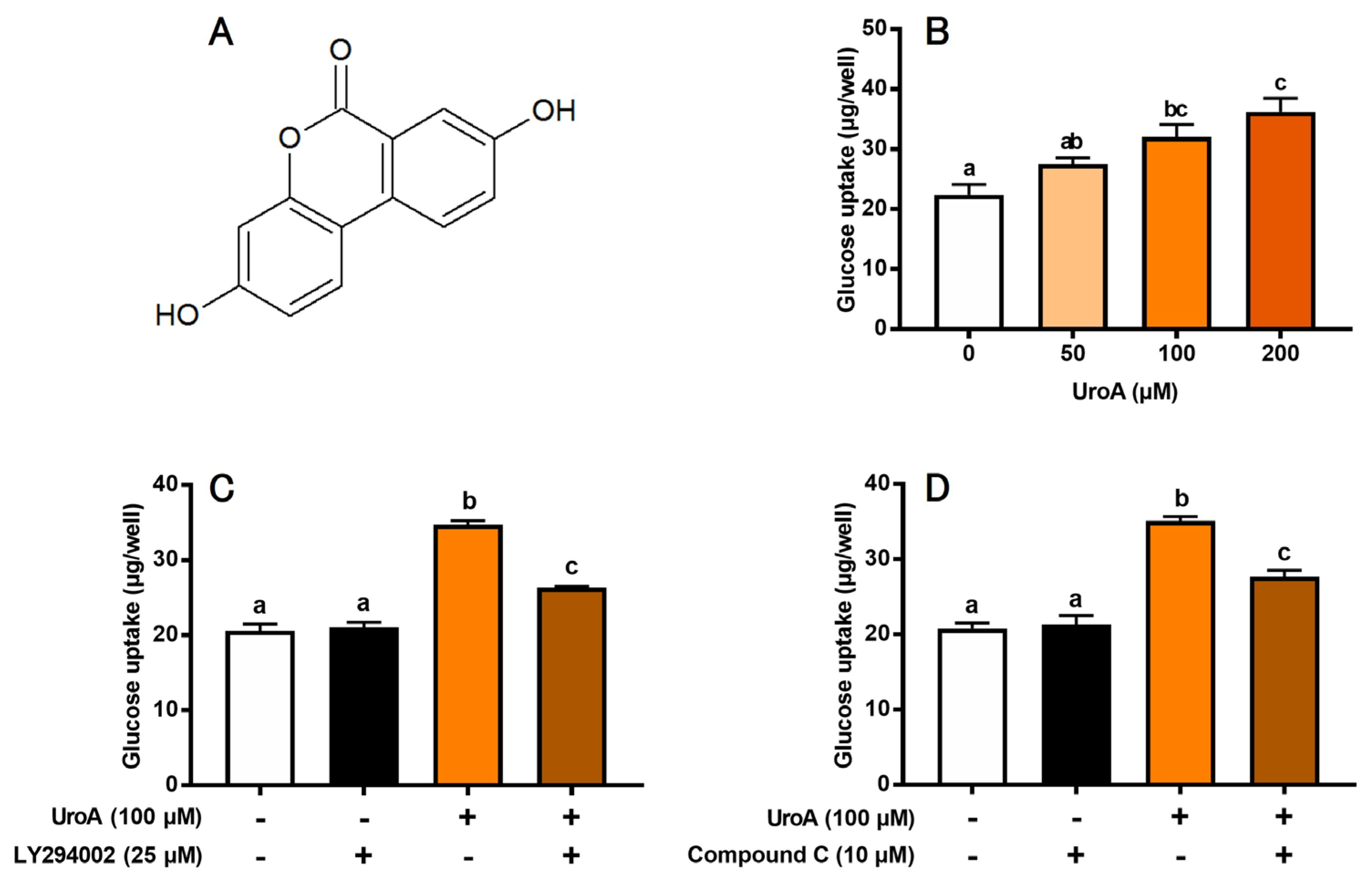
3.1. Effect of UroA on Glucose Uptake in Cultured L6 Myotubes
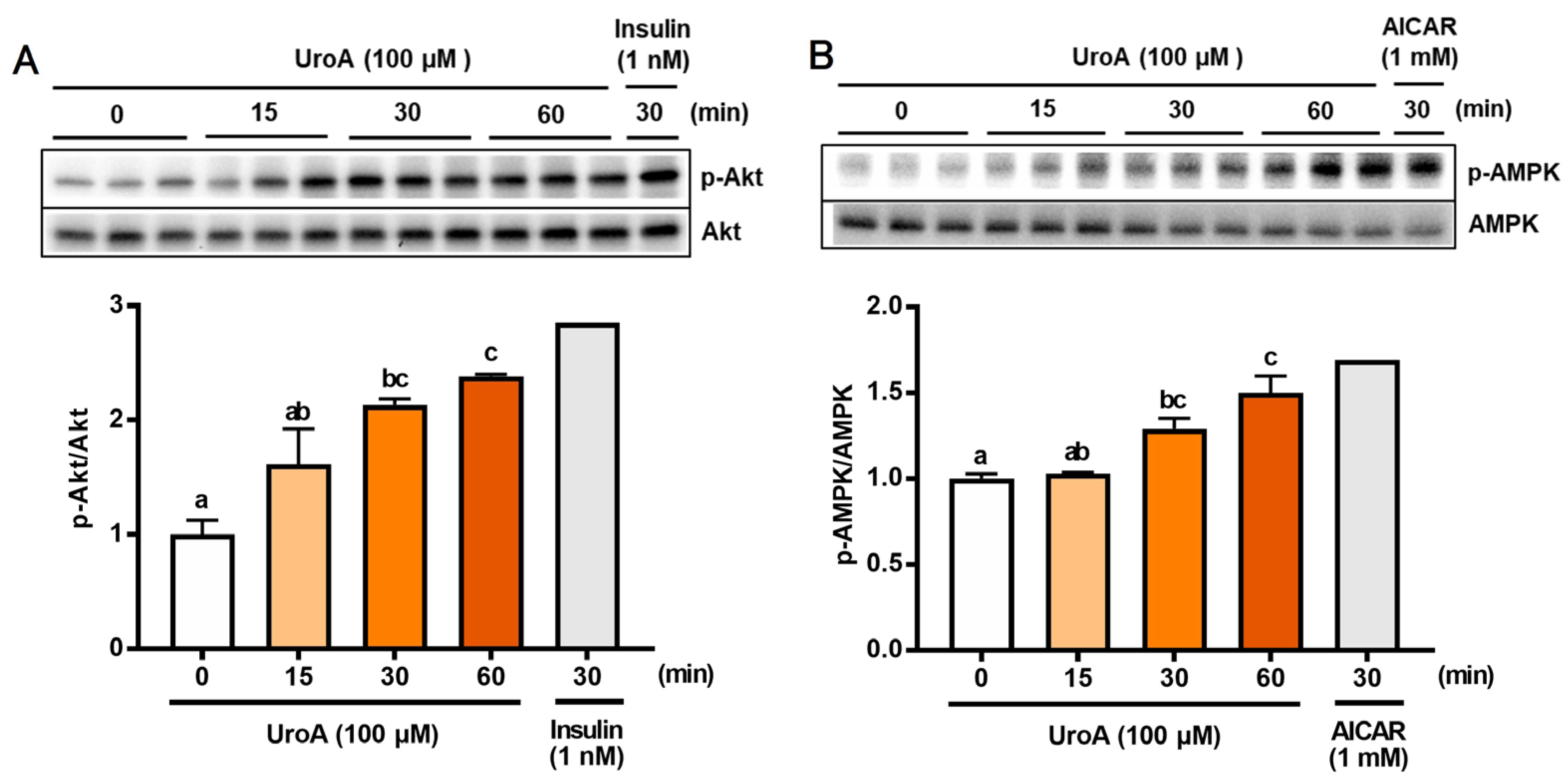
3.2. Effect of UroA on Phosphorylation of Akt and AMPK in Cultured L6 Myotubes
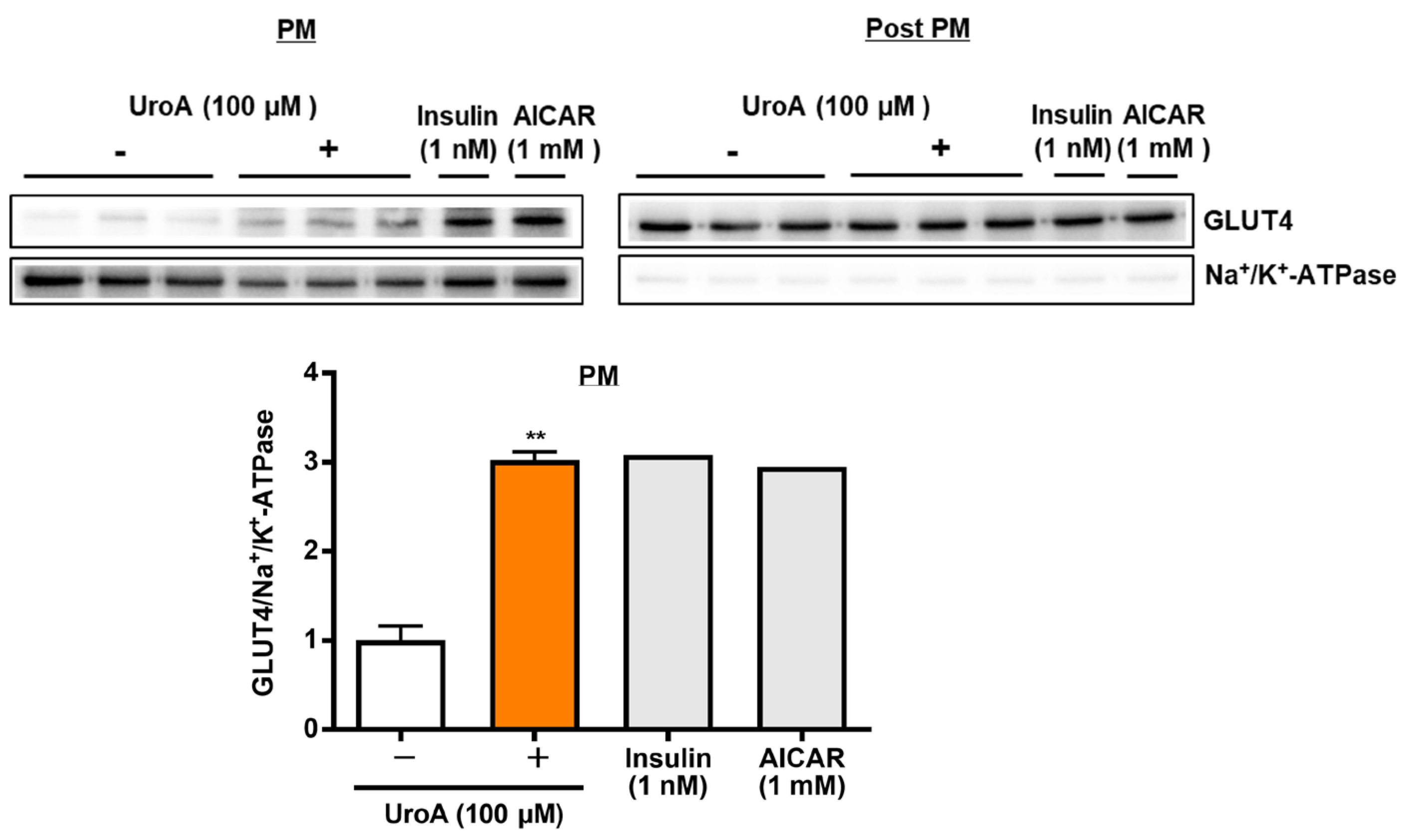
3.3. Effect of UroA on GLUT4 Translocation to the Plasma Membrane in L6 Myotubes
3.4. Effect of UroA on Intraperitoneal Glucose Tolerance Test in KK-Ay/Ta Mice
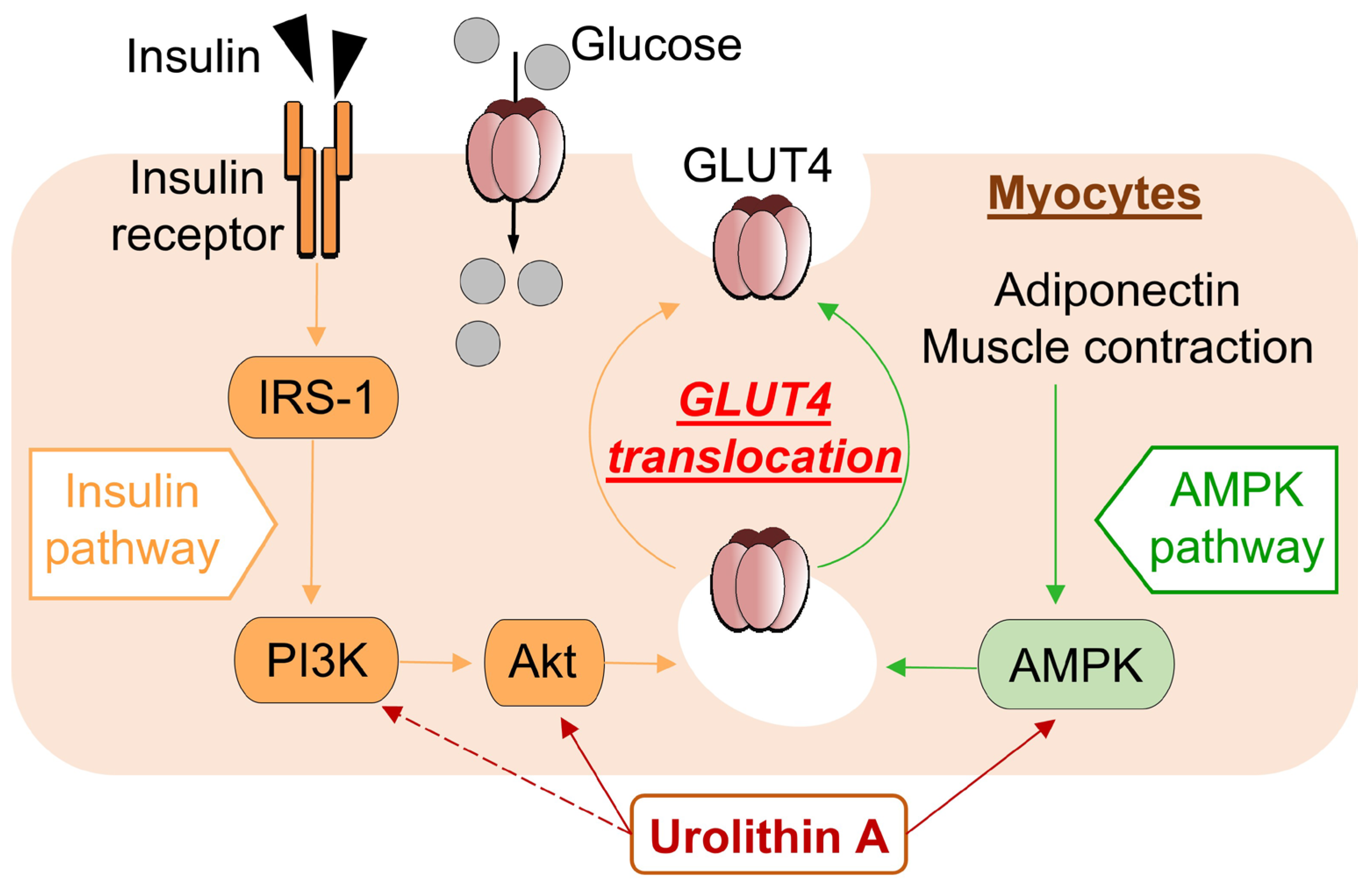
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AICAR | 5-aminoimidazole-4-carboxamide 1-β-D-ribofuranoside |
| Akt | protein kinase B |
| AMPK | 5′-adenosine monophosphate-activated protein kinase |
| BSA | bovine serum albumin |
| DTT | dithiothreitol |
| DMEM | Dulbecco’s modified Eagle’s medium |
| FBS | fetal bovine serum |
| GLUT4 | glucose transporter 4 |
| GU | glucose uptake |
| IPGTT | intraperitoneal glucose tolerance test |
| KHH buffer | Krebs–Henseleit-HEPES buffer |
| PI3K | phosphatidylinositol 3-kinase |
| TBST | Tris-buffered saline with Tween 20 |
| T2D | type 2 diabetes |
| UroA | urolithin A |
References
- Saeedi, P.; Petersohn, I.; Salpea, P.; Malanda, B.; Karuranga, S.; Unwin, N.; Colagiuri, S.; Guariguata, L.; Motala, A.A.; Ogurtsova, K.; et al. Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: Results from the International Diabetes Federation Diabetes Atlas, 9th edition. Diabetes Res. Clin. Pract. 2019, 157, 107843. [Google Scholar] [CrossRef]
- Sun, H.; Saeedi, P.; Karuranga, S.; Pinkepank, M.; Ogurtsova, K.; Duncan, B.B.; Stein, C.; Basit, A.; Chan, J.C.N.; Mbanya, J.C.; et al. IDF Diabetes Atlas: Global, regional and country-level diabetes prevalence estimates for 2021 and projections for 2045. Diabetes Res. Clin. Pract. 2022, 183, 109119. [Google Scholar] [CrossRef] [PubMed]
- Han, S.B.; Yang, H.K.; Hyon, J.Y. Influence of diabetes mellitus on anterior segment of the eye. Clin. Interv. Aging 2018, 14, 53–63. [Google Scholar] [CrossRef] [PubMed]
- Yan, L.; Vaghari-Tabari, M.; Malakoti, F.; Moein, S.; Qujeq, D.; Yousefi, B.; Asemi, Z. Quercetin: An effective polyphenol in alleviating diabetes and diabetic complications. Crit. Rev. Food Sci. Nutr. 2023, 63, 9163–9186. [Google Scholar] [CrossRef] [PubMed]
- Szałabska-Rąpała, K.; Borymska, W.; Kaczmarczyk-Sedlak, I. Effectiveness of Magnolol, a Lignan from Magnolia Bark, in Diabetes, Its Complications and Comorbidities—A Review. Int. J. Mol. Sci. 2021, 22, 10050. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.J.; Chilakala, R.; Jo, H.G.; Lee, S.-J.; Lee, D.-S.; Cheong, S.H. Anti-Obesity and Anti-Hyperglycemic Effects of Meretrix lusoria Protamex Hydrolysate in ob/ob Mice. Int. J. Mol. Sci. 2022, 23, 4015. [Google Scholar] [CrossRef]
- Shahin, D.H.H.; Sultana, R.; Farooq, J.; Taj, T.; Khaiser, U.F.; Alanazi, N.S.A.; Alshammari, M.K.; Alshammari, M.N.; Alsubaie, F.H.; Asdaq, S.M.B.; et al. Insights into the Uses of Traditional Plants for Diabetes Nephropathy: A Review. Curr. Issues Mol. Biol. 2022, 44, 2887–2902. [Google Scholar] [CrossRef]
- Kondash, M.E.; Ananthakumar, A.; Khodabukus, A.; Bursac, N.; Truskey, G.A. Glucose Uptake and Insulin Response in Tissue-engineered Human Skeletal Muscle. Tissue Eng. Regen. Med. 2020, 17, 801–813. [Google Scholar] [CrossRef]
- Rey, D.; Fernandes, T.A.; Sulis, P.M.; Gonçalves, R.; Sepúlveda, R.M.; Silva Frederico, M.J.; Aragon, M.; Ospina, L.F.; Costa, G.M.; Silva, F. Cellular target of isoquercetin from Passiflora ligularis Juss for glucose uptake in rat soleus muscle. Chem. Biol. Interact. 2020, 330, 109198. [Google Scholar] [CrossRef]
- Issac, P.K.; Karan, R.; Guru, A.; Pachaiappan, R.; Arasu, M.V.; Al-Dhabi, N.A.; Choi, K.C.; Harikrishnan, R.; Raj, J.A. Insulin signaling pathway assessment by enhancing antioxidant activity due to morin using in vitro rat skeletal muscle L6 myotubes cells. Mol. Biol. Rep. 2021, 48, 5857–5872. [Google Scholar] [CrossRef]
- Kawano, A.; Nakamura, H.; Hata, S.-I.; Minakawa, M.; Miura, Y.; Yagasaki, K. Hypoglycemic effect of aspalathin, a rooibos tea component from Aspalathus linearis, in type 2 diabetic model db/db mice. Phytomedicine 2009, 16, 437–443. [Google Scholar] [CrossRef] [PubMed]
- Yagasaki, K. Chapter 26: Phytochemicals, Their Intestinal Metabolites, and Skeletal Muscle Function. In Nutrition and Skeletal Muscle, 1st ed.; Walrand, S., Ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2019; pp. 421–438. [Google Scholar]
- Zhao, P.; Ming, Q.; Qiu, J.; Tian, D.; Liu, J.; Shen, J.; Liu, Q.-H.; Yang, X. Ethanolic Extract of Folium Sennae Mediates the Glucose Uptake of L6 Cells by GLUT4 and Ca2+. Molecules 2018, 23, 2934. [Google Scholar] [CrossRef]
- Yao, Y.; Yang, X.; Shen, J.; Zhao, P. Glucose Uptake Is Increased by Estradiol Dipropionate in L6 Skeletal Muscle Cells. Pharmaceuticals 2023, 16, 25. [Google Scholar] [CrossRef] [PubMed]
- Shijina, B.N.; Radhika, A.; Sherin, S.; Biju, P.G. Vindoline Exhibits Anti-Diabetic Potential in Insulin-Resistant 3T3-L1 Adipocytes and L6 Skeletal Myoblasts. Nutrients 2023, 15, 2865. [Google Scholar] [CrossRef] [PubMed]
- Minakawa, M.; Kawano, A.; Miura, Y.; Yagasaki, K. Hypoglycemic effect of resveratrol in type 2 diabetic model db/db mice and its actions in cultured L6 myotubes and RIN-5F pancreatic β-cells. J. Clin. Biochem. Nutr. 2011, 48, 237–244. [Google Scholar] [CrossRef] [PubMed]
- Kondo, S.; Adachi, S.-I.; Yoshizawa, F.; Yagasaki, K. Antidiabetic Effect of Taxifolin in Cultured L6 Myotubes and Type 2 Diabetic Model KK-Ay/Ta Mice with Hyperglycemia and Hyperuricemia. Curr. Issues Mol. Biol. 2021, 43, 1293–1306. [Google Scholar] [CrossRef]
- Djedjibegovic, J.; Marjanovic, A.; Panieri, E.; Saso, L. Ellagic Acid-Derived Urolithins as Modulators of Oxidative Stress. Oxidative Med. Cell. Longev. 2020, 2020, 5194508. [Google Scholar] [CrossRef]
- Tomás-Barberán, F.A.; González-Sarrías, A.; García-Villalba, R.; Núñez-Sánchez, M.A.; Selma, M.V.; García-Conesa, M.T.; Espín, J.C. Urolithins, the rescue of “old” metabolites to understand a “new” concept: Metabotypes as a nexus among phenolic metabolism, microbiota dysbiosis, and host health status. Mol. Nutr. Food Res. 2017, 61, 1500901. [Google Scholar] [CrossRef]
- Heilman, J.; Andreux, P.; Tran, N.; Rinsch, C.; Blanco-Bose, W. Safety assessment of Urolithin A, a metabolite produced by the human gut microbiota upon dietary intake of plant derived ellagitannins and ellagic acid. Food Chem. Toxicol. 2017, 108, 289–297. [Google Scholar] [CrossRef]
- Andreux, P.A.; Blanco-Bose, W.; Ryu, D.; Burdet, F.; Ibberson, M.; Aebischer, P.; Auwerx, J.; Singh, A.; Rinsch, C. The mitophagy activator urolithin A is safe and induces a molecular signature of improved mitochondrial and cellular health in humans. Nat. Metab. 2019, 1, 595–603. [Google Scholar] [CrossRef]
- Liu, S.; D’Amico, D.; Shankland, E.; Bhayana, S.; Garcia, J.M.; Aebischer, P.; Rinsch, C.; Singh, A.; Marcinek, D.J. Effect of Urolithin A Supplementation on Muscle Endurance and Mitochondrial Health in Older Adults. JAMA Netw. Open 2022, 5, e2144279. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Yuan, Q.; Ma, Y.; Zhou, X.; Wang, G.; Wang, S.; Li, S.; Shi, J.; Wang, D. Dietary Intervention with the Gut Microbial Metabolite Urolithin A Attenuates Lipopolysaccharide-Induced Neuroinflammation and Cognitive Deficits via the Sirt1/acetyl-NF-κB Signaling Pathway. Mol. Nutr. Food Res. 2023, 67, e2200401. [Google Scholar] [CrossRef] [PubMed]
- Piwowarski, J.P.; Granica, S.; Zwierzyńska, M.; Stefańska, J.; Schopohl, P.; Melzig, M.F.; Kiss, A.K. Role of human gut microbiota metabolism in the anti-inflammatory effect of traditionally used ellagitannin-rich plant materials. J. Ethnopharmacol. 2014, 155, 801–809. [Google Scholar] [CrossRef] [PubMed]
- Komatsu, W.; Kishi, H.; Yagasaki, K.; Ohhira, S. Urolithin A attenuates pro-inflammatory mediator production by suppressing PI3-K/Akt/NF-κB and JNK/AP-1 signaling pathways in lipopolysaccharide-stimulated RAW264 macrophages: Possible involvement of NADPH oxidase-derived reactive oxygen species. Eur. J. Pharmacol. 2018, 833, 411–424. [Google Scholar] [CrossRef] [PubMed]
- Adachi, S.-I.; Sasaki, K.; Kondo, S.; Komatsu, W.; Yoshizawa, F.; Isoda, H.; Yagasaki, K. Antihyperuricemic Effect of Urolithin A in Cultured Hepatocytes and Model Mice. Molecules 2020, 25, 5136. [Google Scholar] [CrossRef]
- Singh, A.; D’Amico, D.; Andreux, P.A.; Fouassier, A.M.; Blanco-Bose, W.; Evans, M.; Aebischer, P.; Auwerx, J.; Rinsch, C. Urolithin A improves muscle strength, exercise performance, and biomarkers of mitochondrial health in a randomized trial in middle-aged adults. Cell Rep. Med. 2022, 3, 100633. [Google Scholar] [CrossRef]
- Yang, J.; Guo, Y.; Henning, S.M.; Chan, B.; Long, J.; Zhong, J.; Acin-Perez, R.; Petcherski, A.; Shirihai, O.; Heber, D.; et al. Ellagic Acid and Its Microbial Metabolite Urolithin A Alleviate Diet-Induced Insulin Resistance in Mice. Mol. Nutr. Food Res. 2020, 64, 2000091. [Google Scholar] [CrossRef]
- Nishiumi, S.; Ashida, H. Rapid preparation of a plasma membrane fraction from adipocytes and muscle cells: Application to detection of translocated glucose transporter 4 on the plasma membrane. Biosci. Biotechnol. Biochem. 2007, 71, 2343–2346. [Google Scholar] [CrossRef]
- Oki, K.; Arias, E.B.; Kanzaki, M.; Cartee, G.D. Prior treatment with the AMPK activator AICAR induces subsequently enhanced glucose uptake in isolated skeletal muscles from 24-month-old rats. Appl. Physiol. Nutr. Metab. 2018, 43, 795–805. [Google Scholar] [CrossRef]
- Alkhateeb, H.; Al-Duais, M.; Qnais, E. Beneficial effects of oleuropein on glucose uptake and on parameters relevant to the normal homeostatic mechanisms of glucose regulation in rat skeletal muscle. Phytother. Res. 2018, 32, 651–656. [Google Scholar] [CrossRef]
- Yang, S.-C.; Hsu, C.-Y.; Chou, W.-L.; Fang, J.-Y.; Chuang, S.-Y. Bioactive Agent Discovery from the Natural Compounds for the Treatment of Type 2 Diabetes Rat Model. Molecules 2020, 25, 5713. [Google Scholar] [CrossRef] [PubMed]
- Saadeldeen, F.S.A.; Niu, Y.; Wang, H.; Zhou, L.; Meng, L.; Chen, S.; Sun-Waterhouse, D.; Waterhouse, G.I.N.; Liu, Z.; Kang, W. Natural products: Regulating glucose metabolism and improving insulin resistance. Food Sci. Hum. Wellness 2020, 9, 214–228. [Google Scholar] [CrossRef]
- Shahwan, M.; Alhumaydhi, F.; Ashraf, G.M.; Hasan, P.M.Z.; Shamsi, A. Role of polyphenols in combating Type 2 Diabetes and insulin resistance. Int. J. Biol. Macromol. 2022, 206, 567–579. [Google Scholar] [CrossRef] [PubMed]
- Sakuma, T.; Nakamura, M.; Chiba, T.; Iwanaga, T.; Kan, M.; Kojima, R.; Ao, J.; Ma, Y.; Unozawa, H.; Fujita, N.; et al. A diet-induced murine model for non-alcoholic fatty liver disease with obesity and insulin resistance that rapidly develops steatohepatitis and fibrosis. Lab. Investig. 2022, 102, 1150–1157. [Google Scholar] [CrossRef] [PubMed]
- Tamura, Y.O.; Sugama, J.; Abe, S.-i.; Shimizu, Y.; Hirose, H.; Watanabe, M. Selective somatostatin receptor 5 inhibition improves hepatic insulin sensitivity. Pharmacol. Res. Perspect. 2023, 11, e01043. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Liu, G.; Guo, J.; Su, Z. The PI3K/AKT pathway in obesity and type 2 diabetes. Int. J. Biol. Sci. 2018, 14, 1483–1496. [Google Scholar] [CrossRef]
- Abou-Samra, M.; Selvais, C.M.; Dubuisson, N.; Brichard, S.M. Adiponectin and Its Mimics on Skeletal Muscle: Insulin Sensitizers, Fat Burners, Exercise Mimickers, Muscling Pills … or Everything Together? Int. J. Mol. Sci. 2020, 21, 2620. [Google Scholar] [CrossRef]
- Ghanbari, M.; Lamuki, M.S.; Habibi, E.; Sadeghimahalli, F. Artemisia annua L. Extracts Improved Insulin Resistance via Changing Adiponectin, Leptin and Resistin Production in HFD/STZ Diabetic Mice. J. Pharmacopunct. 2022, 25, 130–137. [Google Scholar] [CrossRef]
- Mohammed Saeed, W.; Nasser Binjawhar, D. Association of Serum Leptin and Adiponectin Concentrations with Type 2 Diabetes Biomarkers and Complications Among Saudi Women. Diabetes Metab. Syndr. Obes. 2023, 16, 2129–2140. [Google Scholar] [CrossRef]
- Li, N.; Qiu, Y.; Wu, Y.; Zhang, M.; Lai, Z.; Wang, Q.; Du, Y.; Guo, L.; Liu, S.; Li, Z. Association of serum total fatty acids with type 2 diabetes. Clin. Chim. Acta 2020, 500, 59–68. [Google Scholar] [CrossRef]
- Xia, B.; Shi, X.C.; Xie, B.C.; Zhu, M.Q.; Chen, Y.; Chu, X.Y.; Cai, G.H.; Liu, M.; Yang, S.Z.; Mitchell, G.A.; et al. Urolithin A exerts antiobesity effects through enhancing adipose tissue thermogenesis in mice. PLoS Biol. 2020, 18, e3000688. [Google Scholar] [CrossRef] [PubMed]
- Toney, A.M.; Fan, R.; Xian, Y.; Chaidez, V.; Ramer-Tait, A.E.; Chung, S. Urolithin A, a Gut Metabolite, Improves Insulin Sensitivity Through Augmentation of Mitochondrial Function and Biogenesis. Obesity 2019, 27, 612–620. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Li, W.; Guo, Q.; Xu, L.; Santhanam, R.K.; Gao, X.; Chen, Y.; Wang, C.; Panichayupakaranant, P.; Chen, H. Anthocyanins from dietary black soybean potentiate glucose uptake in L6 rat skeletal muscle cells via up-regulating phosphorylated Akt and GLUT4. J. Funct. Foods 2019, 52, 663–669. [Google Scholar] [CrossRef]
- Bhattacharjee, N.; Dua, T.K.; Khanra, R.; Joardar, S.; Nandy, A.; Saha, A.; De Feo, V.; Dewanjee, S. Protocatechuic Acid, a Phenolic from Sansevieria roxburghiana Leaves, Suppresses Diabetic Cardiomyopathy via Stimulating Glucose Metabolism, Ameliorating Oxidative Stress, and Inhibiting Inflammation. Front. Pharmacol. 2017, 8, 251. [Google Scholar] [CrossRef] [PubMed]
- Kunz, H.E.; Hart, C.R.; Gries, K.J.; Parvizi, M.; Laurenti, M.; Dalla Man, C.; Moore, N.; Zhang, X.; Ryan, Z.; Polley, E.C.; et al. Adipose tissue macrophage populations and inflammation are associated with systemic inflammation and insulin resistance in obesity. Am. J. Physiol. Endocrinol. Metab. 2021, 321, E105–E121. [Google Scholar] [CrossRef] [PubMed]
- Shou, J.; Chen, P.-J.; Xiao, W.-H. Mechanism of increased risk of insulin resistance in aging skeletal muscle. Diabetol. Metab. Syndr. 2020, 12, 14. [Google Scholar] [CrossRef]
- Shabani, M.; Sadeghi, A.; Hosseini, H.; Teimouri, M.; Babaei Khorzoughi, R.; Pasalar, P.; Meshkani, R. Resveratrol alleviates obesity-induced skeletal muscle inflammation via decreasing M1 macrophage polarization and increasing the regulatory T cell population. Sci. Rep. 2020, 10, 3791. [Google Scholar] [CrossRef]
- Gong, L.; Guo, S.; Zou, Z. Resveratrol ameliorates metabolic disorders and insulin resistance in high-fat diet-fed mice. Life Sci. 2020, 242, 117212. [Google Scholar] [CrossRef]
- Kjøbsted, R.; Hingst, J.R.; Fentz, J.; Foretz, M.; Sanz, M.N.; Pehmøller, C.; Shum, M.; Marette, A.; Mounier, R.; Treebak, J.T.; et al. AMPK in skeletal muscle function and metabolism. FASEB J. 2018, 32, 1741–1777. [Google Scholar] [CrossRef]
- Xiao, Y.; Li, K.; Bian, J.; Zhang, Y.; Li, J.; Liu, H.; Ye, Y.; Han, L.; Gong, L.; Wang, M. Urolithin A Protects Neuronal Cells against Stress Damage and Apoptosis by Atp2a3 Inhibition. Mol. Nutr. Food Res. 2023, 67, 2300146. [Google Scholar] [CrossRef]

| Measurement | Normal | Control | UroA |
|---|---|---|---|
| Initial body weight (g) | 19.9 ± 0.3 ** | 28.8 ± 0.2 | 28.3 ± 0.7 |
| Final body weight (g) | 22.3 ± 0.4 ** | 36.9 ± 0.4 | 36.1 ± 1.0 |
| Weight gain (g/3 weeks) | 2.4 ± 0.4 ** | 8.0 ± 0.4 | 8.0 ± 0.5 |
| Food intake (g/3 weeks) | 60.8 ± 1.4 ** | 111.3 ± 2.6 | 110.8 ± 4.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kondo, S.; Adachi, S.-i.; Komatsu, W.; Yoshizawa, F.; Yagasaki, K. Antidiabetic Effect of Urolithin A in Cultured L6 Myotubes and Type 2 Diabetic Model KK-Ay/Ta Mice with Glucose Intolerance. Curr. Issues Mol. Biol. 2024, 46, 1078-1090. https://doi.org/10.3390/cimb46020068
Kondo S, Adachi S-i, Komatsu W, Yoshizawa F, Yagasaki K. Antidiabetic Effect of Urolithin A in Cultured L6 Myotubes and Type 2 Diabetic Model KK-Ay/Ta Mice with Glucose Intolerance. Current Issues in Molecular Biology. 2024; 46(2):1078-1090. https://doi.org/10.3390/cimb46020068
Chicago/Turabian StyleKondo, Shinji, Shin-ichi Adachi, Wataru Komatsu, Fumiaki Yoshizawa, and Kazumi Yagasaki. 2024. "Antidiabetic Effect of Urolithin A in Cultured L6 Myotubes and Type 2 Diabetic Model KK-Ay/Ta Mice with Glucose Intolerance" Current Issues in Molecular Biology 46, no. 2: 1078-1090. https://doi.org/10.3390/cimb46020068
APA StyleKondo, S., Adachi, S.-i., Komatsu, W., Yoshizawa, F., & Yagasaki, K. (2024). Antidiabetic Effect of Urolithin A in Cultured L6 Myotubes and Type 2 Diabetic Model KK-Ay/Ta Mice with Glucose Intolerance. Current Issues in Molecular Biology, 46(2), 1078-1090. https://doi.org/10.3390/cimb46020068






