Genetics of 21-OH Deficiency and Genotype–Phenotype Correlation: Experience of the Hellenic National Referral Center
Abstract
1. Introduction
2. Materials and Methods
2.1. Subjects
2.2. DNA Extraction
2.3. CYP21A2 Gene Sequencing
2.4. MLPA
2.5. Evaluation of Novel Variants
2.6. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Classic Form of 21-OHD | Compound Heterozygotes | Homozygotes |
|---|---|---|
| SW | Group Null Variants in Both Alleles (2 Cases) | Group A Variant (3 Cases) |
| c.[1069C>T];[949C>T], c.[955C>T;1360C>T];[(?_-163)_(939 + 39_?)del] | c.[293-13C>G];[293-13C>G] | |
| Group Null variant in trans with a Group A variant (2 cases) | ||
| c.[1069C>T];[293-13C>G], c.[293-13C>G];[(?_-163)_(939 + 39_?)del] | ||
| SV | Group B variant in trans with a Group A variant (3 cases) | Group B variant (1 case) |
| c.[518T>A];[293-13C>G] | c.[518T>A];[518T>A] | |
| Group B variant in trans with a null variant (3 cases) | ||
| c.[518T>A];[332_339del], c.[332_339del];[1451G>C], c.[518T>A];[(?_-163)_(939 + 39_?)del] |

References
- El-Maouche, D.; Arlt, W.; Merke, D.P. Congenital adrenal hyperplasia. Lancet 2017, 390, 2194–2210. [Google Scholar] [CrossRef]
- White, P.C.; New, M.I.; Dupont, B. HLA-linked congenital adrenal hyperplasia results from a defective gene encoding a cytochrome P-450 specific for steroid 21-hydroxylation. Proc. Natl. Acad. Sci. USA 1984, 81, 7505–7509. [Google Scholar] [CrossRef]
- Krone, N.; Dhir, V.; Ivison, H.E.; Arlt, W. Congenital adrenal hyperplasia and P450 oxidoreductase deficiency. Clin. Endocrinol. 2007, 66, 162–172. [Google Scholar] [CrossRef]
- Claahsen-van der Grinten, H.L.; Speiser, P.W.; Ahmed, S.F.; Arlt, W.; Auchus, R.J.; Falhammar, H.; Fluck, C.E.; Guasti, L.; Huebner, A.; Kortmann, B.B.M.; et al. Congenital Adrenal Hyperplasia-Current Insights in Pathophysiology, Diagnostics, and Management. Endocr. Rev. 2022, 43, 91–159. [Google Scholar] [CrossRef]
- Hannah-Shmouni, F.; Morissette, R.; Sinaii, N.; Elman, M.; Prezant, T.R.; Chen, W.; Pulver, A.; Merke, D.P. Revisiting the prevalence of nonclassic congenital adrenal hyperplasia in US Ashkenazi Jews and Caucasians. Genet. Med. 2017, 19, 1276–1279. [Google Scholar] [CrossRef]
- Speiser, P.W.; Dupont, B.; Rubinstein, P.; Piazza, A.; Kastelan, A.; New, M.I. High frequency of nonclassical steroid 21-hydroxylase deficiency. Am. J. Hum. Genet. 1985, 37, 650–667. [Google Scholar] [CrossRef]
- New, M.I.; Abraham, M.; Gonzalez, B.; Dumic, M.; Razzaghy-Azar, M.; Chitayat, D.; Sun, L.; Zaidi, M.; Wilson, R.C.; Yuen, T. Genotype-phenotype correlation in 1,507 families with congenital adrenal hyperplasia owing to 21-hydroxylase deficiency. Proc. Natl. Acad. Sci. USA 2013, 110, 2611–2616. [Google Scholar] [CrossRef]
- Livadas, S.; Bothou, C. Management of the Female With Non-classical Congenital Adrenal Hyperplasia (NCCAH): A Patient-Oriented Approach. Front. Endocrinol. 2019, 10, 366. [Google Scholar] [CrossRef]
- Speiser, P.W.; Arlt, W.; Auchus, R.J.; Baskin, L.S.; Conway, G.S.; Merke, D.P.; Meyer-Bahlburg, H.F.L.; Miller, W.L.; Murad, M.H.; Oberfield, S.E.; et al. Congenital Adrenal Hyperplasia Due to Steroid 21-Hydroxylase Deficiency: An Endocrine Society Clinical Practice Guideline. J. Clin. Endocrinol. Metab. 2018, 103, 4043–4088. [Google Scholar] [CrossRef]
- Livadas, S.; Dracopoulou, M.; Dastamani, A.; Sertedaki, A.; Maniati-Christidi, M.; Magiakou, A.M.; Kanaka-Gantenbein, C.; Chrousos, G.P.; Dacou-Voutetakis, C. The spectrum of clinical, hormonal and molecular findings in 280 individuals with nonclassical congenital adrenal hyperplasia caused by mutations of the CYP21A2 gene. Clin. Endocrinol. 2015, 82, 543–549. [Google Scholar] [CrossRef]
- Azziz, R.; Hincapie, L.A.; Knochenhauer, E.S.; Dewailly, D.; Fox, L.; Boots, L.R. Screening for 21-hydroxylase-deficient nonclassic adrenal hyperplasia among hyperandrogenic women: A prospective study. Fertil. Steril. 1999, 72, 915–925. [Google Scholar] [CrossRef] [PubMed]
- Baumgartner-Parzer, S.; Witsch-Baumgartner, M.; Hoeppner, W. EMQN best practice guidelines for molecular genetic testing and reporting of 21-hydroxylase deficiency. Eur. J. Hum. Genet. 2020, 28, 1341–1367. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Mendoza, A.R.; Welch, T.R.; Zipf, W.B.; Yu, C.Y. Modular variations of the human major histocompatibility complex class III genes for serine/threonine kinase RP, complement component C4, steroid 21-hydroxylase CYP21, and tenascin TNX (the RCCX module). A mechanism for gene deletions and disease associations. J. Biol. Chem. 1999, 274, 12147–12156. [Google Scholar] [CrossRef]
- Yang, S.Y.; Levine, L.S.; Zachmann, M.; New, M.I.; Prader, A.; Oberfield, S.E.; O'Neill, G.J.; Pollack, M.S.; Dupont, B. Mapping of the 21-hydroxylase deficiency gene within the HLA linkage group. Transplant. Proc. 1978, 10, 753–755. [Google Scholar] [PubMed]
- White, P.C.; New, M.I.; Dupont, B. Structure of human steroid 21-hydroxylase genes. Proc. Natl. Acad. Sci. USA 1986, 83, 5111–5115. [Google Scholar] [CrossRef] [PubMed]
- Levine, L.S.; Zachmann, M.; New, M.I.; Prader, A.; Pollack, M.S.; O'Neill, G.J.; Yang, S.Y.; Oberfield, S.E.; Dupont, B. Genetic mapping of the 21-hydroxylase-deficiency gene within the HLA linkage group. N. Engl. J. Med. 1978, 299, 911–915. [Google Scholar] [CrossRef]
- Tusie-Luna, M.T.; White, P.C. Gene conversions and unequal crossovers between CYP21 (steroid 21-hydroxylase gene) and CYP21P involve different mechanisms. Proc. Natl. Acad. Sci. USA 1995, 92, 10796–10800. [Google Scholar] [CrossRef]
- White, P.C.; Speiser, P.W. Congenital Adrenal Hyperplasia due to 21-Hydroxylase Deficiency. Endocr. Rev. 2000, 21, 245–291. [Google Scholar] [CrossRef]
- Concolino, P.; Costella, A. Congenital Adrenal Hyperplasia (CAH) due to 21-Hydroxylase Deficiency: A Comprehensive Focus on 233 Pathogenic Variants of CYP21A2 Gene. Mol. Diagn. Ther. 2018, 22, 261–280. [Google Scholar] [CrossRef]
- Wedell, A.; Stengler, B.; Luthman, H. Characterization of mutations on the rare duplicated C4/CYP21 haplotype in steroid 21-hydroxylase deficiency. Hum. Genet. 1994, 94, 50–54. [Google Scholar] [CrossRef]
- Koppens, P.F.; Hoogenboezem, T.; Degenhart, H.J. CYP21 and CYP21P variability in steroid 21-hydroxylase deficiency patients and in the general population in the Netherlands. Eur. J. Hum. Genet. 2000, 8, 827–836. [Google Scholar] [CrossRef] [PubMed]
- Kleinle, S.; Lang, R.; Fischer, G.F.; Vierhapper, H.; Waldhauser, F.; Fodinger, M.; Baumgartner-Parzer, S.M. Duplications of the functional CYP21A2 gene are primarily restricted to Q318X alleles: Evidence for a founder effect. J. Clin. Endocrinol. Metab. 2009, 94, 3954–3958. [Google Scholar] [CrossRef] [PubMed]
- Kharrat, M.; Riahi, A.; Maazoul, F.; M'Rad, R.; Chaabouni, H. Detection of a frequent duplicated CYP21A2 gene carrying a Q318X mutation in a general population with quantitative PCR methods. Diagn. Mol. Pathol. 2011, 20, 123–127. [Google Scholar] [CrossRef] [PubMed]
- Parajes, S.; Quinteiro, C.; Dominguez, F.; Loidi, L. High frequency of copy number variations and sequence variants at CYP21A2 locus: Implication for the genetic diagnosis of 21-hydroxylase deficiency. PLoS ONE 2008, 3, e2138. [Google Scholar] [CrossRef]
- Ezquieta, B.; Beneyto, M.; Munoz-Pacheco, R.; Barrio, R.; Oyarzabal, M.; Lechuga, J.L.; Luzuriaga, C.; Hermoso, F.; Quinteiro, S.; Martinez, S. Gene duplications in 21-hydroxylase deficiency: The importance of accurate molecular diagnosis in carrier detection and prenatal diagnosis. Prenat. Diagn. 2006, 26, 1172–1178. [Google Scholar] [CrossRef]
- Wilson, R.C.; Mercado, A.B.; Cheng, K.C.; New, M.I. Steroid 21-hydroxylase deficiency: Genotype may not predict phenotype. J. Clin. Endocrinol. Metab. 1995, 80, 2322–2329. [Google Scholar] [CrossRef]
- Nimkarn, S.; Cerame, B.I.; Wei, J.Q.; Dumic, M.; Zunec, R.; Brkljacic, L.; Skrabic, V.; New, M.I.; Wilson, R.C. Congenital adrenal hyperplasia (21-hydroxylase deficiency) without demonstrable genetic mutations. J. Clin. Endocrinol. Metab. 1999, 84, 378–381. [Google Scholar] [CrossRef][Green Version]
- Krone, N.; Braun, A.; Roscher, A.A.; Knorr, D.; Schwarz, H.P. Predicting phenotype in steroid 21-hydroxylase deficiency? Comprehensive genotyping in 155 unrelated, well defined patients from southern Germany. J. Clin. Endocrinol. Metab. 2000, 85, 1059–1065. [Google Scholar] [CrossRef]
- Richards, S.; Aziz, N.; Bale, S.; Bick, D.; Das, S.; Gastier-Foster, J.; Grody, W.W.; Hegde, M.; Lyon, E.; Spector, E.; et al. Standards and guidelines for the interpretation of sequence variants: A joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet. Med. 2015, 17, 405–424. [Google Scholar] [CrossRef]
- Karczewski, K.J.; Francioli, L.C.; Tiao, G.; Cummings, B.B.; Alfoldi, J.; Wang, Q.; Collins, R.L.; Laricchia, K.M.; Ganna, A.; Birnbaum, D.P.; et al. The mutational constraint spectrum quantified from variation in 141,456 humans. Nature 2020, 581, 434–443. [Google Scholar] [CrossRef]
- Bruque, C.D.; Delea, M.; Fernandez, C.S.; Orza, J.V.; Taboas, M.; Buzzalino, N.; Espeche, L.D.; Solari, A.; Luccerini, V.; Alba, L.; et al. Structure-based activity prediction of CYP21A2 stability variants: A survey of available gene variations. Sci. Rep. 2016, 6, 39082. [Google Scholar] [CrossRef] [PubMed]
- Menassa, R.E.M. Functional and Structural Studies of cyp21a2 gene mutants in Congenital Adrenal Hyperplasia (Études Fonctionnelles et Structurales des Mutants du gène CYP21A2 dans l’Hyperplasie Congénitale des Surrénales). Ph.D. Thesis, Université Claude Bernard—Lyon I, Villeurbanne, France, 2009. [Google Scholar]
- Felix-Lopez, X.; Riba, L.; Ordonez-Sanchez, M.L.; Ramirez-Jimenez, S.; Ventura-Gallegos, J.L.; Zentella-Dehesa, A.; Tusie-Luna, M.T. Steroid 21-hydroxylase (P450c21) naturally occurring mutants I172N, V281L and I236n/V237E/M239K exert a dominant negative effect on enzymatic activity when co-expressed with the wild-type protein. J. Pediatr. Endocrinol. Metab 2003, 16, 1017–1024. [Google Scholar] [CrossRef] [PubMed]
- Neocleous, V.; Shammas, C.; Phedonos, A.A.; Phylactou, L.A.; Skordis, N. Phenotypic variability of hyperandrogenemia in females heterozygous for CYP21A2 mutations. Indian J. Endocrinol. Metab. 2014, 18, S72–S79. [Google Scholar] [CrossRef] [PubMed]
- Simonetti, L.; Bruque, C.D.; Fernandez, C.S.; Benavides-Mori, B.; Delea, M.; Kolomenski, J.E.; Espeche, L.D.; Buzzalino, N.D.; Nadra, A.D.; Dain, L. CYP21A2 mutation update: Comprehensive analysis of databases and published genetic variants. Hum. Mutat. 2018, 39, 5–22. [Google Scholar] [CrossRef]
- Dacou-Voutetakis, C.; Dracopoulou, M. High incidence of molecular defects of the CYP21 gene in patients with premature adrenarche. J. Clin. Endocrinol. Metab. 1999, 84, 1570–1574. [Google Scholar] [CrossRef]
- Dracopoulou-Vabouli, M.; Maniati-Christidi, M.; Dacou-Voutetakis, C. The spectrum of molecular defects of the CYP21 gene in the Hellenic population: Variable concordance between genotype and phenotype in the different forms of congenital adrenal hyperplasia. J. Clin. Endocrinol. Metab. 2001, 86, 2845–2848. [Google Scholar] [CrossRef][Green Version]
- Nan, M.N.; Roig, R.; Martinez, S.; Rives, J.; Urgell, E.; Espinos, J.J.; Tirado, M.; Carreras, G.; Aulinas, A.; Webb, S.M.; et al. Comprehensive Genetic Testing of CYP21A2: A Retrospective Analysis in Patients with Suspected Congenital Adrenal Hyperplasia. J. Clin. Med. 2021, 10, 1183. [Google Scholar] [CrossRef]
- Phedonos, A.A.; Shammas, C.; Skordis, N.; Kyriakides, T.C.; Neocleous, V.; Phylactou, L.A. High carrier frequency of 21-hydroxylase deficiency in Cyprus. Clin. Genet. 2013, 84, 585–588. [Google Scholar] [CrossRef]
- Neocleous, V.; Fanis, P.; Toumba, M.; Stylianou, C.; Picolos, M.; Andreou, E.; Kyriakou, A.; Iasonides, M.; Nicolaou, S.; Kyriakides, T.C.; et al. The Spectrum of Genetic Defects in Congenital Adrenal Hyperplasia in the Population of Cyprus: A Retrospective Analysis. Horm. Metab. Res. 2019, 51, 586–594. [Google Scholar] [CrossRef]
- Santos-Silva, R.; Cardoso, R.; Lopes, L.; Fonseca, M.; Espada, F.; Sampaio, L.; Brandao, C.; Antunes, A.; Braganca, G.; Coelho, R.; et al. CYP21A2 Gene Pathogenic Variants: A Multicenter Study on Genotype-Phenotype Correlation from a Portuguese Pediatric Cohort. Horm. Res. Paediatr. 2019, 91, 33–45. [Google Scholar] [CrossRef]
- Concolino, P.; Perrucci, A.; Carrozza, C.; Urbani, A. Genetic Characterization of a Cohort of Italian Patients with Congenital Adrenal Hyperplasia Due to 21-Hydroxylase Deficiency. Mol. Diagn. Ther. 2023, 27, 621–630. [Google Scholar] [CrossRef] [PubMed]
- Sadeghi, F.; Yurur-Kutlay, N.; Berberoglu, M.; Cetinkaya, E.; Aycan, Z.; Kara, C.; Ilgin Ruhi, H.; Ocal, G.; Siklar, Z.; Elhan, A.; et al. Identification of frequency and distribution of the nine most frequent mutations among patients with 21-hydroxylase deficiency in Turkey. J. Pediatr. Endocrinol. Metab. 2008, 21, 781–787. [Google Scholar] [CrossRef] [PubMed]
- Karaoglan, M. The distribution of intrafamilial CYP21A2 mutant alleles and investigation of clinical features in Turkish children and their siblings in Southeastern Anatolia. J. Pediatr. Endocrinol. Metab. 2019, 32, 1311–1320. [Google Scholar] [CrossRef] [PubMed]
- Karaoglan, M.; Nacarkahya, G.; Aytac, E.H.; Keskin, M. Challenges of CYP21A2 genotyping in children with 21-hydroxylase deficiency: Determination of genotype-phenotype correlation using next generation sequencing in Southeastern Anatolia. J. Endocrinol. Investig. 2021, 44, 2395–2405. [Google Scholar] [CrossRef]
- Dumic, K.K.; Grubic, Z.; Yuen, T.; Wilson, R.C.; Kusec, V.; Barisic, I.; Stingl, K.; Sansovic, I.; Skrabic, V.; Dumic, M.; et al. Molecular genetic analysis in 93 patients and 193 family members with classical congenital adrenal hyperplasia due to 21-hydroxylase deficiency in Croatia. J. Steroid Biochem. Mol. Biol. 2017, 165, 51–56. [Google Scholar] [CrossRef]
- Haider, S.; Islam, B.; D'Atri, V.; Sgobba, M.; Poojari, C.; Sun, L.; Yuen, T.; Zaidi, M.; New, M.I. Structure-phenotype correlations of human CYP21A2 mutations in congenital adrenal hyperplasia. Proc. Natl. Acad. Sci. USA 2013, 110, 2605–2610. [Google Scholar] [CrossRef]
- Carvalho, B.; Marques, C.J.; Santos-Silva, R.; Fontoura, M.; Carvalho, D.; Carvalho, F. Congenital Adrenal Hyperplasia Due to 21-Hydroxylase Deficiency: An Update on Genetic Analysis of CYP21A2 Gene. Exp. Clin. Endocrinol. Diabetes 2021, 129, 477–481. [Google Scholar] [CrossRef]
- Koppens, P.F.; Hoogenboezem, T.; Degenhart, H.J. Duplication of the CYP21A2 gene complicates mutation analysis of steroid 21-hydroxylase deficiency: Characteristics of three unusual haplotypes. Hum. Genet. 2002, 111, 405–410. [Google Scholar] [CrossRef]
- Baumgartner-Parzer, S.M.; Fischer, G.; Vierhapper, H. Predisposition for de novo gene aberrations in the offspring of mothers with a duplicated CYP21A2 gene. J. Clin. Endocrinol. Metab. 2007, 92, 1164–1167. [Google Scholar] [CrossRef]
- Speiser, P.W.; White, P.C. Congenital adrenal hyperplasia. N. Engl. J. Med. 2003, 349, 776–788. [Google Scholar] [CrossRef]
- Baumgartner-Parzer, S.M.; Nowotny, P.; Waldhausl, W.; Vierhapper, H. A rare duplicated 21-hydroxylase haplotype and a de novo mutation: A family analysis. J. Clin. Endocrinol. Metab. 2003, 88, 2794–2796. [Google Scholar] [CrossRef] [PubMed]
- Nandagopal, R.; Sinaii, N.; Avila, N.A.; Van Ryzin, C.; Chen, W.; Finkielstain, G.P.; Mehta, S.P.; McDonnell, N.B.; Merke, D.P. Phenotypic profiling of parents with cryptic nonclassic congenital adrenal hyperplasia: Findings in 145 unrelated families. Eur. J. Endocrinol. 2011, 164, 977–984. [Google Scholar] [CrossRef] [PubMed]
- Carrera, P.; Bordone, L.; Azzani, T.; Brunelli, V.; Garancini, M.P.; Chiumello, G.; Ferrari, M. Point mutations in Italian patients with classic, non-classic, and cryptic forms of steroid 21-hydroxylase deficiency. Hum. Genet. 1996, 98, 662–665. [Google Scholar] [CrossRef] [PubMed]
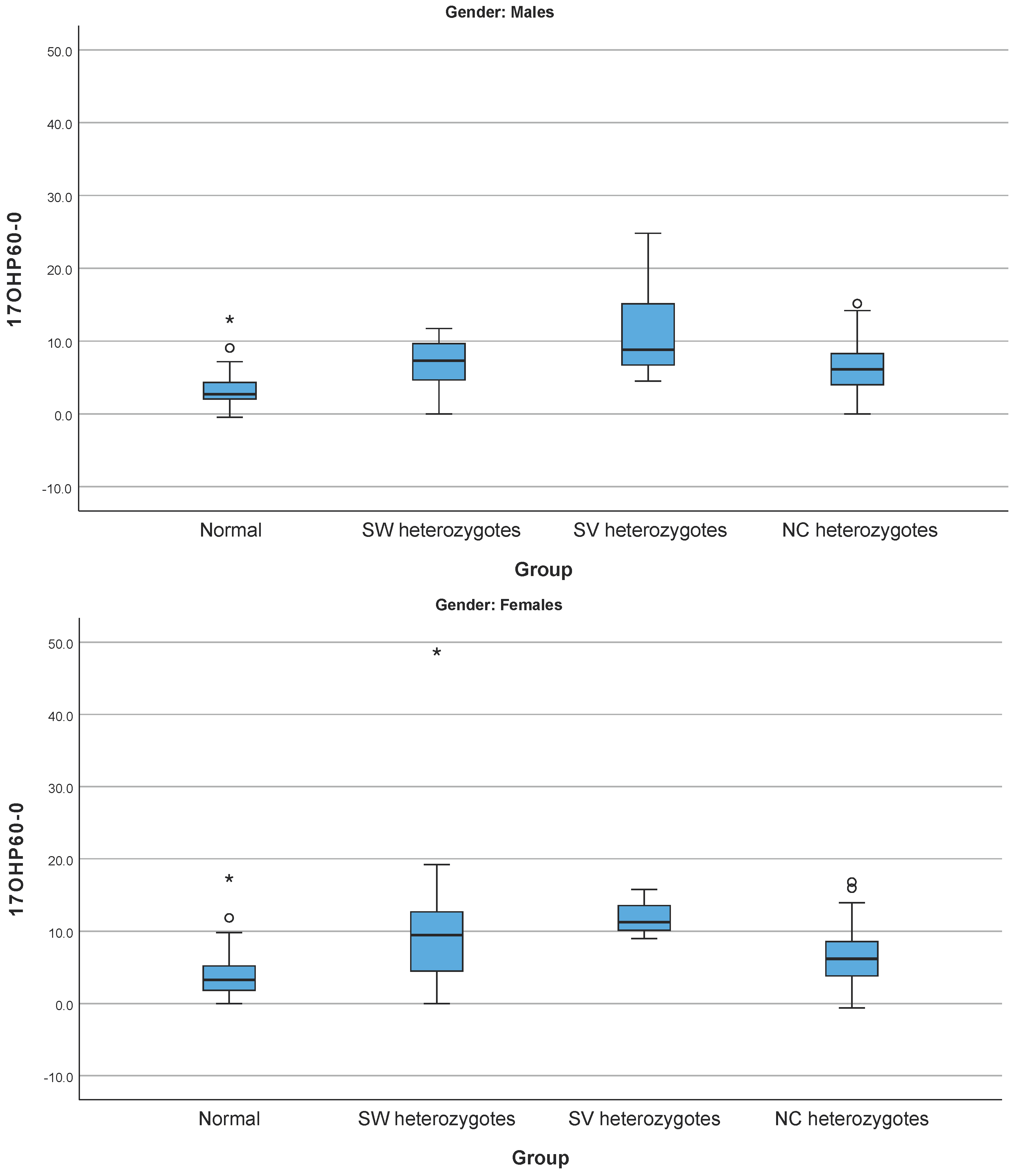
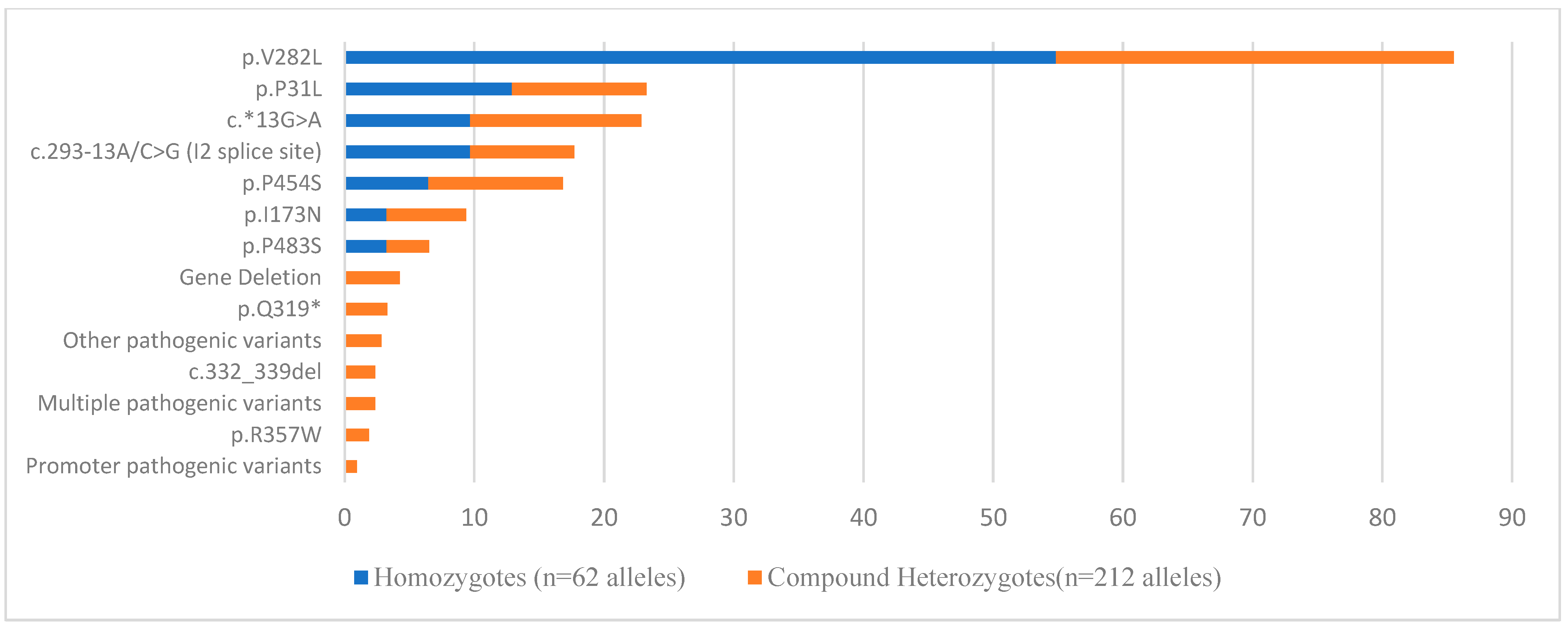


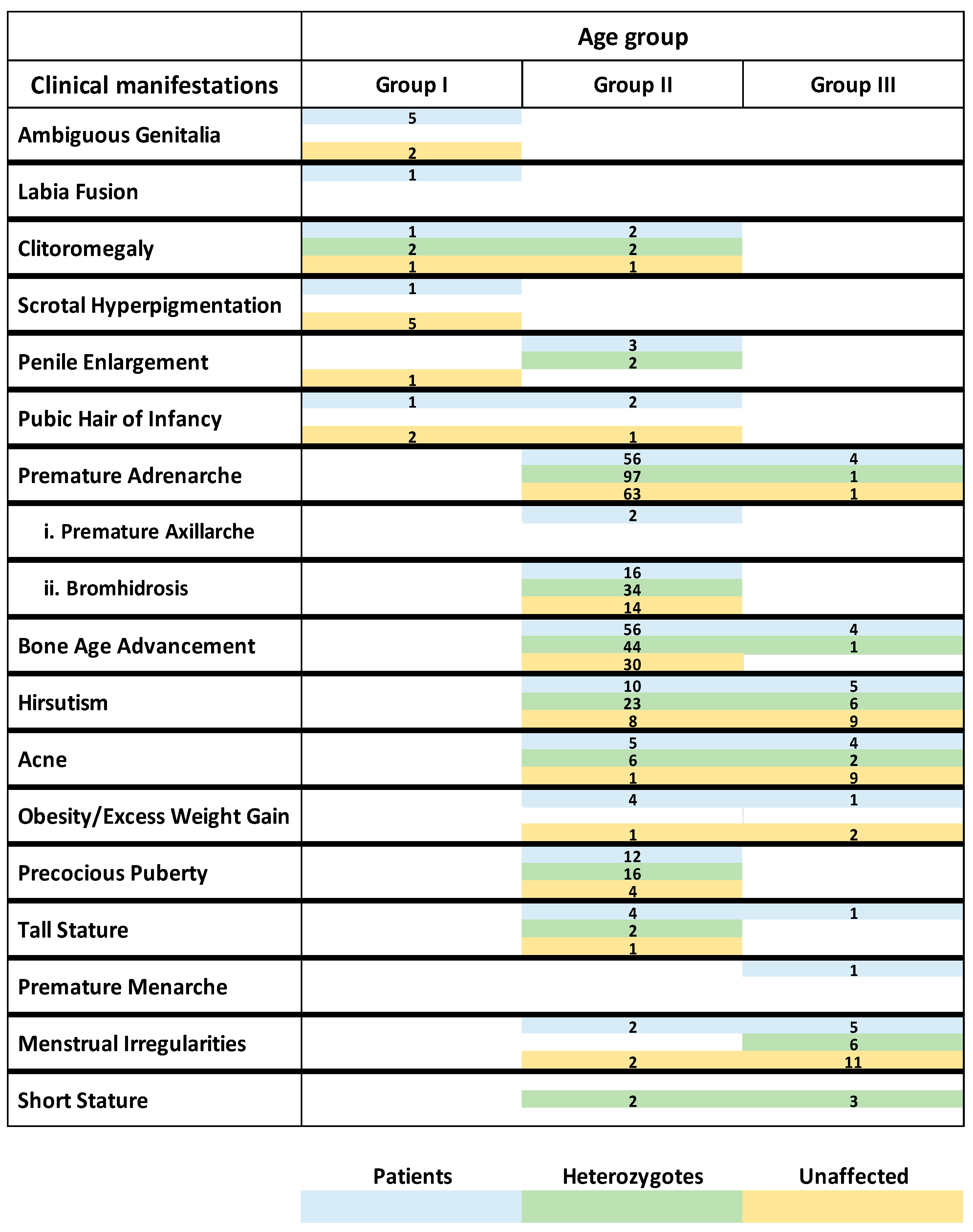
| Age Group I Patients | Age Group I Heterozygotes | Age Group I Cases with No Pathogenic Variants Identified | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Total | Males | Females | Total | Males | Females | Total | Males | Females | |
| 17-OHP concentrations(ng/mL) Valid % Median [min–max] IQR Mean ± SD | n = 7 63.6 35.9 [0.8–1.793] 64.9 287.7 ± 664.9 | n = 1 25 23 [23–23] 0 23 | n = 6 85.7 38.1 [0.8–1.793] 85.5 331.8 ± 717 | n = 2 50 11 [4.3–17.6] 6.6 11 ± 9.4 | n = 0 | n = 2 66.7 11 [4.3–17.6] 6.6 11 ± 9.4 | n = 13 100 3.8 [1.7–19] 8.2 6.8 ± 5.7 | n = 9 100 4.8 [1.7–19] 8.5 8.1 ± 6.4 | n = 4 100 3.7 [3.3–4.3] 0.4 3.8 ± 0.4 |
| F concentrations (μg/dL) Valid % Median [min–max] IQR Mean ± SD | n = 3 27.3 5.4 [3.3–19.4] 8.1 9.4 ± 8.8 | n = 0 | n = 3 42.9 5.4 [3.3–19.4] 8.1 9.4 ± 8.8 | n = 3 75 15.9 [14.6–18.9] 2.2 16.5 ± 2.2 | n = 0 | n = 3 100 15.9 [14.6–18.9] 2.2 16.5 ± 2.2 | n = 13 100 8 [0.9–23.1] 10.2 9.4 ± 7.3 | n = 9 100 6.5 [0.9–20.2] 7.9 8.5 ± 6.8 | n = 4 100 10.6 [1.7–23.1] 9.3 11.5 ± 9 |
| Δ4-A (ng/mL) Valid % Median [min–max] IQR Mean ± SD | n = 4 36.4 6 [0.4–12.5] 5.6 6.2 ± 5.1 | n = 0 | n = 4 57.1 6 [0.4–12.5] 5.6 6.2 ± 5.1 | n = 2 50 2.6 [0.1–5.2] 2.5 2.6 ± 3.6 | n = 0 | n = 2 66.7 2.6 [0.1–5.2] 2.5 2.6 ± 3.6 | n = 8 61.5 0.4 [0–2.9] 2 1 ± 1.2 | n = 4 44.4 0.2 [0–2.8] 0.9 0.8 ± 1.4 | n = 4 100 1.1 [0.1–2.9] 1.6 1.3 ± 1.3 |
| Τ (ng/dL) Valid % Median [min–max] IQR Mean ± SD | n = 3 27.3 36.5 [0.1–500] 249.9 178.9 ± 278.7 | n = 0 | n = 3 42.9 36.5 [0.1–500] 249.9 178.9 ± 278.7 | n = 1 25 1.1 [1.1–1.1] 0 1.1 | n = 0 | n = 1 33.3 1.1 [1.1–1.1] 0 1.1 | n = 10 76.9 62 [1.1–193] 74.4 69.2 ± 62.3 | n = 6 66.7 68 [1.1–193] 108.6 78.8 ± 76.3 | n = 4 100 61.5 [2.5–94] 31.1 54.9 ± 38.4 |
| ACTH (pg/mL) Valid % Median [min–max] IQR Mean ± SD | n = 4 36.4 34.5 [2.5–163] 65.2 58.6 ± 72.5 | n = 0 | n = 4 57.1 34.5 [2.5–163] 65.2 58.6 ± 72.5 | n = 1 25 130.9 [130.9–130.9] 0 130.9 | n = 0 | n = 1 33.3 130.9 [130.9–130.9] 0 130.9 | n = 8 61.5 53.1 [9.9–91.4] 42.3 49.8 ± 29.8 | n = 4 44.4 60.6 [17–91.4] 21.8 57.4 ± 30.7 | n = 4 100 38 [9.9–82.8] 32.9 42.1 ± 31.2 |
| DHEAS (μg/dL) Valid % Median [min–max] IQR Mean ± SD | n = 4 36.4 809 [2–2208.6] 1588.2 957.2 ± 1076.6 | n = 0 | n = 4 57.1 809 [2–2208.6] 1588.2 957.2 ± 1076.6 | n = 2 50 61 [4.1–118] 57.0 61 ± 80.6 | n = 0 | n = 2 66.7 61 [4.1–118] 57 61 ± 80.6 | n = 9 69.2 24 [0.2–2458.5] 978.5 563.4 ± 894.9 | n = 6 66.7 5.2 [0.2–980] 74.1 181.4 ± 393.1 | n = 3 75 1500 [24–2458.5] 1.217.2 1327.5 ± 1226.4 |
| Age Group II Patients | Age Group II Heterozygotes | Age Group II Cases with No Pathogenic Variants Identified | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Total | Males | Females | Total | Males | Females | Total | Males | Females | |
| 17-OHP concentrations (ng/mL) Valid % Median [min–max] IQR Mean ± SD | n = 100 90.9 11 [0.8–200] 18.8 25.7 ± 40.1 | n = 30 88.2 13.6 [1.9–200] 39.6 37.7 ± 52.3 | n = 70 92.1 9.6 [0.8–200] 18.2 20.5 ± 32.7 | n = 183 98.9 2.6 [0.3–13.7] 2.4 3.1 ± 2.1 | n = 47 97.9 2.1 [0.4–7.5] 1.6 2.5 ± 1.5 | n = 136 99.3 2.7 [0.3–13.7] 2.6 3.4 ± 2.3 | n = 94 97.9 2.1 [0.5–25] 1.7 2.7 ± 2.8 | n = 10 100 1.5 [0.5–25] 2.7 4.2 ± 7.5 | n = 84 97.7 2.1 [0.5–8.9] 1.6 2.5 ± 1.5 |
| F concentrations (μg/dL) Valid % Median [min–max] IQR Mean ± SD | n = 91 82.7 12.3 [4.2–26.5] 6.7 12.9 ± 4.9 | n = 30 88.2 12.2 [4.2–91.5] 6.7 14.8 ± 15.5 | n = 61 80.3 12.5 [5.2–26.5] 6.7 13.3 ± 4.6 | n = 170 91.9 13.8 [3.9–29.5] 7.7 14.5 ± 5.6 | n = 43 89.6 14 [5.3–23.8] 8.3 13.8 ± 5.3 | n = 127 92.7 13.7 [3.9–29.5] 7.6 14.8 ± 5.6 | n = 90 93.8 14.8 [0.6–31.5] 9.8 15.5 ± 6.5 | n = 10 100 16.6 [2.9–26.7] 10.1 15.7 ± 7.9 | n = 80 93 14.8 [0.6–31.5] 9.5 15.5 ± 6.4 |
| Δ4-A (ng/mL) Valid % Median [min–max] IQR Mean ± SD | n = 71 64.5 0.8 [0–35.3] 1.4 1.7 ± 4.3 | n = 21 61.8 0.9 [0.1–35.3] 1.7 3.1 ± 7.6 | n = 50 65.8 0.8 [0–6.7] 1.1 1.2 ± 1.3 | n = 117 63.2 0.4 [0–76] 0.6 1.2 ± 7 | n = 29 60.4 0.4 [0–1.4] 0.6 0.5 ± 0.4 | n = 88 64.2 0.5 [0–76] 0.6 1.5 ± 8.1 | n = 73 76 0.4 [0.1–22] 0.5 0.9 ± 2.6 | n = 7 70 0.3 [0.1–0.7] 0.3 0.4 ± 0.2 | n = 66 76.7 0.4 [0.1–22] 0.6 1 ± 2.7 |
| Τ (ng/dL) Valid % Median [min–max] IQR Mean ± SD | n = 88 80 8 [0–309] 16.5 20.6 ± 49.1 | n = 31 91.2 3 [0–309] 22.6 36.0 ± 79.0 | n = 57 75 8 [0–78.9] 15 12.3 ± 14.3 | n = 144 77.8 5 [0–126] 12 10.6 ± 18.2 | n = 41 85.4 7 [0–126] 16 18.2 ± 30.1 | n = 103 75.2 3 [0–47] 11 7.6 ± 8.7 | n = 83 86.5 2.5 [0–250] 5 9.5 ± 29.1 | n = 10 100 2 [0–19] 2.2 3.5 ± 5.6 | n = 73 84.9 2.5 [0–250] 5 10.4 ± 30.9 |
| ACTH (pg/mL) Valid % Median [min–max] IQR Mean ± SD | n = 51 46.4 38.9 [2.3–357] 64.8 73.7 ± 80.6 | n = 16 47.1 45.6 [2.3–357] 109.4 101 ± 104.8 | n = 35 46.1 36.5 [10–310] 36.5 61.2 ± 64.7 | n = 80 43.2 28.2 [0.6–400] 24.8 42.2 ± 49.8 | n = 18 37.5 27.9 [8.8–120] 9.5 32.9 ± 24.7 | n = 62 45.3 29.4 [0.6–400] 30 44.9 ± 54.9 | n = 55 57.3 33 [0.7–229] 35.3 46.8 ± 44.3 | n = 8 80 34.5 [13.8–153] 39.4 53.3 ± 46.4 | n = 47 54.7 33 [0.7–229] 30.8 45.7 ± 44.4 |
| DHEAS (μg/dL) Valid % Median [min–max] IQR Mean ± SD | n = 94 85.5 79.9 [0.1–3,742] 118.1 142.1 ± 390.1 | n = 30 88.2 83.8 [0.4–448] 195.2 130.4 ± 135.6 | n = 64 84.2 79.9 [0.1–3,742] 109.6 147.6 ± 464.9 | n = 153 82.7 76.3 [0–964] 82 82.6 ± 93 | n = 37 71.1 78 [1.2–252] 102 95.1 ± 68.9 | n = 116 84.7 73.4 [0–964] 74.5 78.6 ± 99.4 | n = 85 88.5 64.9 [0.3–341] 70.5 80.9 ± 60.8 | n = 9 90 65 [0.5–195] 88.8 84.3 ± 63.6 | n = 76 88.4 64.4 [0.3–341] 69.4 80.5 ± 60.9 |
| Age Group III Patients | Age Group III Heterozygotes | Age Group III Cases with No Pathogenic Variants Identified | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Total | Males | Females | Total | Males | Females | Total | Males | Females | |
| 17-OHP concentrations (ng/mL) Valid % Median [min–max] IQR Mean ± SD | n = 14 93.3 13.1 [1.7–119.5] 10.8 23.3 ± 31.8 | n = 3 100 12 [1.7–20.6] 9.4 11.4 ± 9.4 | n = 11 91.7 13.5 [3.4–119.5] 10 26.6 ± 35.2 | n = 18 100 3.6 [0.8–7.8] 1.7 3.8 ± 1.7 | n = 4 100 4.3 [3.3–5.2] 1.3 4.2 ± 0.9 | n = 14 100 3.3 [0.8–7.8] 1.4 3.7 ± 1.9 | n = 24 96 3.6 [0.6–20.9] 2.8 4.9 ± 4.5 | n = 6 100 4.1 [2.6–13.1] 1.5 5.4 ± 3.9 | n = 18 94.7 3.2 [0.6–20.9] 3 4.8 ± 4.8 |
| F concentrations (μg/dL) Valid % Median [min–max] IQR Mean ± SD | n = 11 73.3 16.2 [4.6–27.9] 5.9 15 ± 6.4 | n = 3 100 16.2 [6.5–17.3] 5.4 13.3 ± 5.9 | n = 8 66.7 15.4 [4.6–27.9] 6 15.6 ± 6.8 | n = 15 83.3 14.9 [9.8–23.2] 4.8 15.5 ± 4.3 | n = 4 100 15.9 [9.8–23.2] 4.8 16.2 ± 5.5 | n = 11 78.6 14.9 [10–23.1] 4.4 15.2 ± 4.1 | n = 22 84.6 18.5 [5.4–29.5] 8.4 17.2 ± 6.1 | n = 5 83.3 12.9 [11.1–18.6] 6.4 14.4 ± 3.6 | n = 18 90 18.7 [5.4–29.5] 8.3 18 ± 6.4 |
| Δ4-A (ng/mL) Valid % Median [min–max] IQR Mean ± SD | n = 9 60 3 [1.1–7.9] 1.7 3.4 ± 2 | n = 3 100 1.3 [1.1–2.4] 0.6 1.6 ± 0.7 | n = 6 50 3.9 [2.7–7.9] 1.1 4.3 ± 1.9 | n = 10 55.6 2.3 [0.9–6.2] 1.6 2.8 ± 1.9 | n = 2 50 2.1 [1.9–2.3] 0.2 2.1 ± 0.3 | n = 8 57.1 2.6 [0.9–6.2] 2.6 3 ± 2.1 | n = 19 76 2.4 [0.2–6.8] 2 2.8 ± 1.7 | n = 4 66.7 0.7 [0.2–2.3] 0.6 1 ± 0.9 | n = 15 78.9 2.6 [1.2–6.8] 2.4 3.3 ± 1.6 |
| Τ (ng/dL) Valid % Median [min–max] IQR Mean ± SD | n = 13 86.7 18 [0.5–173] 70.3 47.3 ± 57.3 | n = 3 100 133 [18–173] 77.5 108 ± 80.5 | n = 10 83.3 15.5 [0.5–110] 38.8 29 ± 36.7 | n = 16 88.9 30 [0.1–621] 55.5 126.6 ± 218.3 | n = 4 100 531 [11.4–621] 228.9 423.6 ± 281.1 | n = 12 85.7 26 [0.1–80] 38 27.6 ± 26 | n = 18 72 26.5 [0.1–560.1] 42.6 78.7 ± 157.9 | n = 3 50 452 [6.8–560.1] 276.6 339.6 ± 293.3 | n = 15 78.9 25 [0.1–68] 36.2 26.5 ± 22.1 |
| ACTH (pg/mL) Valid % Median [min–max] IQR Mean ± SD | n = 8 53.3 30.4 [15.4–142] 21.4 45.6 ± 40.8 | n = 0 | n = 8 66.7 30.4 [15.4–142] 21.4 45.6 ± 40.8 | n = 10 55.6 28.6 [16.6–59.2] 14.9 32.3 ± 12.6 | n = 1 25 31.6 [31.6–31.6] 0 31.6 | n = 9 64.3 26 [16.6–59.2] 17.9 32.4 ± 13.3 | n = 9 36 26.2 [10–84.8] 29.5 34.3 ± 24.6 | n = 0 | n = 9 47.4 26.2 [10–84.8] 29.5 34.3 ± 24.6 |
| DHEAS (μg/dL) Valid % Median [min–max] IQR Mean ± SD | n = 13 86.7 243 [0.6–704] 275.6 280.6 ± 212.3 | n = 3 100 243 [113.8–260] 73.1 205.6 ± 80 | n = 10 83.3 246.5 [0.6–704] 274.6 303.1 ± 237.1 | n = 16 88.9 209 [1.3–453] 164.5 189 ± 123.2 | n = 3 75 255 [212–298] 43 255 ± 43 | n = 13 92.9 131 [1.3–453] 159.5 173.8 ± 131.6 | n = 22 88 196.5 [0.8–702] 247 227.9 ± 184.1 | n = 4 66.7 139 [72–702] 219 263 ± 296.3 | n = 18 94.7 229.5 [0.8–573.5] 241 220.1 ± 161.3 |
| Variant | Location CYP21A2 | Genotype | Clinical Manifestation | Sex | Hormonal Profile | Frequency (GnomAD) | ACMG Classification | Remarks | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Age | Hormonal Findings (before Treatment, if Required) | ACTH Stimulation Test | |||||||||||
| 0′ | 30′ | 60′ | |||||||||||
| c.-127G>A | 5’UTR | c.[-127G>A];[=] | PA | f | 7 yrs | 17-OHP: 4.98 ng/mL T: <0.025 ng/dL, DHEA-S: 61 μg/dL Δ4-A: 0.4 ng/mL | 17-OHP (ng/mL) | 4.91 | 11.88 | -(not efficiently covered) | VUS (PM2, BP7) | Promoter region (c.-76 to c.-126) | |
| F (μg/dL) | 18.3 | 26 | |||||||||||
| c.-115G>T | 5’UTR | c.[-115G>T];[92C>T] | PA | f | 6 yrs | - | 17-OHP (ng/mL) | 9.22 | 33.6 | 0.00003188 | VUS (PM2, PM3, BP7) | Promoter region (c.-76 to c.-126) | |
| F(μg/dL) | 12.99 | 23.43 | |||||||||||
| c.-82C>T | 5’UTR | c.[-82C>T];[=] | PA | f | 7 yrs | Δ4-A: 0.96 ng/mL, T: 6 ng/dL, DHEA-S: 178 μg/dL | 17-OHP (ng/mL) | 3.64 | 4.13 | 5.69 | 0.00223 | VUS (BS1, BP7) | Promoter region (c.-76 to c.-126) |
| F (μg/dL) | 31.1 | 37.7 | 39.9 | ||||||||||
| c.764G>A# (p.R255K) | Exon 7 | c.[764G>A];[=] | PHI | m | 6 mos | 17-OHP: 4 ng/mL, Cortisol: 14.7 μg/dL, ACTH: 99 pg/mL, PRA: 4.18 ng/mL/h, T: 0.88 ng/dL, DHEA-S: 246.8 μg/dL, Δ4-A: 0.76, Aldosterone: 71.5 ng/dL | 17-OHP (ng/mL) | 4.39 | 9.03 | 0.000004032 | VUS (PM2) | [31] * | |
| F (μg/dL) | 10.2 | 24.8 | |||||||||||
| c.844G>A (p.V282M) | Exon 7 | p.[V282M];[L308Ffs*6] | PA | f | 11 yrs | 17-OHP: 17.1 ng/mL, DHEA-S: 121 μg/dL, Δ4-A: 1.68 ng/mL | 17-OHP (ng/mL) | 6.5 | 57.6 | 71.6 | 0.00001238 | LP (PM2, PP2, PM3, PM5) | [32] |
| F (μg/dL) | 6.56 | 11.7 | 13.64 | ||||||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fylaktou, I.; Mertzanian, A.; Farakla, I.; Gryparis, A.; Vasilakis, I.A.; Binou, M.; Charmandari, E.; Kanaka-Gantenbein, C.; Sertedaki, A. Genetics of 21-OH Deficiency and Genotype–Phenotype Correlation: Experience of the Hellenic National Referral Center. Curr. Issues Mol. Biol. 2024, 46, 10696-10713. https://doi.org/10.3390/cimb46100635
Fylaktou I, Mertzanian A, Farakla I, Gryparis A, Vasilakis IA, Binou M, Charmandari E, Kanaka-Gantenbein C, Sertedaki A. Genetics of 21-OH Deficiency and Genotype–Phenotype Correlation: Experience of the Hellenic National Referral Center. Current Issues in Molecular Biology. 2024; 46(10):10696-10713. https://doi.org/10.3390/cimb46100635
Chicago/Turabian StyleFylaktou, Irene, Anny Mertzanian, Ioanna Farakla, Alexandros Gryparis, Ioannis Anargyros Vasilakis, Maria Binou, Evangelia Charmandari, Christina Kanaka-Gantenbein, and Amalia Sertedaki. 2024. "Genetics of 21-OH Deficiency and Genotype–Phenotype Correlation: Experience of the Hellenic National Referral Center" Current Issues in Molecular Biology 46, no. 10: 10696-10713. https://doi.org/10.3390/cimb46100635
APA StyleFylaktou, I., Mertzanian, A., Farakla, I., Gryparis, A., Vasilakis, I. A., Binou, M., Charmandari, E., Kanaka-Gantenbein, C., & Sertedaki, A. (2024). Genetics of 21-OH Deficiency and Genotype–Phenotype Correlation: Experience of the Hellenic National Referral Center. Current Issues in Molecular Biology, 46(10), 10696-10713. https://doi.org/10.3390/cimb46100635







