Homosalate and ERK Knockdown in the Modulation of Aurelia coerulea Metamorphosis by Regulating the PI3K Pathway and ERK Pathway
Abstract
1. Introduction
2. Materials and Methods
2.1. Regents and Animals
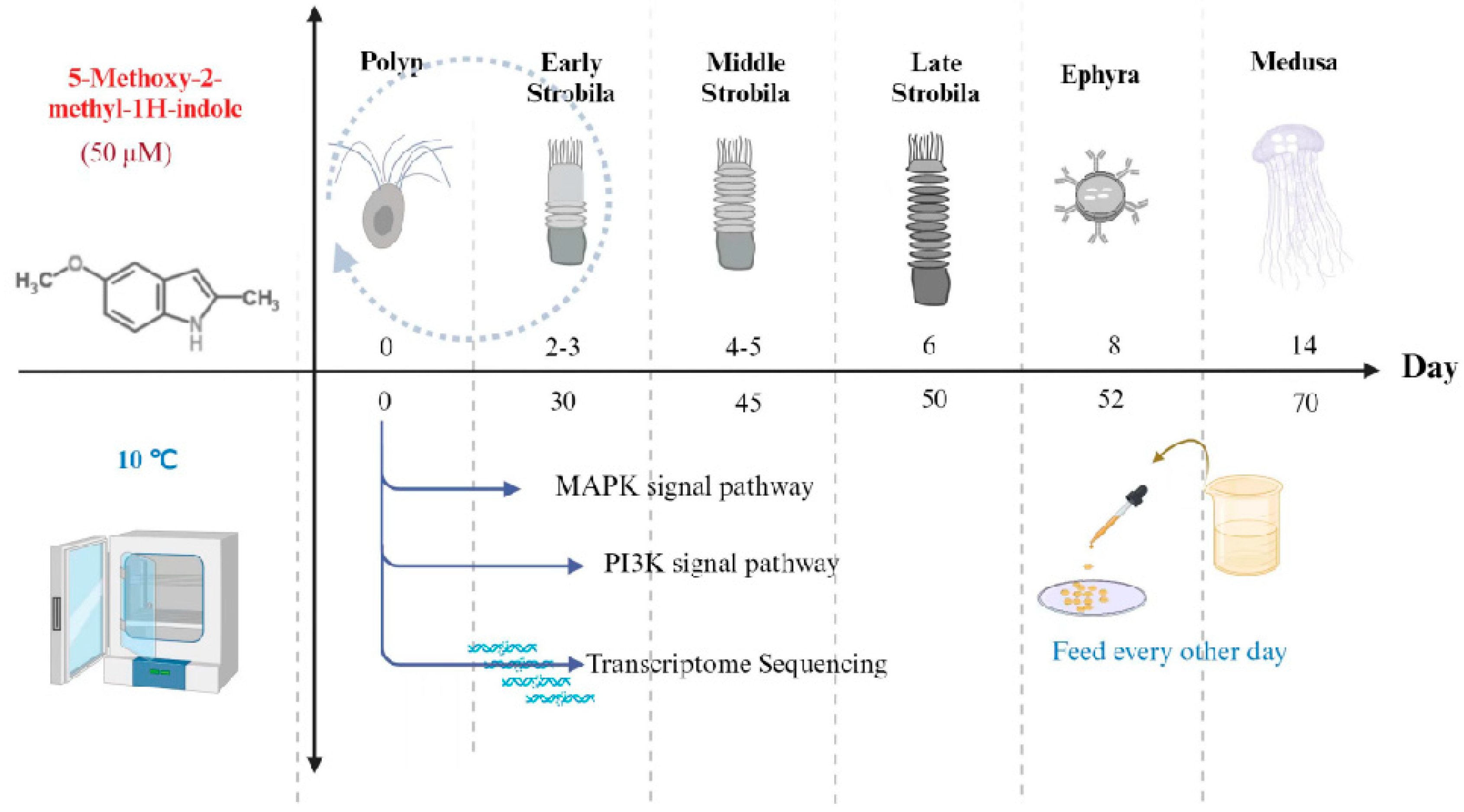
2.2. Culture of the Polyps and Induction of the Metamorphosis
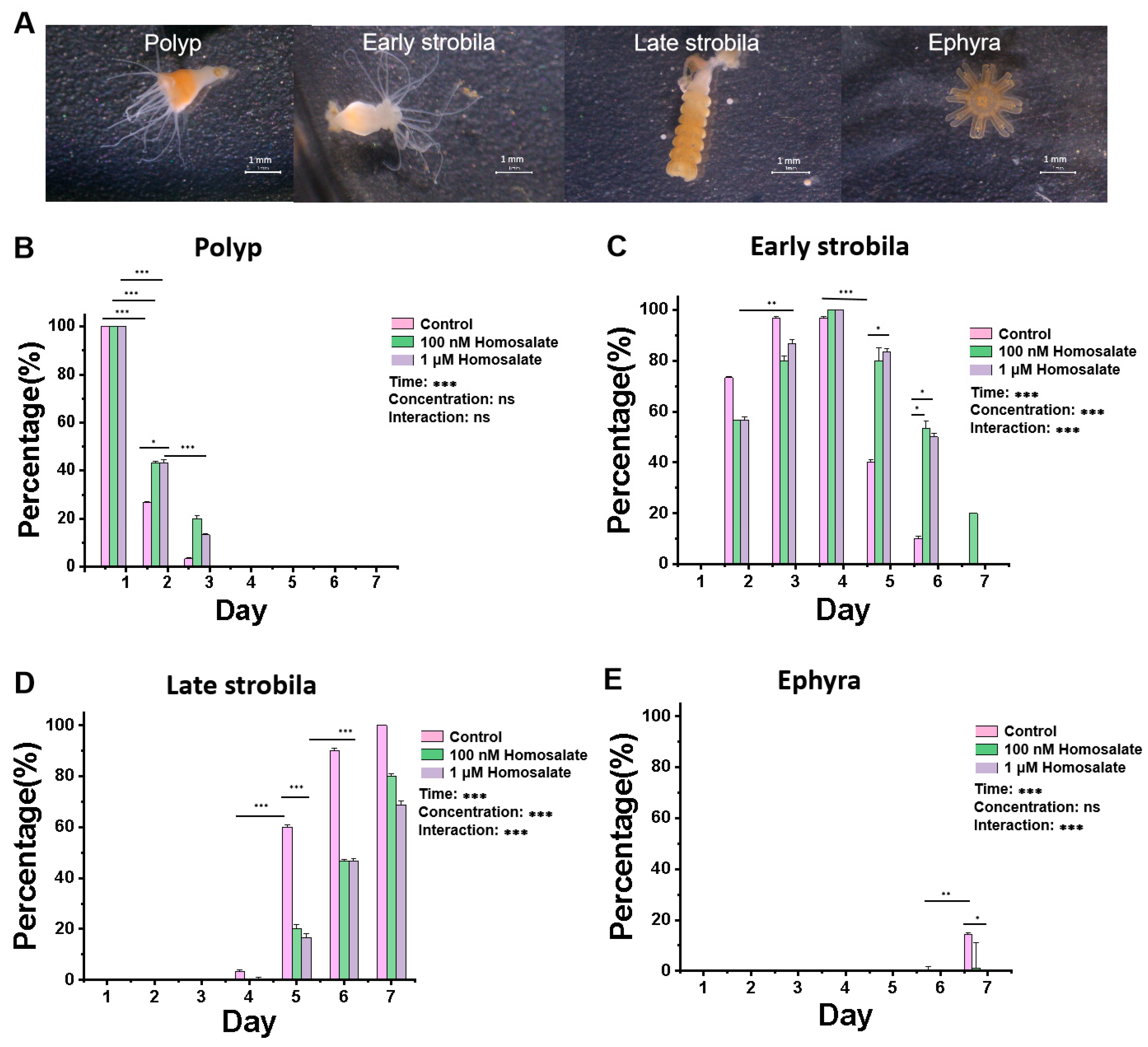
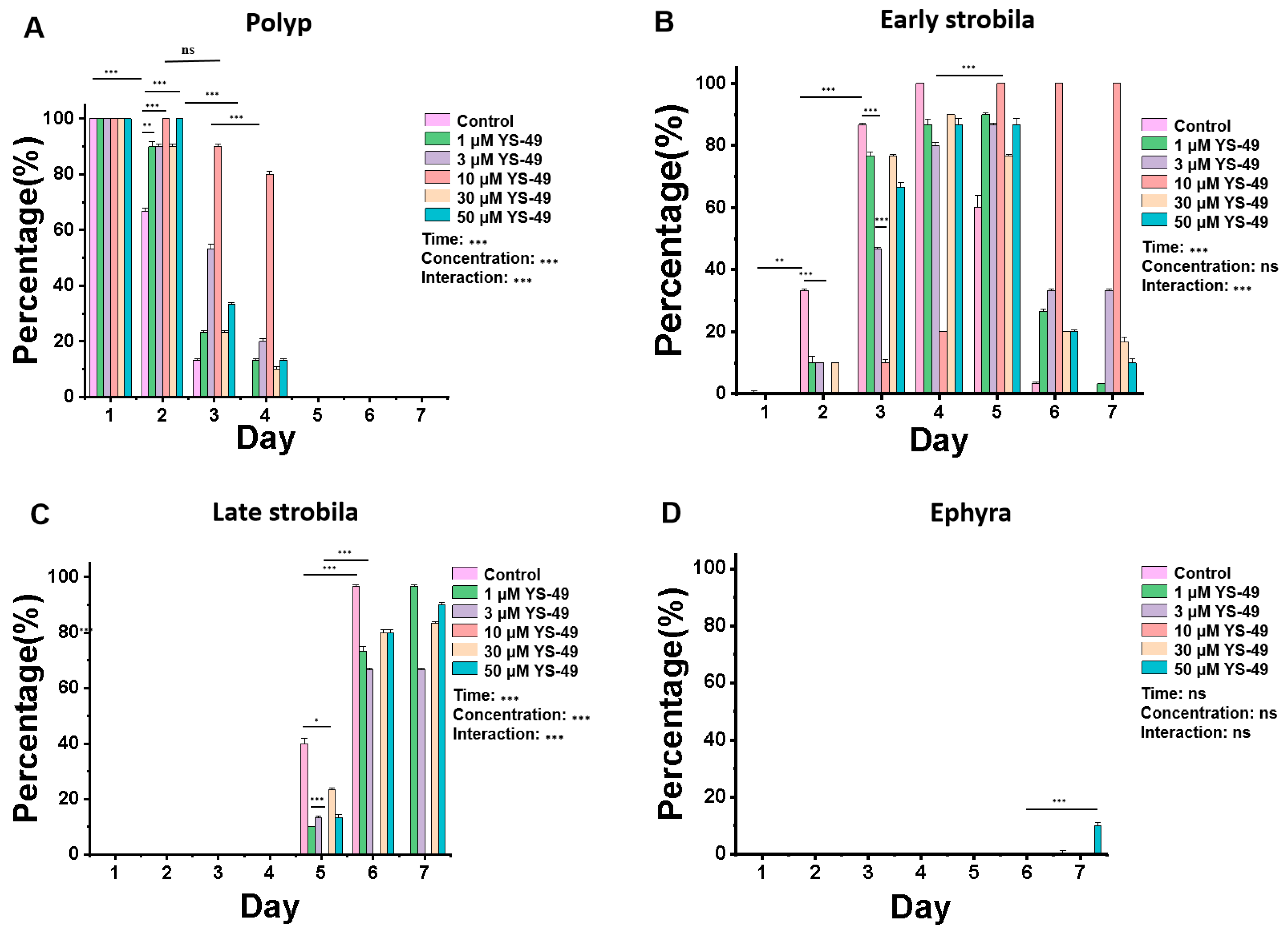
2.3. Effects of Signal Pathway Agonists Homosalate and YS-49 on the Metamorphosis of Aurelia coerulea
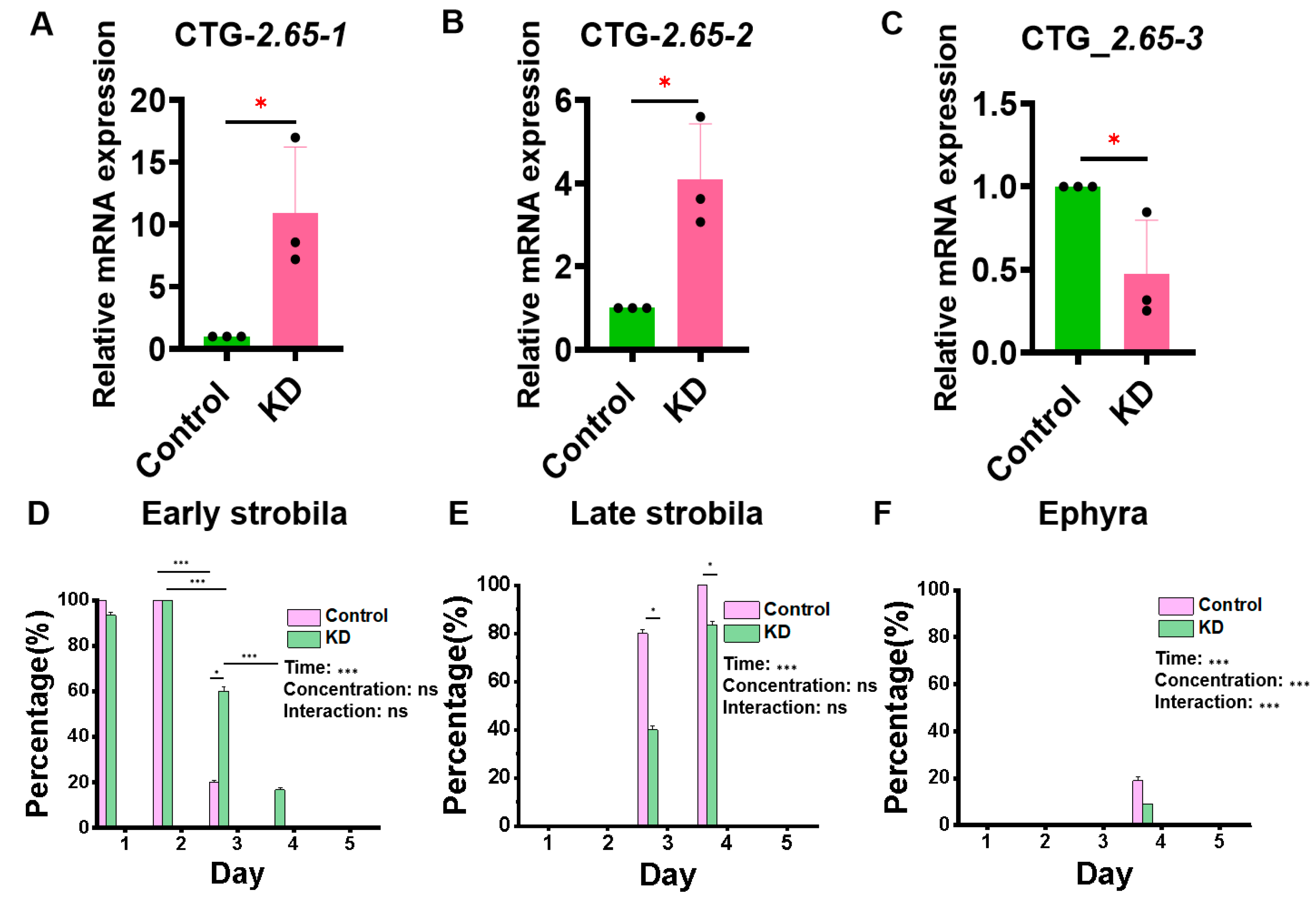
2.4. The Effect of ERK Molecular Knockdown on the Development of Metamorphosis
2.5. Extraction of Total RNA from the Samples
2.6. RT-qPCR
2.7. Quality Control, De Novo Assembly, and Functional Annotation
2.8. Statistical Analysis
3. Results
3.1. Homosalate Inhibits the Metamorphosis of Aurelia coerulea Polyps
3.2. YS-49 Inhibits the Metamorphosis of Aurelia coerulea Polyps
3.3. Effect of ERK Molecular Knockdown on Development and Metamorphosis of Aurelia coerulea Polyps
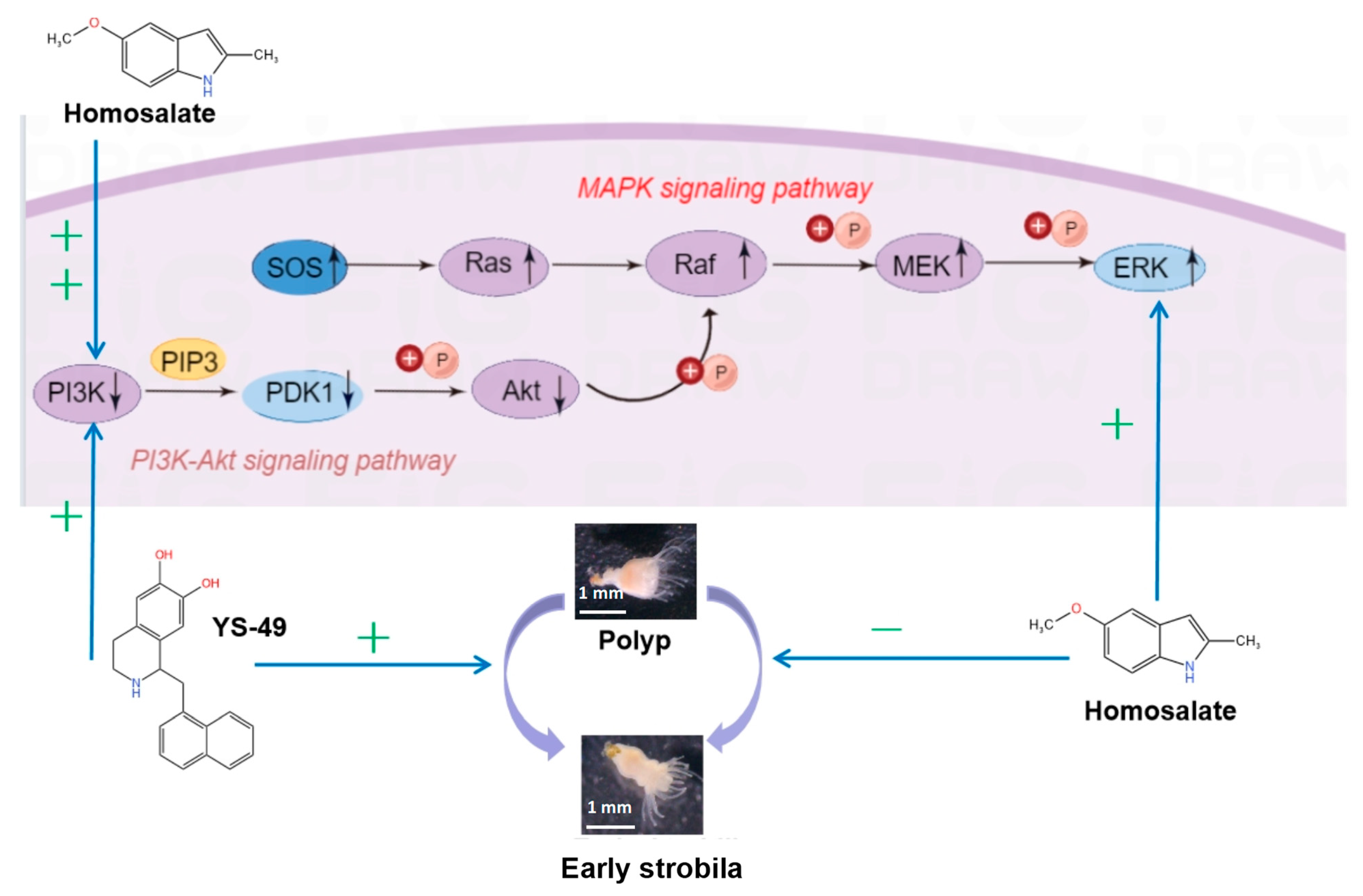
3.4. Signaling Pathway Map of Metamorphosis of Aurelia coerulea Polyps
4. Discussion
4.1. Control of Jellyfish Outbreaks from the Perspective of Regulating Metamorphic Development
4.2. Signaling Pathways in Jellyfish Metamorphosis
4.3. Implications for Environmental Management and Conservation
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
- Prepare artificial seawater. Weigh 0.5 kg of sea salt, evenly dissolve it in 5 L water, stir with a glass rod, wait for 15 min, and use a salinometer to test the salinity as 2.8–3.2‰. If the salinity is outside this range, increase the amount of water or sea salt based on the measurement results to adjust the salinity within the required range.
- Artemia incubation. Pour 2 g (a full spoon) of Artemia, and add 800 mL artificial seawater into the automatic beaker with the oxygen pumps. In summer, it takes 12 h to incubate fully, and 24–48 h in winter. After incubation, turn off the inflation pumps, wait for 10 min until there is obvious stratification (the top is brown shrimp shells, the middle is transparent seawater, and the bottom is orange Artemia), then pour out the floating shrimp shells on the upper layer quickly, and the orange particles at the bottom are the incubated Artemia. Collect the Artemia in a beaker and fill it with seawater again, wait for 10 min until the shrimp shells float and the Artemia sink, and then, pour out the floating shrimp shells quickly. The fresh Artemia is stored in a refrigerator at 4 °C, and should be used up within 2 days after marking the date.
- Feeding. Turn off the oxygen pumps and filtration devices in the large water tank for raising polyps. It is necessary to ensure that the Artemia is fresh, and feed it directly to the tentacles of the polyps with a disposable Pasteur pipette so that each polyp can eat fresh Artemia. After 2 h, the polyps will be fed, and it is time to change the water in the water tank for artificial seawater; then, the oxygen pumps are turned on. They are fed every two days.
- Environmental control of the water tank. In order to ensure that the salinity and specific gravity are within the normal range, water should be added to the water tank every day to replenish the evaporated seawater; the filter cotton should be cleaned every two days; the water should be changed thoroughly and the tank wall and bottom cleared every week.
References
- Purcell, J.E.; Uye, S.I.; Lo, W.T. Anthropogenic causes of jellyfish blooms and their direct consequences for humans: A review. Mar. Ecol. Prog. Ser. 2007, 350, 153–174. [Google Scholar] [CrossRef]
- Brotz, L.; Cheung, W.L.; Kleisner, K.; Pakhomov, E.; Pauly, D. Increasing jellyfish populations: Trends in Large Marine Ecosystems. Hydrobiologia 2012, 690, 3–20. [Google Scholar] [CrossRef]
- Boulware, D.R. A Randomized, Controlled Field Trial for the Prevention of Jellyfish Stings with a Topical Sting Inhibitor. J. Travel Med. 2010, 2, 166–171. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Yang, K.; Wang, Y.; Yao, J.; Hua, X.; Danso, B.; Wang, Y.; Liang, H.; Wang, M.; Chen, J.; et al. Insights into the discovery and intervention of metalloproteinase in marine hazardous jellyfish. J. Hazard. Mater. 2024, 472, 134526. [Google Scholar] [CrossRef]
- McIver, L.J.; Tjhung, I.G.; Parish, S.T.; Derkenne, R.C.; Kippin, A.N. Irukandji sydrome in the Torres Strait: A series of 8 cases. Wilderness Environ. Med. 2011, 22, 338–342. [Google Scholar] [CrossRef]
- Li, R.F.; Yu, H.H.; Yue, Y.; Xing, R.; Chen, X.L.; Wang, X.Q.; Li, P.C. In depth analysis of the in vivo toxicity of venom from the jellyfish Stomolophus meleagris. Toxicon 2014, 92, 60–65. [Google Scholar] [CrossRef] [PubMed]
- Lippmann, J.M.; Fenner, P.J.; Winkel, K.; Gershwin, L.A. Fatal and severe box jellyfish stings, including Irukandji stings, in Malaysia, 2000–2010. J. Travel Med. 2011, 18, 275–281. [Google Scholar] [CrossRef]
- Fenner, P.J.; Williamson, J.A.; Burnett, J.W.; Rifkin, J. First aid treatment of jellyfish stings in Australia. Response to a newly differentiated species. Med. J. Aust. 1993, 158, 498–501. [Google Scholar] [CrossRef]
- Cegolon, L.; Heymann, W.C.; Lange, J.H.; Mastrangelo, G. Jellyfish stings and their management: A review. Mar. Drugs 2013, 11, 523–550. [Google Scholar] [CrossRef]
- Peng, X.; Liu, K.T.; Chen, J.B.; Yan, Z.H.; Danso, B.; Wang, M.K.; Peng, Z.Y.; Xiao, L. Jellyfish Stings: A Review of Skin Symptoms, Pathophysiology, and Management. Med. Sci. Monit. 2024, 30, e944265. [Google Scholar] [CrossRef]
- Simmons, B.J.; Griffith, R.D.; Falto-Aizpurua, L.A.; Nouri, K. Moon jellyfish stings. JAMA Dermatol. 2015, 151, 454–456. [Google Scholar] [CrossRef] [PubMed][Green Version]
- DeLoughery, E.P. There’s something in the water: An overview of jellyfish, their stings, and treatment. Int. Marit. Health 2022, 73, 199–202. [Google Scholar] [CrossRef] [PubMed]
- Koch, T.L.; Grimmelikhuijzen, C.J.P. A comparative genomics study of neuropeptide genes in the cnidarian subclasses Hexacorallia and Ceriantharia. BMC Genom. 2020, 21, 666. [Google Scholar] [CrossRef]
- Nordstrom, B.; James, M.C.; Worm, B. Jellyfish distribution in space and time predicts leatherback sea turtle hot spots in the Northwest Atlantic. PLoS ONE 2020, 15, e0232628. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.P.; Zhang, F.; Sun, S. Predation effect on copepods by the giant jellyfish Nemopilema nomurai during the early occurrence stage in May in the northern East China Sea and southern Yellow Sea, China. Mar. Pollut. Bull. 2023, 186, 114462. [Google Scholar] [CrossRef]
- Yu, C.; Yue, Y.; Yin, X.J.; Li, R.F.; Yu, H.H.; Li, P.C. Identifying and revealing the geographical variation in Nemopilema nomurai venom metalloprotease and phospholipase A(2) activities. Chemosphere 2021, 266, 129164. [Google Scholar] [CrossRef]
- Kogovšek, T.; Vodopivec, M.; Raicich, F.; Uye, S.I.; Malej, A. Comparative analysis of the ecosystems in the northern Adriatic Sea and the Inland Sea of Japan: Can anthropogenic pressures disclose jellyfish outbreaks? Sci. Total Environ. 2018, 626, 982–994. [Google Scholar] [CrossRef]
- Cillari, T.; Allegra, A.; Berto, D.; Bosch-belmar, M.; Falautano, M.; Maggio, T.; Milisenda, G.; Perzia, P.; Rampazzo, F.; Sinopoli, M.; et al. Snapshot of the Distribution and Biology of Alien Jellyfish Cassiopea andromeda (Forsskål, 1775) in a Mediterranean Touristic Harbour. Biology 2022, 11, 319. [Google Scholar] [CrossRef]
- De Rinaldis, G.; Leone, A.; Domenico, S.D.; Bosch-belmar, M.; Slizyte, R.; Milisenda, G.; Santucci, A.; Albano, C.; Piraino, S. Biochemical Characterization of Cassiopea andromeda (Forsskål, 1775), Another Red Sea Jellyfish in the Western Mediterranean Sea. Mar. Drugs 2021, 19, 498. [Google Scholar] [CrossRef]
- Weissbourd, B.; Momose, T.; Nair, A.; Kennedy, A.; Hunt, B.; Anderson, D. A genetically tractable jellyfish model for systems and evolutionary neuroscience. Cell 2021, 184, 5854–5868.e5820. [Google Scholar] [CrossRef]
- Lewis, S. New model jellyfish? Nat. Rev. Neurosci. 2022, 23, 69. [Google Scholar] [CrossRef] [PubMed]
- Helm, R.R. Evolution and development of scyphozoan jellyfish. Biol. Rev. Camb. Philos. Soc. 2018, 93, 1228–1250. [Google Scholar] [CrossRef] [PubMed]
- Gengel, E.; Kuplik, Z.; Angel, D.; Heifetz, E. A physics-based model of swarming jellyfish. PLoS ONE 2023, 18, e0288378. [Google Scholar] [CrossRef] [PubMed]
- Costello, J.H.; Colin, S.P.; Dabiri, J.O.; Gemmell, B.J.; Lucas, K.N.; Sutherland, K.R. The Hydrodynamics of Jellyfish Swimming. Ann. Rev. Mar. Sci. 2021, 13, 375–396. [Google Scholar] [CrossRef]
- McAfee, J.S.; Benson, C.; Spangenberg, D.; Lattanzio, F.; Strasnick, B. Jellyfish model for ototoxicity. Otol. Neurotol. 2015, 36, 329–335. [Google Scholar] [CrossRef]
- Fujita, S.; Kuranaga, E.; Nakajima, Y.I. Regeneration Potential of Jellyfish: Cellular Mechanisms and Molecular Insights. Genes 2021, 12, 758. [Google Scholar] [CrossRef]
- Sinigaglia, C.; Person, S.; Eichelbrenner, J.; Chevalier, S.; Steger, J.; Barreau, C.; Houliston, E.; Leclère, L. Pattern regulation in a regenerating jellyfish. Elife 2020, 9, e54868. [Google Scholar] [CrossRef]
- Wang, B.; Zhang, B.; Huo, H.; Wang, T.; Wu, Y.; Xiao, L.; Ren, Y.Q.; Zhang, L.M. Detection of microvasculature alterations by synchrotron radiation in murine with delayed jellyfish envenomation syndrome. Toxicon 2014, 81, 48–53. [Google Scholar] [CrossRef]
- Nabipour, I.; Mohebbi, G.; Vatanpour, H.; Vazirizadeh, A. Hematological parameters on the effect of the jellyfish venom Cassiopea andromeda in animal models. Data Brief 2017, 11, 517–521. [Google Scholar] [CrossRef]
- Kang, C.; Jin, Y.B.; Kwak, J.; Yoon, W.D.; Yoon, T.J.; Kim, J.S.; Kim, E. Protective effect of tetracycline against dermal toxicity induced by Jellyfish venom. PLoS ONE 2013, 8, e57658. [Google Scholar] [CrossRef]
- Bellingeri, A.; Battocchio, C.; Faleri, C.; Protano, G.; Venditti, L.; Corsi, I. Sensitivity of Hydra vulgaris to Nanosilver for Environmental Applications. Toxics 2022, 10, 693. [Google Scholar] [CrossRef] [PubMed]
- Dong, Z.; Liu, D.; Keesing, J.K. Jellyfish blooms in China: Dominant species, causes and consequences. Mar. Pollut. Bull. 2010, 60, 954–963. [Google Scholar] [CrossRef] [PubMed]
- Yuezhu, S.; Zhengtao, Q.; Caixia, X.; Mingxia, L.; Lizhuang, M.; Honglan, Z. Clinical analysis of 136 cases of acute sea moon jellyfish sting. Chin. J. Ind. Hyg. Occup. Dis. 2002, 1, 68–69. [Google Scholar]
- Yuezhu, S.; Jinrong, W.; Shuxiang, G.; Lijing, W.; Xiuhua, B.; Chunxia, W.; Mingxia, L. Effect of acute sea moon jellyfish sting on heart. Chin. J. Emerg. Med. 2001, 3, 194–195. [Google Scholar]
- Geng, X.Y.; Wang, M.K.; Hou, X.C.; Wang, Z.F.; Wang, Y.; Zhang, D.Y.; Danso, B.; Wei, D.B.; Shou, Z.Y.; Xiao, L.; et al. Comparative Analysis of Tentacle Extract and Nematocyst Venom: Toxicity, Mechanism, and Potential Intervention in the Giant Jellyfish Nemopilema nomurai. Mar. Drugs 2024, 22, 362. [Google Scholar] [CrossRef]
- Li, L.; Zhu, Y.; Wu, F.; Shen, Y.; Wang, Y.; Hofer, J.; Pozzolini, M.; Wang, M.; Xiao, L.; Dai, X. Microbial Diversity and Screening for Potential Pathogens and Beneficial Bacteria of Five Jellyfish Species-Associated Microorganisms Based on 16S rRNA Sequencing. Pol. J. Microbiol. 2024, 73, 297–314. [Google Scholar] [CrossRef]
- Wang, J.Y. Effects of Temperature and Food Level on Asexual Reproduction of Sea Moon Jellyfish and Molecular Detection of Jellyfish, Ocean University of China. 2013. Available online: https://d.wanfangdata.com.cn/thesis/Y2657283 (accessed on 30 September 2024).
- Leclère, L.; Horin, C.; Chevalier, S.; Lapébie, P.; Dru, P.; Peron, S.; Jager, M.; Condamine, T.; Pottin, K.; Romano, S.; et al. The genome of the jellyfish Clytia hemisphaerica and the evolution of the cnidarian life-cycle. Nat. Ecol. Evol. 2019, 3, 801–810. [Google Scholar] [CrossRef]
- Fuchs, B.; Wang, W.; Graspeuntner, S.; Li, Y.Z.; Insua, S.; Herbst, E.; Dirken, P.; Böhm, A.; Hemmrich, G.; Sommer, F.; et al. Regulation of polyp-to-jellyfish transition in Aurelia aurita. Curr. Biol. 2014, 24, 263–273. [Google Scholar] [CrossRef] [PubMed]
- Kuniyoshi, H.; Okumura, I.; Kuroda, R.; Tsujita, N.; Arakawa, K.; Shoji, J.; Saito, T.; Osada, H. Indomethacin induction of metamorphosis from the asexual stage to sexual stage in the moon jellyfish, Aurelia aurita. Biosci. Biotechnol. Biochem. 2012, 76, 1397–1400. [Google Scholar] [CrossRef]
- Ge, J.; Liu, C.; Tan, J.; Bian, L.; Chen, S. Transcriptome analysis of scyphozoan jellyfish Rhopilema esculentum from polyp to medusa identifies potential genes regulating strobilation. Dev. Genes Evol. 2018, 228, 243–254. [Google Scholar] [CrossRef]
- Gufler, S.; Artes, B.; Bielen, H.; Krainer, I.; Eder, M.K.; Falschlunger, J.; Bollmann, A.; Ostermann, T.; Valovka, T.; Hartl, M.; et al. beta-Catenin acts in a position-independent regeneration response in the simple eumetazoan Hydra. Dev. Biol. 2018, 433, 310–323. [Google Scholar] [CrossRef]
- Zhong, Z.; Jiao, Z.; Yu, F.X. The Hippo signaling pathway in development and regeneration. Cell Rep. 2024, 43, 113926. [Google Scholar] [CrossRef] [PubMed]
- Cardenas, M.M.; Salgado, L.M. STK, the src homologue, is responsible for the initial commitment to develop head structures in Hydra. Dev. Biol. 2003, 264, 495–505. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Fabila, Y.; Navarro, L.; Fujisawa, T.; Bode, H.R.; Salgado, L.M. Selective inhibition of protein kinases blocks the formation of a new axis, the beginning of budding, in Hydra. Mech. Dev. 2002, 119, 157–164. [Google Scholar] [CrossRef] [PubMed]
- Münder, S.; Käsbauer, T.; Prexl, A.; Aufschnaiter, R.; Zhang, X.; Towb, P.; Böttger, A. Notch signalling defines critical boundary during budding in Hydra. Dev. Biol. 2010, 344, 331–345. [Google Scholar] [CrossRef]
- Ishii, A.; Furusho, M.; Bansal, R. Mek/ERK1/2-MAPK and PI3K/Akt/mTOR signaling plays both independent and cooperative roles in Schwann cell differentiation, myelination and dysmyelination. Glia 2021, 69, 2429–2446. [Google Scholar] [CrossRef]
- Liu, M.; Zhang, S. Amphioxus IGF-like peptide induces mouse muscle cell development via binding to IGF receptors and activating MAPK and PI3K/Akt signaling pathways. Mol. Cell. Endocrinol. 2011, 343, 45–54. [Google Scholar] [CrossRef]
- Xu, Y.; Li, N.; Xiang, R.; Sun, P. Emerging roles of the p38 MAPK and PI3K/AKT/mTOR pathways in oncogene-induced senescence. Trends Biochem. Sci. 2014, 39, 268–276. [Google Scholar] [CrossRef]
- Chambon, J.P.; Nakayama, A.; Takamura, K.; McDougall, A.; Satoh, N. ERK- and JNK-signalling regulate gene networks that stimulate metamorphosis and apoptosis in tail tissues of ascidian tadpoles. Development 2007, 134, 1203–1219. [Google Scholar] [CrossRef]
- Castellano, I.; Ercolesi, E.; Palumbo, A. Nitric oxide affects ERK signaling through down-regulation of MAP kinase phosphatase levels during larval development of the ascidian Ciona intestinalis. PLoS ONE 2014, 9, e102907. [Google Scholar] [CrossRef][Green Version]
- Wang, B.; Liu, D.; Wang, C.; Wang, Q.Q.; Zhang, H.; Liu, G.Y.; He, Q.; Zhang, L.M. Tentacle extract from the jellyfish Cyanea capillata increases proliferation and migration of human umbilical vein endothelial cells through the ERK1/2 signaling pathway. PLoS ONE 2017, 12, e0189920. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Wang, B.B.; Wang, B.; Wang, Q.Q.; Liu, G.Y.; Fan, C.; Zhang, L.M. A novel granulin homologue isolated from the jellyfish Cyanea capillata promotes proliferation and migration of human umbilical vein endothelial cells through the ERK1/2-signaling pathway. Int. J. Biol. Macromol. 2019, 135, 212–225. [Google Scholar] [CrossRef] [PubMed]
- Krause, M.; Klit, A.; Jensen, M.B.; Søeborg, T.; Frederiksen, H.; Schlumpf, M.; Lichtensteiger, W.; Skakkebaek, N.E.; Drzewiecki, K.T. Sunscreens: Are they beneficial for health? An overview of endocrine disrupting properties of UV-filters. Int. J. Androl. 2012, 35, 424–436. [Google Scholar] [CrossRef]
- Gago-Ferrero, P.; Díaz-Cruz, M.S.; Barceló, D. An overview of UV-absorbing compounds (organic UV filters) in aquatic biota. Anal. Bioanal. Chem. 2012, 404, 2597–2610. [Google Scholar] [CrossRef] [PubMed]
- Schlumpf, M.; Cotton, B.; Haller, V.; Steinmann, B.; Lichetensteger, W. In vitro and in vivo estrogenicity of UV screens. Environ. Health Perspect. 2001, 109, 239–244. [Google Scholar] [CrossRef]
- Sarveiya, V.; Risk, S.; Benson, H.A. Liquid chromatographic assay for common sunscreen agents: Application to in vivo assessment of skin penetration and systemic absorption in human volunteers. J. Chromatogr. B Analyt Technol. Biomed. Life Sci. 2004, 803, 225–231. [Google Scholar] [CrossRef]
- Yazar, S.; Kara Ertekin, S. Assessment of the cytotoxicity and genotoxicity of homosalate in MCF-7. J. Cosmet. Dermatol. 2020, 19, 246–252. [Google Scholar] [CrossRef]
- Erol, M.; Cok, I.; Gayret, Ö.B.; Günes, P.; Yigit, Ö.; Sayman, E.; Günes, A.; Celik, D.S.; Hamilcikan, S.; Altinay, S.; et al. Evaluation of the endocrine-disrupting effects of homosalate (HMS) and 2-ethylhexyl 4-dimethylaminobenzoate (OD-PABA) in rat pups during the prenatal, lactation, and early postnatal periods. Toxicol. Ind. Health 2017, 33, 775–791. [Google Scholar] [CrossRef]
- Jiménez-Díaz, I.; Molina-Molina, J.M.; Molina-Molina, A.; Ballesteros, O.; Navalón, A.; Real, M.; Sáenz, J.M.; Fernández, M.F.; Olea, N. Simultaneous determination of the UV-filters benzyl salicylate, phenyl salicylate, octyl salicylate, homosalate, 3-(4-methylbenzylidene) camphor and 3-benzylidene camphor in human placental tissue by LC-MS/MS. Assessment of their in vitro endocrine activity. J. Chromatogr. B Analyt Technol. Biomed. Life Sci. 2013, 936, 80–87. [Google Scholar] [CrossRef]
- Palioura, E.; Diamanti-Kandarakis, E. Polycystic ovary syndrome (PCOS) and endocrine disrupting chemicals (EDCs). Rev. Endocr. Metab. Disord. 2015, 16, 365–371. [Google Scholar] [CrossRef]
- Hain, E.; He, K.; Batista-Andrade, J.A.; Feerick, A.; Tarnowski, M.; Timm, A.; Blaney, L. Geospatial and co-occurrence analysis of antibiotics, hormones, and UV filters in the Chesapeake Bay (USA) to confirm inputs from wastewater treatment plants, septic systems, and animal feeding operations. J. Hazard. Mater. 2023, 460, 132405. [Google Scholar] [CrossRef] [PubMed]
- Thorel, E.; Clergeaud, F.; Jaugeon, L.; Rodrigues, A.M.; Lucas, J.; Stien, D.; Lebaron, P. Effect of 10 UV Filters on the Brine Shrimp Artemia salina and the Marine Microalga Tetraselmis sp. Toxics 2020, 8, 29. [Google Scholar] [CrossRef] [PubMed]
- Kunz, P.Y.; Fent, K. Multiple hormonal activities of UV filters and comparison of in vivo and in vitro estrogenic activity of ethyl-4-aminobenzoate in fish. Aquat. Toxicol. 2006, 79, 305–324. [Google Scholar] [CrossRef] [PubMed]
- Corinaldesi, C.; Damiani, E.; Marcellini, F.; Falugi, C.; Tiano, L.; Bruge, F.; Danovaro, R. Sunscreen products impair the early developmental stages of the sea urchin Paracentrotus lividus. Sci. Rep. 2017, 7, 7815. [Google Scholar] [CrossRef]
- Liu, J.; Chang, Y.-T.; Kou, Y.-Y.; Zhang, P.-P.; Dong, Q.-L.; Guo, R.-Y.; Liu, L.-Y.; Lin, H.-W.; Yang, F. Marine sponge-derived alkaloid inhibits the PI3K/AKT/mTOR signaling pathway against diffuse large B-cell lymphoma. Med. Oncol. 2024, 41, 212. [Google Scholar] [CrossRef]
- Xinhui, Z.; Fan, Z.; Yan, L.; Na, F.; Ke, Z.; Anding, Z.; Jiefang, K.; Yan, L.; Xiaochang, X.; Xun, J. Blockade of PI3K/AKT signaling pathway by Astragaloside IV attenuates ulcerative colitis via improving the intestinal epithelial barrier. J. Transl. Med. 2024, 22, 406. [Google Scholar] [CrossRef]
- Xintong, C.; Xiaoya, L.; Zheng, X.; Qing, L.; ZhaoYun, P.; YiNa, Z.; JianPing, H.; Wei, L.; Jianguo, C.; Liang, X. The distinct microbial community in Aurelia coerulea polyps versus medusae and its dynamics after exposure to 60Co-γ radiation. Environ. Res. 2020, 188, 109843. [Google Scholar] [CrossRef]
- Hou, J.; Zhou, Y.; Zheng, Y.; Fan, J.; Zhou, W.; Ng, I.O.; Sun, H.; Qin, L.; Qiu, S.; Lee, J.M.; et al. Hepatic RIG-I predicts survival and interferon-alpha Therapeutic Response in Hepatocellular Carcinoma. Cancer Cell 2014, 25, 49–63. [Google Scholar] [CrossRef]
- Yan, D.; Ming, S.; Nan, L.; Xiang, X.; Aiyong, W.; Jing, D. Effects of temperature and salinity on the growth and survival of moon jellyfish (Aurelia coerulea) ephyrae. Acta Ecol. Sin. 2022, 42, 6356–6367. [Google Scholar]
- Jingyu, L.; Xiaohua, D.; Jiqun, S.; Xin, C.; Xu, W.; Xueqing, J.; Xin, T.; Zhaohui, Z.; Yan, B.; Mugent, L.; et al. Identification and functional characterization of a novel splicing mutation in RP gene PRPF31. Biochem. Biophys. Res. Commun. 2008, 367, 420–426. [Google Scholar] [CrossRef]
- Asati, V.; Mahapatra, D.K.; Bharti, S.K. PI3K/Akt/mTOR and Ras/Raf/MEK/ERK signaling pathways inhibitors as anticancer agents: Structural and pharmacological perspectives. Eur. J. Med. Chem. 2016, 109, 314–341. [Google Scholar] [CrossRef] [PubMed]
- Chappell, W.H.; Steelman, L.S.; Long, J.M.; Kempf, R.C.; Abrams, S.; Franklin, R.A.; Bäsecke, J.; Stivala, F.; Donia, M.; Fagone, P.; et al. Ras/Raf/MEK/ERK and PI3K/PTEN/Akt/mTOR inhibitors: Rationale and importance to inhibiting these pathways in human health. Oncotarget 2011, 2, 135–164. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Lim, W.; Bazer, F.W.; Song, G. Homosalate aggravates the invasion of human trophoblast cells as well as regulates intracellular signaling pathways including PI3K/AKT and MAPK pathways. Environ. Pollut. 2018, 243, 1263–1273. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.; Chen, L.; Yang, J. Network pharmacology and in vitro experiments reveal that Noscapine induces ROS-mediated apoptosis and cell cycle arrest via PI3K/Akt/FoxO3a signaling pathway in human bladder cancer cells. Curr. Cancer Drug Targets. 2023. online ahead of print. [Google Scholar] [CrossRef]
- Setia, S.; Nehru, B.; Sanyal, S.N. Upregulation of MAPK/Erk and PI3K/Akt pathways in ulcerative colitis-associated colon cancer. Biomed. Pharmacother. 2014, 68, 1023–1029. [Google Scholar] [CrossRef]
- Fojtík, P.; Beckerová, D.; Holomková, K.; Šenfluk, M.; Rotrekl, V. Both Hypoxia-Inducible Factor 1 and MAPK Signaling Pathway Attenuate PI3K/AKT via Suppression of Reactive Oxygen Species in Human Pluripotent Stem Cells. Front. Cell Dev. Biol. 2020, 8, 607444. [Google Scholar] [CrossRef]
- Liu, L.; Zhu, W.; Liu, J.; Wang, S.; Jiang, J. Identification and differential regulation of microRNAs during thyroid hormone-dependent metamorphosis in Microhyla fissipes. BMC Genom. 2018, 19, 507. [Google Scholar] [CrossRef]
- Jiang, Y.; Zhao, X.; Chen, J.; Aniagu, S.; Chen, T. PM2.5 induces cardiac malformations via PI3K/akt2/mTORC1 signaling pathway in zebrafish larvae. Environ. Pollut. 2023, 323, 121306. [Google Scholar] [CrossRef]
- Li, H.Y.; Wang, T.; Yang, Y.P.; Geng, S.L.; Xu, W.H. TGF-β signaling regulates p-Akt levels via PP2A during diapause entry in the cotton bollworm, Helicoverpa armigera. Insect Biochem. Mol. Biol. 2017, 87, 165–173. [Google Scholar] [CrossRef]
- Gu, S.H.; Lin, P.L.; Hsieh, H.Y. Bombyxin/Akt signaling in relation to the embryonic diapause process of the silkworm, Bombyx mori. J. Insect Physiol. 2019, 116, 32–40. [Google Scholar] [CrossRef]
- Shikuma, N.J.; Antoshechkin, I.; Medeiros, J.M.; Pilhofer, M.; Newman, D.K. Stepwise metamorphosis of the tubeworm Hydroides elegans is mediated by a bacterial inducer and MAPK signaling. Proc. Natl. Acad. Sci. USA 2016, 113, 10097–10102. [Google Scholar] [CrossRef] [PubMed]
- Faimali, M.; Garaventa, F.; Piazza, V.; Costa, E.; Greco, G.; Mazzola, V.; Beltrandi, M.; Bongiovanni, E.; Lavorano, S.; Gnone, G. Ephyra jellyfish as a new model for ecotoxicological bioassays. Mar. Environ. Res. 2014, 93, 93–101. [Google Scholar] [CrossRef] [PubMed]
- Costa, E.; Gambardella, C.; Piazza, V.; Vassalli, M.; Sbrana, F.; Lavorano, S.; Garaventa, F.; Famimali, M. Microplastics ingestion in the ephyra stage of Aurelia sp. triggers acute and behavioral responses. Ecotoxicol. Environ. Saf. 2020, 189, 109983. [Google Scholar] [CrossRef]
- Echols, B.S.; Smith, A.J.; Gardinali, P.R.; Rand, G.M. The use of ephyrae of a scyphozoan jellyfish, Aurelia aurita, in the aquatic toxicological assessment of Macondo oils from the Deepwater Horizon incident. Chemosphere 2016, 144, 1893–1900. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Qian, Y. Clinical analysis of 48 cases of jellyfish dermatitis. J. Nanjing Mil. Med. Coll. 2003, 332. [Google Scholar]
- Li, R.; Yu, H.; Li, A.; Yu, C.; Li, P. Refinement and Neutralization Evaluation of the F(ab’)(2) Type of Antivenom against the Deadly Jellyfish Nemopilema nomurai Toxins. Int. J. Mol. Sci. 2021, 22, 12672. [Google Scholar] [CrossRef]
| Number | Query ID | Forward | Reverse |
|---|---|---|---|
| A | evm.model.CTG_2.65 | ACCTCACAGAACTTGTATCCACTCG | CGCATCCACTACTCCAAACATCAAC |
| B | GAPGH | CCGTGTTCCAGTCCCAGATGTTTC | CCTTGCTCTCTGATGCTGCCTTC |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, J.; Geng, X.; Li, B.; Xie, J.; Ma, J.; Qin, Z.; Wang, M.; Yang, J. Homosalate and ERK Knockdown in the Modulation of Aurelia coerulea Metamorphosis by Regulating the PI3K Pathway and ERK Pathway. Curr. Issues Mol. Biol. 2024, 46, 11630-11645. https://doi.org/10.3390/cimb46100690
Chen J, Geng X, Li B, Xie J, Ma J, Qin Z, Wang M, Yang J. Homosalate and ERK Knockdown in the Modulation of Aurelia coerulea Metamorphosis by Regulating the PI3K Pathway and ERK Pathway. Current Issues in Molecular Biology. 2024; 46(10):11630-11645. https://doi.org/10.3390/cimb46100690
Chicago/Turabian StyleChen, Jinhong, Xiaoyu Geng, Bingbing Li, Jinyao Xie, Jieying Ma, Zhen Qin, Mingke Wang, and Jishun Yang. 2024. "Homosalate and ERK Knockdown in the Modulation of Aurelia coerulea Metamorphosis by Regulating the PI3K Pathway and ERK Pathway" Current Issues in Molecular Biology 46, no. 10: 11630-11645. https://doi.org/10.3390/cimb46100690
APA StyleChen, J., Geng, X., Li, B., Xie, J., Ma, J., Qin, Z., Wang, M., & Yang, J. (2024). Homosalate and ERK Knockdown in the Modulation of Aurelia coerulea Metamorphosis by Regulating the PI3K Pathway and ERK Pathway. Current Issues in Molecular Biology, 46(10), 11630-11645. https://doi.org/10.3390/cimb46100690



_Kim.png)




