Mouse Model of Anti-Obesity Effects of Blautia hansenii on Diet-Induced Obesity
Abstract
:1. Introduction
2. Materials and Methods
2.1. Samples and Reagents
2.2. Animal Diet and Care
2.3. Glucose Tolerance Tests
2.4. Quantitative Real-Time PCR Analysis
2.5. Analysis of Metabolic Parameters in Feces and Cecal Contents
2.6. Microbiome Analysis
2.7. Statistical Analysis
3. Results
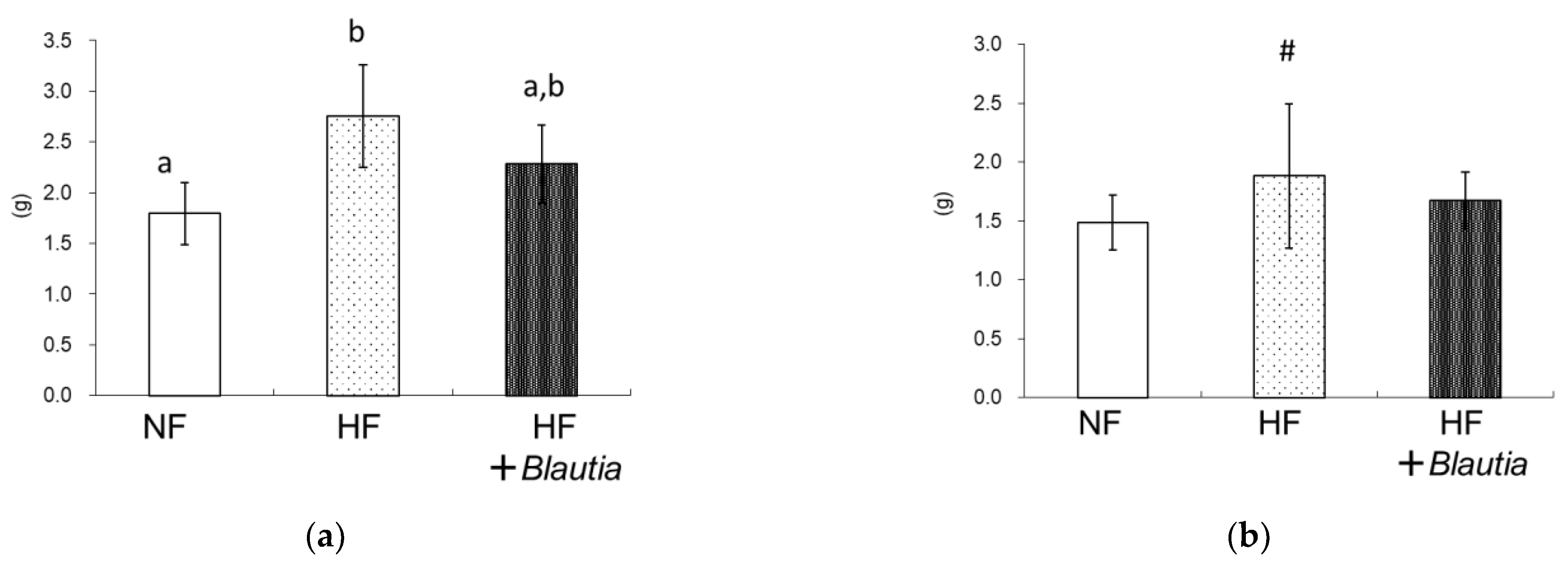
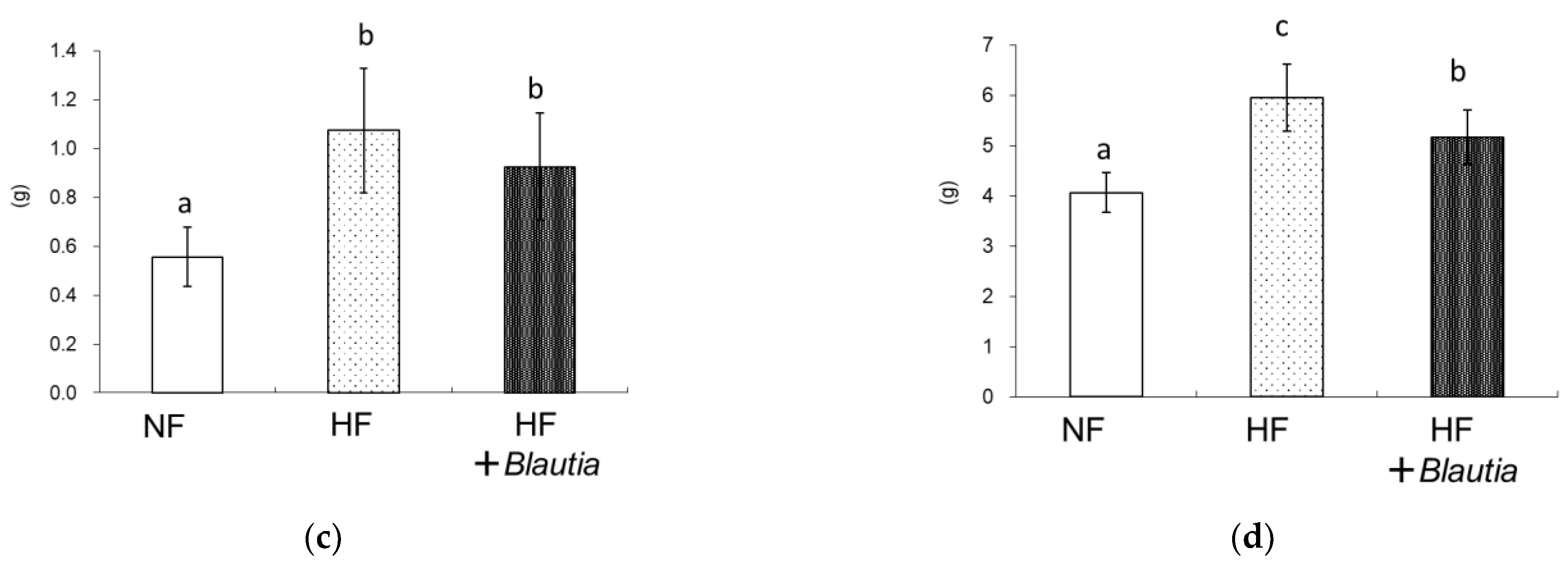
3.1. Growth Parameters and Relative Organ Weights
3.2. Serum Parameters
3.3. Oral Glucose Tolerance Test
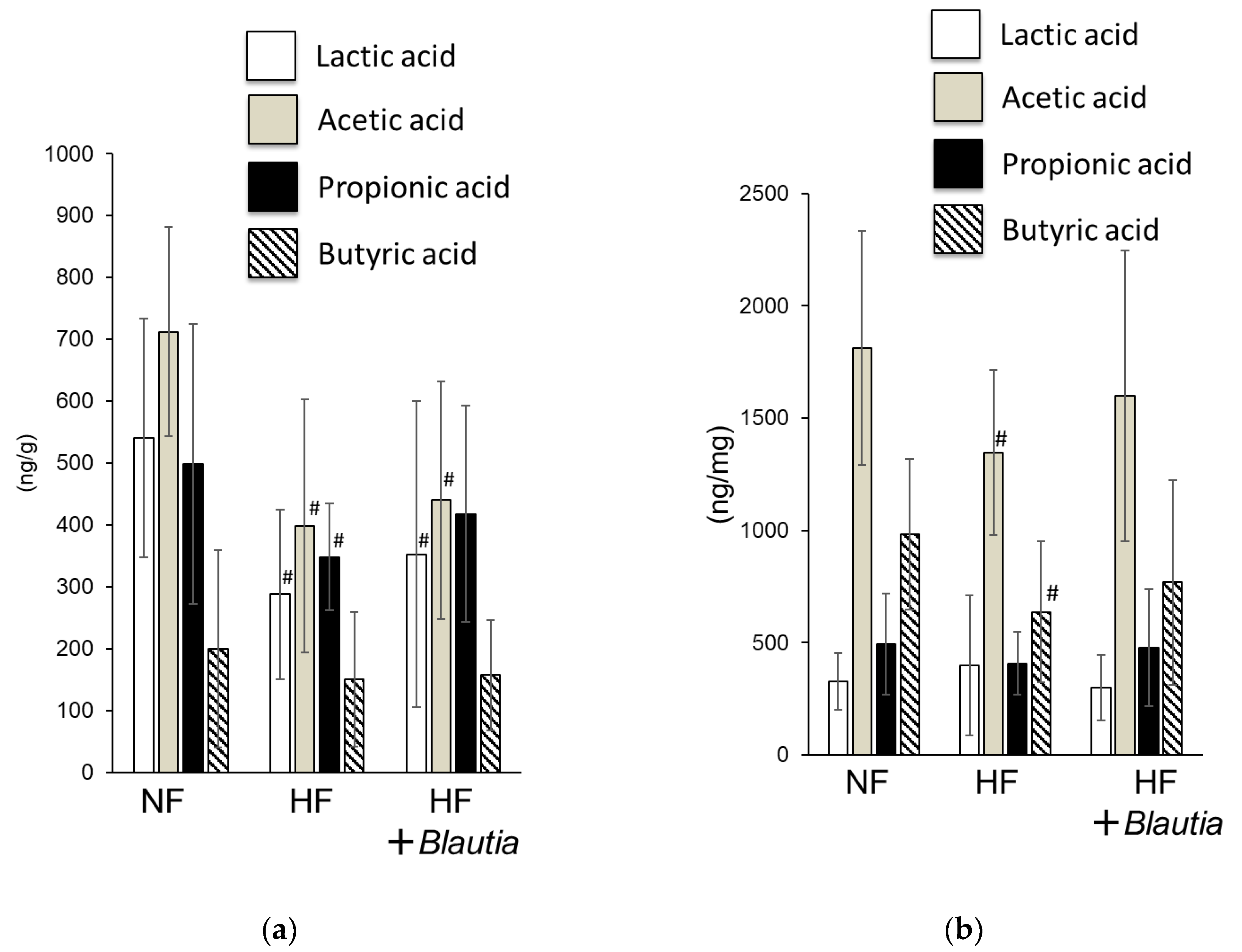
3.4. Short-Chain Fatty Acid Concentrations in Feces and Cecum
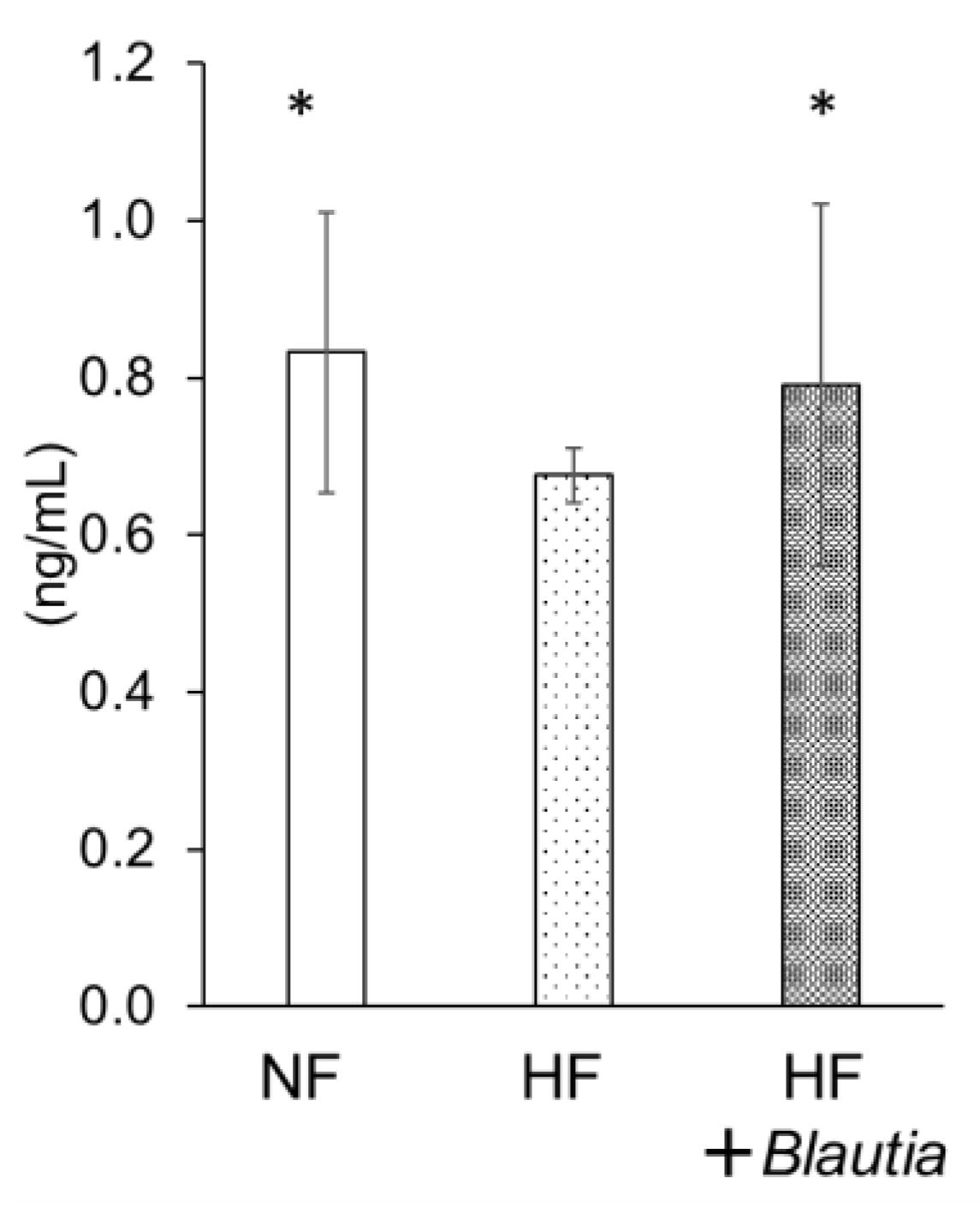
3.5. Serum Peptide YY Concentration
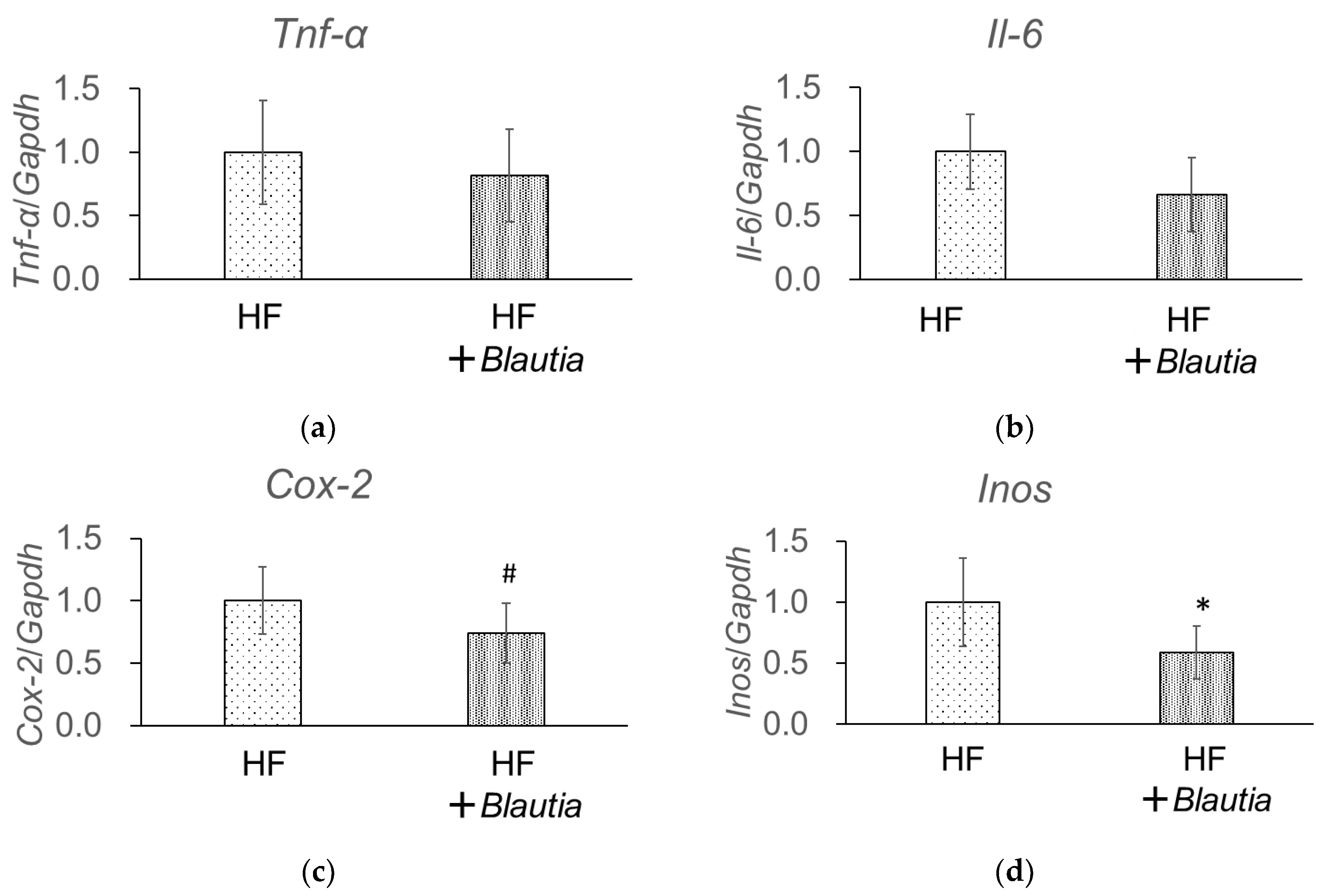
3.6. Gene Expression Related to Low-Grade Chronic Inflammation in the Liver
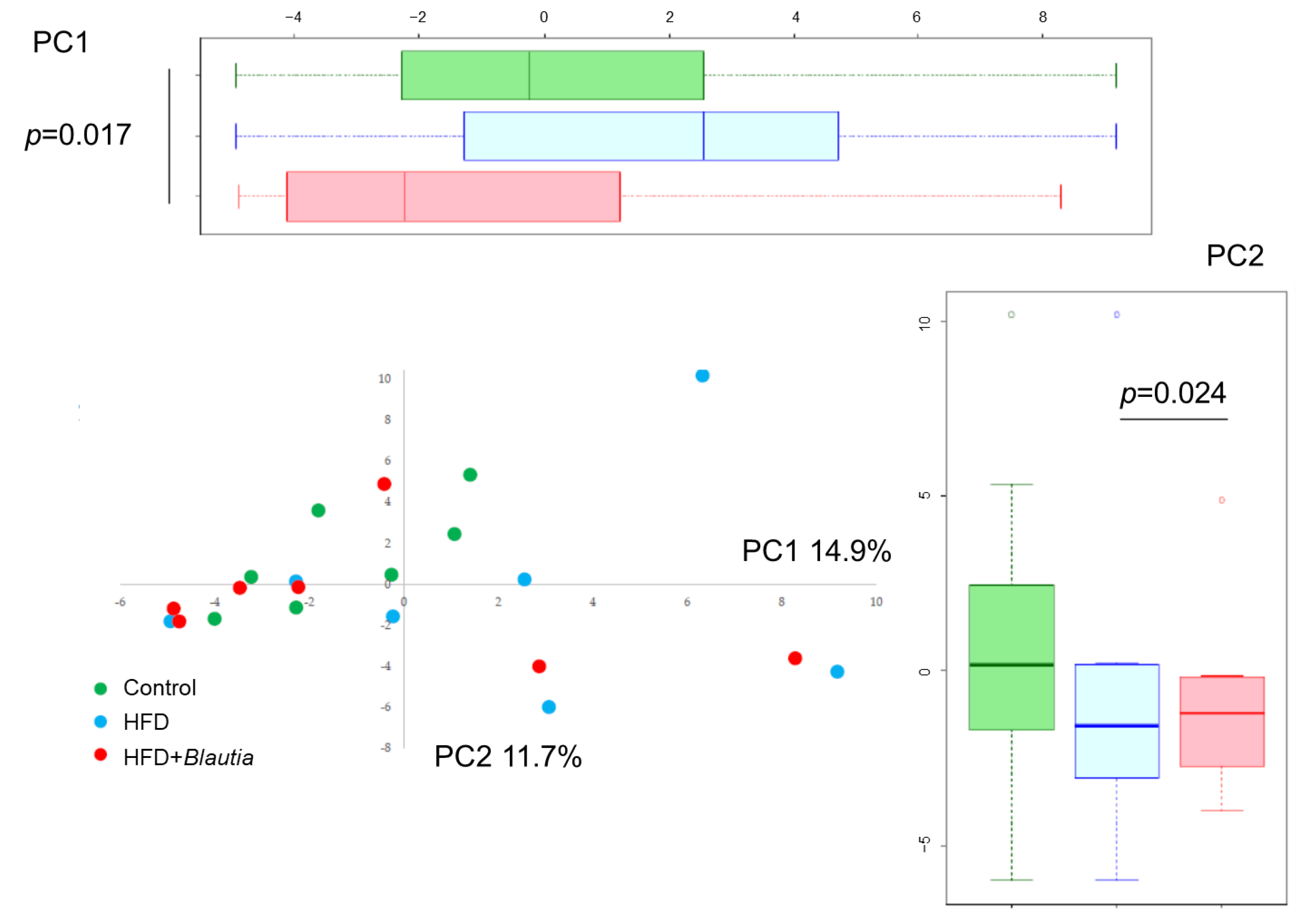
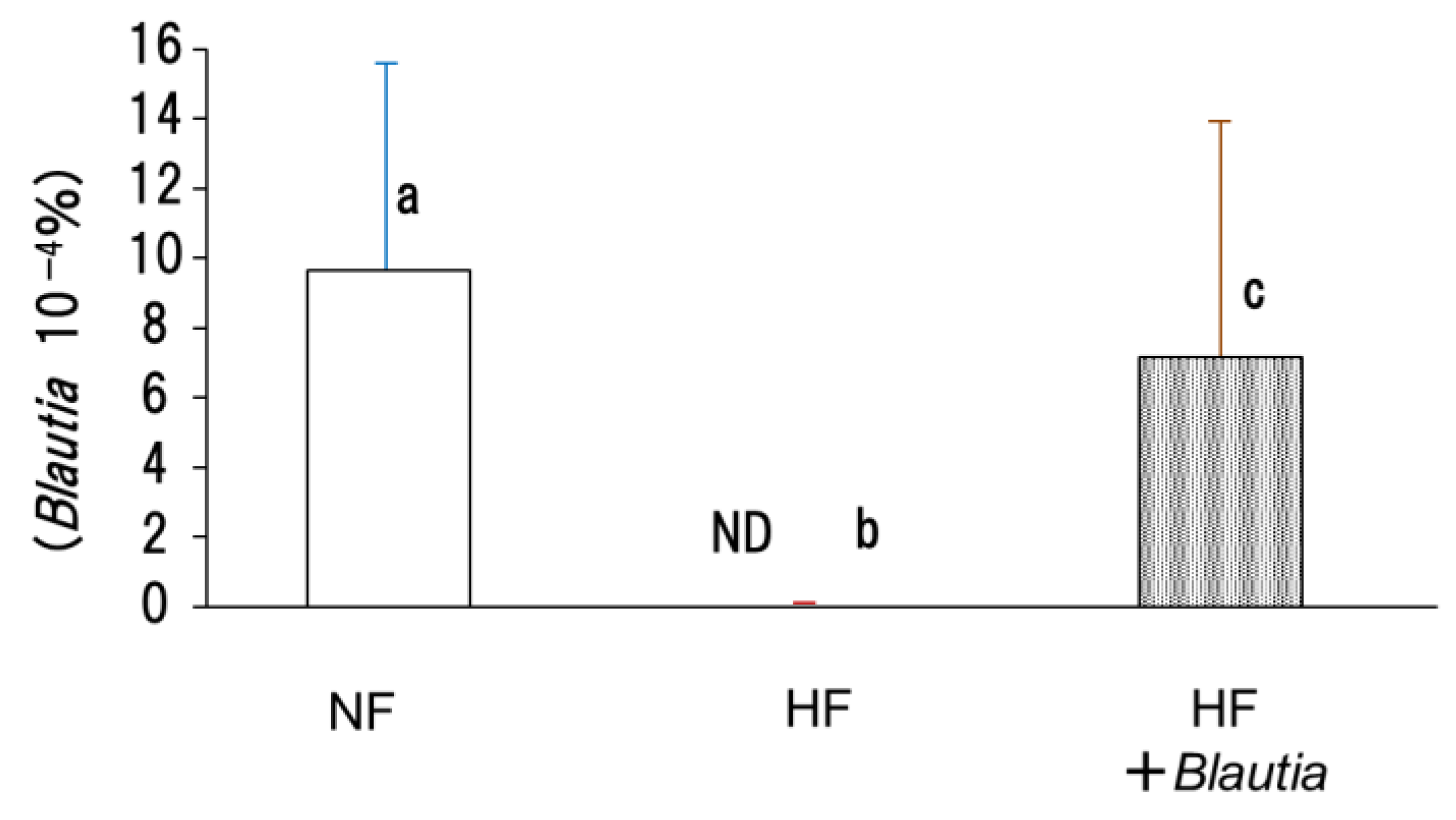
3.7. Microbiota in Cecal Contents
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Flier, J.S. Obesity wars: Molecular progress confronts an expanding epidemic. Cell 2004, 116, 337–350. [Google Scholar] [CrossRef]
- Kahn, S.E.; Hull, R.L.; Utzschneider, K.M. Mechanisms linking obesity to insulin resistance and type 2 diabetes. Nature 2006, 444, 840–846. [Google Scholar] [CrossRef] [PubMed]
- Matsuzawa, Y.; Funahashi, T.; Nakamura, T. Molecular mechanism of metabolic syndrome X: Contribution of adipocytokines adipocyte-derived bioactive substances. Ann. N. Y. Acad. Sci. 1999, 892, 146–154. [Google Scholar] [CrossRef]
- Mirjana, R.S.; Willem, M.V. The first 1000 cultured species of the human gastrointestinal microbiota. FEMS Microbiol. Rev. 2014, 38, 996–1047. [Google Scholar]
- Caitriona, M.G.; Paul, D.C. Role of the gut microbiota in health and chronic gastrointestinal disease: Understanding a hidden metabolic organ. Therap. Adv. Gastroenterol. 2013, 6, 295–308. [Google Scholar]
- Ley, R.E.; Peterson, D.A.; Gordon, J.I. Ecological and evolutionary forces shaping microbial diversity in the human intestine. Cell 2006, 124, 837–848. [Google Scholar] [CrossRef]
- Santacruz, A.; Collado, M.C.; García-Valdés, L.; Segura, M.T.; Martín-Lagos, J.A.; Anjos, T.; Martí-Romero, M.; Lopez, R.M.; Florido, J.; Campoy, C.; et al. Gut microbiota composition is associated with body weight, weight gain and biochemical parameters in pregnant women. Br. J. Nutr. 2010, 104, 83–92. [Google Scholar] [CrossRef] [PubMed]
- Lawrence, A.D.; Corinne, F.M.; Rachel, N.C.; David, B.G.; Julie, E.B.; Benjamin, E.W.; Alisha, V.L.; Devlin, A.S.; Varma, Y.; Michael, A.F.; et al. Diet rapidly and reproducibly alters the human gut microbiome. Nature 2014, 505, 559–563. [Google Scholar]
- Odamaki, T.; Kato, K.; Sugahara, H.; Hashikura, N.; Takahashi, S.; Xiao, J.Z.; Abe, F.; Osawa, R. Age-related changes in gut microbiota composition from newborn to centenarian: A cross-sectional study. BMC Microbiol. 2016, 16, 90. [Google Scholar] [CrossRef] [PubMed]
- Emily, R.D.; Darren, A.C.; Katelyn, M.; Luis, B.B.; Carole, O.; Yoav, G. Genome-Wide Association Studies of the Human Gut Microbiota. PLoS ONE 2015, 10, e0140301. [Google Scholar]
- Joanne, S. Fiber and Prebiotics: Mechanisms and Health Benefits. Nutrients 2013, 4, 1417–1435. [Google Scholar]
- Zhao, L.; Zhang, F.; Ding, X.; Wu, G.; Lam, Y.Y.; Wang, X.; Fu, H.; Xue, X.; Lu, C.; Ma, J. Gut bacteria selectively promoted by dietary fibers alleviate type 2 diabetes. Science 2018, 359, 1151–1156. [Google Scholar] [CrossRef]
- Nagendra, P.S. Functional cultures and health benefits. Int. Dairy J. 2007, 17, 1262–1277. [Google Scholar]
- Mekkes, M.C.; Weenen, T.C.; Brummer, R.J.; Claassen, E. The development of probiotic treatment in obesity: A review. Benef. Microbes 2014, 5, 19–28. [Google Scholar] [CrossRef]
- Fukushima, Y.; Kawata, Y.; Hara, H.; Terada, A.; Mitsuoka, T. Effect of a probiotic formula on intestinal immunoglobulin A production in healthy children. Int. J. Food Microbiol. 1998, 42, 39–44. [Google Scholar] [CrossRef]
- Nakamura, F.; Ishida, Y.; Aihara, K.; Sawada, D.; Ashida, N.; Sugawara, T.; Aoki, Y.; Takehara, I.; Takano, K.; Fujiwara, S. Effect of fragmented Lactobacillus amylovorus CP1563 on lipid metabolism in overweight and mildly obese individuals: A randomized controlled trial. Microb. Ecol. Health Dis. 2016, 27, 30312. [Google Scholar] [PubMed]
- Minami, J.; Kondo, S.; Yanagisawa, N.; Odamaki, T.; Xiao, J.; Abe, F.; Nakajima, S.; Hamamoto, Y.; Saitoh, S.; Shimoda, T. Oral administration of Bifidobacterium breve B-3 modifies metabolic functions in adults with obese tendencies in a randomized controlled trial. J. Nutr. Sci. 2015, 4, e17. [Google Scholar] [CrossRef]
- Kadooka, Y.; Sato, M.; Imaizumi, K.; Ogawa, A.; Ikuyama, K.; Akai, Y.; Okano, M.; Kagoshima, M.; Tsuchida, T. Regulation of abdominal adiposity by probiotics (Lactobacillus gasseri SBT2055) in adults with obese tendencies in a randomized controlled trial. Eur. J. Clin. Nutr. 2010, 64, 636–643. [Google Scholar] [CrossRef]
- Peter, J.T.; Ruth, E.L.; Michael, A.M.; Vincent, M.; Elaine, R.M.; Jeffrey, I. Gordon. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature 2006, 444, 1027–1031. [Google Scholar]
- Schwiertz, A.; Taras, D.; Schäfer, K.; Beijer, S.; Bos, N.A.; Donus, C.; Hardt, P.D. Microbiota and SCFA in lean and overweight healthy subjects. Obesity 2010, 18, 190–195. [Google Scholar] [CrossRef]
- Le Chatelier, E.; Nielsen, T.; Qin, J.; Prifti, E.; Hildebrand, F.; Falony, G.; Almeida, M.; Arumugam, M.; Batto, J.-M.; Kennedy, S.; et al. Richness of human gut microbiome correlates with metabolic markers. Nature 2013, 500, 541–546. [Google Scholar] [CrossRef]
- Duncan, S.H.; Lobley, G.E.; Holtrop, G.; Ince, J.; Johnstone, A.M.; Louis, P.; Flint, H.J. Human colonic microbiota associated with diet, obesity and weight loss. Int. J. Obes. 2008, 32, 1720–1724. [Google Scholar] [CrossRef]
- Walker, A.W.; Ince, J.; Duncan, S.H.; Webster, L.M.; Holtrop, G.; Ze, X.; Brown, D.; Stares, M.D.; Scott, P.; Bergerat, A.; et al. Dominant and diet-responsive groups of bacteria within the human colonic microbiota. ISME J. 2011, 5, 220–230. [Google Scholar] [CrossRef]
- Ozato, N.; Saito, S.; Yamaguchi, T.; Katashima, M.; Tokuda, I.; Sawada, K.; Katsuragi, Y.; Kakuta, M.; Imoto, S.; Ihara, K.; et al. Blautia genus associated with visceral fat accumulation in adults 20–76 years of age. NPJ Biofilms Microbiomes 2019, 5, 28. [Google Scholar] [CrossRef]
- Ozato, N.; Yamaguchi, T.; Mori, K.; Katashima, M.; Kumagai, M.; Murashita, K.; Katsuragi, Y.; Tamada, Y.; Kakuta, M.; Imoto, S.; et al. Two Blautia Species Associated with Visceral Fat Accumulation: A One-Year Longitudinal Study. Biology 2022, 11, 318. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Mao, B.; Gu, J.; Wu, J.; Cui, S.; Wang, G.; Zhao, J.; Zhang, H.; Chen, W. Blautia—A new functional genus with potential probiotic properties? Gut Microbes 2021, 13, 1875796. [Google Scholar] [CrossRef]
- Nishijima, S.; Suda, W.; Oshima, K.; Kim, S.W.; Hirose, Y.; Morita, H.; Hattori, M. The gut microbiome of healthy Japanese and its microbial and functional uniqueness. DNA Res. 2016, 23, 125–133. [Google Scholar] [CrossRef] [PubMed]
- Bamberger, C.; Rossmeier, A.; Lechner, K.; Wu, L.; Waldmann, E.; Fischer, S.; Stark, R.G.; Altenhofer, J.; Henze, K.; Parhofer, K.G. A Walnut-Enriched Diet Affects Gut Microbiome in Healthy Caucasian Subjects: A Randomized, Controlled Trial. Nutrients 2018, 10, 244. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Li, J.; Zhang, Y.; Philip, A.; Shi, E.; Chi, X.; Meng, J. Influence of glucose fermentation on CO2 assimilation to acetate in homoacetogen Blautia coccoides GA-1. J. Ind. Microbiol. Biotechnol. 2015, 42, 1217–1224. [Google Scholar] [CrossRef]
- Kimura, I.; Inoue, D.; Maeda, T.; Hara, T.; Ichimura, A.; Miyauchi, S.; Kobayashi, M.; Hirasawa, A.; Tsujimoto, G. Short-chain fatty acids and ketones directly regulate sympathetic nervous system via G protein-coupled receptor 41 (GPR41). Proc. Natl. Acad. Sci. USA 2011, 108, 8030–8035. [Google Scholar] [CrossRef]
- Kimura, I.; Ozawa, K.; Inoue, D.; Imamura, T.; Kimura, K.; Maeda, T.; Terasawa, K.; Kashihara, D.; Hirano, K.; Tani, T.; et al. The gut microbiota suppresses insulin-mediated fat accumulation via the short-chain fatty acid receptor GPR43. Nat. Commun. 2013, 4, 1829. [Google Scholar] [CrossRef] [PubMed]
- Endo, A.; Pärtty, A.; Kalliomäki, M.; Isolauri, E.; Salminen, S. Long-term monitoring of the human intestinal microbiota from the second week to 13 years of age. Anaerobe 2014, 28, 149–156. [Google Scholar] [CrossRef]
- Bai, G.; Ni, K.; Tsuruta, T.; Nishino, N. Dietary Casein and Soy Protein Isolate Modulate the Effects of Raffinose and Fructooligosaccharides on the Composition and Fermentation of Gut Microbiota in Rats. J. Food Sci. 2016, 81, H2093–H2098. [Google Scholar] [CrossRef] [PubMed]
- Hosomi, K.; Saito, M.; Park, J.; Murakami, H.; Shibata, N.; Ando, M.; Nagatake, T.; Konishi, K.; Ohno, H.; Tanisawa, K.; et al. Oral administration of Blautia wexlerae ameliorates obesity and type 2 diabetes via metabolic remodeling of the gut microbiota. Nat. Commun. 2022, 13, 4477. [Google Scholar] [CrossRef]
- Zeng, Y.; Zhang, H.; Zong, L.; Tsao, R.; Arie, H.; Izumo, T.; Shibata, H.; Mine, Y. Lactobacillus pentosus S-PT84 prevents LPS-induced low-grade chronic inflammation in a C57BL/6J mouse model. J. Funct. Foods 2019, 62, 103526. [Google Scholar] [CrossRef]
- Takahashi, S.; Tomita, J.; Nishioka, K.; Hisada, T.; Nishijima, M. Development of a prokaryotic universal primer for simultaneous analysis of Bacteria and Archaea using next-generation sequencing. PLoS ONE 2014, 9, e105592. [Google Scholar] [CrossRef]
- Hisada, T.; Endoh, K.; Kuriki, K. Inter- and intra-individual variations in seasonal and daily stabilities of the human gut microbiota in Japanese. Arch. Microbiol. 2015, 197, 919–934. [Google Scholar] [CrossRef]
- Kasai, C.; Sugimoto, K.; Moritani, I.; Tanaka, J.; Oya, Y.; Inoue, H.; Tameda, M.; Shiraki, K.; Ito, M.; Takei, Y.; et al. Comparison of the gut microbiota composition between obese and non-obese individuals in a Japanese population, as analyzed by terminal restriction fragment length polymorphism and next-generation sequencing. BMC Gastroenterol. 2015, 15, 100. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Real, J.M.; Ricart, W. Insulin resistance and chronic cardiovascular inflammatory syndrome. Endocr. Rev. 2003, 24, 278–301. [Google Scholar] [CrossRef]
- Hotamisligil, G.S. Inflammation and metabolic disorders. Nature 2006, 444, 860–867. [Google Scholar] [CrossRef]
- Suganami, T.; Ogawa, Y. Adipose tissue macrophages: Their role in adipose tissue remodeling. J. Leuko. Biol. 2010, 88, 33–39. [Google Scholar] [CrossRef]
- Shimizu, H.; Ohue-Kitano, R.; Kimura, I. Regulation of host energy metabolism by gut microbiota-derived short-chain fatty acids. Glycative Stress Res. 2019, 6, 181–191. [Google Scholar]
- Lu, Y.; Fan, C.; Li, P.; Lu, Y.; Chang, X.; Qi, K. Short Chain Fatty Acids Prevent High-fat-diet-induced Obesity in Mice by Regulating G Protein-coupled Receptors and Gut Microbiota. Sci. Rep. 2016, 6, 37589. [Google Scholar] [CrossRef]
- Chambers, E.S.; Viardot, A.; Psichas, A.; Morrison, D.J.; Murphy, K.G.; Zac-Varghese, S.E.; MacDougall, K.; Preston, T.; Tedford, C.; Finlayson, G.S. Effects of targeted delivery of propionate to the human colon on appetite regulation, body weight maintenance and adiposity in overweight adults. Gut 2015, 64, 1744–1754. [Google Scholar] [CrossRef]
- Xu, L.; Zhang, J. Bacterial glucans: Production, properties, and applications. Appl. Microbiol. Biotechnol. 2016, 100, 9023–9036. [Google Scholar] [CrossRef] [PubMed]
- Hamajima, H.; Matsunaga, H.; Fujikawa, A.; Sato, T.; Mitsutake, S.; Yanagita, T.; Nagao, K.; Nakayama, J.; Kitagaki, H. Japanese traditional dietary fungus koji Aspergillus oryzae functions as a prebiotic for Blautia coccoides through glycosylceramide: Japanese dietary fungus koji is a new prebiotic. Springerplus 2016, 5, 1321. [Google Scholar] [CrossRef] [PubMed]
- Plovier, H.; Everard, A.; Druart, C.; Depommier, C.; Van Hul, M.; Geurts, L.; Chilloux, J.; Ottman, N.; Duparc, T.; Lichtenstein, L.; et al. A purified membrane protein from Akkermansia muciniphila or the pasteurized bacterium improves metabolism in obese and diabetic mice. Nat. Med. 2017, 23, 107–113. [Google Scholar] [CrossRef]
- Depommier, C.; Everard, A.; Druart, C.; Plovier, H.; Van Hul, M.; Vieira-Silva, S.; Falony, G.; Raes, J.; Maiter, D.; Delzenne, N.M.; et al. Supplementation with Akkermansia muciniphila in overweight and obese human volunteers: A proof-of-concept exploratory study. Nat. Med. 2019, 25, 1096–1103. [Google Scholar] [CrossRef]

| Ingredients | NF | HF | HF + Blautia |
|---|---|---|---|
| Lard 1 | 130 | 130 | |
| Soybean oil 2 | 70 | 70 | 70 |
| Corn starch 3 | 397.49 | 277.37 | 277.37 |
| Casein 3 | 200 | 230.00 | 230.00 |
| Dextrinized cornstarch 3 | 132.00 | 92.11 | 92.11 |
| Sucrose 4 | 100 | 100 | 100 |
| AIN-93 mineral mixture 3 | 35 | 35 | 35 |
| AIN-93 vitamin mixture 3 | 10 | 10 | 10 |
| L-cystine 5 | 3 | 3 | 3 |
| Choline bitartrate 5 | 2.5 | 2.5 | 2.5 |
| Cellulose 3 | 50 | 50 | 50 |
| Tert-Butylhydroquinone 5 | 0.014 | 0.014 | 0.014 |
| Blautia cells dry powder | 0.04 | ||
| Total | 1000 | 1000 | 1000 |
| NF | HF | HF + Blautia | |
|---|---|---|---|
| Initial body weight (g) | 16.76 ± 1.40 | 16.78 ± 1.72 | 16.94 ± 1.42 |
| Final body weight (g) | 25.88 ± 1.25 | 28.13 ± 1.75 * | 27.11 ± 1.75 |
| Total food intake (g) | 219.9 ± 9.6 | 204.0 ± 16.6 | 201.3 ± 15.3 |
| Total water intake (g) | 441.5 ± 51.1 | 406.8 ± 51.8 | 420.1 ± 45.7 |
| Feces weight (g/2 days) | 1.11 ± 0.18 | 0.99 ± 0.19 | 1.02 ± 0.15 |
| NF | HF | HF + Blautia | |
|---|---|---|---|
| Liver | 3.81 ± 0.55 | 3.62 ± 0.49 | 3.77 ± 0.57 |
| Spleen | 0.28 ± 0.08 | 0.24 ± 0.07 | 0.25 ± 0.06 |
| Kidney | 1.28 ± 0.11 | 1.27 ± 0.18 | 1.27 ± 0.11 |
| Cecum | 1.73 ± 0.39 | 1.42 ± 0.30 | 1.51 ± 0.31 |
| Brown adipose tissue | 0.48 ± 0.09 | 0.53 ± 0.09 | 0.60 ± 0.11 |
| NF | HF | HF + Blautia | |
|---|---|---|---|
| Triglyceride (mg/dL) | 27.8 ± 14.5 | 25.6 ± 9.9 | 25.1 ± 12.7 |
| Phospholipid (mg/dL) | 258.1 ± 22.9 | 289.6 ± 22.9 | 285.0 ± 25.8 |
| Total cholesterol (mg/dL) | 123.4 ± 11.4 | 153.7 ± 10.5 | 148.7 ± 11.4 |
| LDL cholesterol (mg/dL) | 11.3 ± 1.7 | 18.0 ± 2.9 | 18.9 ± 3.2 |
| HDL cholesterol (mg/dL) | 105.7 ± 9.2 | 126.4 ± 6.8 | 122.9 ± 10.4 |
| GOT (IU/L) | 97.1 ± 45.0 | 66.6 ± 13.7 | 78.4 ± 34.8 |
| GPT (IU/L) | 19.0 ± 4.1 | 15.6 ± 2.1 | 19.0 ± 6.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shibata, M.; Ozato, N.; Tsuda, H.; Mori, K.; Kinoshita, K.; Katashima, M.; Katsuragi, Y.; Nakaji, S.; Maeda, H. Mouse Model of Anti-Obesity Effects of Blautia hansenii on Diet-Induced Obesity. Curr. Issues Mol. Biol. 2023, 45, 7147-7160. https://doi.org/10.3390/cimb45090452
Shibata M, Ozato N, Tsuda H, Mori K, Kinoshita K, Katashima M, Katsuragi Y, Nakaji S, Maeda H. Mouse Model of Anti-Obesity Effects of Blautia hansenii on Diet-Induced Obesity. Current Issues in Molecular Biology. 2023; 45(9):7147-7160. https://doi.org/10.3390/cimb45090452
Chicago/Turabian StyleShibata, Masaki, Naoki Ozato, Harutoshi Tsuda, Kenta Mori, Keita Kinoshita, Mitsuhiro Katashima, Yoshihisa Katsuragi, Shigeyuki Nakaji, and Hayato Maeda. 2023. "Mouse Model of Anti-Obesity Effects of Blautia hansenii on Diet-Induced Obesity" Current Issues in Molecular Biology 45, no. 9: 7147-7160. https://doi.org/10.3390/cimb45090452
APA StyleShibata, M., Ozato, N., Tsuda, H., Mori, K., Kinoshita, K., Katashima, M., Katsuragi, Y., Nakaji, S., & Maeda, H. (2023). Mouse Model of Anti-Obesity Effects of Blautia hansenii on Diet-Induced Obesity. Current Issues in Molecular Biology, 45(9), 7147-7160. https://doi.org/10.3390/cimb45090452







