Cloning and Identification of Common Carp (Cyprinus carpio) PI3KC3 and Its Expression in Response to CyHV-3 Infection
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Animals
2.2. Virus Challenge Experiment
2.3. RNA Extraction and cDNA Synthesis
2.4. Cloning and Sequencing of the CcPI3KC3 Gene
2.5. Bioinformatics Analysis
2.6. Real-Time Polymerase Chain Reaction (RT—PCR)
2.7. Data Analysis
3. Results
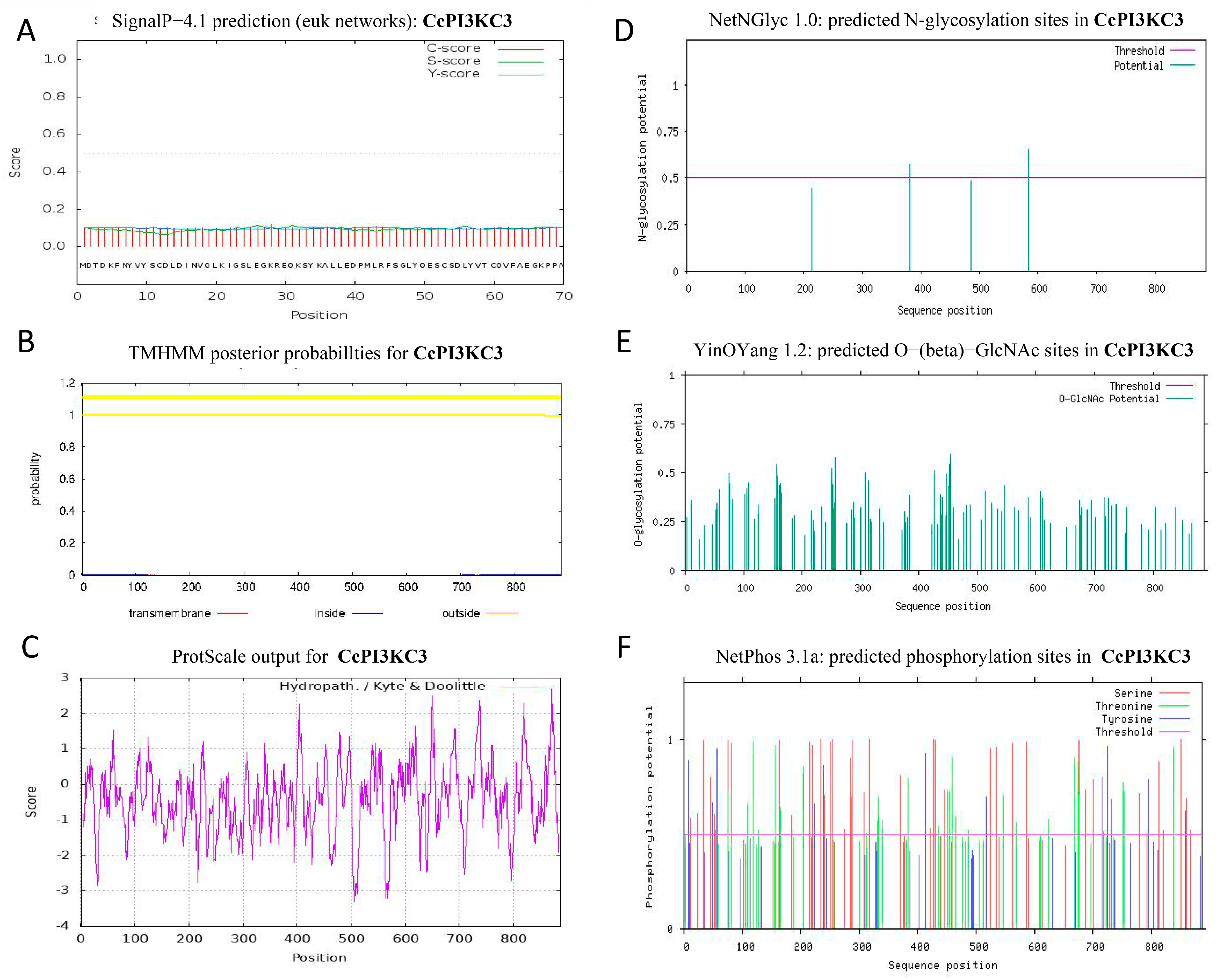
3.1. Sequence and Characteristic Analyses
3.2. Phylogenetic Analysis
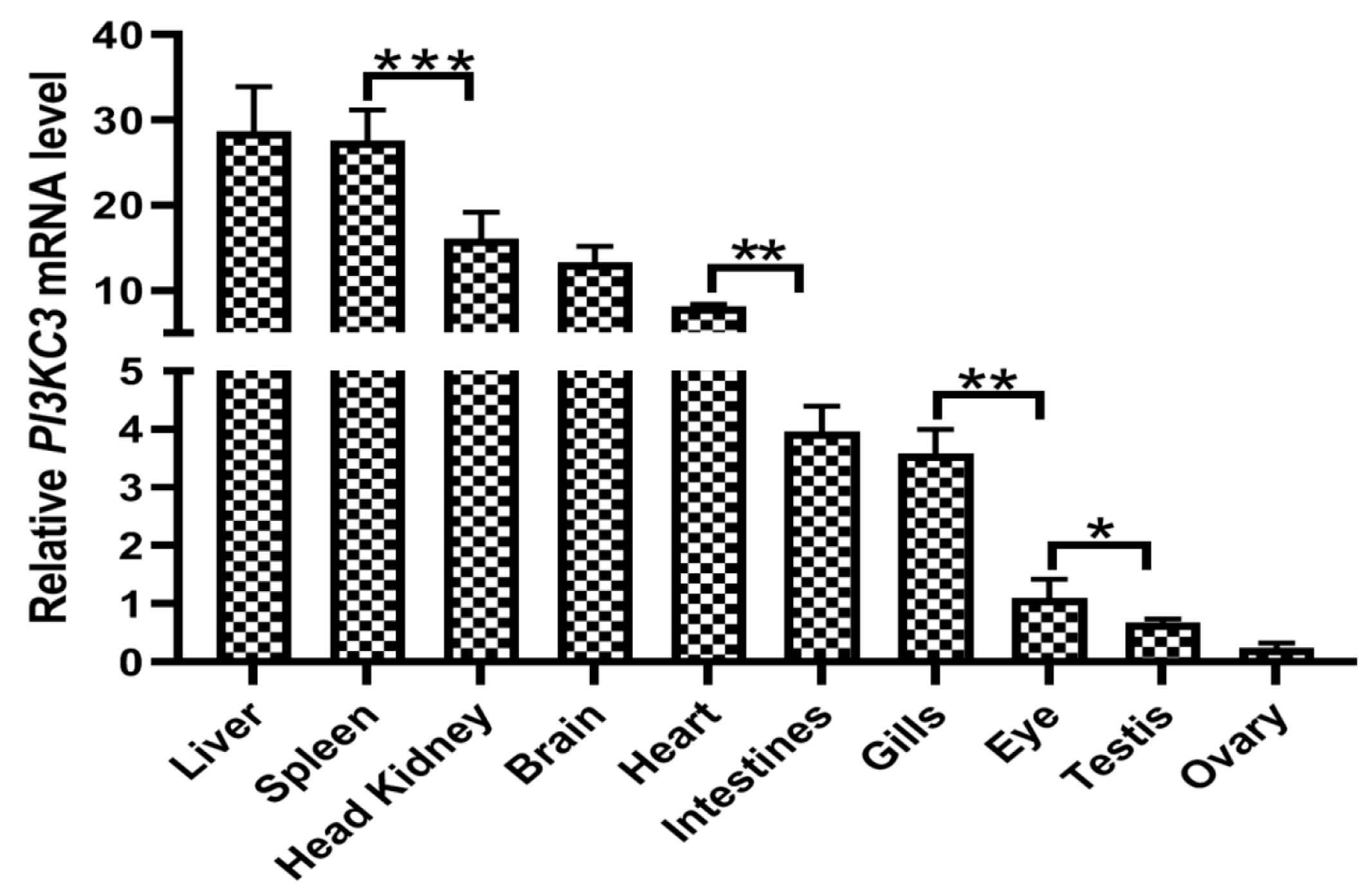
3.3. Expression of the CcPI3KC3 mRNA in Common Carp Tissues
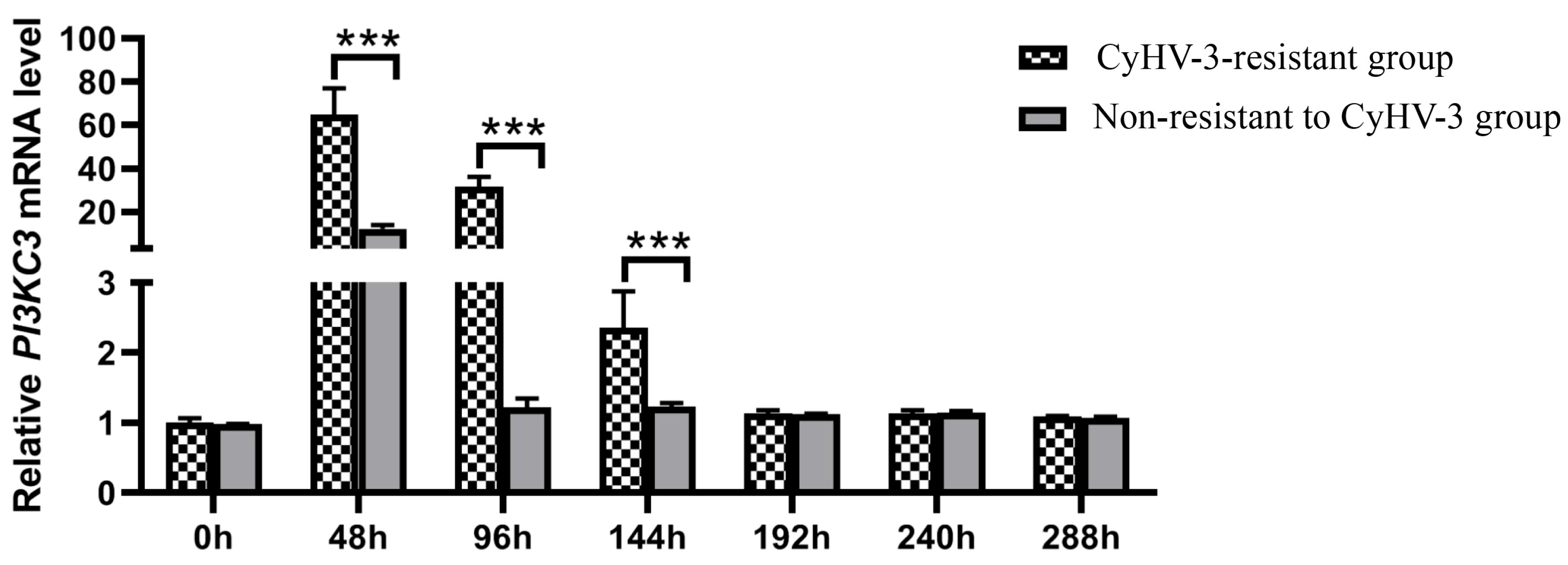
3.4. CcPI3KC3 mRNA Expression Patterns at Different Times after CyHV-3 Infection
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhao, Y.; Qian, Y.; Sun, Z.; Shen, X.; Cai, Y.; Li, L.; Wang, Z. Role of PI3K in the Progression and Regression of Atherosclerosis. Front. Pharmacol. 2021, 12, 632378. [Google Scholar] [CrossRef] [PubMed]
- Okkenhaug, K. Signaling by the Phosphoinositide 3-Kinase Family in Immune Cells. Annu. Rev. Immunol. 2013, 31, 675–704. [Google Scholar] [CrossRef] [PubMed]
- Perino, A.; Ghigo, A.; Ferrero, E.; Morello, F.; Santulli, G.; Baillie, G.S.; Damilano, F.; Dunlop, A.J.; Pawson, C.; Walser, R.; et al. Integrating Cardiac PIP3 and cAMP Signaling through a PKA Anchoring Function of p110γ. Mol. Cell 2011, 42, 84–95. [Google Scholar] [CrossRef] [PubMed]
- Gulluni, F.; De Santis, M.C.; Margaria, J.P.; Martini, M.; Hirsch, E. Class II PI3K Functions in Cell Biology and Disease. Trends Cell Biol. 2019, 29, 339–359. [Google Scholar] [CrossRef]
- Jaber, N.; Zong, W.-X. Class III PI3K Vps34: Essential roles in autophagy, endocytosis, and heart and liver function. Ann. N. Y. Acad. Sci. 2013, 1280, 48–51. [Google Scholar] [CrossRef]
- Kihara, A.; Noda, T.; Ishihara, N.; Ohsumi, Y. Two Distinct Vps34 Phosphatidylinositol 3–Kinase Complexes Function in Autophagy and Carboxypeptidase Y Sorting in Saccharomyces cerevisiae. J. Cell Biol. 2001, 152, 519–530. [Google Scholar] [CrossRef]
- Ohashi, Y.; Tremel, S.; Williams, R.L. VPS34 complexes from a structural perspective. J. Lipid Res. 2019, 60, 229–241. [Google Scholar] [CrossRef]
- Stjepanovic, G.; Baskaran, S.; Lin, M.G.; Hurley, J.H. Vps34 Kinase Domain Dynamics Regulate the Autophagic PI 3-Kinase Complex. Mol. Cell 2017, 67, 528–534.e3. [Google Scholar] [CrossRef]
- Fan, W.; Nassiri, A.; Zhong, Q. Autophagosome targeting and membrane curvature sensing by Barkor/Atg14(L). Proc. Natl. Acad. Sci. USA 2011, 108, 7769–7774. [Google Scholar] [CrossRef]
- Tremel, S.; Ohashi, Y.; Morado, D.R.; Bertram, J.; Perisic, O.; Brandt, L.T.L.; von Wrisberg, M.-K.; Chen, Z.A.; Maslen, S.L.; Kovtun, O.; et al. Structural basis for VPS34 kinase activation by Rab1 and Rab5 on membranes. Nat. Commun. 2021, 12, 1564. [Google Scholar] [CrossRef]
- Xie, Z.; Klionsky, D.J. Autophagosome formation: Core machinery and adaptations. Nat. Cell Biol. 2007, 9, 1102–1109. [Google Scholar] [CrossRef] [PubMed]
- Deretic, V. Autophagy as an innate immunity paradigm: Expanding the scope and repertoire of pattern recognition receptors. Curr. Opin. Immunol. 2012, 24, 21–31. [Google Scholar] [CrossRef] [PubMed]
- FAO. FAO Yearbook. Fishery and Aquaculture Statistics; FAO: Rome, Italy, 2020. [Google Scholar]
- Troszok, A.; Roszko, M. Thyme essential oil inhibits intracellular replication of CyHV-3 and inactivates extracellular virus. An in vitro study. J. Fish Dis. 2023, 46, 663–677. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Ming, Y.; Fu, X.; Niu, Y.; Lin, Q.; Liang, H.; Luo, X.; Liu, L.; Li, N. PI3K/AKT/p53 pathway inhibits infectious spleen and kidney necrosis virus infection by regulating autophagy and immune responses. Fish Shellfish Immunol. 2021, 120, 648–657. [Google Scholar] [CrossRef] [PubMed]
- Kaur, S.; Katsoulidis, E.; Platanias, L.C. Akt and mRNA translation by interferons. Cell Cycle 2008, 7, 2112–2116. [Google Scholar] [CrossRef]
- Ehrhardt, C.; Marjuki, H.; Wolff, T.; Nurnberg, B.; Planz, O.; Pleschka, S.; Ludwig, S. Bivalent role of the phosphatidylinositol-3-kinase (PI3K) during influenza virus infection and host cell defence. Cell. Microbiol. 2006, 8, 1336–1348. [Google Scholar] [CrossRef]
- Jia, Z.; Wu, N.; Jiang, X.; Li, H.; Sun, J.; Shi, M.; Li, C.; Ge, Y.; Hu, X.; Ye, W.; et al. Integrative Transcriptomic Analysis Reveals the Immune Mechanism for a CyHV-3-Resistant Common Carp Strain. Front. Immunol. 2021, 12, 687151. [Google Scholar] [CrossRef]
- Boutier, M.; Gao, Y.; Donohoe, O.; Vanderplasschen, A. Current knowledge and future prospects of vaccines against cyprinid herpesvirus 3 (CyHV-3). Fish Shellfish Immunol. 2019, 93, 531–541. [Google Scholar] [CrossRef]
- SC/T 7212.1-2011; Detection Methods of Cyprinid Herpesvirus (CyHv)—Part 1:Koi Herpevirus. Ministry of Agriculture of the People’s Republic of China: Beijing, China, 2011.
- Jiang, X.; Sun, J.; Li, C.; Hu, X.; Ge, Y.; Li, B.; Shi, L.; Jia, Z. Molecular cloning and sequence characterization of common carp (Cyprinus carpio) integrin β1 (ITGβ1) and its temporal expression in response to CyHV-3. Aquac. Int. 2021, 29, 1869–1884. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Tamura, K. MEGA7: Molecular Evolutionary Genetics Analysis Version 7.0 for Bigger Datasets. Mol. Biol. Evol. 2016, 33, 1870–1874. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Wang, B.; Xiao, Y. PI3K: A potential therapeutic target for cancer. J. Cell. Physiol. 2012, 227, 2818–2821. [Google Scholar] [CrossRef] [PubMed]
- Safaroghli-Azar, A.; Sanaei, M.-J.; Pourbagheri-Sigaroodi, A.; Bashash, D. Phosphoinositide 3-kinase (PI3K) classes: From cell signaling to endocytic recycling and autophagy. Eur. J. Pharmacol. 2023, 953, 175827. [Google Scholar] [CrossRef]
- Ji, W.-T.; Liu, H. PI3K-Akt Signaling and Viral Infection. Recent Pat. Biotechnol. 2008, 2, 218–226. [Google Scholar] [CrossRef] [PubMed]
- Kozakov, D.; Hall, D.R.; Chuang, G.-Y.; Cencic, R.; Brenke, R.; Grove, L.E.; Beglov, D.; Pelletier, J.; Whitty, A.; Vajda, S. Structural conservation of druggable hot spots in protein–protein interfaces. Proc. Natl. Acad. Sci. USA 2011, 108, 13528–13533. [Google Scholar] [CrossRef]
- Conibear, A.C.; Rosengren, K.J.; Becker, C.F.W.; Kaehlig, H. Random coil shifts of posttranslationally modified amino acids. J. Biomol. NMR 2019, 73, 587–599. [Google Scholar] [CrossRef]
- Schultz, U.; Köck, J.; Schlicht, H.-J.; Staeheli, P. Recombinant Duck Interferon: A New Reagent for Studying the Mode of Interferon Action against Hepatitis B Virus. Virology 1995, 212, 641–649. [Google Scholar] [CrossRef]
- Levine, B.; Kroemer, G. Biological Functions of Autophagy Genes: A Disease Perspective. Cell 2019, 176, 11–42. [Google Scholar] [CrossRef]
- Li, Z. Cloning and Expression Analysis of Akt, Pi3k and STAT1 in Sebastes schlegelii. Master’s Thesis, Shanghai Ocean University, Shanghai, China, 2023. [Google Scholar]
- Zhuo, M.-Q.; Pan, Y.-X.; Wu, K.; Xu, Y.-H.; Luo, Z. Characterization and mechanism of phosphoinositide 3-kinases (PI3Ks) members in insulin-induced changes of protein metabolism in yellow catfish Pelteobagrus fulvidraco. Gen. Comp. Endocrinol. 2017, 247, 34–45. [Google Scholar] [CrossRef]
- Condello, M.; Pellegrini, E.; Caraglia, M.; Meschini, S. Targeting Autophagy to Overcome Human Diseases. Int. J. Mol. Sci. 2019, 20, 725. [Google Scholar] [CrossRef]
- Olsen, J.V.; Vermeulen, M.; Santamaria, A.; Kumar, C.; Miller, M.L.; Jensen, L.J.; Gnad, F.; Cox, J.; Jensen, T.S.; Nigg, E.A.; et al. Quantitative Phosphoproteomics Reveals Widespread Full Phosphorylation Site Occupancy During Mitosis. Sci. Signal. 2010, 3, ra3. [Google Scholar] [CrossRef] [PubMed]
- Nishi, H.; Hashimoto, K.; Panchenko, A.R. Phosphorylation in Protein-Protein Binding: Effect on Stability and Function. Structure 2011, 19, 1807–1815. [Google Scholar] [CrossRef] [PubMed]
- Ta, H.X.; Holm, L. Evaluation of different domain-based methods in protein interaction prediction. Biochem. Biophys. Res. Commun. 2009, 390, 357–362. [Google Scholar] [CrossRef]
- Ulusoy, E.; Doğan, T. Mutual annotation-based prediction of protein domain functions with Domain2GO. Protein Sci. 2024, 33, e4988. [Google Scholar] [CrossRef]
- Pacold, M.E.; Suire, S.; Perisic, O.; Lara-Gonzalez, S.; Davis, C.T.; Walker, E.H.; Hawkins, P.; Stephens, L.R.; Eccleston, J.F.; Williams, R.L. Crystal Structure and Functional Analysis of Ras Binding to Its Effector Phosphoinositide 3-Kinase γ. Cell 2000, 103, 931–944. [Google Scholar] [CrossRef]
- Walker, E.H.; Perisic, O.; Ried, C.; Stephens, L.; Williams, R.L. Structural insights into phosphoinositide 3-kinase catalysis and signalling. Nature 1999, 402, 313–320. [Google Scholar] [CrossRef]
- Zhang, L. Molecular Cloning and Expression Analysis of piwil2 and pik3r1 Gene in the Half-Smooth Tongue-Sole, Cynoglossus semilaevis. Ph.D. Thesis, College of Fisheries and Life Science, Dalian Ocean University, Dalian, China, 2014. [Google Scholar]
- Li, S.; Xie, J.; Zhang, D.; Zhao, G.; Bai, Y.; Li, K.; Li, X.; Li, Q.; Tang, X.; Ge, X. Lycopene abolishes typical polyhalogenated carbazoles (PHCZs)-induced hepatic injury in yellow catfish (Pelteobagrus fulvidraco): Involvement of ROS/PI3K-AKT/NF-κB signaling. Fish Shellfish Immunol. 2023, 139, 108897. [Google Scholar] [CrossRef]
- Surai, P.F. Silymarin as a Natural Antioxidant: An Overview of the Current Evidence and Perspectives. Antioxidants 2015, 4, 204–247. [Google Scholar] [CrossRef]
- Wen, C.-M. Development and characterization of a cell line from tilapia head kidney with melanomacrophage characteristics. Fish Shellfish Immunol. 2016, 49, 442–449. [Google Scholar] [CrossRef]
- Adamek, M.; Steinhagen, D.; Irnazarow, I.; Hikima, J.-I.; Jung, T.-S.; Aoki, T. Biology and host response to Cyprinid herpesvirus 3 infection in common carp. Dev. Comp. Immunol. 2014, 43, 151–159. [Google Scholar] [CrossRef]
- Lee, X.; Fan, Z.; Huang, Z.; Guo, M.; Peng, D.; Luo, W.; Qin, Q.; Wang, S.; Wei, S.; Yang, M. Common carp (Cyprinus carpio) CD81 promoting CyHV-3 virus replication via regulating autophagy and RLRs-interferon signaling pathway. Fish Shellfish Immunol. 2023, 143, 109181. [Google Scholar] [CrossRef] [PubMed]
- Luo, W.; Qi, H.; Huang, Z.; Guo, M.; Peng, D.; Yang, Z.; Fan, Z.; Wang, Q.; Qin, Q.; Yang, M.; et al. Autophagy induced by Cyprinid herpesvirus 3 (CyHV-3) facilitated intracellular viral replication and extracellular viral yields in common carp brain cells. Fish Shellfish Immunol. 2023, 141, 109049. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Liu, J.; Zhang, X.; Yu, Y.; Huang, X.; Wei, J.; Qin, Q. Red grouper nervous necrosis virus (RGNNV) induces autophagy to promote viral replication. Fish Shellfish Immunol. 2019, 98, 908–916. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Li, J.; Du, Y.; Xu, Y.; Wang, Y.; Zhang, Z.; Xu, Z.; Zeng, Y.; Mao, X.; Cao, B. The Class I PI3K inhibitor S14161 induces autophagy in malignant blood cells by modulating the Beclin 1/Vps34 complex. J. Pharmacol. Sci. 2017, 134, 197–202. [Google Scholar] [CrossRef]
- Karim, R.; Fisher, C.R.; Kapphahn, R.J.; Polanco, J.R.; Ferrington, D.A. Investigating AKT activation and autophagy in immunoproteasome-deficient retinal cells. PLoS ONE 2020, 15, e0231212. [Google Scholar] [CrossRef]
- Kma, L.; Baruah, T.J. The interplay of ROS and the PI3K/Akt pathway in autophagy regulation. Biotechnol. Appl. Biochem. 2021, 69, 248–264. [Google Scholar] [CrossRef]
- Wang, B.; Zhong, Y.; Li, Q.; Cui, L.; Huang, G. Autophagy of macrophages is regulated by PI3k/Akt/mTOR signalling in the development of diabetic encephalopathy. Aging 2018, 10, 2772–2782. [Google Scholar] [CrossRef]
- Jia, Z.; Wang, S.; Bai, S.; Ge, Y.; Li, C.; Hu, X.; Shang, M.; Zhang, J.; Li, B.; Shi, L. Survival Rate and Immunological Responses of Mirror Carp Selective Breeding Generations to CyHV-3. J. World Aquac. Soc. 2018, 49, 388–395. [Google Scholar] [CrossRef]
- Sun, J.; Shi, L.; Jiang, X.; Li, C.; Ge, Y.; Hu, X.; Zhang, X.; Jia, Z. Mirror carp anti-herpesvirus (CyHV-3) F(4) virus expression level evaluation. Shanghai Ocean. Univ. J. 2021, 30, 1–10. Available online: http://kns.cnki.net/kcms/detail/31.2024.S.20200612.1514.002.html (accessed on 30 September 2024).




| Primers | Primer Sequences (5′-3′) | Application |
|---|---|---|
| CcPI3KC3–1 | F:ATTCAAGTCACTGCGGTTATCGA | Conserved sequence amplification |
| R:TGCATTAGGTTTGAGATCGTGGT | ||
| CcPI3KC3–2 | F:CCCTCGTGTCAAAAACGGTG | Conserved sequence amplification |
| R:ATGTTTGAAGATGACAGGATATTGC | ||
| CcPI3KC3–3 | F:CCTGCTGGCTGATAATGAGAAG | Conserved sequence amplification |
| R:GTTGGAATCTTTATTTGGCTGTCTT | ||
| 5′adaptor | F:GCTGTCAACGATACGCTACGTAACG | Nested 5′-race PCR |
| R:GCATGACAGTGGGIIGGGIIGGGIIG | ||
| 3′adaptor | F:GCTGTCAACGATACGCTACGTAACGGCA | Nested 3′-racePCR |
| R:TGACAGTGTTTTTTTTTTTTTTTTTT | ||
| 5.3′outer | GCTGTCAACGATACGCTACGTAAC | Nested PCR |
| 5.3′inner | GCTACGTAACGGCATGACAGTG | Nested PCR |
| RC947-F3 | AGGAGGCGGTGCATTATATGC | Amplification of the partial cDNA and nested 3′-race PCR |
| RC947-F4 | ATTGATGAGAGTGTAGGTGCTCTGT | Amplification of the partial cDNA and nested 5′-race PCR |
| RC947-RT1 | GACGTACAGGTCGGAGCAGC | 5′RACE Amplification of the partial cDNA |
| RC947-RT2 | GAGTGCTGAAGGCCTTGTAAGAC | 5′RACE Amplification of the partial cDNA |
| RC947-R3 | TTCTTGATACGACTCTGTCTGATTAATG | 5′RACE Amplification of the partial cDNA |
| RC947-R4 | GCTGATCAAAAGTACTGTCAGTCTTAAT | 5′RACE Amplification of the partial cDNA |
| qPI3KC3 | F:GGATTCCTTGGAGCTGCTGT | RT–PCR |
| R:CACCAGCTGGAGCAAGTACA | ||
| β-actin | F:GCCGTGACCTGACTGACTACCT | RT–PCR |
| R:GCCACATAGCAGAGCTTCTCCTTG |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, X.; Tian, L.; Ren, W.; Li, C.; Hu, X.; Ge, Y.; Cheng, L.; Shi, X.; Jia, Z. Cloning and Identification of Common Carp (Cyprinus carpio) PI3KC3 and Its Expression in Response to CyHV-3 Infection. Curr. Issues Mol. Biol. 2024, 46, 11714-11728. https://doi.org/10.3390/cimb46100696
Jiang X, Tian L, Ren W, Li C, Hu X, Ge Y, Cheng L, Shi X, Jia Z. Cloning and Identification of Common Carp (Cyprinus carpio) PI3KC3 and Its Expression in Response to CyHV-3 Infection. Current Issues in Molecular Biology. 2024; 46(10):11714-11728. https://doi.org/10.3390/cimb46100696
Chicago/Turabian StyleJiang, Xiaona, Lijing Tian, Wanying Ren, Chitao Li, Xuesong Hu, Yanlong Ge, Lei Cheng, Xiaodan Shi, and Zhiying Jia. 2024. "Cloning and Identification of Common Carp (Cyprinus carpio) PI3KC3 and Its Expression in Response to CyHV-3 Infection" Current Issues in Molecular Biology 46, no. 10: 11714-11728. https://doi.org/10.3390/cimb46100696
APA StyleJiang, X., Tian, L., Ren, W., Li, C., Hu, X., Ge, Y., Cheng, L., Shi, X., & Jia, Z. (2024). Cloning and Identification of Common Carp (Cyprinus carpio) PI3KC3 and Its Expression in Response to CyHV-3 Infection. Current Issues in Molecular Biology, 46(10), 11714-11728. https://doi.org/10.3390/cimb46100696





