Curcumin Mitigates Muscle Atrophy Potentially by Attenuating Calcium Signaling and Inflammation in a Spinal Nerve Ligation Model
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Spinal Nerve Ligation Procedure
2.3. Dietary Treatments
2.4. Sample Collection
2.5. Cross-Sectional Area (CSA) Analysis
2.6. Western Blot Analysis
2.7. Statistical Analysis
3. Results
3.1. Muscle Fiber Cross-Sectional Area (CSA)
3.2. Protein Analysis
3.2.1. Acetylcholine Receptor (AChR)
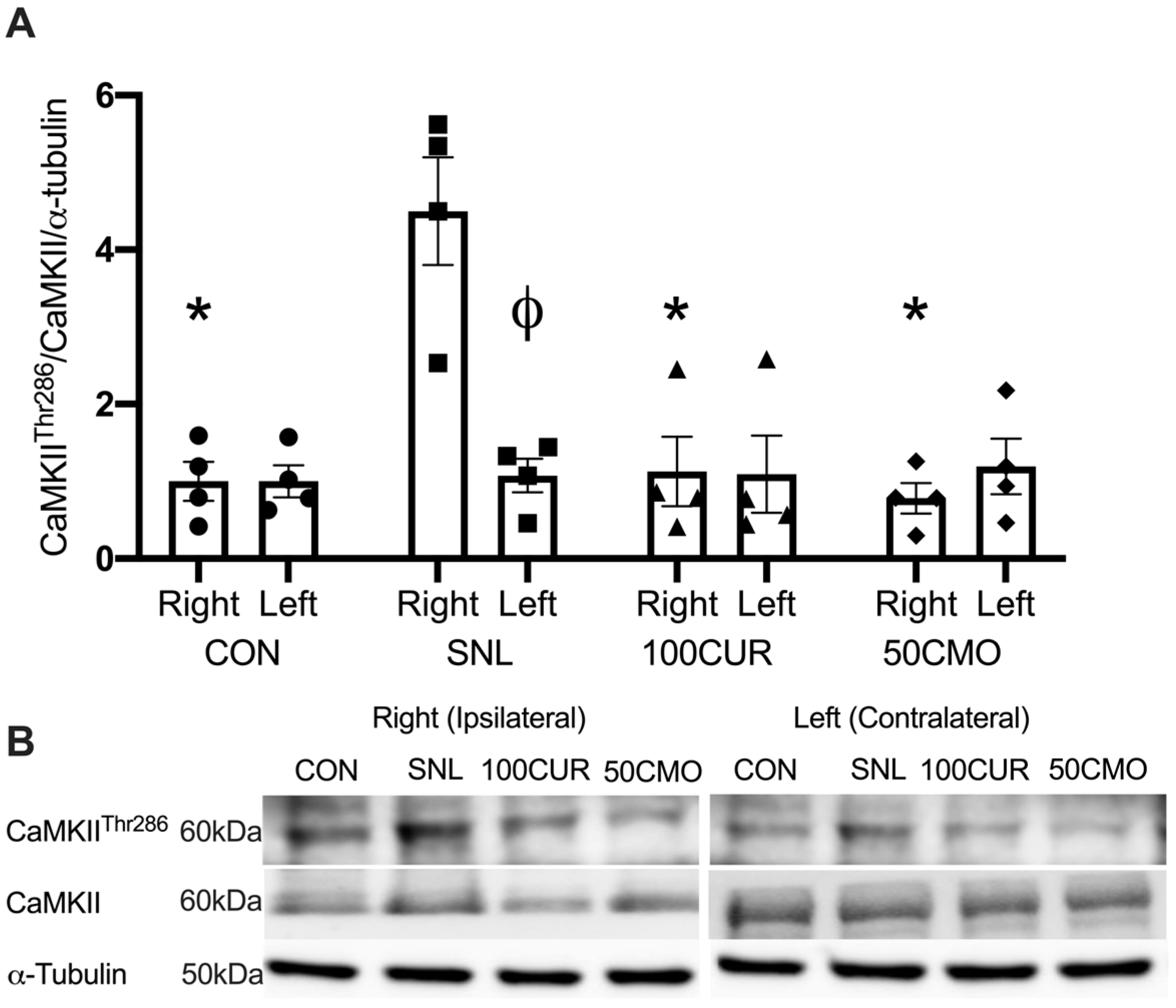
3.2.2. Calcium/Calmodulin-Dependent Protein Kinase II (CaMKII)
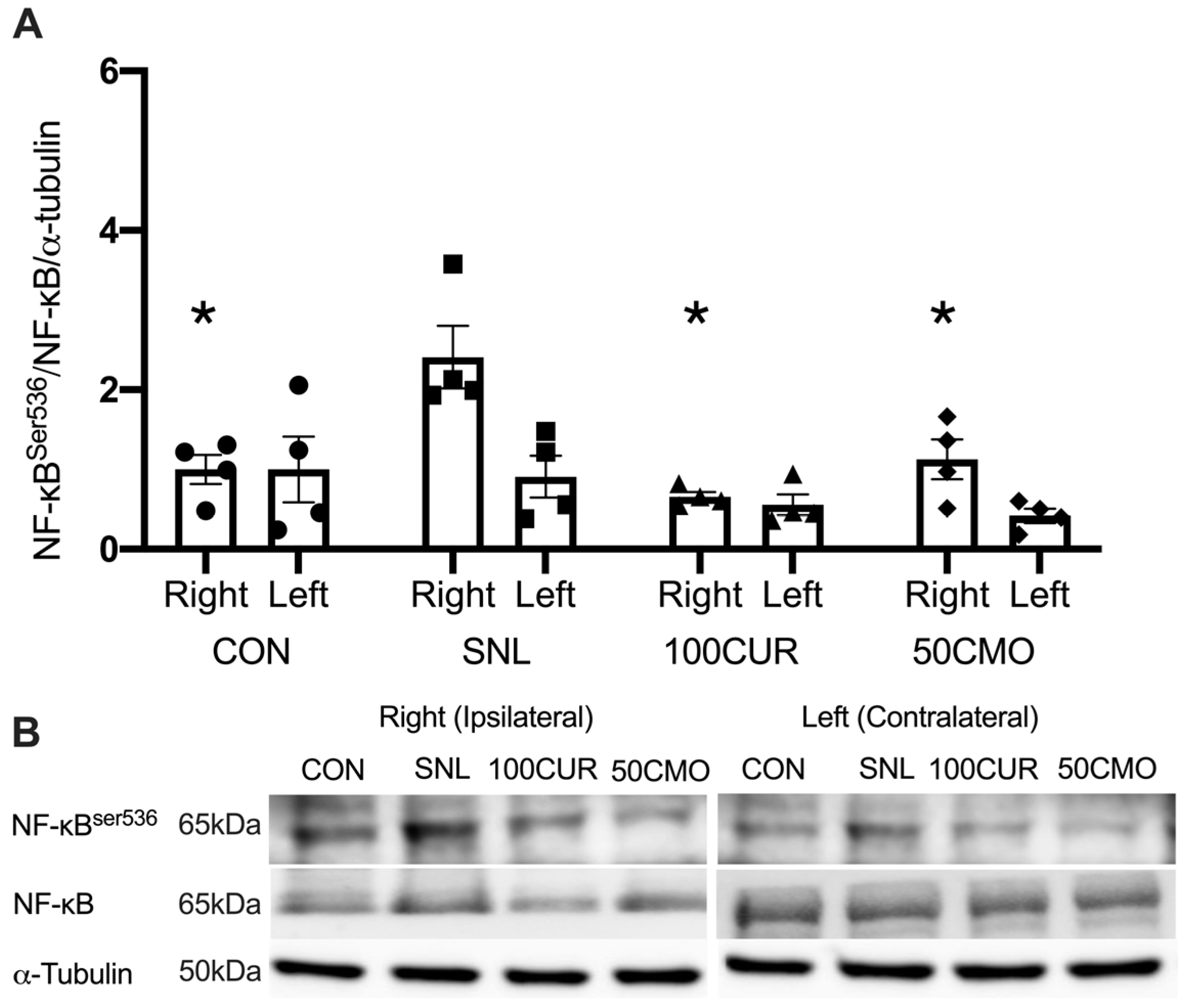
3.2.3. Nuclear Factor-κB (NF-κB)
3.2.4. Interleukin-1β (IL-1β)
4. Discussion
4.1. Muscle Fiber Cross-Sectional Area
4.2. AChR Content
4.3. CaMKII Activation, NF-κB Activation, and IL-1β
4.4. Limitations
4.5. Translational Implications
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Andersen, H.; Gadeberg, P.; Brock, B.; Jakobsen, J. Muscular Atrophy in Diabetic Neuropathy: A Stereological Magnetic Resonance Imaging Study. Diabetologia 1997, 40, 1062–1069. [Google Scholar] [CrossRef] [PubMed]
- Andersen, H.; Gjerstad, M.D.; Jakobsen, J. Atrophy of Foot Muscles: A Measure of Diabetic Neuropathy. Diabetes Care 2004, 27, 2382–2385. [Google Scholar] [CrossRef] [PubMed]
- Bus, S.A.; Yang, Q.X.; Wang, J.H.; Smith, M.B.; Wunderlich, R.; Cavanagh, P.R. Intrinsic Muscle Atrophy and Toe Deformity in the Diabetic Neuropathic Foot: A Magnetic Resonance Imaging Study. Diabetes Care 2002, 25, 1444–1450. [Google Scholar] [CrossRef] [PubMed]
- Hicks, C.W.; Wang, D.; Matsushita, K.; Windham, B.G.; Selvin, E. Peripheral Neuropathy and All-Cause and Cardiovascular Mortality in US Adults: A Prospective Cohort Study. Ann. Intern. Med. 2021, 174, 167–174. [Google Scholar] [CrossRef]
- Bennett, G.J.; Chung, J.M.; Honore, M.; Seltzer, Z. Models of Neuropathic Pain in the Rat. Curr. Protoc. Neurosci. 2003, 22, 9–14. [Google Scholar] [CrossRef]
- Borisov, A.B.; Dedkov, E.I.; Carlson, B.M. Interrelations of Myogenic Response, Progressive Atrophy of Muscle Fibers, and Cell Death in Denervated Skeletal Muscle. Anat. Rec. Off. Publ. Am. Assoc. Anat. 2001, 264, 203–218. [Google Scholar] [CrossRef]
- Grossman, E.J.; Roy, R.R.; Talmadge, R.J.; Zhong, H.; Edgerton, V.R. Effects of Inactivity on Myosin Heavy Chain Composition and Size of Rat Soleus Fibers. Muscle Nerve Off. J. Am. Assoc. Electrodiagn. Med. 1998, 21, 375–389. [Google Scholar] [CrossRef]
- Bodine, S.C.; Stitt, T.N.; Gonzalez, M.; Kline, W.O.; Stover, G.L.; Bauerlein, R.; Zlotchenko, E.; Scrimgeour, A.; Lawrence, J.C.; Glass, D.J. Akt/mTOR Pathway Is a Crucial Regulator of Skeletal Muscle Hypertrophy and Can Prevent Muscle Atrophy In Vivo. Nat. Cell Biol. 2001, 3, 1014–1019. [Google Scholar] [CrossRef]
- MacDonald, E.M.; Andres-Mateos, E.; Mejias, R.; Simmers, J.L.; Mi, R.; Park, J.-S.; Ying, S.; Hoke, A.; Lee, S.-J.; Cohn, R.D. Denervation Atrophy Is Independent from Akt and mTOR Activation and Is Not Rescued by Myostatin Inhibition. Dis. Model. Mech. 2014, 7, 471–481. [Google Scholar] [CrossRef]
- Castets, P.; Rion, N.; Théodore, M.; Falcetta, D.; Lin, S.; Reischl, M.; Wild, F.; Guérard, L.; Eickhorst, C.; Brockhoff, M. mTORC1 and PKB/Akt Control the Muscle Response to Denervation by Regulating Autophagy and HDAC4. Nat. Commun. 2019, 10, 3187. [Google Scholar] [CrossRef]
- Wang, Y.; Tang, Y.; Gao, X.; Liu, J.; Zhang, N.; Liang, Z.; Li, Y.; Pan, L. The Anti-Inflammatory and Analgesic Effects of Intraperitoneal Melatonin after Spinal Nerve Ligation Are Mediated by Inhibition of the NF-κB/NLRP3 Inflammasome Signaling Pathway. Brain Res. Bull. 2021, 169, 156–166. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Xue, P.; Liu, X.; Xu, X.; Chen, Z. HMGB1/Autophagy Pathway Mediates the Atrophic Effect of TGF-Β1 in Denervated Skeletal Muscle. Cell Commun. Signal. 2018, 16, 97. [Google Scholar] [CrossRef] [PubMed]
- Shen, C.-L.; Wang, R.; Yakhnitsa, V.; Santos, J.M.; Watson, C.; Kiritoshi, T.; Ji, G.; Hamood, A.N.; Neugebauer, V. Gingerol-Enriched Ginger Supplementation Mitigates Neuropathic Pain via Mitigating Intestinal Permeability and Neuroinflammation: Gut-Brain Connection. Front. Pharmacol. 2022, 13, 912609. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Luo, F.; Yang, C.; Kirkmire, C.M.; Wang, Z.J. Acute Inhibition of Ca2+/Calmodulin-Dependent Protein Kinase II Reverses Experimental Neuropathic Pain in Mice. J. Pharmacol. Exp. Ther. 2009, 330, 650–659. [Google Scholar] [CrossRef]
- Garry, E.M.; Moss, A.; Delaney, A.; O’Neill, F.; Blakemore, J.; Bowen, J.; Husi, H.; Mitchell, R.; Grant, S.G.; Fleetwood-Walker, S.M. Neuropathic Sensitization of Behavioral Reflexes and Spinal NMDA Receptor/CaM Kinase II Interactions Are Disrupted in PSD-95 Mutant Mice. Curr. Biol. 2003, 13, 321–328. [Google Scholar] [CrossRef]
- Dai, Y.; Wang, H.; Ogawa, A.; Yamanaka, H.; Obata, K.; Tokunaga, A.; Noguchi, K. Ca2+/Calmodulin-dependent Protein Kinase II in the Spinal Cord Contributes to Neuropathic Pain in a Rat Model of Mononeuropathy. Eur. J. Neurosci. 2005, 21, 2467–2474. [Google Scholar] [CrossRef]
- Komatsu, M.; Nakada, T.; Kawagishi, H.; Kato, H.; Yamada, M. Increase in Phospholamban Content in Mouse Skeletal Muscle after Denervation. J. Muscle Res. Cell Motil. 2018, 39, 163–173. [Google Scholar] [CrossRef]
- Yuasa, K.; Okubo, K.; Yoda, M.; Otsu, K.; Ishii, Y.; Nakamura, M.; Itoh, Y.; Horiuchi, K. Targeted Ablation of P38α MAPK Suppresses Denervation-Induced Muscle Atrophy. Sci. Rep. 2018, 8, 9037. [Google Scholar] [CrossRef]
- Staunton, C.A.; Pollock, N.; Lightfoot, A.; Vasilaki, A.; Barrett-Jolley, R.; McArdle, A.; Jackson, M. The Role of Denervation in Cytokine-mediated Muscle Dysfunction in Muscles of Old Mice. FASEB J. 2016, 30, 1009.4. [Google Scholar] [CrossRef]
- Shao, W.; Yu, Z.; Chiang, Y.; Yang, Y.; Chai, T.; Foltz, W.; Lu, H.; Fantus, I.G.; Jin, T. Curcumin Prevents High Fat Diet Induced Insulin Resistance and Obesity via Attenuating Lipogenesis in Liver and Inflammatory Pathway in Adipocytes. PLoS ONE 2012, 7, e28784. [Google Scholar] [CrossRef]
- Alappat, L.; Awad, A.B. Curcumin and Obesity: Evidence and Mechanisms. Nutr. Rev. 2010, 68, 729–738. [Google Scholar] [CrossRef] [PubMed]
- Weisberg, S.P.; Leibel, R.; Tortoriello, D.V. Dietary Curcumin Significantly Improves Obesity-Associated Inflammation and Diabetes in Mouse Models of Diabesity. Endocrinology 2008, 149, 3549–3558. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, B.B. Targeting Inflammation-Induced Obesity and Metabolic Diseases by Curcumin and Other Nutraceuticals. Annu. Rev. Nutr. 2010, 30, 173–199. [Google Scholar] [CrossRef] [PubMed]
- Caillaud, M.; Chantemargue, B.; Richard, L.; Vignaud, L.; Favreau, F.; Faye, P.-A.; Vignoles, P.; Sturtz, F.; Trouillas, P.; Vallat, J.-M. Local Low Dose Curcumin Treatment Improves Functional Recovery and Remyelination in a Rat Model of Sciatic Nerve Crush through Inhibition of Oxidative Stress. Neuropharmacology 2018, 139, 98–116. [Google Scholar] [CrossRef]
- Ma, J.; Liu, J.; Yu, H.; Wang, Q.; Chen, Y.; Xiang, L. Curcumin Promotes Nerve Regeneration and Functional Recovery in Rat Model of Nerve Crush Injury. Neurosci. Lett. 2013, 547, 26–31. [Google Scholar] [CrossRef]
- Mayadevi, M.; Sherin, D.; Keerthi, V.; Rajasekharan, K.; Omkumar, R. Curcumin Is an Inhibitor of Calcium/Calmodulin Dependent Protein Kinase II. Bioorg. Med. Chem. 2012, 20, 6040–6047. [Google Scholar] [CrossRef]
- Cangiano, A. Denervation Supersensitivity as a Model for the Neural Control of Muscle. Neuroscience 1985, 14, 963–971. [Google Scholar] [CrossRef]
- Séguéla, P.; Wadiche, J.; Dineley-Miller, K.; Dani, J.A.; Patrick, J.W. Molecular Cloning, Functional Properties, and Distribution of Rat Brain Alpha 7: A Nicotinic Cation Channel Highly Permeable to Calcium. J. Neurosci. 1993, 13, 596–604. [Google Scholar] [CrossRef]
- Zhang, Z.; Vijayaraghavan, S.; Berg, D.K. Neuronal Acetylcholine Receptors That Bind α-Bungarotoxin with High Affinity Function as Ligand-Gated Ion Channels. Neuron 1994, 12, 167–177. [Google Scholar] [CrossRef]
- Castro, N.G.; Albuquerque, E.X. Alpha-Bungarotoxin-Sensitive Hippocampal Nicotinic Receptor Channel Has a High Calcium Permeability. Biophys. J. 1995, 68, 516–524. [Google Scholar] [CrossRef]
- Salpeter, M.M.; Marchaterre, M.; Harris, R. Distribution of Extrajunctional Acetylcholine Receptors on a Vertebrate Muscle: Evaluated by Using a Scanning Electron Microscope Autoradiographic Procedure. J. Cell Biol. 1988, 106, 2087–2093. [Google Scholar] [CrossRef] [PubMed]
- Chin, E.R. Role of Ca2+/Calmodulin-Dependent Kinases in Skeletal Muscle Plasticity. J. Appl. Physiol. 2005, 99, 414–423. [Google Scholar] [CrossRef] [PubMed]
- Lilienbaum, A.; Israël, A. From Calcium to NF-κB Signaling Pathways in Neurons. Mol. Cell. Biol. 2003, 23, 2680–2698. [Google Scholar] [CrossRef] [PubMed]
- Powers, S.K.; Morton, A.B.; Ahn, B.; Smuder, A.J. Redox Control of Skeletal Muscle Atrophy. Free Radic. Biol. Med. 2016, 98, 208–217. [Google Scholar] [CrossRef]
- Bong, P.H. Spectral and Photophysical Behaviors of Curcumin and Curcuminoids. Bull. Korean Chem. Soc. 2000, 21, 81–86. [Google Scholar]
- Jayaprakasha, G.K.; Jena, B.S.; Negi, P.S.; Sakariah, K.K. Evaluation of Antioxidant Activities and Antimutagenicity of Turmeric Oil: A Byproduct from Curcumin Production. Z. Naturforschung C 2002, 57, 828–835. [Google Scholar] [CrossRef]
- Anand, P.; Kunnumakkara, A.B.; Newman, R.A.; Aggarwal, B.B. Bioavailability of Curcumin: Problems and Promises. Mol. Pharm. 2007, 4, 807–818. [Google Scholar] [CrossRef]
- Priyadarsini, K.I. The Chemistry of Curcumin: From Extraction to Therapeutic Agent. Molecules 2014, 19, 20091–20112. [Google Scholar] [CrossRef]
- Xiao, L.; Ding, M.; Fernandez, A.; Zhao, P.; Jin, L.; Li, X. Curcumin Alleviates Lumbar Radiculopathy by Reducing Neuroinflammation, Oxidative Stress and Nociceptive Factors. Eur. Cells Mater. 2017, 33, 279. [Google Scholar] [CrossRef]
- Daugherty, D.J.; Marquez, A.; Calcutt, N.A.; Schubert, D. A Novel Curcumin Derivative for the Treatment of Diabetic Neuropathy. Neuropharmacology 2018, 129, 26–35. [Google Scholar] [CrossRef]
- Maher, P.; Akaishi, T.; Schubert, D.; Abe, K. A Pyrazole Derivative of Curcumin Enhances Memory. Neurobiol. Aging 2010, 31, 706–709. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Lin, D.; Li, S.; Li, G.; Shyamala, S.G.; Barish, P.A.; Vernon, M.M.; Pan, J.; Ogle, W.O. Curcumin Reverses Impaired Cognition and Neuronal Plasticity Induced by Chronic Stress. Neuropharmacology 2009, 57, 463–471. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Wang, P.; Ying, J.; Chen, Z.; Yu, S. Curcumin Attenuates Retinal Vascular Leakage by Inhibiting Calcium/Calmodulin-Dependent Protein Kinase II Activity in Streptozotocin-Induced Diabetes. Cell. Physiol. Biochem. 2016, 39, 1196–1208. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Shang, Y.; Li, M.; Han, X.; Wang, J.; Wang, J. Curcumin Ameliorates Asthmatic Airway Inflammation by Activating Nuclear factor-E2-related Factor 2/Haem Oxygenase (HO)-1 Signalling Pathway. Clin. Exp. Pharmacol. Physiol. 2015, 42, 520–529. [Google Scholar] [CrossRef]
- Oh, S.-W.; Cha, J.-Y.; Jung, J.-E.; Chang, B.-C.; Kwon, H.-J.; Lee, B.-R.; Kim, D.-Y. Curcumin Attenuates Allergic Airway Inflammation and Hyper-Responsiveness in Mice through NF-κB Inhibition. J. Ethnopharmacol. 2011, 136, 414–421. [Google Scholar] [CrossRef]
- Fu, Y.; Gao, R.; Cao, Y.; Guo, M.; Wei, Z.; Zhou, E.; Li, Y.; Yao, M.; Yang, Z.; Zhang, N. Curcumin Attenuates Inflammatory Responses by Suppressing TLR4-Mediated NF-κB Signaling Pathway in Lipopolysaccharide-Induced Mastitis in Mice. Int. Immunopharmacol. 2014, 20, 54–58. [Google Scholar] [CrossRef]
- Jin, F.; Chen, X.; Yan, H.; Xu, Z.; Yang, B.; Luo, P.; He, Q. Bisdemethoxycurcumin Attenuates Cisplatin-Induced Renal Injury through Anti-Apoptosis, Anti-Oxidant and Anti-Inflammatory. Eur. J. Pharmacol. 2020, 874, 173026. [Google Scholar] [CrossRef]
- Cavaleri, F. Curcuminoid Analogs Differentially Modulate Nuclear Factor Kappa-Light-Chain-Enhancer, P65 Serine276, Mitogen-and Stress-Activated Protein Kinase 1 and MicroRNA 148a Status. Prog. Prev. Med. 2019, 4, e0024. [Google Scholar] [CrossRef]
- Sandur, S.K.; Pandey, M.K.; Sung, B.; Ahn, K.S.; Murakami, A.; Sethi, G.; Limtrakul, P.; Badmaev, V.; Aggarwal, B.B. Curcumin, Demethoxycurcumin, Bisdemethoxycurcumin, Tetrahydrocurcumin and Turmerones Differentially Regulate Anti-Inflammatory and Anti-Proliferative Responses through a ROS-Independent Mechanism. Carcinogenesis 2007, 28, 1765–1773. [Google Scholar] [CrossRef]
- Ruby, A.J.; Kuttan, G.; Babu, K.D.; Rajasekharan, K.; Kuttan, R. Anti-Tumour and Antioxidant Activity of Natural Curcuminoids. Cancer Lett. 1995, 94, 79–83. [Google Scholar] [CrossRef]
- Hoehle, S.I.; Pfeiffer, E.; Sólyom, A.M.; Metzler, M. Metabolism of Curcuminoids in Tissue Slices and Subcellular Fractions from Rat Liver. J. Agric. Food Chem. 2006, 54, 756–764. [Google Scholar] [CrossRef] [PubMed]
- Sudeep, V.H.; Gouthamchandra, K.; Chandrappa, S.; Naveen, P.; Reethi, B.; Venkatakrishna, K.; Shyamprasad, K. In Vitro Gastrointestinal Digestion of a Bisdemethoxycurcumin-Rich Curcuma longa Extract and Its Oral Bioavailability in Rats. Bull. Natl. Res. Cent. 2021, 45, 84. [Google Scholar] [CrossRef]
- Ji, G.; Yakhnitsa, V.; Kiritoshi, T.; Presto, P.; Neugebauer, V. Fear Extinction Learning Ability Predicts Neuropathic Pain Behaviors and Amygdala Activity in Male Rats. Mol. Pain 2018, 14, 1744806918804441. [Google Scholar] [CrossRef] [PubMed]
- Ji, G.; Zhang, W.; Mahimainathan, L.; Narasimhan, M.; Kiritoshi, T.; Fan, X.; Wang, J.; Green, T.A.; Neugebauer, V. 5-HT2C Receptor Knockdown in the Amygdala Inhibits Neuropathic-Pain-Related Plasticity and Behaviors. J. Neurosci. 2017, 37, 1378–1393. [Google Scholar] [CrossRef] [PubMed]
- Zimmermann, M. Ethical Guidelines for Investigations of Experimental Pain in Conscious Animals. Pain 1983, 16, 109–110. [Google Scholar] [CrossRef]
- GRAS-Affirmed—USFDA—Curcumin C3 Complex®. Available online: https://curcuminoids.com/gras-affirmed-usfda/ (accessed on 31 October 2024).
- Santos, J.M.; Wang, R.; Bhakta, V.; Driver, Z.; Vadim, Y.; Kiritoshi, T.; Ji, G.; Neugebauer, V.; Shen, C.-L. Turmeric Bioactive Compounds Alleviate Spinal Nerve Ligation-Induced Neuropathic Pain by Suppressing Glial Activation and Improving Mitochondrial Function in Spinal Cord and Amygdala. Nutrients 2023, 15, 4403. [Google Scholar] [CrossRef]
- Armstrong, R.B.; Phelps, R.O. Muscle Fiber Type Composition of the Rat Hindlimb. Am. J. Anat. 1984, 171, 259–272. [Google Scholar] [CrossRef]
- Borrell, J.A.; Frost, S.B.; Peterson, J.; Nudo, R.J. A 3D Map of the Hindlimb Motor Representation in the Lumbar Spinal Cord in Sprague Dawley Rats. J. Neural Eng. 2016, 14, 016007. [Google Scholar] [CrossRef]
- Jiwan, N.C.; Appell, C.R.; Wang, R.; Shen, C.-L.; Luk, H.-Y. Geranylgeraniol Supplementation Mitigates Soleus Muscle Atrophy via Changes in Mitochondrial Quality in Diabetic Rats. In Vivo 2022, 36, 2638–2649. [Google Scholar] [CrossRef]
- Engel, W.K.; Brocke, M.H.; Nelson, P.G. Histochemical Studies of Denervated or Tenotomized Cat Muscle: Illustrating Difficulties in Relating Experimental Animal Conditions to Human Neuromuscular Diseases. Ann. N. Y. Acad. Sci. 1966, 138, 160–185. [Google Scholar] [CrossRef]
- Karpati, G.; Engel, W.K. Correlative Histochemical Study of Skeletal Muscle after Suprasegmental Denervation, Peripheral Nerve Section, and Skeletal Fixation. Neurology 1968, 18, 681. [Google Scholar] [CrossRef] [PubMed]
- Karpati, G.; Engel, W.K. Histochemical Investigation of Fiber Type Ratios with the Myofibrillar ATP-ASE Reaction in Normal and Denervated Skeletal Muscles of Guinea Pig. Am. J. Anat. 1968, 122, 145–155. [Google Scholar] [CrossRef] [PubMed]
- Guth, L.; Dempsey, P.J.; Cooper, T. Maintenance of Neurotrophically Regulated Proteins in Denervated Skeletal and Cardiac Muscle. Exp. Neurol. 1971, 32, 478–488. [Google Scholar] [CrossRef]
- Beehler, B.C.; Sleph, P.G.; Benmassaoud, L.; Grover, G.J. Reduction of Skeletal Muscle Atrophy by a Proteasome Inhibitor in a Rat Model of Denervation. Exp. Biol. Med. 2006, 231, 335–341. [Google Scholar] [CrossRef]
- Hutchinson, K.J.; Linderman, J.K.; Basso, D.M. Skeletal Muscle Adaptations Following Spinal Cord Contusion Injury in Rat and the Relationship to Locomotor Function: A Time Course Study. J. Neurotrauma 2001, 18, 1075–1089. [Google Scholar] [CrossRef]
- Leterme, D.; Tyč, F. Re-innervation and Recovery of Rat Soleus Muscle and Motor Unit Function after Nerve Crush. Exp. Physiol. 2004, 89, 353–361. [Google Scholar] [CrossRef]
- Choe, M.-A.; Kim, K.H.; An, G.J.; Lee, K.-S.; Heitkemper, M. Hindlimb Muscle Atrophy Occurs from Peripheral Nerve Damage in a Rat Neuropathic Pain Model. Biol. Res. Nurs. 2011, 13, 44–54. [Google Scholar] [CrossRef]
- Moes, J.R.; Holden, J.E. Characterizing Activity and Muscle Atrophy Changes in Rats with Neuropathic Pain: A Pilot Study. Biol. Res. Nurs. 2014, 16, 16–22. [Google Scholar] [CrossRef]
- KANAYA, F.; TAJIMA, T. Effect of Electrostimulation on Denervated Muscle. Clin. Orthop. Relat. Res. 1992, 283, 296–301. [Google Scholar] [CrossRef]
- Lindboe, C.; Presthus, J. Effects of Denervation, Immobilization and Cachexia on Fibre Size in the Anterior Tibial Muscle of the Rat. Acta Neuropathol. 1985, 66, 42–51. [Google Scholar] [CrossRef]
- Chin, E.R. The Role of Calcium and Calcium/Calmodulin-Dependent Kinases in Skeletal Muscle Plasticity and Mitochondrial Biogenesis. Proc. Nutr. Soc. 2004, 63, 279–286. [Google Scholar] [CrossRef] [PubMed]
- Mortreux, M.; Rosa-Caldwell, M.E.; Stiehl, I.D.; Sung, D.; Thomas, N.T.; Fry, C.S.; Rutkove, S.B. Hindlimb Suspension in Wistar Rats: Sex-based Differences in Muscle Response. Physiol. Rep. 2021, 9, e15042. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, A.N.; Memme, J.M.; Wong, J.; Hood, D.A. Dimorphic Effect of TFE3 in Determining Mitochondrial and Lysosomal Content in Muscle Following Denervation. Skelet. Muscle 2024, 14, 7. [Google Scholar] [CrossRef] [PubMed]
- Witzemann, V.; Brenner, H.R.; Sakmann, B. Neural Factors Regulate AChR Subunit mRNAs at Rat Neuromuscular Synapses. J. Cell Biol. 1991, 114, 125–141. [Google Scholar] [CrossRef]
- Witzemann, V.; Barg, B.; Nishikawa, Y.; Sakmann, B.; Numa, S. Differential Regulation of Muscle Acetylcholine Receptor Γ- and Ε-subunit mRNAs. FEBS Lett. 1987, 223, 104–112. [Google Scholar] [CrossRef]
- Goldman, D.; Brenner, H.R.; Heinemann, S. Acetylcholine Receptor α-, β-, γ-, and δ-Subunit mRNA Levels Are Regulated by Muscle Activity. Neuron 1988, 1, 329–333. [Google Scholar] [CrossRef]
- Gundersen, K.; Sanes, J.R.; Merlie, J.P. Neural Regulation of Muscle Acetylcholine Receptor Epsilon-and Alpha-Subunit Gene Promoters in Transgenic Mice. J. Cell Biol. 1993, 123, 1535–1544. [Google Scholar] [CrossRef]
- Witzemann, V.; Barg, B.; Criado, M.; Stein, E.; Sakmann, B. Developmental Regulation of Five Subunit Specific mRNAs Encoding Acetylcholine Receptor Subtypes in Rat Muscle. FEBS Lett. 1989, 242, 419–424. [Google Scholar] [CrossRef]
- Baldwin Jr, A.S. The NF-κB and IκB Proteins: New Discoveries and Insights. Annu. Rev. Immunol. 1996, 14, 649–681. [Google Scholar] [CrossRef]
- Baeuerle, P.A.; Henkel, T. Function and Activation of NF-kappaB in the Immune System. Annu. Rev. Immunol. 1994, 12, 141–179. [Google Scholar] [CrossRef]
- Ghosh, S.; May, M.J.; Kopp, E.B. NF-κB and Rel Proteins: Evolutionarily Conserved Mediators of Immune Responses. Annu. Rev. Immunol. 1998, 16, 225–260. [Google Scholar] [CrossRef] [PubMed]
- El-Habta, R.; Andersson, G.; Kingham, P.J.; Backman, L.J. Anti-Apoptotic Effect of Adipose Tissue-Derived Stromal Vascular Fraction in Denervated Rat Muscle. Stem Cell Res. Ther. 2021, 12, 162. [Google Scholar] [CrossRef] [PubMed]
- Kruppa, G.; Thoma, B.; Machleidt, T.; Wiegmann, K.; Krönke, M. Inhibition of Tumor Necrosis Factor (TNF)-Mediated NF-Kappa B Activation by Selective Blockade of the Human 55-kDa TNF Receptor. J. Immunol. 1992, 148, 3152–3157. [Google Scholar] [CrossRef] [PubMed]
- Bonaldo, P.; Sandri, M. Cellular and Molecular Mechanisms of Muscle Atrophy. Dis. Model. Mech. 2013, 6, 25–39. [Google Scholar] [CrossRef]
- Zhu, Y.-G.; Chen, X.-C.; Chen, Z.-Z.; Zeng, Y.-Q.; Shi, G.-B.; Su, Y.-H.; Peng, X. Curcumin Protects Mitochondria from Oxidative Damage and Attenuates Apoptosis in Cortical Neurons. Acta Pharmacol. Sin. 2004, 25, 1606–1612. [Google Scholar]
- Li, X.; Zhu, J.; Lin, Q.; Yu, M.; Lu, J.; Feng, J.; Hu, C. Effects of Curcumin on Mitochondrial Function, Endoplasmic Reticulum Stress, and Mitochondria-Associated Endoplasmic Reticulum Membranes in the Jejunum of Oxidative Stress Piglets. J. Agric. Food Chem. 2022, 70, 8974–8985. [Google Scholar] [CrossRef]
- Wang, W.; Xu, J. Curcumin Attenuates Cerebral Ischemia-Reperfusion Injury through Regulating Mitophagy and Preserving Mitochondrial Function. Curr. Neurovasc. Res. 2020, 17, 113–122. [Google Scholar] [CrossRef]
- de Oliveira, M.R.; Jardim, F.R.; Setzer, W.N.; Nabavi, S.M.; Nabavi, S.F. Curcumin, Mitochondrial Biogenesis, and Mitophagy: Exploring Recent Data and Indicating Future Needs. Biotechnol. Adv. 2016, 34, 813–826. [Google Scholar] [CrossRef]
- Chainani-Wu, N. Safety and Anti-Inflammatory Activity of Curcumin: A Component of Tumeric (Curcuma longa). J. Altern. Complement. Med. 2003, 9, 161–168. [Google Scholar] [CrossRef]
- Huang, Q.; Riviere, J.E. The Application of Allometric Scaling Principles to Predict Pharmacokinetic Parameters across Species. Expert Opin. Drug Metab. Toxicol. 2014, 10, 1241–1253. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Appell, C.; Jiwan, N.C.; Shen, C.-L.; Luk, H.-Y. Curcumin Mitigates Muscle Atrophy Potentially by Attenuating Calcium Signaling and Inflammation in a Spinal Nerve Ligation Model. Curr. Issues Mol. Biol. 2024, 46, 12497-12511. https://doi.org/10.3390/cimb46110742
Appell C, Jiwan NC, Shen C-L, Luk H-Y. Curcumin Mitigates Muscle Atrophy Potentially by Attenuating Calcium Signaling and Inflammation in a Spinal Nerve Ligation Model. Current Issues in Molecular Biology. 2024; 46(11):12497-12511. https://doi.org/10.3390/cimb46110742
Chicago/Turabian StyleAppell, Casey, Nigel C. Jiwan, Chwan-Li Shen, and Hui-Ying Luk. 2024. "Curcumin Mitigates Muscle Atrophy Potentially by Attenuating Calcium Signaling and Inflammation in a Spinal Nerve Ligation Model" Current Issues in Molecular Biology 46, no. 11: 12497-12511. https://doi.org/10.3390/cimb46110742
APA StyleAppell, C., Jiwan, N. C., Shen, C.-L., & Luk, H.-Y. (2024). Curcumin Mitigates Muscle Atrophy Potentially by Attenuating Calcium Signaling and Inflammation in a Spinal Nerve Ligation Model. Current Issues in Molecular Biology, 46(11), 12497-12511. https://doi.org/10.3390/cimb46110742









