Wound Healing, Metabolite Profiling, and In Silico Studies of Aspergillus terreus
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Fungus
2.2. Fermentation and Extraction
2.3. In Vitro Cell Migration Assay
2.4. Assessment of In Vivo Burn Wound Healing Potential
2.5. Histopathological Assessment
2.6. Statistical Analysis
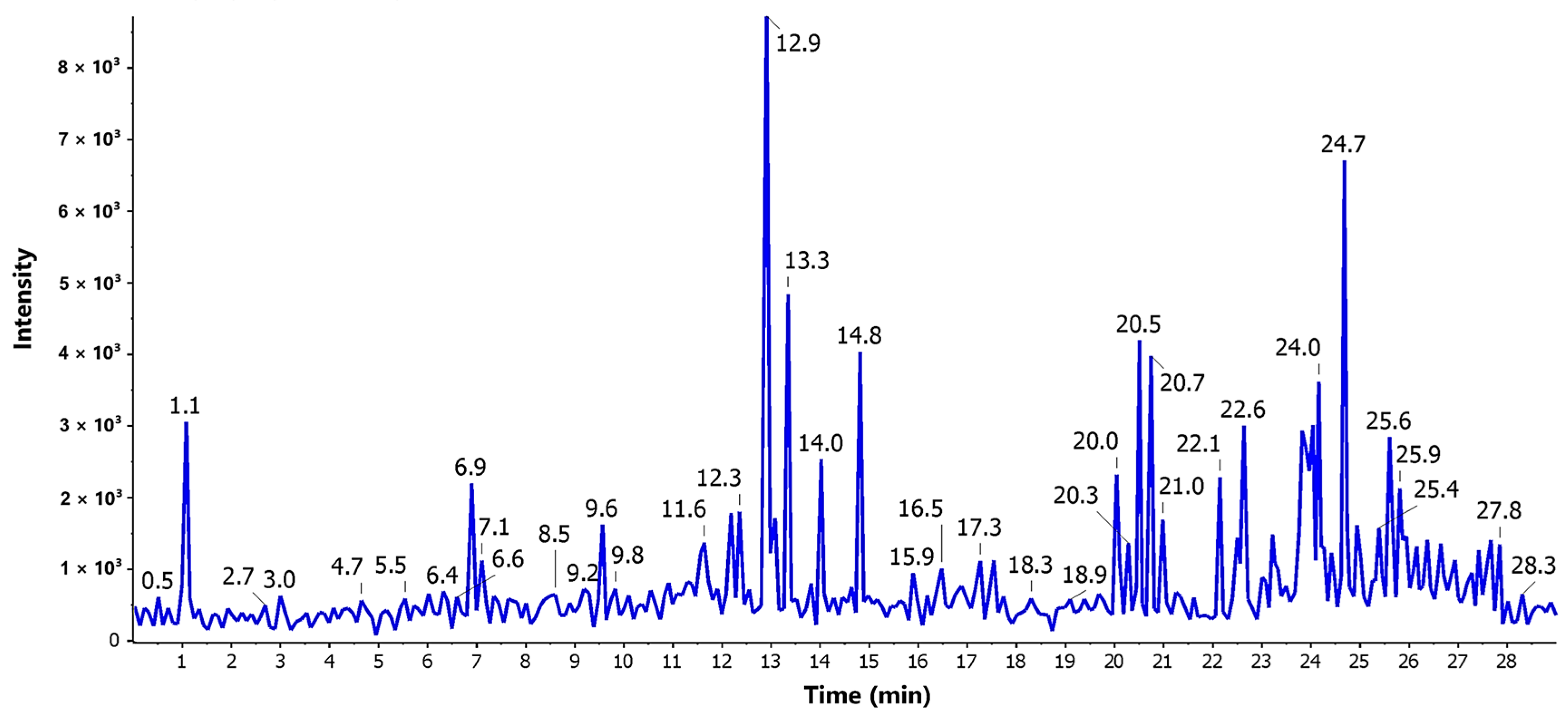
2.7. LC-ESI-MS/MS Profiling
2.8. Molecular Docking Simulation
2.9. Structure Drug-like Properties
3. Results
3.1. In Vitro Cell Migration Assay
3.2. Assessment of In Vivo Burn Wound Healing Potential
3.3. Histopathological Results
3.4. LC-ESI-MS/MS Profiling
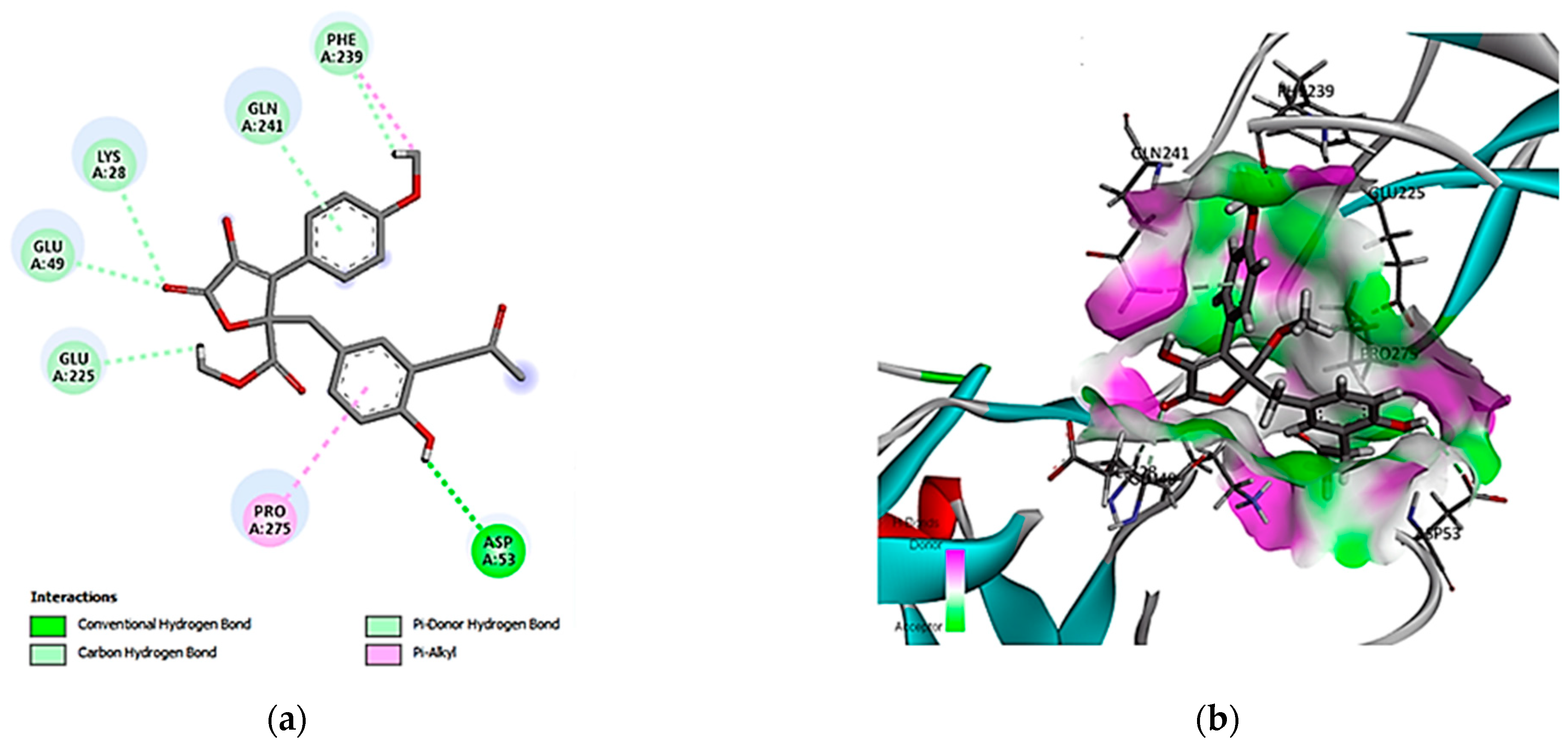
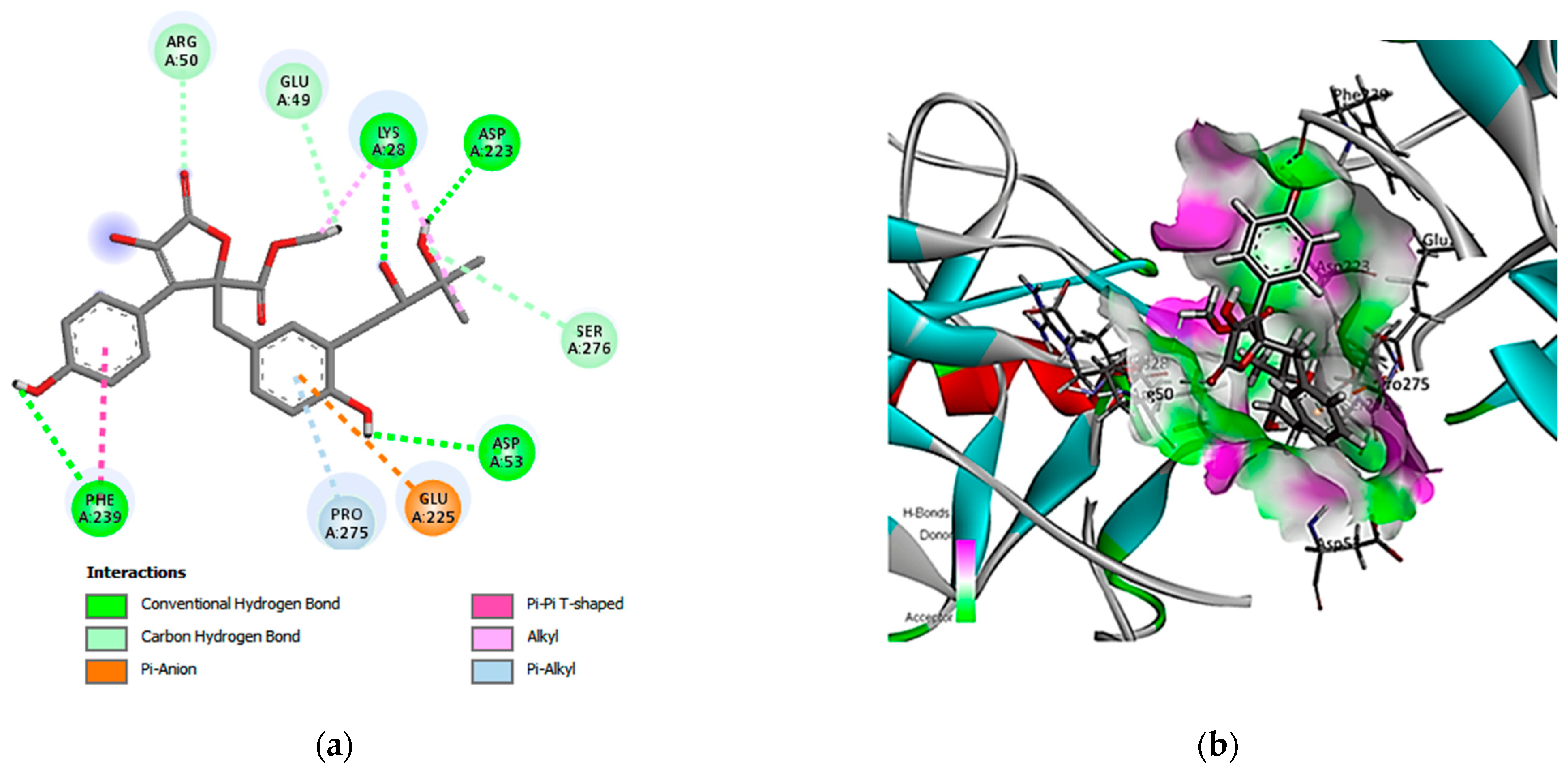
3.5. Molecular Docking Simulation and Drug-like Properties
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zulkefli, N.; Che Zahari, C.N.M.; Sayuti, N.H.; Kamarudin, A.A.; Saad, N.; Hamezah, H.S.; Bunawan, H.; Baharum, S.N.; Mediani, A.; Ahmed, Q.U.; et al. Flavonoids as potential wound-healing molecules: Emphasis on pathways perspective. Int. J. Mol. Sci. 2023, 24, 4607. [Google Scholar] [CrossRef] [PubMed]
- Schilrreff, P.; Alexiev, U. Chronic inflammation in non-healing skin wounds and promising natural bioactive compounds treatment. Int. J. Mol. Sci. 2022, 23, 4928. [Google Scholar] [CrossRef]
- Das, M.; Kityania, S.; Nath, R.; Nath, R.; Nath, D.; Talukdar, A.D. Endophytic Fungi and the Health Benefits from Their Potential Bioactive Secondary Metabolites. In Endophytic Fungi: The Hidden Sustainable Jewels for the Pharmaceutical and Agricultural Industries, 1st ed.; Singh, B.P., Abdel-Azeem, A.M., Gautam, V., Singh, G., Singh, S.K., Eds.; Springer: Cham, Switzerland, 2024; pp. 295–324. [Google Scholar] [CrossRef]
- Mahmoudi, F.; Jalayeri, M.H.T.; Montaseri, A.; Khosroshahi, L.M.; Baradaran, B. Microbial natural compounds and secondary metabolites as Immunomodulators: A review. Int. J. Biol. Macromol. 2024, 278, 134778. [Google Scholar] [CrossRef]
- Yao, G.; Bai, X.; Zhang, B.; Wang, L.; Chen, S.; Wang, Z. Enhanced production of terrein in marine-derived Aspergillus terreus by refactoring both global and pathway-specific transcription factors. Microb. Cell Fact. 2022, 21, 136. [Google Scholar] [CrossRef] [PubMed]
- Hamed, A.; Abdel-Razek, A.S.; Abdelwahab, A.B.; El Taweel, A.; GabAllah, M.; Sewald, N.; Shaaban, M. Diverse bioactive secondary metabolites from Aspergillus terreus: Antimicrobial, anticancer, and anti-SARS-CoV-2 activity studies. Z. Naturforsch. C 2024, 0083. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Wang, Y.; Li, G.; Shao, Z.; Xia, J.; Qin, J.-J.; Wang, W. Secondary metabolites from the deep-sea derived fungus Aspergillus terreus MCCC M28183. Front. Microbiol. 2024, 15, 1361550. [Google Scholar] [CrossRef]
- Ahmed, T.; Juhász, A.; Bose, U.; Terefe, N.S.; Colgrave, M.L. Research trends in production, separation, and identification of bioactive peptides from fungi—A critical review. J. Funct. Foods 2024, 119, 106343. [Google Scholar] [CrossRef]
- Ye, L.; Zhang, B.; Zhou, J.; Yang, X.; Zhang, X.; Tan, W.; Li, X. LC-MS/MS-based targeted amino acid metabolic profile of Auricularia cornea grown on pinecone substrate. Food Chem. 2024, 432, 137247. [Google Scholar] [CrossRef] [PubMed]
- Ismail, F.M.; Nahar, L.; Sarker, S.D. High throughput screening of phytochemicals: Application of computational methods. In Computational Phytochemistry; Elsevier: Amsterdam, The Netherlands, 2024; pp. 219–253. [Google Scholar] [CrossRef]
- Ashtekar, N.; Anand, G.; Prakash, P.Y.; Rajeshkumar, K.C. Aspergillus terreus: Taxonomy, biology, and bioactive secondary metabolites with potential applications. In New and Future Developments in Microbial Biotechnology and Bioengineering, 1st ed.; Singh, J., Gehlot, P., Eds.; Elsevier: Amsterdam, The Netherlands, 2021; pp. 215–223. [Google Scholar] [CrossRef]
- Al Mousa, A.A.; Abouelela, M.E.; Al Ghamidi, N.S.; Abo-Dahab, Y.; Mohamed, H.; Abo-Dahab, N.F.; Hassane, A.M.A. Anti-Staphylococcal, Anti-Candida, and Free-Radical Scavenging Potential of Soil Fungal Metabolites: A Study Supported by Phenolic Characterization and Molecular Docking Analysis. Curr. Issues Mol. Biol. 2023, 46, 221–243. [Google Scholar] [CrossRef]
- Al Mousa, A.A.; Mohamed, H.; Hassane, A.M.; Abo-Dahab, N.F. Antimicrobial and cytotoxic potential of an endophytic fungus Alternaria tenuissima AUMC14342 isolated from Artemisia judaica L. growing in Saudi Arabia. J. King Saud Univ. Sci. 2021, 33, 101462. [Google Scholar] [CrossRef]
- Mohamed, H.; Hassane, A.; Atta, O.; Song, Y. Deep learning strategies for active secondary metabolites biosynthesis from fungi: Harnessing artificial manipulation and application. Biocatal. Agric. Biotechnol. 2021, 38, 102195. [Google Scholar] [CrossRef]
- Main, K.A.; Mikelis, C.M.; Doci, C.L. In vitro wound healing assays to investigate epidermal migration. In Epidermal Cells, 1st ed.; Turksen, K., Ed.; Methods in Molecular Biology; Humana: New York, NY, USA, 2019; pp. 147–154. [Google Scholar] [CrossRef]
- El-Sayed, M.E.; Atwa, A.; Sofy, A.R.; Helmy, Y.A.; Amer, K.; Seadawy, M.G.; Bakry, S. Mesenchymal stem cell transplantation in burn wound healing: Uncovering the mechanisms of local regeneration and tissue repair. Histochem. Cell Biol. 2024, 161, 165–181. [Google Scholar] [CrossRef] [PubMed]
- Suvarna, K.S.; Layton, C.; Bancroft, J.D. Bancroft’s Theory and Practice of Histological Techniques, 1st ed.; Elsevier Health Sciences: Amsterdam, The Netherlands, 2018; pp. 126–138. [Google Scholar] [CrossRef]
- Al Mousa, A.A.; Abouelela, M.E.; Hassane, A.M.A.; Al-Khattaf, F.S.; Hatamleh, A.A.; Alabdulhadi, H.S.; Dahmash, N.D.; Abo-Dahab, N.F. Cytotoxic potential of Alternaria tenuissima AUMC14342 mycoendophyte extract: A study combined with LC-MS/MS metabolic profiling and molecular docking simulation. Curr. Issues Mol. Biol. 2022, 44, 5067–5085. [Google Scholar] [CrossRef]
- Banaganapalli, B.; Mulakayala, C.; Mulakayala, N.; Pulaganti, M.; Shaik, N.A.; CM, A.; Rao, R.M.; Al-Aama, J.Y.; Chitta, S.K. Synthesis and biological activity of new resveratrol derivative and molecular docking: Dynamics studies on NFkB. Appl. Biochem. Biotechnol. 2013, 171, 1639–1657. [Google Scholar] [CrossRef] [PubMed]
- Daina, A.; Michielin, O.; Zoete, V. SwissADME: A free web tool to evaluate pharmacokinetics, drug-likeness and medicinal chemistry friendliness of small molecules. Sci. Rep. 2017, 7, 42717. [Google Scholar] [CrossRef] [PubMed]
- Sheehan, J.C.; Lawson, W.B.; Gaul, R.J. The structure of terreic acid. J. Am. Chem. Soc. 1958, 80, 5536–5538. [Google Scholar] [CrossRef]
- Petterson, G. Toluquinones from Aspergillus fumigatus. Acta. Chem. Scand. 1963, 17, 1771–1776. [Google Scholar] [CrossRef]
- Yuan, W.; Zhu, H.; Cheng, K.; Huang, Z.; Qin, Y.; Yang, J.; Zhu, P. A novel butenoate derivative from Aspergillus niger. Nat. Prod. Res. 2006, 20, 573–577. [Google Scholar] [CrossRef]
- Chen, X.-W.; Li, C.-W.; Cui, C.-B.; Hua, W.; Zhu, T.-J.; Gu, Q.-Q. Nine new and five known polyketides derived from a deep sea-sourced Aspergillus sp. 16-02-1. Mar. Drugs 2014, 12, 3116–3137. [Google Scholar] [CrossRef]
- Zhang, X.; Wu, Z.; Lai, Y.; Li, D.; Wang, J.; Luo, Z.; Xue, Y.; Zhu, H.; Chen, C.; Zhang, Y. (±)-Terreinlactone A, a pair of 3-Substituted δ-lactone enantiomers derived from Terrein from the fungus Aspergillus terreus. Chem. Pharm. Bull. 2018, 66, 764–767. [Google Scholar] [CrossRef]
- Chaudhary, N.K.; Pitt, J.I.; Lacey, E.; Crombie, A.; Vuong, D.; Piggott, A.M.; Karuso, P. Banksialactones and banksiamarins: Isochromanones and isocoumarins from an Australian fungus, Aspergillus banksianus. J. Nat. Prod. 2018, 81, 1517–1526. [Google Scholar] [CrossRef] [PubMed]
- Du, X.; Liu, D.; Huang, J.; Zhang, C.; Proksch, P.; Lin, W. Polyketide derivatives from the sponge associated fungus Aspergillus europaeus with antioxidant and NO inhibitory activities. Fitoterapia 2018, 130, 190–197. [Google Scholar] [CrossRef] [PubMed]
- Ahuja, M.; Chiang, Y.-M.; Chang, S.-L.; Praseuth, M.B.; Entwistle, R.; Sanchez, J.F.; Lo, H.-C.; Yeh, H.-H.; Oakley, B.R.; Wang, C.C. Illuminating the diversity of aromatic polyketide synthases in Aspergillus nidulans. J. Am. Chem. Soc. 2012, 134, 8212–8221. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.; Gai, C.; Cai, C.; Chen, L.; Liu, S.; Zeng, Y.; Yuan, J.; Mei, W.; Dai, H. Metabolites with insecticidal activity from Aspergillus fumigatus JRJ111048 isolated from mangrove plant Acrostichum specioum endemic to Hainan Island. Mar. Drugs 2017, 15, 381. [Google Scholar] [CrossRef]
- Tang, S.; Zhang, W.; Li, Z.; Li, H.; Geng, C.; Huang, X.; Lu, X. Discovery and characterization of a PKS–NRPS hybrid in Aspergillus terreus by genome mining. J. Nat. Prod. 2020, 83, 473–480. [Google Scholar] [CrossRef]
- Ma, L.-Y.; Zhang, H.-B.; Kang, H.-H.; Zhong, M.-J.; Liu, D.-S.; Ren, H.; Liu, W.-Z. New butenolides and cyclopentenones from saline soil-derived fungus Aspergillus sclerotiorum. Molecules 2019, 24, 2642. [Google Scholar] [CrossRef]
- Capon, R.J.; Ratnayake, R.; Stewart, M.; Lacey, E.; Tennant, S.; Gill, J.H. Aspergillazines A–E: Novel heterocyclic dipeptides from an Australian strain of Aspergillus unilateralis. Org. Biomol. Chem. 2005, 3, 123–129. [Google Scholar] [CrossRef]
- Antia, B.S.; Aree, T.; Kasettrathat, C.; Wiyakrutta, S.; Ekpa, O.D.; Ekpe, U.J.; Mahidol, C.; Ruchirawat, S.; Kittakoop, P. Itaconic acid derivatives and diketopiperazine from the marine-derived fungus Aspergillus aculeatus CRI322-03. Phytochemistry 2011, 72, 816–820. [Google Scholar] [CrossRef]
- Zhou, M.; Du, G.; Yang, H.-Y.; Xia, C.-F.; Yang, J.-X.; Ye, Y.-Q.; Gao, X.-M.; Li, X.-N.; Hu, Q.-F. Antiviral butyrolactones from the endophytic fungus Aspergillus versicolor. Planta Med. 2015, 81, 235–240. [Google Scholar] [CrossRef]
- Wang, J.-T.; Zhang, P.-L.; Liu, J.-S.; Wang, G.-K.; Xu, F.-Q.; Chen, L.; Yu, Y.; Wang, G. Aspergilates A to E, second metabolites from Aspergillus sp. isolated from Paeonia ostii. Fitoterapia 2018, 131, 204–208. [Google Scholar] [CrossRef]
- Nuclear, P.; Sommit, D.; Boonyuen, N.; Pudhom, K. Butenolide and furandione from an endophytic Aspergillus terreus. Chem. Pharm. Bull. 2010, 58, 1221–1223. [Google Scholar] [CrossRef] [PubMed]
- Li, H.-L.; Li, X.-M.; Yang, S.-Q.; Meng, L.-H.; Li, X.; Wang, B.-G. Prenylated phenol and benzofuran derivatives from Aspergillus terreus EN-539, an endophytic fungus derived from marine red alga Laurencia okamurai. Mar. Drugs 2019, 17, 605. [Google Scholar] [CrossRef] [PubMed]
- Vertesy, L.; Burger, H.-J.; Kenja, J.; Knauf, M.; Kogler, H.; Paulus, E.R.; Ramakrishna, N.V.S.; Swamy, K.H.; Vuayakumar, E.K.; Hammann, P. Kodaistatins, novel inhibitors of glucose-6-phosphate translocase T1 from Aspergillus terreus thorn DSM 11247 isolation and structural elucidation. J. Antibiot. 2000, 53, 677–686. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Yurchenko, A.; Smetanina, O.; Kalinovsky, A.; Pivkin, M.; Dmitrenok, P.; Kuznetsova, T. A new meroterpenoid from the marine fungus Aspergillus versicolor (Vuill.) Tirab. Russ. Chem. Bull. 2010, 59, 852–856. [Google Scholar] [CrossRef]
- Ishikawa, K.; Sato, F.; Itabashi, T.; Wachi, H.; Takeda, H.; Wakana, D.; Yaguchi, T.; Kawai, K.-I.; Hosoe, T. Asnovolins A–G, spiromeroterpenoids isolated from the fungus Aspergillus novofumigatus, and suppression of fibronectin expression by Asnovolin E. J. Nat. Prod. 2016, 79, 2167–2174. [Google Scholar] [CrossRef]
- Lu, Z.; Wang, Y.; Miao, C.; Liu, P.; Hong, K.; Zhu, W. Sesquiterpenoids and benzofuranoids from the marine-derived fungus Aspergillus ustus 094102. J. Nat. Prod. 2009, 72, 1761–1767. [Google Scholar] [CrossRef]
- Cueto, M.; MacMillan, J.B.; Jensen, P.R.; Fenical, W. Tropolactones AD, four new meroterpenoids from a marine-derived fungus of the genus Aspergillus. Phytochemistry 2006, 67, 1826–1831. [Google Scholar] [CrossRef] [PubMed]
- Pang, X.; Zhao, J.-Y.; Fang, X.-M.; Zhang, T.; Zhang, D.-W.; Liu, H.-Y.; Su, J.; Cen, S.; Yu, L.-Y. Metabolites from the plant endophytic fungus Aspergillus sp. CPCC 400735 and their anti-HIV activities. J. Nat. Prod. 2017, 80, 2595–2601. [Google Scholar] [CrossRef]
- Li, G.-Y.; Li, B.-G.; Yang, T.; Yin, J.-H.; Qi, H.-Y.; Liu, G.-Y.; Zhang, G.-L. Sesterterpenoids, Terretonins A–D, and an Alkaloid, Asterrelenin, from Aspergillus terreus. J. Nat. Prod. 2005, 68, 1243–1246. [Google Scholar] [CrossRef]
- Wu, Q.; Jin, X.; Draskovic, M.; Crews, M.; Tenney, K.; Valeriote, F.; Yao, X.; Crews, P. Unraveling the numerous biosynthetic products of the marine sediment-derived fungus, Aspergillus insulicola. Phytochem. Lett. 2012, 5, 114–117. [Google Scholar] [CrossRef]
- Jiang, T.; Li, T.; Li, J.; Fu, H.-Z.; Pei, Y.-H.; Lin, W.-H. Cerebroside analogues from marine-derived fungus Aspergillus flavipes. J. Asian Nat. Prod. Res. 2004, 6, 249–257. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wei, X.; Qin, X.; Tian, X.; Liao, L.; Li, K.; Zhou, X.; Yang, X.; Wang, F.; Zhang, T.; et al. Antiviral merosesquiterpenoids produced by the antarctic fungus Aspergillus ochraceopetaliformis SCSIO 05702. J. Nat. Prod. 2016, 79, 59–65. [Google Scholar] [CrossRef] [PubMed]
- Jonkman, J.E.; Cathcart, J.A.; Xu, F.; Bartolini, M.E.; Amon, J.E.; Stevens, K.M.; Colarusso, P. An introduction to the wound healing assay using live-cell microscopy. Cell Adhes. Migr. 2014, 8, 440–451. [Google Scholar] [CrossRef]
- Sreedevi, R.; Sujith, S.; Yakoob, R.; Pradeep, B.V. Potential wound healing activity of bornane dominating fraction extracted from Aspergillus terreus. Bull. Environ. Pharmacol. Life Sci. 2020, 10, 139–145. [Google Scholar]
- Salem, S.H.; El-Maraghy, S.S.; Abdel-Mallek, A.Y.; Abdel-Rahman, M.A.; Hassanein, E.H.; Al-Bedak, O.A.; El-Aziz, F.E.-Z.A.A. The antimicrobial, antibiofilm, and wound healing properties of ethyl acetate crude extract of an endophytic fungus Paecilomyces sp. (AUMC 15510) in earthworm model. Sci. Rep. 2022, 12, 19239. [Google Scholar] [CrossRef]
- Gurtner, G.C.; Werner, S.; Barrandon, Y.; Longaker, M.T. Wound repair and regeneration. Nature 2008, 453, 314–321. [Google Scholar] [CrossRef]
- Fu, Y.; Xie, B.; Ben, D.; Lv, K.; Zhu, S.; Lu, W.; Tang, H.; Cheng, D.; Ma, B.; Wang, G.; et al. Pathogenic alteration in severe burn wounds. Burns 2012, 38, 90–94. [Google Scholar] [CrossRef] [PubMed]
- Shady, N.H.; Mostafa, N.M.; Fayez, S.; Abdel-Rahman, I.M.; Maher, S.A.; Zayed, A.; Saber, E.A.; Khowdiary, M.M.; Elrehany, M.A.; Alzubaidi, M.A.; et al. Mechanistic wound healing and antioxidant potential of Moringa oleifera seeds extract supported by metabolic profiling, in silico network design, molecular docking, and in vivo studies. Antioxidants 2022, 11, 1743. [Google Scholar] [CrossRef]
- Liu, C.; Liu, H.; Wen, Y.; Huang, H.; Hao, J.; Lv, Y.; Qin, R.; Yang, X. Aspernolide a inhibits the proliferation of human laryngeal carcinoma cells through the mitochondrial apoptotic and STAT3 signaling pathways. Molecules 2019, 24, 1074. [Google Scholar] [CrossRef]
- El-Sayed, A.S.; ElSayed, A.I.; Wadan, K.M.; El-Saadany, S.S.; Abd El-Hady, N.A. Camptothecin bioprocessing from Aspergillus terreus, an endophyte of Catharanthus roseus: Antiproliferative activity, topoisomerase inhibition and cell cycle analysis. Microb. Cell Fact. 2024, 23, 15. [Google Scholar] [CrossRef]
- Eldeghidy, A.; Abdel-Fattah, G.; El-Sayed, A.S.; Abdel-Fattah, G.G. Production, bioprocessing and antiproliferative activity of camptothecin from Aspergillus terreus, endophyte of Cinnamomum camphora: Restoring their biosynthesis by indigenous microbiome of C. camphora. Microb. Cell Fact. 2023, 22, 143. [Google Scholar] [CrossRef] [PubMed]
- Parvatkar, R.R.; D’Souza, C.; Tripathi, A.; Naik, C.G. Aspernolides A and B, butenolides from a marine-derived fungus Aspergillus terreus. Phytochemistry 2009, 70, 128–132. [Google Scholar] [CrossRef] [PubMed]
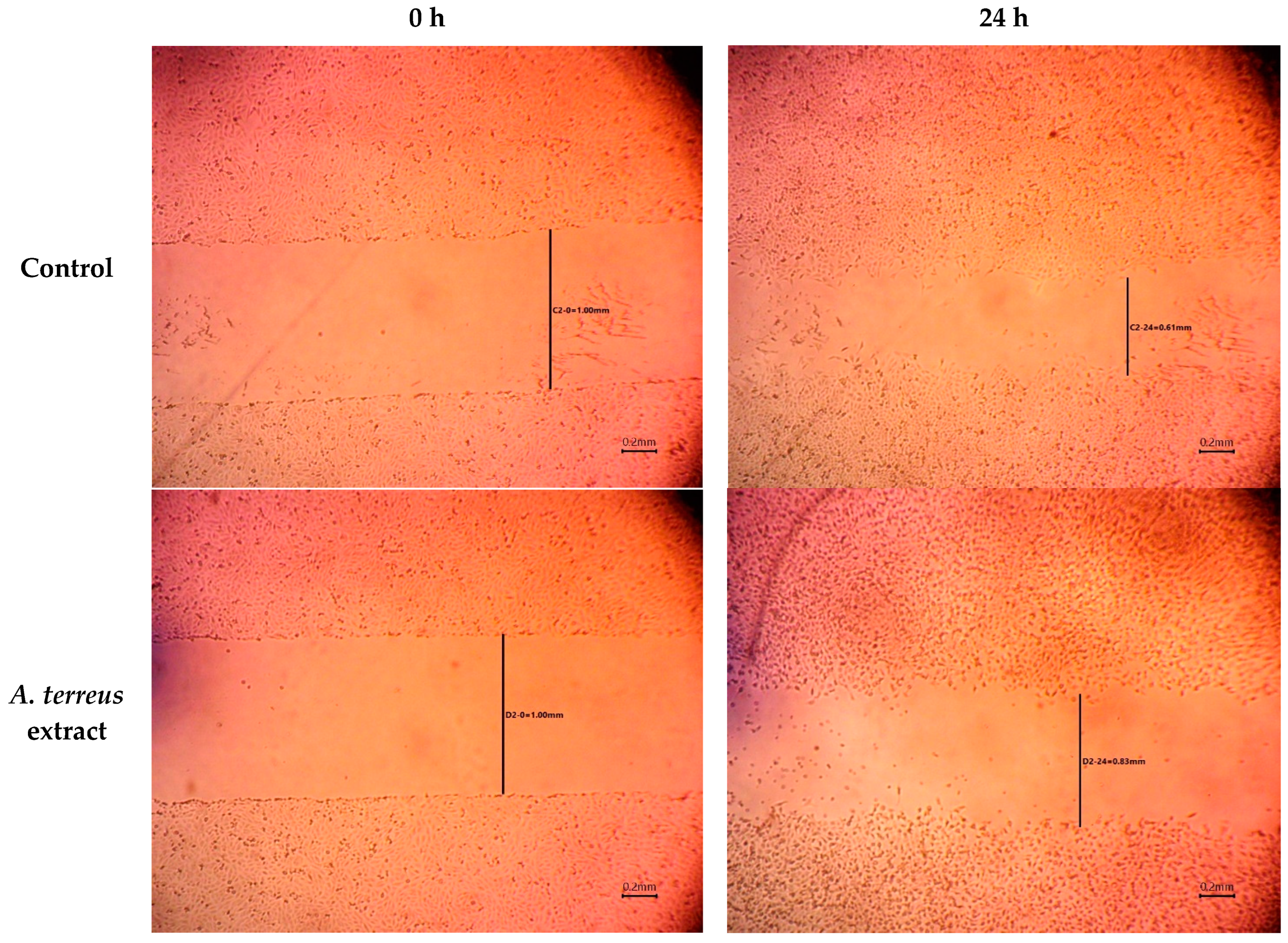

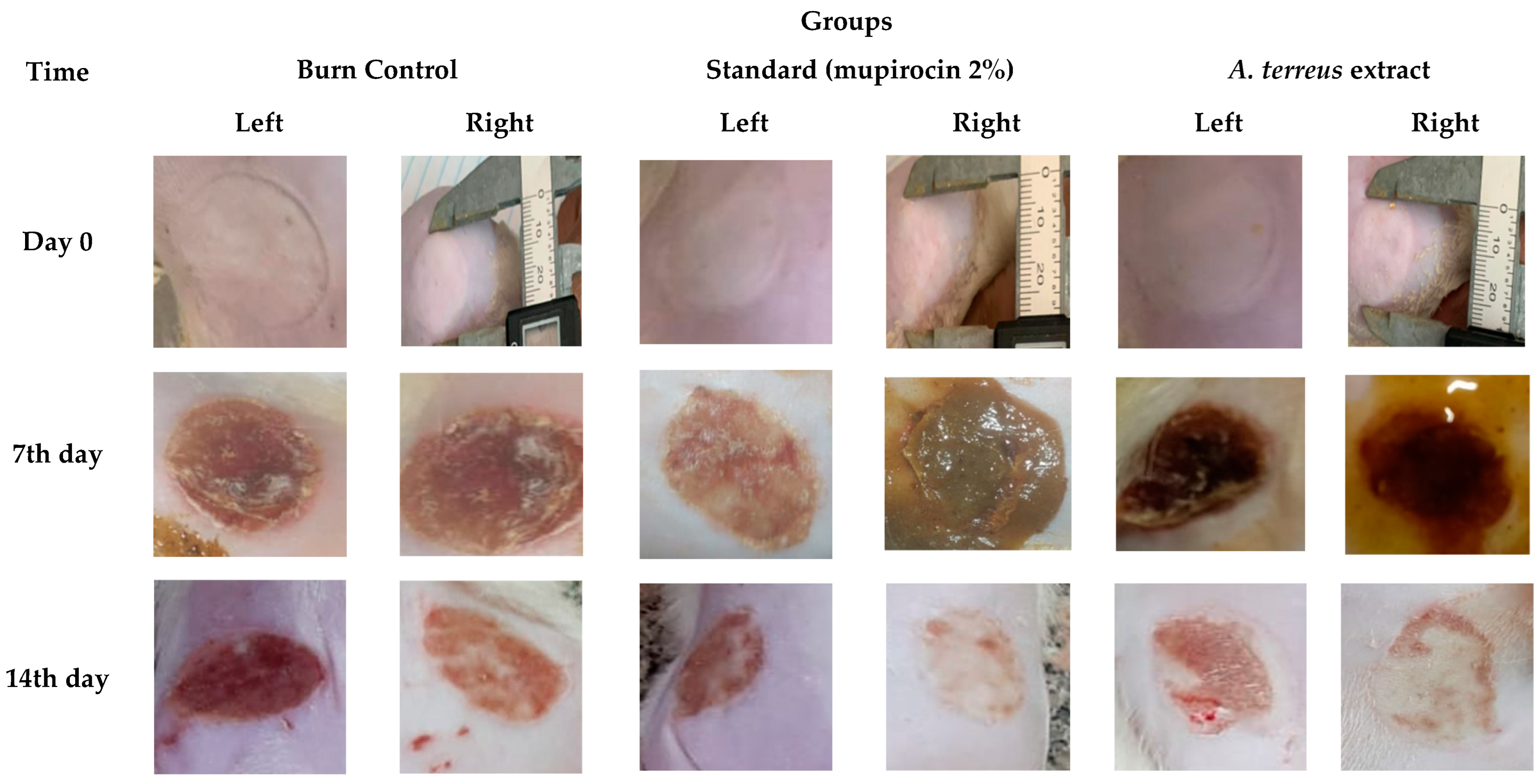
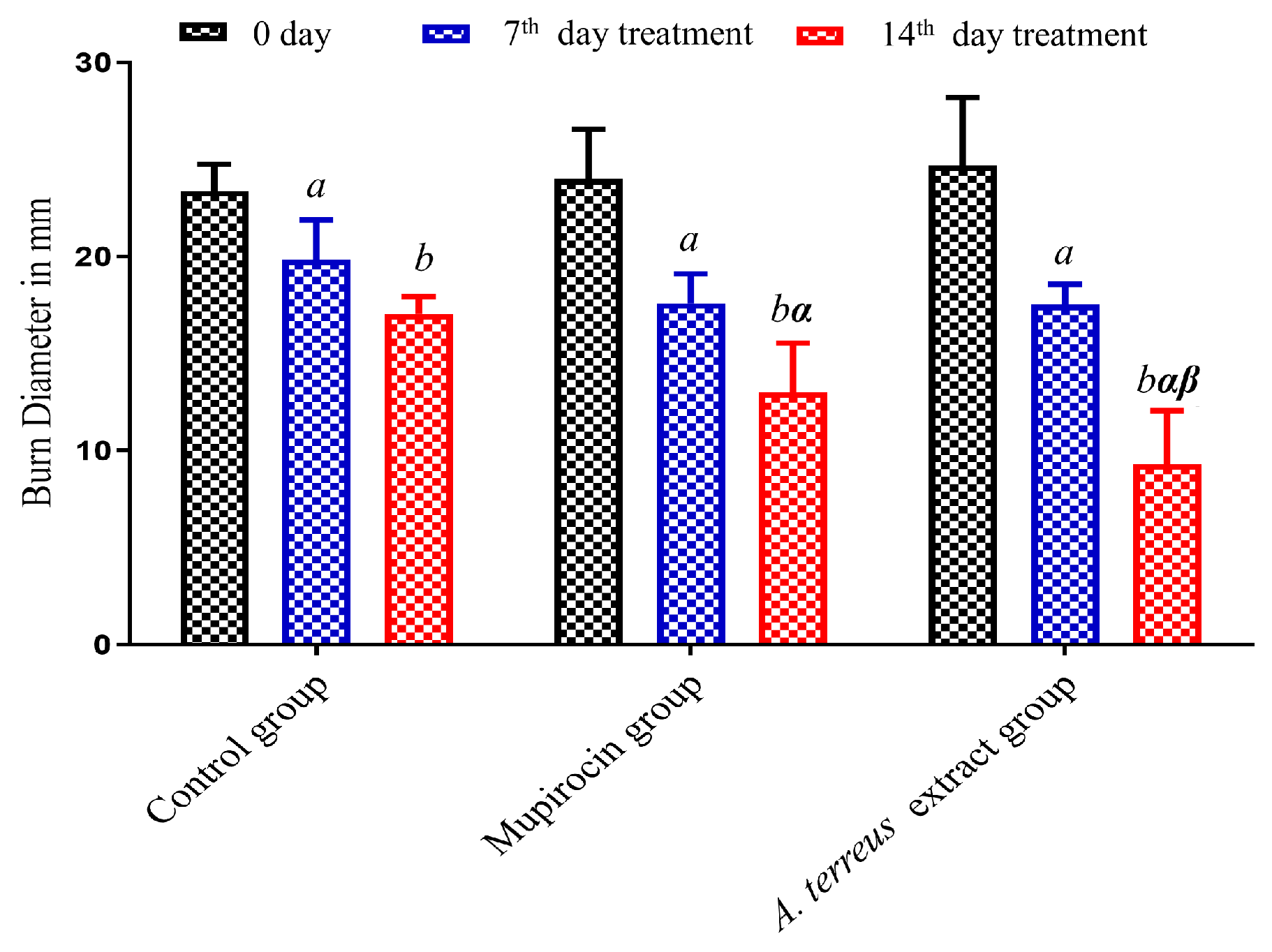


| Time (h) | Wound Width (mm) | |
|---|---|---|
| Control | A. terreus Extract | |
| 0 | 0.99 ± 0.027 | 1.02 ± 0.040 |
| 24 | 0.60 ± 0.015 | 0.84 ± 0.006 |
| 48 | 0.31 ± 0.012 | 0.82 ± 0.006 |
| 72 | 0.00 ± 0.00 | 0.30 ± 0.0252 |
| Time (day) | Groups–Burn Diameter (mm) | ||
|---|---|---|---|
| Control | Mupirocin | A. terreus Extract | |
| Day 0 | 23.13 ± 0.55 | 24.08 ± 0.93 | 24.41 ± 1.30 |
| 7th day | 20.21 ± 0.82 a | 17.08 ± 0.75 a | 16.95 ± 0.68 a |
| 14th day | 16.78 ± 0.41 b | 12.91 ± 0.93 b, α | 9.60 ± 1.05 b, α, β |
| No. | tR | M + H | MF | MS2 | Compound Name | Reported Source | Ref. |
|---|---|---|---|---|---|---|---|
| 1 | 5.65 | 155.05 | C7H6O4 | 137, 127 | Terreic acid | A. terreus | [21] |
| 2 | 8.57 | 139.07 | C7H6O3 | 121, 111 | 3-hydroxy-2,5-toluquinone | A. fumigatus | [22] |
| 3 | 8.94 | 262.16 | C14H15NO4 | 244, 216, 200, 132, 115 | Methyl (Z)-4-[(Z)-1-(hydroxymethyl)-2-phenyl-1-ethenyl] amino-4-oxo-2-butenoate | A. niger | [23] |
| 4 | 10.17 | 171.10 | C9H14O3 | 153, 125, 111, 107, 103 | Aspilactonol A | Aspergillus sp. 16-02-1 | [24] |
| 5 | 10.59 | 139.07 | C8H10O2 | 121, 111, 109 | Terreinlactone B | A. terreus | [25] |
| 6 | 10.84 | 239.03 | C12H14O5 | 221, 193, 177, 163, 149, 135 | Banksialactone C | A. banksianus | [26] |
| 7 | 11.58 | 423.01 | C19H18O11 | 387, 329, 313, 287 | Eurobenzophenone A | A. europaeus WZXY-SX-4-1 | [27] |
| 8 | 11.97 | 139.08 | C8H10O2 | 123, 121, 111, 103 | 2,5-Dimethylresorcinol | A. nidulans | [28] |
| 9 | 13.02 | 230.19 | C13H27NO2 | 213, 212, 171, 167, 137, 129 | 11-methyl-11-hydroxyldodecanoic acid amide | A. fumigatus JRJ111048 | [29] |
| 10 | 13.85 | 274.07 | C15H15NO4 | 256, 232, 218, 200 | Pyranterrone A/B | A. terreus | [30] |
| 11 | 14.20 | 189.05 | C8H12O5 | 171, 153, 141, 115, 105 | Aspersclerolide B | A. sclerotiorum | [31] |
| 12 | 14.65 | 449.10 | C20H20N2O8S | 431, 393, 363, 315, 285, 212 | Aspergillazine A | A. unilateralis | [32] |
| 13 | 17.37 | 235.12 | C11H14N4O2 | 219, 191, 179, 175, 219, 191, 179, 175, 135, 119 | Pre-aurantiamine | A. aculeatus CRI322-03 | [33] |
| 14 | 17.57 | 427.14 | C23H22O8 | 325, 295 | Versicolactone A | A. versicolor | [34] |
| 15 | 18.03 | 299.11 | C14H18O7 | 281, 267, 223, 197, 167 | Aspergilate D | Aspergillus sp. | [35] |
| 16 | 18.05 | 459.17 | C24H26O9 | 441, 401, 357, 221 | Aspernolide D | A. terreus RCBC1002 | [36] |
| 17 | 18.80 | 207.11 | C12H14O3 | 189, 171, 149, 135, 123, 121,105 | Terreprenphenol C | A. terreus EN-539 | [37] |
| 18 | 19.11 | 631.08 | C35H34O11 | 613, 603, 585, 575, 557, 529, 463 | Kodaistatin A | A. terreus DSM 11247 | [38] |
| 19 | 20.29 | 393.20 | C21H28O7 | 375, 335, 323, 281, 267, 253 | Asperdemin | A. versicolor | [39] |
| 20 | 20.45 | 463.27 | C26H38O7 | 445, 403, 389, 359, 305, 223, 207 | Asnovolin B | A. novofumigatus CBS117520 | [40] |
| 21 | 22.87 | 391.25 | C23H34O5 | 373, 363, 356,253, 237, 225 | Ustusolate A | A. ustus 094102 | [41] |
| 22 | 23.61 | 501.26 | C28H36O8 | 483, 469, 379, 273 | Tropolactone B | Aspergillus sp. | [42] |
| 23 | 23.73 | 577.31 | C35H44O7 | 559, 545, 531, 491, 451, 449, 447, 437, 421, 285 | Asperphenalenone A | Aspergillus sp. CPCC 400735 | [43] |
| 24 | 24.64 | 475.23 | C26H34O8 | 219, 137, 131, 121 | Terretonin D | A. terreus | [44] |
| 25 | 25.64 | 407.22 | C20H30N4O5 | 389, 295, 282 | Pre-sclerotiotide F | A. insulicola | [45] |
| 26 | 25.69 | 754.38 | C43H79NO9 | 736, 718, 574, 556, 294 | Flavicerebroside B | A. flavipes | [46] |
| 27 | 27.55 | 391.21 | C22H30O6 | 356, 251, 237, 223, 149, 121 | Ochraceopone E | A. ochraceopetaliformis SCSIO 05702 | [47] |
| No. | Compound Name | Pose Score (kcal/mol) | No. | Compound Name | Pose Score (kcal/mol) |
|---|---|---|---|---|---|
| 1 | Terreic acid | −9.63 | 15 | Aspergilate D | −12.45 |
| 2 | 3-hydroxy-2,5-toluquinone | −10.14 | 16 | Aspernolide D | −14.65 |
| 3 | Methyl (Z)-4-[(Z)-1-(hydroxymethyl)-2-phenyl-1-ethenyl] amino-4-oxo-2-butenoate | −10.58 | 17 | Terreprenphenol C | −9.53 |
| 4 | Aspilactonol A | −8.64 | 18 | Kodaistatin A | −12.19 |
| 5 | Terreinlactone B | −6.82 | 19 | Asperdemin | −9.62 |
| 6 | Banksialactone C | −11.33 | 20 | Asnovolin B | −8.11 |
| 7 | Eurobenzophenone A | −16.86 | 21 | Ustusolate A | −8.41 |
| 8 | 2,5-Dimethylresorcinol | −8.71 | 22 | Tropolactone B | −11.59 |
| 9 | 11-methyl-11-hydroxyldodecanoic acid amide | −9.59 | 23 | Asperphenalenone A | −12.65 |
| 10 | Pyranterrone A/B | −11.09 | 24 | Terretonin D | −9.61 |
| 11 | Aspersclerolide B | −11.18 | 25 | Pre-sclerotiotide F | −8.64 |
| 12 | Aspergillazine A | −9.86 | 26 | Flavicerebroside B | −10.71 |
| 13 | Pre-aurantiamine | −9.47 | 27 | Ochraceopone E | −8.73 |
| 14 | Versicolactone A | −12.08 | 28 | (E)-2-Fluoro-4′-methoxystilbene | −8.24 |
| No. | Compound Name | MW | Heavy Atoms | Aromatic Heavy Atoms | Rotatable Bonds | H-Bond Acceptors | H-Bond Donors | TPSA | Lipinski Violations | Bioavailability Score |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Terreic acid | 154.12 | 11 | 0 | 0 | 4 | 1 | 66.9 | 0 | 0.85 |
| 2 | 3-hydroxy-2,5-toluquinone | 138.12 | 10 | 0 | 0 | 3 | 1 | 54.37 | 0 | 0.85 |
| 3 | Methyl (Z)-4-[(Z)-1-(hydroxymethyl)-2-phenyl-1-ethenyl] amino-4-oxo-2-butenoate | 261.27 | 19 | 6 | 7 | 4 | 2 | 75.63 | 0 | 0.55 |
| 4 | Aspilactonol A | 170.21 | 12 | 0 | 3 | 3 | 1 | 46.53 | 0 | 0.55 |
| 5 | Terreinlactone B | 138.16 | 10 | 0 | 1 | 2 | 0 | 26.3 | 0 | 0.55 |
| 6 | Banksialactone C | 238.24 | 17 | 6 | 0 | 5 | 3 | 86.99 | 0 | 0.55 |
| 7 | Eurobenzophenone A | 422.34 | 30 | 12 | 8 | 11 | 7 | 202.05 | 2 | 0.11 |
| 8 | 2,5-Dimethylresorcinol | 138.16 | 10 | 6 | 0 | 2 | 2 | 40.46 | 0 | 0.55 |
| 9 | 11-methyl-11-hydroxyldodecanoic acid amide | 229.36 | 16 | 0 | 10 | 2 | 2 | 63.32 | 0 | 0.55 |
| 10 | Pyranterrone A/B | 273.28 | 20 | 9 | 3 | 4 | 2 | 87.23 | 0 | 0.55 |
| 11 | Aspersclerolide B | 188.18 | 13 | 0 | 3 | 5 | 2 | 75.99 | 0 | 0.55 |
| 12 | Aspergillazine A | 448.45 | 31 | 10 | 5 | 9 | 4 | 164.79 | 0 | 0.55 |
| 13 | Pre-aurantiamine | 234.25 | 17 | 5 | 2 | 3 | 3 | 86.88 | 0 | 0.55 |
| 14 | Versicolactone A | 426.42 | 31 | 12 | 8 | 8 | 2 | 119.36 | 0 | 0.55 |
| 15 | Aspergilate D | 298.29 | 21 | 6 | 7 | 7 | 3 | 113.29 | 0 | 0.55 |
| 16 | Aspernolide D | 458.46 | 33 | 12 | 8 | 9 | 5 | 153.75 | 0 | 0.55 |
| 17 | Terreprenphenol C | 206.24 | 15 | 6 | 3 | 3 | 1 | 49.83 | 0 | 0.55 |
| 18 | Kodaistatin A | 630.64 | 46 | 12 | 10 | 11 | 6 | 198.89 | 3 | 0.11 |
| 19 | Asperdemin | 392.44 | 28 | 6 | 0 | 7 | 2 | 106.2 | 0 | 0.55 |
| 20 | Asnovolin B | 462.58 | 33 | 0 | 3 | 7 | 1 | 99.13 | 0 | 0.56 |
| 21 | Ustusolate A | 390.51 | 28 | 0 | 7 | 5 | 3 | 86.99 | 0 | 0.55 |
| 22 | Tropolactone B | 500.58 | 36 | 7 | 4 | 8 | 0 | 105.2 | 1 | 0.55 |
| 23 | Asperphenalenone A | 576.72 | 42 | 10 | 12 | 7 | 5 | 135.29 | 1 | 0.55 |
| 24 | Terretonin D | 474.54 | 34 | 0 | 2 | 8 | 1 | 124.04 | 0 | 0.55 |
| 25 | Pre-sclerotiotide F | 406.48 | 29 | 0 | 6 | 5 | 3 | 124.68 | 0 | 0.55 |
| 26 | Flavicerebroside B | 754.09 | 53 | 0 | 34 | 9 | 7 | 168.94 | 2 | 0.17 |
| 27 | Ochraceopone E | 390.47 | 28 | 6 | 0 | 6 | 2 | 96.97 | 0 | 0.55 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al Mousa, A.A.; Abouelela, M.E.; Mansour, A.; Nasr, M.; Ali, Y.H.; Al Ghamidi, N.S.; Abo-Dahab, Y.; Mohamed, H.; Abo-Dahab, N.F.; Hassane, A.M.A. Wound Healing, Metabolite Profiling, and In Silico Studies of Aspergillus terreus. Curr. Issues Mol. Biol. 2024, 46, 11681-11699. https://doi.org/10.3390/cimb46100694
Al Mousa AA, Abouelela ME, Mansour A, Nasr M, Ali YH, Al Ghamidi NS, Abo-Dahab Y, Mohamed H, Abo-Dahab NF, Hassane AMA. Wound Healing, Metabolite Profiling, and In Silico Studies of Aspergillus terreus. Current Issues in Molecular Biology. 2024; 46(10):11681-11699. https://doi.org/10.3390/cimb46100694
Chicago/Turabian StyleAl Mousa, Amal A., Mohamed E. Abouelela, Ahmed Mansour, Mohamed Nasr, Yasser H. Ali, Nadaa S. Al Ghamidi, Youssef Abo-Dahab, Hassan Mohamed, Nageh F. Abo-Dahab, and Abdallah M. A. Hassane. 2024. "Wound Healing, Metabolite Profiling, and In Silico Studies of Aspergillus terreus" Current Issues in Molecular Biology 46, no. 10: 11681-11699. https://doi.org/10.3390/cimb46100694
APA StyleAl Mousa, A. A., Abouelela, M. E., Mansour, A., Nasr, M., Ali, Y. H., Al Ghamidi, N. S., Abo-Dahab, Y., Mohamed, H., Abo-Dahab, N. F., & Hassane, A. M. A. (2024). Wound Healing, Metabolite Profiling, and In Silico Studies of Aspergillus terreus. Current Issues in Molecular Biology, 46(10), 11681-11699. https://doi.org/10.3390/cimb46100694


_Kim.png)








