Integrated MicroRNA-mRNA Analyses of the Osteogenic Differentiation of Human Dental Pulp Stem Cells by a Helioxanthin Derivative
Abstract
1. Introduction
2. Materials and Methods
2.1. Isolation and Culture of DPSCs
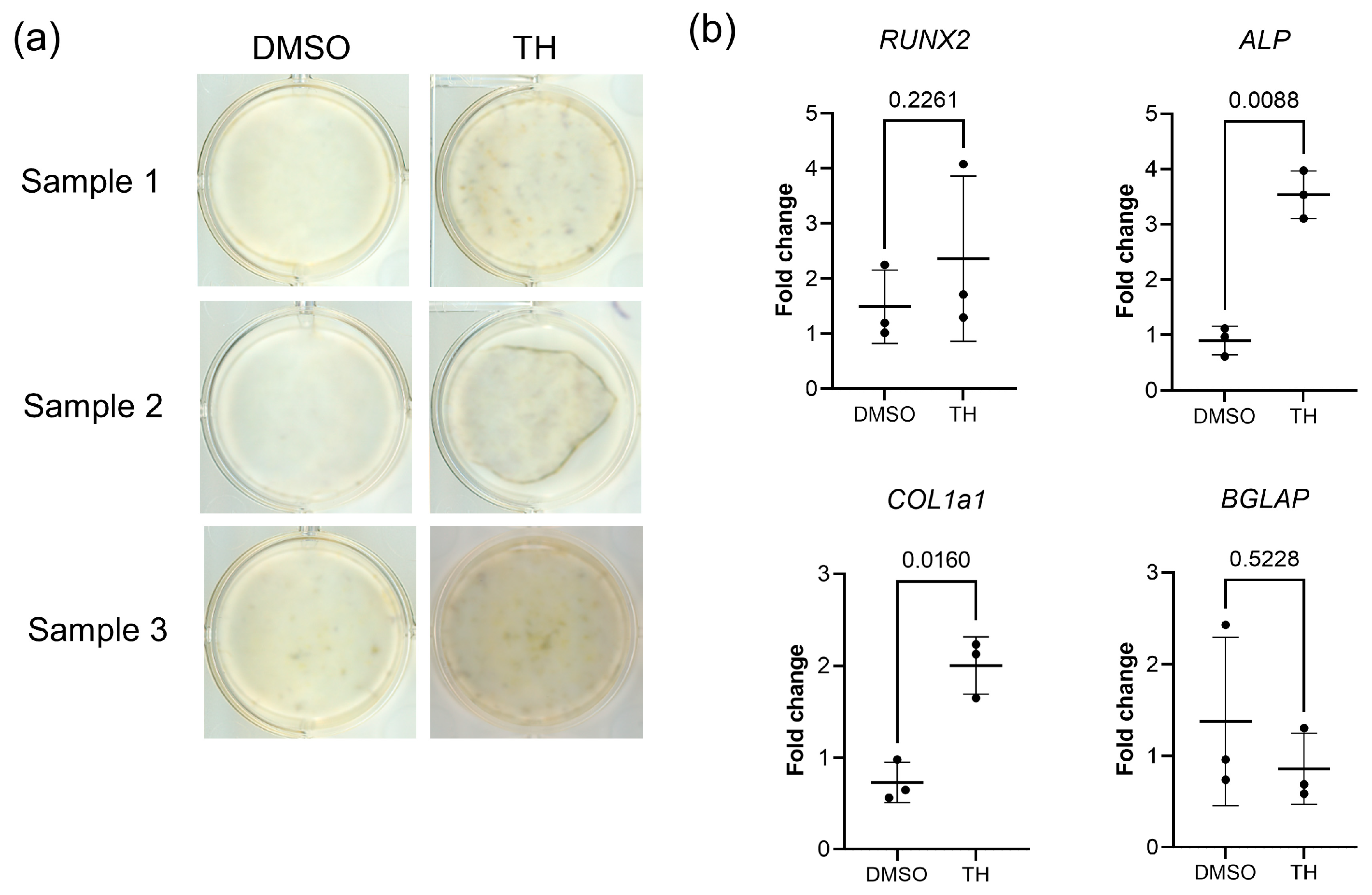
2.2. Alkaline Phosphatase (ALP) Staining
2.3. Extraction of DPSC-Derived EVs
2.4. RNA Isolation and Quantitative Real-Time PCR (qPCR)
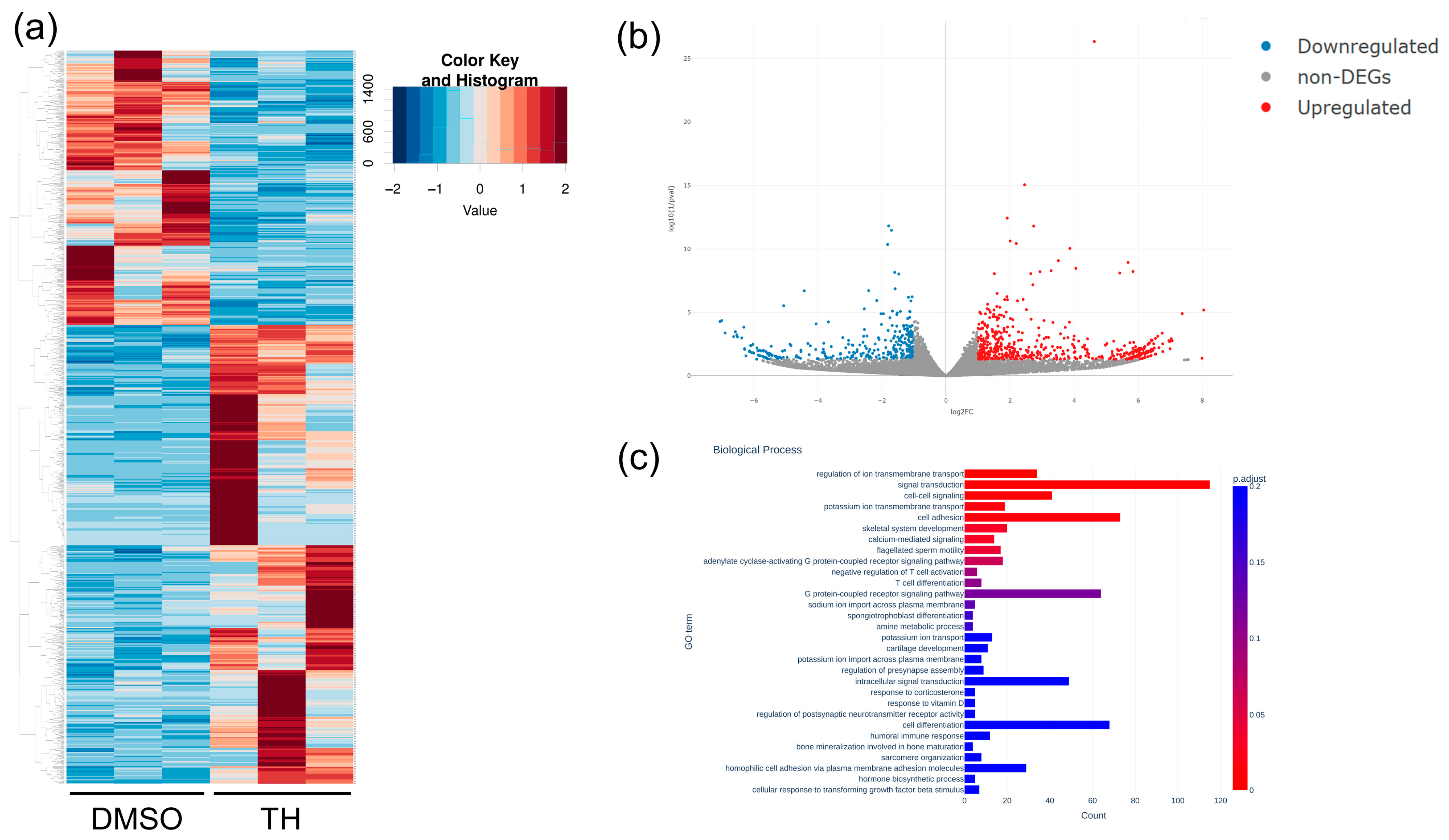
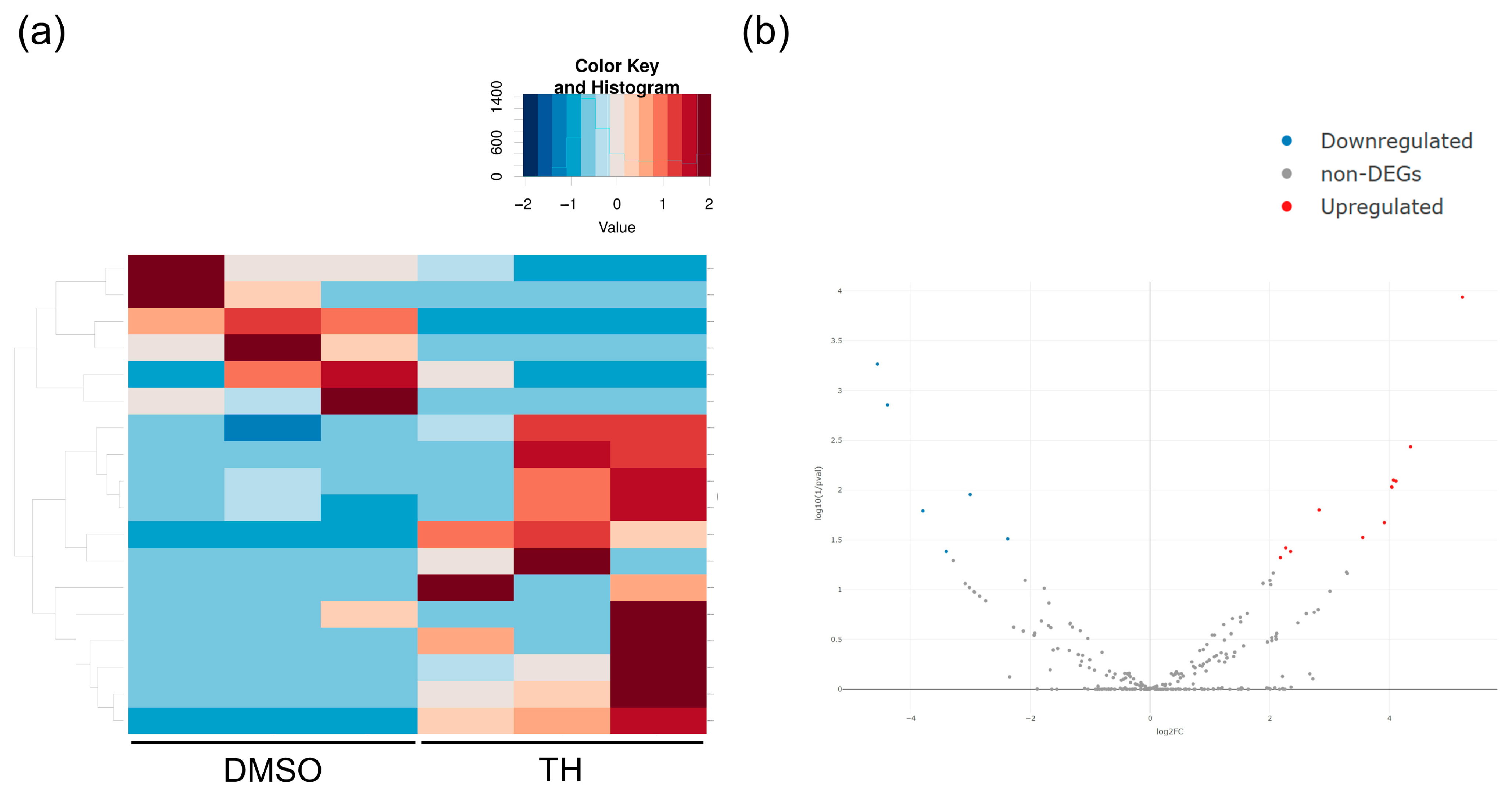
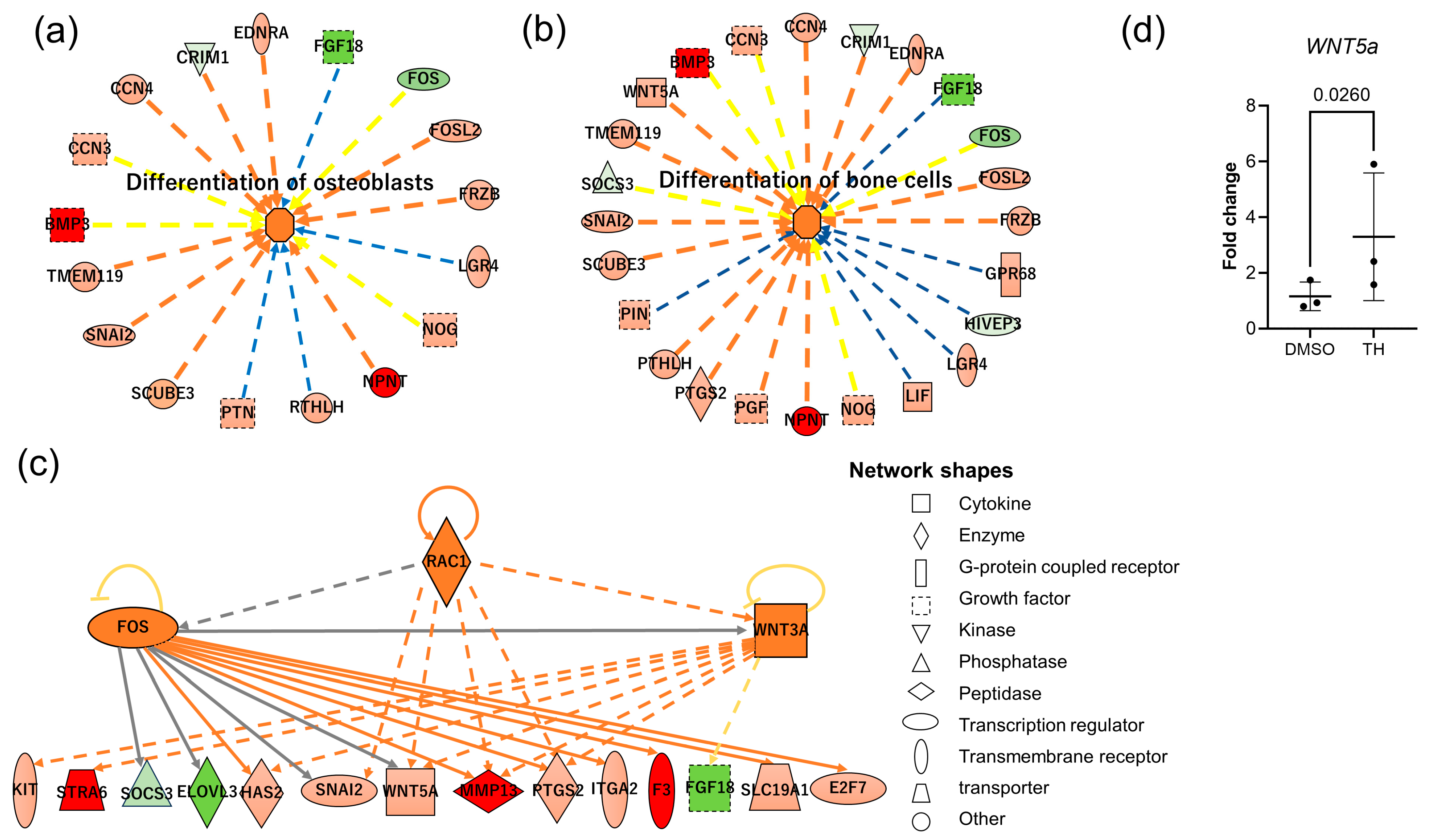
2.5. RNA Sequencing (RNA-Seq), miRNA Sequencing (miRNA-Seq), and Ingenuity Pathway Analysis (IPA)
2.6. Statistical Analysis
3. Results
3.1. Effects of TH on the Osteogenic Differentiation of DPSCs
3.2. mRNA and miRNA Expression Patterns in DPSCs Cultured with or without TH
3.3. Predicted mRNA and Pathways Targeted by EV-Derived miRNAs of TH-Induced DPSCs
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gronthos, S.; Mankani, M.; Brahim, J.; Robey, P.G.; Shi, S. Postnatal human dental pulp stem cells (dpscs) in vitro and in vivo. Proc. Natl. Acad. Sci. USA 2000, 97, 13625–13630. [Google Scholar] [CrossRef]
- Fujii, Y.; Kawase-Koga, Y.; Hojo, H.; Yano, F.; Sato, M.; Chung, U.-I.; Ohba, S.; Chikazu, D. Bone regeneration by human dental pulp stem cells using a helioxanthin derivative and cell-sheet technology. Stem Cell Res. Ther. 2018, 9, 24. [Google Scholar] [CrossRef] [PubMed]
- Yamakawa, D.; Kawase-Koga, Y.; Fujii, Y.; Kanno, Y.; Sato, M.; Ohba, S.; Kitaura, Y.; Kashiwagi, M.; Chikazu, D. Effects of helioxanthin derivative-treated human dental pulp stem cells on fracture healing. Int. J. Mol. Sci. 2020, 21, 9158. [Google Scholar] [CrossRef] [PubMed]
- Sato, M.; Kawase-Koga, Y.; Yamakawa, D.; Fujii, Y.; Chikazu, D. Bone regeneration potential of human dental pulp stem cells derived from elderly patients and osteo-induced by a helioxanthin derivative. Int. J. Mol. Sci. 2020, 21, 7731. [Google Scholar] [CrossRef] [PubMed]
- Kanke, K.; Masaki, H.; Saito, T.; Komiyama, Y.; Hojo, H.; Nakauchi, H.; Lichtler, A.C.; Takato, T.; Chung, U.-I.; Ohba, S. Stepwise differentiation of pluripotent stem cells into osteoblasts using four small molecules under serum-free and feeder-free conditions. Stem Cell Rep. 2014, 2, 751–760. [Google Scholar] [CrossRef]
- Maeda, Y.; Hojo, H.; Shimohata, N.; Choi, S.; Yamamoto, K.; Takato, T.; Chung, U.-I.; Ohba, S. Bone healing by sterilizable calcium phosphate tetrapods eluting osteogenic molecules. Biomaterials 2013, 34, 5530–5537. [Google Scholar] [CrossRef]
- Ohba, S.; Nakajima, K.; Komiyama, Y.; Kugimiya, F.; Igawa, K.; Itaka, K.; Moro, T.; Nakamura, K.; Kawaguchi, H.; Takato, T.; et al. A novel osteogenic helioxanthin-derivative acts in a bmp-dependent manner. Biochem. Biophys. Res. Commun. 2007, 357, 854–860. [Google Scholar] [CrossRef] [PubMed]
- Amano, H.; Iwaki, F.; Oki, M.; Aoki, K.; Ohba, S. An osteogenic helioxanthin derivative suppresses the formation of bone-resorbing osteoclasts. Regen. Ther. 2019, 11, 290–296. [Google Scholar] [CrossRef]
- Tang, Y.; Zhou, Y.; Li, H.J. Advances in mesenchymal stem cell exosomes: A review. Stem Cell Res. Ther. 2021, 12, 71. [Google Scholar] [CrossRef]
- Huang, Y.; He, B.; Wang, L.; Yuan, B.; Shu, H.; Zhang, F.; Sun, L. Bone marrow mesenchymal stem cell-derived exosomes promote rotator cuff tendon-bone healing by promoting angiogenesis and regulating m1 macrophages in rats. Stem Cell Res. Ther. 2020, 11, 496. [Google Scholar] [CrossRef]
- Zhou, Y.; Deng, G.; She, H.; Bai, F.; Xiang, B.; Zhou, J.; Zhang, S. Polydopamine-coated biomimetic bone scaffolds loaded with exosomes promote osteogenic differentiation of bmsc and bone regeneration. Regen. Ther. 2023, 23, 25–36. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Guo, S.; Tong, S.; Sun, X. Exosomal mir-130a-3p regulates osteogenic differentiation of human adipose-derived stem cells through mediating sirt7/wnt/β-catenin axis. Cell Prolif. 2020, 53, e12890. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Tang, Y.; Liu, Y.; Zhang, P.; Lv, L.; Zhang, X.; Jia, L.; Zhou, Y. Exosomes derived from mir-375-overexpressing human adipose mesenchymal stem cells promote bone regeneration. Cell Prolif. 2019, 52, e12669. [Google Scholar] [CrossRef] [PubMed]
- Lu, G.-D.; Cheng, P.; Liu, T.; Wang, Z. Bmsc-derived exosomal mir-29a promotes angiogenesis and osteogenesis. Front. Cell Dev. Biol. 2020, 8, 608521. [Google Scholar] [CrossRef]
- Ogasawara, T.; Kawaguchi, H.; Jinno, S.; Hoshi, K.; Itaka, K.; Takato, T.; Nakamura, K.; Okayama, H. Bone morphogenetic protein 2-induced osteoblast differentiation requires smad-mediated down-regulation of cdk6. Mol. Cell. Biol. 2004, 24, 6560–6568. [Google Scholar] [CrossRef]
- Bonafede, R.; Scambi, I.; Peroni, D.; Potrich, V.; Boschi, F.; Benati, D.; Bonetti, B.; Mariotti, R. Exosome derived from murine adipose-derived stromal cells: Neuroprotective effect on in vitro model of amyotrophic lateral sclerosis. Exp. Cell Res. 2016, 340, 150–158. [Google Scholar] [CrossRef]
- Kawakubo-Yasukochi, T.; Morioka, M.; Hayashi, Y.; Nishinakagawa, T.; Hazekawa, M.; Kawano, S.; Nakamura, S.; Nakashima, M. The squu-b cell line spreads its metastatic properties to nonmetastatic clone squu-a from the same patient through exosomes. J. Oral Biosci. 2016, 58, 33–38. [Google Scholar] [CrossRef]
- Gong, Y.; Slee, R.B.; Fukai, N.; Rawadi, G.; Roman-Roman, S.; Reginato, A.M.; Wang, H.; Cundy, T.; Glorieux, F.H.; Lev, D.; et al. Ldl receptor-related protein 5 (lrp5) affects bone accrual and eye development. Cell 2001, 107, 513–523. [Google Scholar] [CrossRef]
- Maeda, K.; Kobayashi, Y.; Udagawa, N.; Uehara, S.; Ishihara, A.; Mizoguchi, T.; Kikuchi, Y.; Takada, I.; Kato, S.; Kani, S.; et al. Wnt5a-ror2 signaling between osteoblast-lineage cells and osteoclast precursors enhances osteoclastogenesis. Nat. Med. 2012, 18, 405–412. [Google Scholar] [CrossRef] [PubMed]
- Takada, I.; Mihara, M.; Suzawa, M.; Ohtake, F.; Kobayashi, S.; Igarashi, M.; Youn, M.-Y.; Takeyama, K.-I.; Nakamura, T.; Mezaki, Y.; et al. A histone lysine methyltransferase activated by non-canonical wnt signalling suppresses ppar-γ transactivation. Nat. Cell Biol. 2007, 9, 1273–1285. [Google Scholar] [CrossRef]
- Kornsuthisopon, C.; Chansaenroj, A.; Manokawinchoke, J.; Tompkins, K.A.; Pirarat, N.; Osathanon, T. Non-canonical wnt signaling participates in jagged1-induced osteo/odontogenic differentiation in human dental pulp stem cells. Sci. Rep. 2022, 12, 7583. [Google Scholar] [CrossRef]
- Lin, M.; Li, L.; Liu, C.; Liu, H.; He, F.; Yan, F.; Zhang, Y.; Chen, Y. Wnt5a regulates growth, patterning, and odontoblast differentiation of developing mouse tooth. Dev. Dyn. 2011, 240, 432–440. [Google Scholar] [CrossRef]
- Peng, L.; Ren, L.B.; Dong, G.; Wang, C.L.; Xu, P.; Ye, L.; Zhou, X.D. Wnt5a promotes differentiation of human dental papilla cells. Int. Endod. J. 2010, 43, 404–412. [Google Scholar] [CrossRef]
- Sakisaka, Y.; Tsuchiya, M.; Nakamura, T.; Tamura, M.; Shimauchi, H.; Nemoto, E. Wnt5a attenuates wnt3a-induced alkaline phosphatase expression in dental follicle cells. Exp. Cell Res. 2015, 336, 85–93. [Google Scholar] [CrossRef]
- Yan, G.; Wang, J.; Fang, Z.; Yan, S.; Zhang, Y. Mir-26a-5p targets wnt5a to protect cardiomyocytes from injury due to hypoxia/reoxygenation through the wnt/β-catenin signaling pathway. Int. Heart J. 2021, 62, 1145–1152. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, Z.; Zhou, M.; Liu, C. Microrna-129-5p inhibits vascular smooth muscle cell proliferation by targeting wnt5a. Exp. Ther. Med. 2016, 12, 2651–2656. [Google Scholar] [CrossRef]
- Wu, X.; Tu, X.; Joeng, K.S.; Hilton, M.J.; Williams, D.A.; Long, F. Rac1 activation controls nuclear localization of beta-catenin during canonical wnt signaling. Cell 2008, 133, 340–353. [Google Scholar] [CrossRef]
- Huck, K.; Sens, C.; Wuerfel, C.; Zoeller, C.; Nakchbandi, I.A. The rho gtpase rac1 in osteoblasts controls their function. Int. J. Mol. Sci. 2020, 21, 385. [Google Scholar] [CrossRef]
- Sebastian, A.; Hum, N.R.; Murugesh, D.K.; Hatsell, S.; Economides, A.N.; Loots, G.G. Wnt co-receptors lrp5 and lrp6 differentially mediate wnt3a signaling in osteoblasts. PLoS ONE 2017, 12, e0188264. [Google Scholar] [CrossRef]
- Grigoriadis, A.E.; Schellander, K.; Wang, Z.Q.; Wagner, E.F. Osteoblasts are target cells for transformation in c-fos transgenic mice. J. Cell Biol. 1993, 122, 685–701. [Google Scholar] [CrossRef]
- Jochum, W.; David, J.-P.; Elliott, C.; Wutz, A.; Plenk, H.; Matsuo, K.; Wagner, E.F. Increased bone formation and osteosclerosis in mice overexpressing the transcription factor fra-1. Nat. Med. 2000, 6, 980–984. [Google Scholar] [CrossRef] [PubMed]
- Liou, S.; Hsu, J.; Chu, H.; Lin, H.; Chen, I.; Yeh, J. Kmup-1 promotes osteoblast differentiation through camp and cgmp pathways and signaling of bmp-2/smad1/5/8 and wnt/β-catenin. J. Cell. Physiol. 2015, 230, 2038–2048. [Google Scholar] [CrossRef] [PubMed]
- Murphy, D.E.; de Jong, O.G.; Brouwer, M.; Wood, M.J.; Lavieu, G.; Schiffelers, R.M.; Vader, P. Extracellular vesicle-based therapeutics: Natural versus engineered targeting and trafficking. Exp. Mol. Med. 2019, 51, 1–12. [Google Scholar] [CrossRef] [PubMed]
| Gene | Primer Sequences (Forward [top] and Reverse [bottom], 5′-3′) | Accession No. |
|---|---|---|
| ALP | ATGAAGGAAAAGCCAAGCAG ATGGAGACATTCTCTCGTTC | NM_000478 |
| BGLAP | GGCAGCGAGGTAGTGAAGAG AGCAGAGCGACACCCTAGAC | NM_199173 |
| COLIA1 | GTGCTAAAGGTGCCAATGGT CTCCTCGCTTTCCTTCCTCT | NM_000088 |
| GAPDH | GAAGGTGAAGGTCGGAGTCA GAAGATGGTGATGGGATTTC | BC023632 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fujii, Y.; Minami, S.; Hatori, A.; Kawase-Koga, Y.; Ogasawara, T.; Chikazu, D. Integrated MicroRNA-mRNA Analyses of the Osteogenic Differentiation of Human Dental Pulp Stem Cells by a Helioxanthin Derivative. Curr. Issues Mol. Biol. 2024, 46, 10960-10968. https://doi.org/10.3390/cimb46100651
Fujii Y, Minami S, Hatori A, Kawase-Koga Y, Ogasawara T, Chikazu D. Integrated MicroRNA-mRNA Analyses of the Osteogenic Differentiation of Human Dental Pulp Stem Cells by a Helioxanthin Derivative. Current Issues in Molecular Biology. 2024; 46(10):10960-10968. https://doi.org/10.3390/cimb46100651
Chicago/Turabian StyleFujii, Yasuyuki, Sakura Minami, Ayano Hatori, Yoko Kawase-Koga, Toru Ogasawara, and Daichi Chikazu. 2024. "Integrated MicroRNA-mRNA Analyses of the Osteogenic Differentiation of Human Dental Pulp Stem Cells by a Helioxanthin Derivative" Current Issues in Molecular Biology 46, no. 10: 10960-10968. https://doi.org/10.3390/cimb46100651
APA StyleFujii, Y., Minami, S., Hatori, A., Kawase-Koga, Y., Ogasawara, T., & Chikazu, D. (2024). Integrated MicroRNA-mRNA Analyses of the Osteogenic Differentiation of Human Dental Pulp Stem Cells by a Helioxanthin Derivative. Current Issues in Molecular Biology, 46(10), 10960-10968. https://doi.org/10.3390/cimb46100651






