Epidermal Growth Factor Downregulates Carbon Anhydrase III (CAIII) in Colon Cancer
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Lines and Cell Culture
2.2. MTT Assay
2.3. RNA Isolation and Quantitative Real Time-PCR (qRT-PCR) Analysis
2.4. Extraction of Proteins and Western Blotting
2.5. Statistical Analysis
3. Results
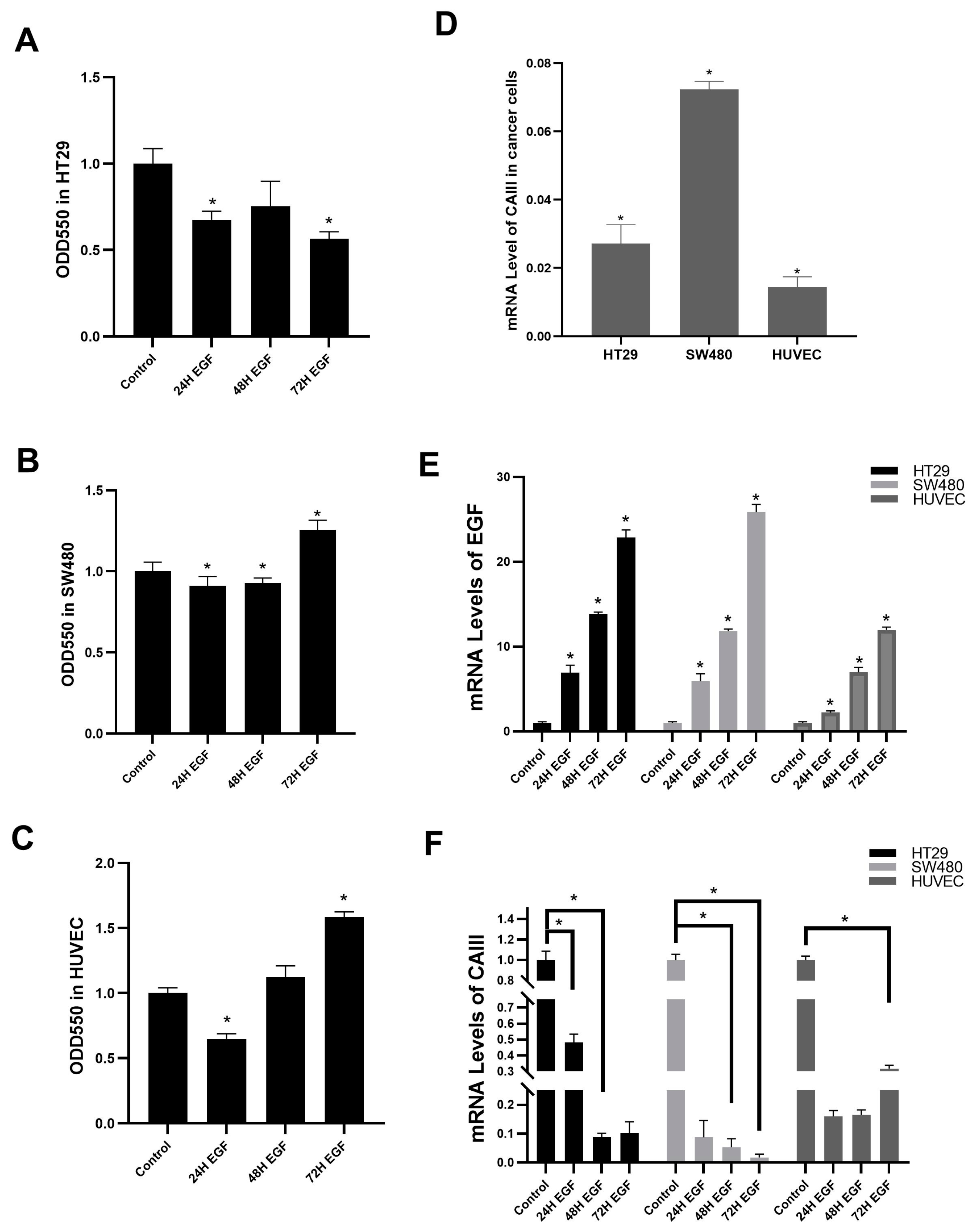
3.1. EGF Decreases CAIII mRNA Expression Level in Colon Cancer and Non-Cancer Cell Lines
3.2. EGF Decreases CAIII Protein Expression Level in Cancer and Non-Cancer Cell Lines
4. Discussion
Supplementary Materials
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Adam, R.; Vinet, E. Regional treatment of metastasis: Surgery of colorectal liver metastases. Ann. Oncol. 2004, 15, 103–106. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Wagle, N.S.; Cercek, A.; Smith, R.A.; Jemal, A. Colorectal cancer statistics, 2023. CA A Cancer J. Clin. 2023, 73, 233–254. [Google Scholar] [CrossRef] [PubMed]
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA A Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef]
- Leporrier, J.; Maurel, J.; Chiche, L.; Bara, S.; Segol, P.; Launoy, G. A population-based study of the incidence, management and prognosis of hepatic metastases from colorectal cancer. J. Br. Surg. 2006, 93, 465–474. [Google Scholar] [CrossRef]
- Bird, N.C.; Mangnall, D.; Majeed, A.W. Biology of colorectal liver metastases: A review. J. Surg. Oncol. 2006, 94, 68–80. [Google Scholar] [CrossRef] [PubMed]
- Koppe, M.J.; Boerman, O.C.; Oyen, W.J.; Bleichrodt, R.P. Peritoneal carcinomatosis of colorectal origin: Incidence and current treatment strategies. Ann. Surg. 2006, 243, 212–222. [Google Scholar] [CrossRef]
- Mishra, J.; Drummond, J.; Quazi, S.H.; Karanki, S.S.; Shaw, J.J.; Chen, B.; Kumar, N. Prospective of colon cancer treatments and scope for combinatorial approach to enhanced cancer cell apoptosis. Crit. Rev. Oncol./Hematol. 2013, 86, 232–250. [Google Scholar] [CrossRef]
- Misra, R.; Acharya, S.; Sahoo, S.K. Cancer nanotechnology: Application of nanotechnology in cancer therapy. Drug Discov. Today 2010, 15, 842–850. [Google Scholar] [CrossRef]
- Daveri, E.; Adamo, A.M.; Alfine, E.; Zhu, W.; Oteiza, P.I. Hexameric procyanidins inhibit colorectal cancer cell growth through both redox and non-redox regulation of the epidermal growth factor signaling pathway. Redox Biol. 2021, 38, 101830. [Google Scholar] [CrossRef]
- Custodio, A.; Feliu, J. Prognostic and predictive biomarkers for epidermal growth factor receptor-targeted therapy in colorectal cancer: Beyond KRAS mutations. Crit. Rev. Oncol./Hematol. 2013, 85, 45–81. [Google Scholar] [CrossRef]
- Khan, K.; Valeri, N.; Dearman, C.; Rao, S.; Watkins, D.; Starling, N.; Cunningham, D. Targeting EGFR pathway in metastatic colorectal cancer-tumour heterogeniety and convergent evolution. Crit. Rev. Oncol./Hematol. 2019, 143, 153–163. [Google Scholar] [CrossRef] [PubMed]
- Mello, R.A.; Marques, A.M.; Araújo, A. Epidermal growth factor receptor and metastatic colorectal cancer: Insights into target therapies. World J. Gastroenterol. 2013, 19, 6315. [Google Scholar] [CrossRef]
- Miyamoto, Y.; Muguruma, N.; Fujimoto, S.; Okada, Y.; Kida, Y.; Nakamura, F.; Takayama, T. Epidermal growth factor receptor-targeted molecular imaging of colorectal tumors: Detection and treatment evaluation of tumors in animal models. Cancer Sci. 2019, 110, 1921–1930. [Google Scholar] [CrossRef]
- Grossmann, A.H.; Samowitz, W.S. Epidermal growth factor receptor pathway mutations and colorectal cancer therapy. Arch. Pathol. Lab. Med. 2011, 135, 1278–1282. [Google Scholar] [CrossRef]
- Imtaiyaz, H.M.; Shajee, B.; Waheed, A.; Ahmad, F.; Sly, W.S. Structure, function and applications of carbonic anhydrase isozymes. Bioorg. Med. Chem. 2013, 21, 1570–1582. [Google Scholar] [CrossRef]
- Supuran, C.T. Carbonic anhydrases: Novel therapeutic applications for inhibitors and activators. Nat. Rev. Drug Disc. 2008, 7, 168. [Google Scholar] [CrossRef] [PubMed]
- Kivelä, A.; Parkkila, S.; Saarnio, J.; Karttunen, T.J.; Kivelä, J.; Parkkila, A.K.; Rajaniemi, H. Expression of a novel transmembrane carbonic anhydrase isozyme XII in normal human gut and colorectal tumors. Am. J. Pathol. 2000, 156, 577–584. [Google Scholar] [CrossRef]
- Kivelã, A.J.; Saarnio, J.; Karttunen, T.J.; Kivelã, J.; Parkkila, A.K.; Pastorekova, S.; Rajaniemi, H. Differential expression of cytoplasmic carbonic anhydrases, CA I and II, and membrane-associated isozymes, CA IX and XII, in normal mucosa of large intestine and in colorectal tumors. Dig. Dis. Sci. 2001, 46, 2179–2186. [Google Scholar] [CrossRef]
- Niemelä, A.M.; Hynninen, P.; Mecklin, J.P.; Kuopio, T.; Kokko, A.; Aaltonen, L.; Kivelä, A.J. Carbonic anhydrase IX is highly expressed in hereditary nonpolyposis colorectal cancer. Cancer Epidemiol. Biomark. Prev. 2007, 16, 1760–1766. [Google Scholar] [CrossRef]
- Saarnio, J.; Parkkila, S.; Parkkila, A.K.; Haukipuro, K.; Pastoreková, S.; Pastorek, J.; Karttunen, T.J. Immunohistochemical study of colorectal tumors for expression of a novel transmembrane carbonic anhydrase, MN/CA IX, with potential value as a marker of cell proliferation. Am. J. Pathol. 1998, 153, 279–285. [Google Scholar] [CrossRef]
- Viikilä, P.; Kivelä, A.J.; Mustonen, H.; Koskensalo, S.; Waheed, A.; Sly, W.S.; Haglund, C. Carbonic anhydrase enzymes II, VII, IX and XII in colorectal carcinomas. World J. Gastroenterol. 2016, 22, 8168. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Tsoi, H.; Li, X.; Wang, H.; Gao, J.; Wang, K.; Yu, J. Carbonic anhydrase IV inhibits colon cancer development by inhibiting the Wnt signalling pathway through targeting the WTAP–WT1–TBL1 axis. Gut 2016, 65, 1482–1493. [Google Scholar] [CrossRef]
- Kuo, W.H.; Chiang, W.L.; Yang, S.F.; Yeh, K.T.; Yeh, C.M.; Hsieh, Y.S.; Chu, S.C. The differential expression of cytosolic carbonic anhydrase in human hepatocellular carcinoma. Life Sci. 2003, 73, 2211–2223. [Google Scholar] [CrossRef]
- Dai, H.Y.; Hong, C.C.; Liang, S.C.; Yan, M.D.; Lai, G.M.; Cheng, A.L.; Chuang, S.E. Carbonic anhydrase III promotes transformation and invasion capability in hepatoma cells through FAK signaling pathway. Mol. Carcinog. 2008, 47, 956–963. [Google Scholar] [CrossRef] [PubMed]
- Turkoglu, S.A.; Okuyan, D.; Kockar, F. TGF-β downregulates CAIII expression via MAPK and PI3K signaling pathways in colon carcinoma and osteosarcoma cells. Arch. Biol. Sci. 2019, 71, 393–401. [Google Scholar]
- Ahmadi, M.; Hajikhani, B.; Shamosi, A.; Yaslianifard, S.; Sameni, F.; Qorbani, M.; Dadashi, M. Cytotoxic and apoptosis-inducing properties of Staphylococcus aureus cytoplasmic extract on lung cancer cells: Insights from MTT assay and bax/bcl-2 gene expression analysis. Gene Rep. 2024, 36, 101955. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Okuyan, D.; Turkoglu, S.A.; Kockar, F. Carbonic anhydrase III is a new target of HIF1α in prostate cancer model. Gene 2020, 762, 145034. [Google Scholar] [CrossRef]
- Schneider, F.; Metz, I.; Khudayberdiev, S.; Rust, M.B. Functional redundancy of cyclase-associated proteins CAP1 and CAP2 in differentiating neurons. Cells 2021, 10, 1525. [Google Scholar] [CrossRef]
- Girden, E.R. ANOVA: Repeated Measures; Sage: Thousand Oaks, CA, USA, 1992. [Google Scholar]
- Owens, L.V.; Xu, L.; Craven, R.J.; Dent, G.A.; Weiner, T.M.; Kornberg, L.; Cance, W.G. Overexpression of the focal adhesion kinase (p125FAK) in invasive human tumors. Cancer Res. 1995, 55, 2752–2755. [Google Scholar]
- Bouchard, V.; Demers, M.J.; Thibodeau, S.; Laquerre, V.; Fujita, N.; Tsuruo, T.; Vachon, P.H. Fak/Src signaling in human intestinal epithelial cell survival and anoikis: Differentiation state-specific uncoupling with the PI3-K/Akt-1 and MEK/Erk pathways. J. Cell. Physiol. 2007, 212, 717–728. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wang, H.; Tu, C.; Shiverick, K.T.; Silverman, D.N.; Frost, S.C. Role of hypoxia and EGF on expression, activity, localization and phosphorylation of carbonic anhydrase IX in MDA-MB-231 breast cancer cells. Biochim. Biophys. Acta (BBA)-Mol. Cell Res. 2011, 1813, 159–167. [Google Scholar] [CrossRef]
- Kallio, H.; Martinez, A.R.; Hilvo, M.; Hyrskyluoto, A.; Parkkila, S. Cancer-associated carbonic anhydrases IX and XII: Effect of growth factors on gene expression in human cancer cell lines. J. Cancer Mol. 2010, 5, 73–78. [Google Scholar]
- Dorai, T.; Sawczuk, I.S.; Pastorek, J.; Wiernik, P.H.; Dutcher, J.P. The role of carbonic anhydrase IX overexpression in kidney cancer. Eur. J. Cancer 2005, 41, 2935–2947. [Google Scholar] [CrossRef] [PubMed]
- Herbst, R.S. Review of epidermal growth factor receptor biology. Int. J. Radiat. Oncol. Biol. Phys. 2004, 59, S21–S26. [Google Scholar] [CrossRef]
- Suzuki, A.; Sekiya, S.; Gunshima, E.; Fujii, S.; Taniguchi, H. EGF signaling activates proliferation and blocks apoptosis of mouse and human intestinal stem/progenitor cells in long-term monolayer cell culture. Lab. Investig. 2010, 90, 1425–1436. [Google Scholar] [CrossRef]
- Abhold, E.L.; Kiang, A.; Rahimy, E.; Kuo, S.Z.; Wang-Rodriguez, J.; Lopez, J.P.; Ongkeko, W.M. EGFR kinase promotes acquisition of stem cell-like properties: A potential therapeutic target in head and neck squamous cell carcinoma stem cells. PLoS ONE 2012, 7, 32459. [Google Scholar] [CrossRef]
- Soeda, A.; Inagaki, A.; Oka, N.; Ikegame, Y.; Aoki, H.; Yoshimura, S.I.; Iwama, T. Epidermal growth factor plays a crucial role in mitogenic regulation of human brain tumor stem cells. J. Biol. Chem. 2008, 283, 10958–10966. [Google Scholar] [CrossRef]
- Alroy, I.; Yarden, Y. The ErbB signaling network in embryogenesis and oncogenesis: Signal diversification through combinatorial ligand-receptor interactions. FEBS Lett. 1997, 410, 83–86. [Google Scholar] [CrossRef]
- Cohen, R.B. Epidermal growth factor receptor as a therapeutic target in colorectal cancer. Clin. Color. Cancer 2003, 2, 246–251. [Google Scholar] [CrossRef]
- Pabla, B.; Bissonnette, M.; Konda, V.J. Colon cancer and the epidermal growth factor receptor: Current treatment paradigms, the importance of diet, and the role of chemoprevention. World J. Clin. Oncol. 2015, 6, 133. [Google Scholar] [CrossRef] [PubMed]
- Cascinu, S.; Staccioli, M.P.; Gasparini, G.; Giordani, P.; Catalano, V.; Ghiselli, R.; Catalano, G. Expression of vascular endothelial growth factor can predict event-free survival in stage II colon cancer. Clin. Cancer Res. 2000, 6, 2803–2807. [Google Scholar]
- Rapisarda, A.; Melillo, G. Role of the VEGF/VEGFR axis in cancer biology and therapy. Adv. Cancer Res. 2012, 114, 237–267. [Google Scholar]
- Gohji, K.; Nomi, M.; Niitani, Y.; Kitazawa, S.; Fujii, A.; Katsuoka, Y.; Nakajima, M. Independent prognostic value of serum hepatocyte growth factor in bladder cancer. J. Clin. Oncol. 2000, 18, 2963–2971. [Google Scholar] [CrossRef]
- Naughton, M.; Picus, J.; Zhu, X.; Catalona, W.J.; Vollmer, R.T.; Humphrey, P.A. Scatter factor-hepatocyte growth factor elevation in the serum of patients with prostate cancer. J. Urol. 2001, 165, 1325–1328. [Google Scholar] [CrossRef] [PubMed]
- Toiyama, Y.; Miki, C.; Inoue, Y.; Okugawa, Y.; Tanaka, K.; Kusunoki, M. Serum hepatocyte growth factor as a prognostic marker for stage II or III colorectal cancer patients. Int. J. Cancer 2009, 125, 1657–1662. [Google Scholar] [CrossRef]
- Abdulla, M.H.; Shaik, A.S.; Vaali-Mohammed, M.A.; Al Khayal, K.A.; Traiki, T.B.; Zubaidi, A.M.; Al-Johani, T.; Shakoor, Z.; Al-Obeed, O.A. Expression of VEGF, EGF and HGF in early-and late-stage colorectal cancer. Mol. Clin. Oncol. 2021, 15, 251. [Google Scholar] [CrossRef] [PubMed]
- Burgering, B.M.T.; Coffer, P.J. Protein kinase B (c-Akt) in phosphatidylinositol-3-OH kinase signal transduction. Nature 1995, 376, 599–602. [Google Scholar] [CrossRef]
- Liu, W.; Li, J.; Roth, R.A. Heregulin regulation of Akt/protein kinase B in breast cancer cells. Biochem. Biophys. Res. Commun. 1999, 261, 897–903. [Google Scholar] [CrossRef]
- Muthuswamy, S.K.; Gilman, M.; Brugge, J.S. Controlled dimerization of ErbB receptors provides evidence for differential signaling by homo-and heterodimers. Mol. Cell. Biol. 1999, 19, 6845–6857. [Google Scholar] [CrossRef]
- Engebraaten, O.; Bjerkvig, R.; Pedersen, P.H.; Laerum, O.D. Effects of EGF, BFGF, NGF and PDGF (bb) on cell proliferative, migratory and invasive capacities of human brain-tumour biopsies In Vitro. Int. J. Cancer 1993, 53, 209–214. [Google Scholar] [CrossRef] [PubMed]
- Shibata, T.; Kawano, T.; Nagayasu, H.; Okumura, K.; Arisue, M.; Hamada, J.I.; Hosokawa, M. Enhancing effects of epidermal growth factor on human squamous cell carcinoma motility and matrix degradation but not growth. Tumor Biol. 1996, 17, 168–175. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Okuyan, D. Epidermal Growth Factor Downregulates Carbon Anhydrase III (CAIII) in Colon Cancer. Curr. Issues Mol. Biol. 2024, 46, 12994-13002. https://doi.org/10.3390/cimb46110774
Okuyan D. Epidermal Growth Factor Downregulates Carbon Anhydrase III (CAIII) in Colon Cancer. Current Issues in Molecular Biology. 2024; 46(11):12994-13002. https://doi.org/10.3390/cimb46110774
Chicago/Turabian StyleOkuyan, Derya. 2024. "Epidermal Growth Factor Downregulates Carbon Anhydrase III (CAIII) in Colon Cancer" Current Issues in Molecular Biology 46, no. 11: 12994-13002. https://doi.org/10.3390/cimb46110774
APA StyleOkuyan, D. (2024). Epidermal Growth Factor Downregulates Carbon Anhydrase III (CAIII) in Colon Cancer. Current Issues in Molecular Biology, 46(11), 12994-13002. https://doi.org/10.3390/cimb46110774




