Hepatotoxicity Induced by Methyl Eugenol: Insights from Toxicokinetics, Metabolomics, and Gut Microbiota
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagentss and Chemicals
2.2. Animal Experiments
2.3. Assessment of Liver Injury Markers
2.4. Histopathological Analysis of Liver Tissue
2.5. Toxicokinetics Investigation
2.6. Metabolomics Profiling
2.7. 16S rRNA Gene Sequencing
2.8. Statistical Analysis
3. Results
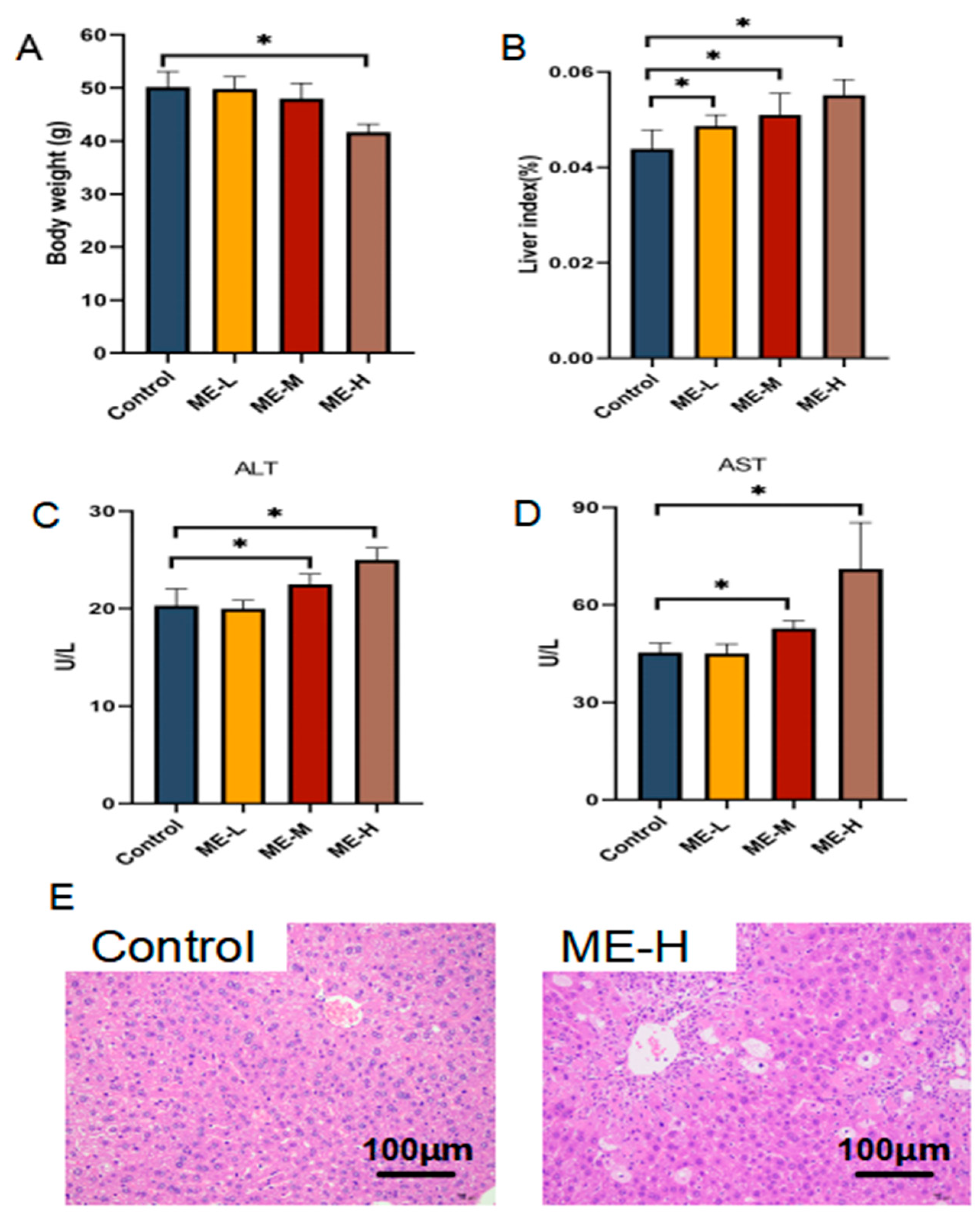
3.1. Hepatotoxic Effects of ME in Mice
3.2. Toxicokinetics Results
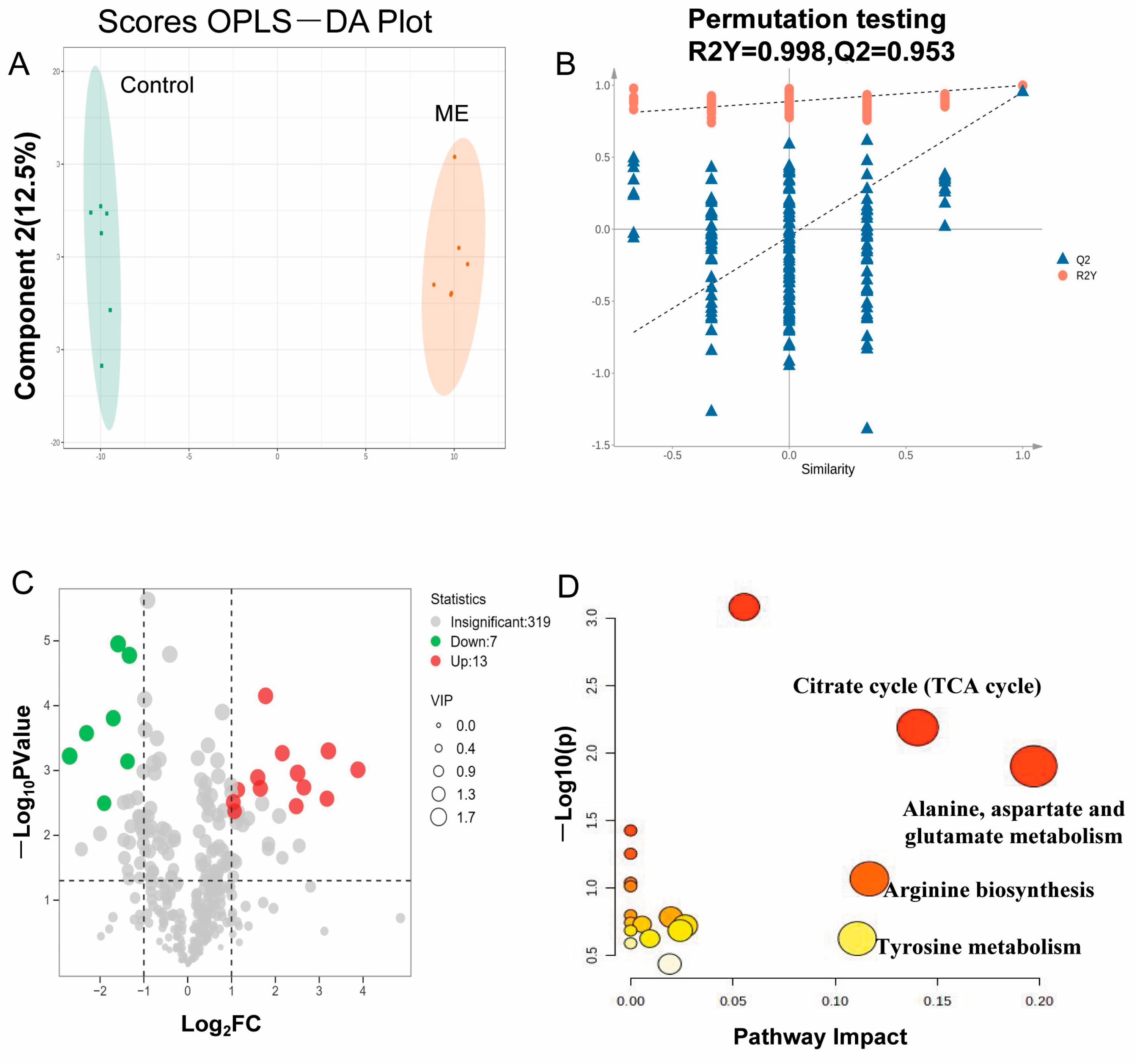
3.3. Metabolomics Analysis Results
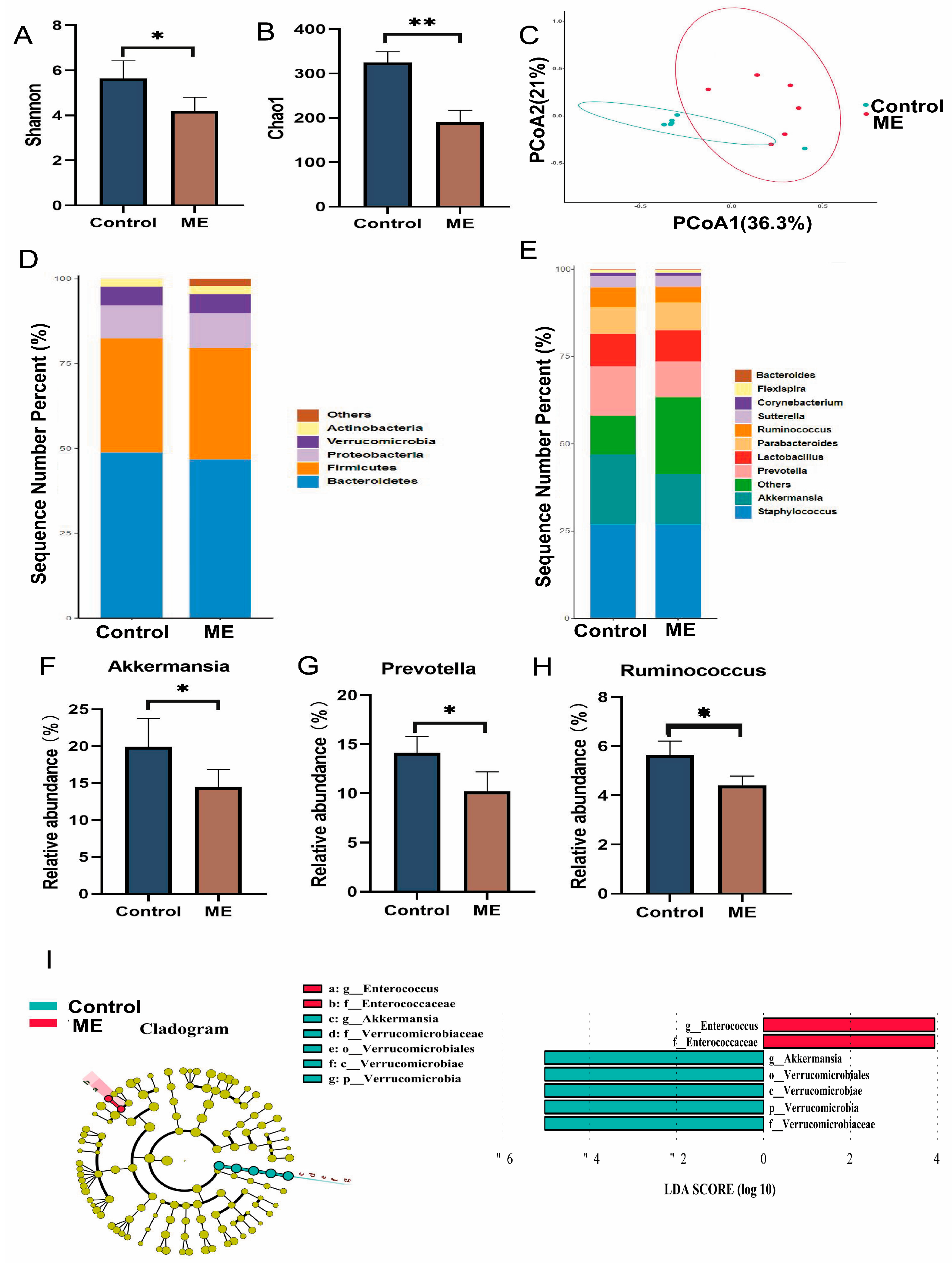
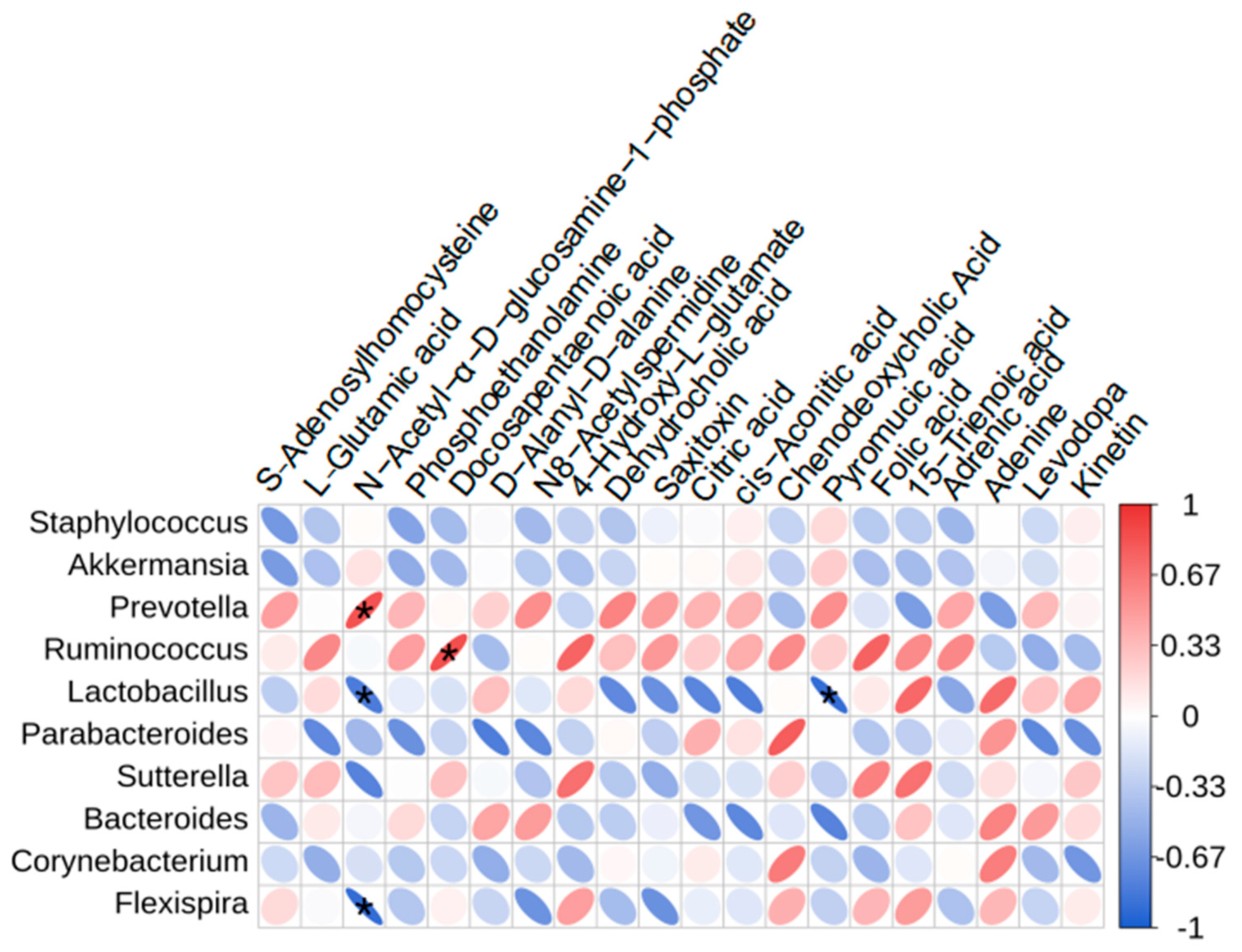
3.4. Impact of ME on Gut Microbiota
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- De Alencar Silva, A.; de Morais, L.P.; de Sena Bastos, C.M.; de Menezes Dantas, D.; Batista, P.R.; Dias, F.J.; Alencar de Menezes, I.R.; Cardoso, J.H.L.; Raposo, A.; Han, H.; et al. Vasorelaxant effect of phenylpropanoids: Methyl eugenol and eugenol in human umbilical cord vein. Biomed. Pharmacother. 2024, 178, 117227. [Google Scholar] [CrossRef] [PubMed]
- Rasool, A.; Fatima, S.; Shah, S.H.; Munis, M.F.H.; Irshad, A.; Shelly, T.E.; Haq, I.U. Methyl eugenol aromatherapy: A delivery system facilitating the simultaneous application of male annihilation and sterile insect technique against the peach fruit fly. Pest Manag. Sci. 2024, 80, 1465–1473. [Google Scholar] [CrossRef]
- Royer, J.E.; Mayer, D.G. Combining cue-lure and methyl eugenol in traps significantly decreases catches of most Bactrocera, Zeugodacus and Dacus species (Diptera: Tephritidae: Dacinae) in Australia and Papua New Guinea. J. Econ. Entomol. 2018, 111, 298–303. [Google Scholar] [CrossRef]
- Oliveira, M.C.N.; Cavalcante, I.L.; de Araújo, A.N.; Ferreira Dos Santos, A.M.; de Menezes, R.P.B.; Herrera-Acevedo, C.; Ferreira de Sousa, N.; de Souza Aquino, J.; Barbosa-Filho, J.M.; de Castro, R.D.; et al. Methyleugenol has an antidepressant effect in a neuroendocrine model: In silico and in vivo evidence. Pharmaceuticals 2023, 16, 1408. [Google Scholar] [CrossRef]
- Fezza, T.; Shelly, T.E.; Fox, A.; Beucke, K.; Rohrig, E.; Aldebron, C.; Manoukis, N.C. Less is more: Fewer attract-and-kill sites improve the male annihilation technique against Bactrocera dorsalis (Diptera: Tephritidae). PLoS ONE 2024, 19, e0300866. [Google Scholar] [CrossRef]
- Huang, J.L.; Xu, Y.H.; Yang, X.W.; Wang, J.; Zhu, Y.; Wu, X.B. Jiawei guomin decoction regulates the degranulation of mast cells in atopic dermatitis mice via the HIS/PAR-2 pathway. J. Ethnopharmacol. 2024, 321, 117485. [Google Scholar] [CrossRef]
- Anwar, M.; Rimsha, G.; Majeed, M.I.; Alwadie, N.; Nawaz, H.; Majeed, M.Z.; Rashid, N.; Zafar, F.; Kamran, A.; Wasim, M.; et al. Rapid identification and quantification of adulteration in methyl eugenol using raman spectroscopy coupled with multivariate data analysis. ACS Omega 2024, 9, 7545–7553. [Google Scholar] [CrossRef]
- Gogoi, R.; Loying, R.; Sarma, N.; Begum, T.; Pandey, S.K.; Lal, M. Comparative analysis of in-vitro biological activities of methyl eugenol rich Cymbopogon Khasianus Hack., leaf essential oil with pure methyl eugenol compound. Curr. Pharm. Biotechnol. 2020, 21, 927–938. [Google Scholar] [CrossRef]
- Ghosh, P.K.; Tamili, D.; Das, A.; Datta, S.; Das, S.; Saha, S.; Kuotsu, K.; Bhattacharjee, P. Valorization of tuberose flower waste through development of therapeutic products using supercritical carbon dioxide extraction and microencapsulation technologies. World J. Microbiol. Biotechnol. 2023, 39, 319. [Google Scholar] [CrossRef]
- Zhang, X.; Felter, S.P.; Api, A.M.; Joshi, K.; Selechnik, D. A cautionary tale for using read-across for cancer hazard classification: Case study of isoeugenol and methyl eugenol. Regul. Toxicol. Pharmacol. 2022, 136, 105280. [Google Scholar] [CrossRef]
- Rivera-Pérez, A.; López-Ruiz, R.; Romero-González, R.; Garrido Frenich, A. A new strategy based on gas chromatography-high resolution mass spectrometry (GC-HRMS-Q-Orbitrap) for the determination of alkenylbenzenes in pepper and its varieties. Food Chem. 2020, 321, 126727. [Google Scholar] [CrossRef]
- Nagarajan, S.R.; Cross, E.; Sanna, F.; Hodson, L. Dysregulation of hepatic metabolism with obesity: Factors influencing glucose and lipid metabolism. Proc. Nutr. Soc. 2022, 81, 1–11. [Google Scholar] [CrossRef]
- Deshmukh, P.U.; Lad, S.B.; Sudarsan, A.; Sudhakar, S.; Aggarwal, T.; Mandal, S.; Bagale, S.S.; Kondabagil, K.; Pradeepkumar, P.I. Human translesion synthesis polymerases polκ and polη perform error-free replication across N2-dG methyleugenol and estragole DNA adducts. Biochemistry 2023, 62, 2391–2406. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Wang, H.; Wang, Q.; Huang, W.; Peng, Y.; Zheng, J. Chemical interaction of protein cysteine residues with reactive metabolites of methyleugenol. Chem. Res. Toxicol. 2017, 30, 564–573. [Google Scholar] [CrossRef]
- Carlsson, M.J.; Vollmer, A.S.; Demuth, P.; Heylmann, D.; Reich, D.; Quarz, C.; Rasenberger, B.; Nikolova, T.; Hofmann, T.G.; Christmann, M.; et al. p53 triggers mitochondrial apoptosis following DNA damage-dependent replication stress by the hepatotoxin methyleugenol. Cell Death Dis. 2022, 13, 1009. [Google Scholar] [CrossRef]
- Carvalho, R.P.R.; Ribeiro, F.C.D.; Lima, T.I.; Ervilha, L.O.G.; de Oliveira, E.L.; Faustino, A.O.; Lima, G.D.A.; Machado-Neves, M. High doses of eugenol cause structural and functional damage to the rat liver. Life Sci. 2022, 304, 120696. [Google Scholar] [CrossRef]
- Joardar, A.; Meher, G.; Bag, B.P.; Chakraborty, H. Host-guest complexation of eugenol in cyclodextrins for enhancing bioavailability. J. Mol. Liq. 2020, 319, 114336. [Google Scholar] [CrossRef]
- Moreau, M.; Jamalpoor, A.; Hall, J.C.; Fisher, J.; Hartvelt, S.; Hendriks, G.; Nong, A. Animal-free assessment of developmental toxicity: Combining PBPK modeling with the ReproTracker assay. Toxicology. 2023, 500, 153684. [Google Scholar] [CrossRef]
- Miao, X.; Chen, J.; Su, Y.; Luo, J.; He, Y.; Ma, J.; He, X. Plasma metabolomic analysis reveals the therapeutic effects of Jiashen tablets on heart failure. Front. Cardiovasc. Med. 2022, 9, 1047322. [Google Scholar] [CrossRef]
- Mattoli, L.; Gianni, M.; Burico, M. Mass spectrometry-based metabolomic analysis as a tool for quality control of natural complex products. Mass Spectrom. Rev. 2023, 42, 1358–1396. [Google Scholar] [CrossRef]
- Shang, L.; Wang, Y.; Li, J.; Zhou, F.; Xiao, K.; Liu, Y.; Zhang, M.; Wang, S.; Yang, S. Mechanism of Sijunzi decoction in the treatment of colorectal cancer based on network pharmacology and experimental validation. J. Ethnopharmacol. 2023, 302 Pt A, 115876. [Google Scholar] [CrossRef]
- Chen, L.; Li, L.; Wang, F.; Hu, S.; Ding, T.; Wang, Y.; Huang, Y.; Fan, B.; Sun, J. Targeted metabolomics study on the effect of vinegar processing on the chemical changes and antioxidant activity of Angelica sinensis. Antioxidants 2023, 12, 2053. [Google Scholar] [CrossRef] [PubMed]
- Hsu, C.L.; Schnabl, B. The gut-liver axis and gut microbiota in health and liver disease. Nat. Rev. Microbiol. 2023, 21, 719–733. [Google Scholar] [CrossRef] [PubMed]
- Kamal, F.D.; Dagar, M.; Reza, T.; Karim Mandokhail, A.; Bakht, D.; Shahzad, M.W.; Silloca-Cabana, E.O.; Mohsin, S.N.; Chilla, S.P.; Bokhari, S.F.H. Beyond diet and exercise: The impact of gut microbiota on control of obesity. Cureus 2023, 15, e49339. [Google Scholar] [CrossRef]
- Yan, M.; Man, S.; Sun, B.; Ma, L.; Guo, L.; Huang, L.; Gao, W. Gut liver brain axis in diseases: The implications for therapeutic interventions. Signal Transduct. Target. Ther. 2023, 8, 43. [Google Scholar] [CrossRef]
- Fan, Y.; Yang, D.; Huang, X.; Yao, G.; Wang, W.; Gao, M.; Jia, X.; Ouyang, H.; Chang, Y.; He, J. Pharmacokinetic study of safrole and methyl eugenol after oral administration of the essential oil extracts of Asarum in rats by GC-MS. Biomed. Res. Int. 2021, 2021, 6699033. [Google Scholar] [CrossRef]
- Gao, S.S.; Shen, Y.L.; Chen, Y.W.; Wei, X.Z.; Hu, J.J.; Wang, J.; Wu, W.J. Liver Metabolomics analysis revealing key metabolites associated with different stages of nonalcoholic fatty liver disease in hamsters. Comb. Chem. High Throughput Screen. 2024, 27, 1303–1317. [Google Scholar] [CrossRef]
- Leontidou, K.; Abad-Recio, I.L.; Rubel, V.; Filker, S.; Däumer, M.; Thielen, A.; Lanzén, A.; Stoeck, T. Simultaneous analysis of seven 16S rRNA hypervariable gene regions increases efficiency in marine bacterial diversity detection. Environ. Microbiol. 2023, 25, 3484–3501. [Google Scholar] [CrossRef]
- Schueller, O.; Willson, A.; Singh, N.; Lohmer, L.; Alabanza, A.; Patel, J. A Phase 1 Pharmacokinetic Drug Interaction Study of Belumosudil Coadministered with CYP3A4 Inhibitors and Inducers and Proton Pump Inhibitors. Clin. Pharmacol. Drug Dev. 2022, 11, 795–806. [Google Scholar] [CrossRef]
- Danek, P.J.; Basińska-Ziobroń, A.; Wójcikowski, J.; Daniel, W.A. Levomepromazine and clozapine induce the main human cytochrome P450 drug metabolizing enzyme CYP3A4. Pharmacol. Rep. 2021, 73, 303–308. [Google Scholar] [CrossRef]
- Janssens, G.E.; Houtkooper, R.H. Identification of longevity compounds with minimized probabilities of side effects. Biogerontology 2020, 21, 709–719. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Fang, C.; Wang, C.; Jin, C.; Qian, M.; Jin, Y. Maternal sodium p-perfluorous nonenoxybenzene sulfonate exposure disturbed lipid metabolism and induced an imbalance in tyrosine metabolism in the F1 generation of mice. Chem. Res. Toxicol. 2022, 35, 651–662. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Slaven, J.E.; Nephew, L.; Patidar, K.R.; Desai, A.P.; Orman, E.; Kubal, C.; Chalasani, N.; Ghabril, M. Severe hepatic encephalopathy with mechanical ventilation may inform waitlist priority in acute liver failure: A UNOS database analysis. Clin. Transplant. 2024, 38, e15215. [Google Scholar] [CrossRef] [PubMed]
- Pearl, N.Z.; Babin, C.P.; Catalano, N.T.; Blake, J.C.; Ahmadzadeh, S.; Shekoohi, S.; Kaye, A.D. Narrative review of topiramate: Clinical uses and pharmacological considerations. Adv. Ther. 2023, 40, 3626–3638. [Google Scholar] [CrossRef]
- Zhao, R.; Zhao, Q.; Wang, X.; Chen, X.; Liang, C.; Xiao, Q.; Yang, S.; Tan, S. Yi-Qi-Jian-Pi formula inhibits hepatocyte pyroptosis through the IDH2-driven tricarboxylic acid cycle to reduce liver injury in acute-on-chronic liver failure. J. Ethnopharmacol. 2023, 317, 116683. [Google Scholar] [CrossRef]
- Pannala, V.R.; Estes, S.K.; Rahim, M.; Trenary, I.; O’Brien, T.P.; Shiota, C.; Printz, R.L.; Reifman, J.; Shiota, M.; Young, J.D.; et al. Toxicant-induced metabolic alterations in lipid and amino acid pathways are predictive of acute liver toxicity in rats. Int. J. Mol. Sci. 2020, 21, 8250. [Google Scholar] [CrossRef]
- Pannala, V.R.; Vinnakota, K.C.; Rawls, K.D.; Estes, S.K.; O’Brien, T.P.; Printz, R.L.; Papin, J.A.; Reifman, J.; Shiota, M.; Young, J.D.; et al. Mechanistic identification of biofluid metabolite changes as markers of acetaminophen-induced liver toxicity in rats. Toxicol. Appl. Pharmacol. 2019, 372, 19–32. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, H.; Liu, X.; Wang, Q.; Zhao, D.; Su, M.; Jia, Z.; Shen, S. Integrating metabolomics and network pharmacology to decipher the hepatoprotective effect mechanisms of magnesium isoglycyrrhizinate injection. Curr. Issues Mol. Biol. 2023, 46, 279–298. [Google Scholar] [CrossRef]
- Xu, J.; Han, J.; Jin, S.; Yu, B.; Li, X.; Ma, X.; Sun, L.; Li, C.; Zhao, L.; Ni, X. Modulation of mercaptopurine intestinal toxicity and pharmacokinetics by gut microbiota. Biomed. Pharmacother. 2024, 177, 116975. [Google Scholar] [CrossRef]
- Chaiyasut, C.; Sivamaruthi, B.S.; Lailerd, N.; Sirilun, S.; Khongtan, S.; Fukngoen, P.; Peerajan, S.; Saelee, M.; Chaiyasut, K.; Kesika, P.; et al. Probiotics supplementation improves intestinal permeability, obesity index and metabolic biomarkers in elderly thai subjects: A randomized controlled trial. Foods 2022, 11, 268. [Google Scholar] [CrossRef]
- Yang, Y.; Liang, M.; Ouyang, D.; Tong, H.; Wu, M.; Su, L. Research progress on the protective effect of brown algae-derived polysaccharides on metabolic diseases and intestinal barrier injury. Int. J. Mol Sci. 2022, 23, 10784. [Google Scholar] [CrossRef] [PubMed]
- Yu, F.; Hu, X.; Ren, H.; Wang, X.; Shi, R.; Guo, J.; Chang, J.; Zhou, X.; Jin, Y.; Li, Y.; et al. Protective effect of synbiotic combination of Lactobacillus plantarum SC-5 and olive oil extract tyrosol in a murine model of ulcerative colitis. J. Transl. Med. 2024, 22, 308. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Wang, J.; Wang, J.; Jiang, R.; Zhao, T. Gut microbiota combined with metabolomics reveal the mechanism of curcumol on liver fibrosis in mice. Biomed. Pharmacother. 2022, 152, 113204. [Google Scholar] [CrossRef] [PubMed]
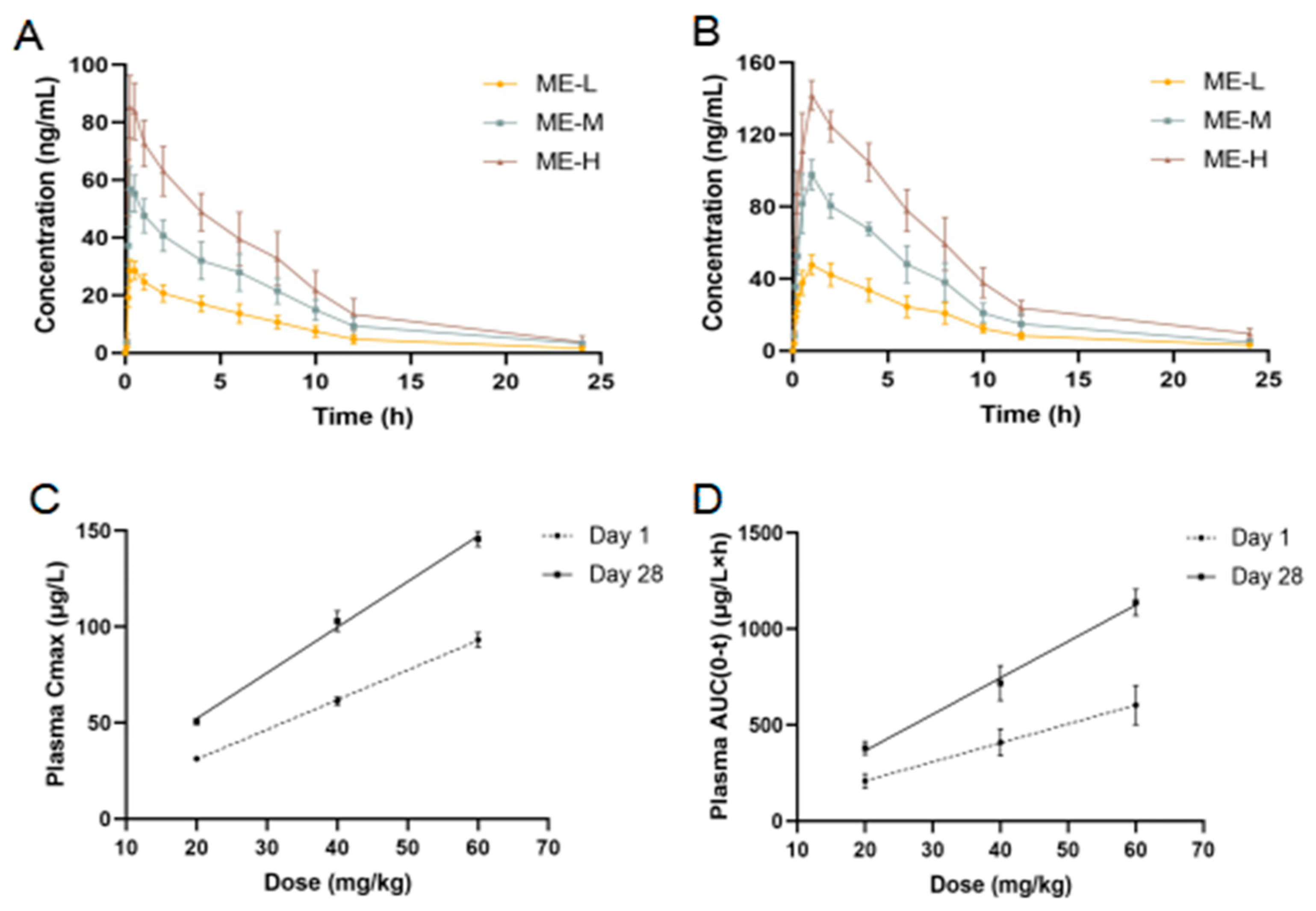
| Time | Dose (mg/kg) | T1/2 (h) | Tmax (h) | Cmax (μg/L) | AUC (0–∞) | AUC (0–t) (μg/L·h) |
|---|---|---|---|---|---|---|
| Day 1 | 20 | 5.56 ± 1.25 | 0.375 ± 0.137 | 31.258 ± 1.249 | 224.215 ± 41.809 | 207.833 ± 35.945 |
| 40 | 6.02 ± 0.74 | 0.375 ± 0.137 | 61.313 ± 2.067 | 444.941 ± 83.842 | 408.773 ± 69.847 | |
| 60 | 4.96 ± 1.98 | 0.375 ± 0.137 | 93.237 ± 3.791 | 641.660 ± 125.788 | 602.684 ± 103.124 | |
| Day 28 | 20 | 5.53 ± 1.84 | 1.083 ± 0.492 * | 50.602 ± 1.557 * | 401.429 ± 36.973 | 379.291 ± 33.738 * |
| 40 | 5.38 ± 1.55 | 0.917 ± 0.204 * | 102.89 ± 5.241 * | 756.641 ± 108.84 | 717.844 ± 90.372 * | |
| 60 | 4.95 ± 1.68 | 0.917 ± 0.204 * | 145.7 ± 3.920 * | 1193.716 ± 111.91 | 1138.405 ± 69.389 * |
| No. | Metabolite | m/z | RT (min) | VIP | Fold Change | Trend |
|---|---|---|---|---|---|---|
| 1 | S-Adenosylhomocysteine | 193.06841 | 2.321 | 1.697 | 0.398 | ↓ * |
| 2 | Adenine | 136.06219 | 4.807 | 1.722 | 0.333 | ↓ * |
| 3 | Dehydrocholic acid | 403.24514 | 6.223 | 1.527 | 0.267 | ↓ * |
| 4 | Saxitoxin | 282.13191 | 5.616 | 1.574 | 0.386 | ↓ * |
| 5 | Folic acid | 440.13283 | 5.075 | 1.615 | 0.202 | ↓ * |
| 6 | Chenodeoxycholic Acid | 437.29076 | 7.700 | 1.718 | 0.155 | ↓ * |
| 7 | 4-Hydroxy-L-glutamate | 162.04129 | 2.371 | 1.620 | 0.308 | ↓ * |
| 8 | L-Glutamic acid | 148.06047 | 1.299 | 1.502 | 2.216 | ↑ * |
| 9 | Phosphoethanolamine | 142.02644 | 1.287 | 1.588 | 4.464 | ↑ * |
| 10 | Levodopa | 215.10301 | 2.159 | 1.562 | 9.043 | ↑ * |
| 11 | D-Alanyl-D-alanine | 161.09236 | 2.150 | 1.634 | 14.759 | ↑ * |
| 12 | N8-Acetylspermidine | 188.17607 | 1.251 | 1.594 | 6.275 | ↑ * |
| 13 | Kinetin | 216.08688 | 2.276 | 1.632 | 3.427 | ↑ * |
| 14 | Citric acid | 191.01896 | 1.802 | 1.685 | 9.249 | ↑ * |
| 15 | cis-Aconitic acid | 173.00828 | 2.149 | 1.640 | 3.153 | ↑ * |
| 16 | Pyromucic acid | 111.00764 | 2.024 | 1.578 | 5.566 | ↑ * |
| 17 | N-Acetyl-α-D-glucosamine-1-phosphate | 300.04853 | 1.303 | 1.576 | 2.058 | ↑ * |
| 18 | 15-Trienoic acid | 293.21237 | 7.342 | 1.538 | 2.098 | ↑ * |
| 19 | Docosapentaenoic acid | 329.24852 | 9.892 | 1.638 | 3.029 | ↑ * |
| 20 | Adrenic acid | 331.26399 | 10.390 | 1.687 | 5.686 | ↑ * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, L.; Li, J.; Li, Q.; Sun, Q. Hepatotoxicity Induced by Methyl Eugenol: Insights from Toxicokinetics, Metabolomics, and Gut Microbiota. Curr. Issues Mol. Biol. 2024, 46, 11314-11325. https://doi.org/10.3390/cimb46100673
Chen L, Li J, Li Q, Sun Q. Hepatotoxicity Induced by Methyl Eugenol: Insights from Toxicokinetics, Metabolomics, and Gut Microbiota. Current Issues in Molecular Biology. 2024; 46(10):11314-11325. https://doi.org/10.3390/cimb46100673
Chicago/Turabian StyleChen, Liang, Jiaxin Li, Qian Li, and Qingwen Sun. 2024. "Hepatotoxicity Induced by Methyl Eugenol: Insights from Toxicokinetics, Metabolomics, and Gut Microbiota" Current Issues in Molecular Biology 46, no. 10: 11314-11325. https://doi.org/10.3390/cimb46100673
APA StyleChen, L., Li, J., Li, Q., & Sun, Q. (2024). Hepatotoxicity Induced by Methyl Eugenol: Insights from Toxicokinetics, Metabolomics, and Gut Microbiota. Current Issues in Molecular Biology, 46(10), 11314-11325. https://doi.org/10.3390/cimb46100673





