1. Introduction
Triple-negative breast cancer (TNBC) is a subtype of breast cancer that lacks targeted treatments and accounts for 15–20% of incident breast cancers [
1]. TNBC is characterized by the absence of human epidermal growth factor receptor 2 (HER2) expression, as well as minimal expression of estrogen receptors (ERs) and progesterone receptors (PRs) based on clinical assays [
2]. This subtype is known for its aggressive nature, with cancer cells exhibiting a high-grade phenotype and rapid proliferation. These aggressive cellular characteristics contribute significantly to the unfavorable prognosis associated with TNBC compared to other breast cancer types. The limited treatment options available for TNBC exacerbate the challenges in managing this disease [
1,
3].
Focal adhesion kinase (FAK) is a cytoplasmic protein tyrosine kinase that is typically responsible for transducing signals from cellular adhesions. It governs a wide array of cellular biological functions, notably the survival, migration, and invasion capabilities of cancer cells [
4,
5]. The interaction of integrin receptors with the extracellular matrix (ECM) leads to the recruitment of FAK to areas of integrin clustering, commonly referred to as ‘focal adhesions’ [
6]. Upon localization to focal adhesions, FAK undergoes a conformational change, enabling autophosphorylation of the tyrosine (Tyr,Y) residue 397. Following this, the phosphorylated Y397 functions as a binding site for the SRC family kinases containing SRC homology 2 [
7]. This event culminates in the creation of an active FAK-SRC signaling complex capable of initiating many downstream signaling pathways [
8].
FAK is central in numerous signaling pathways that drive cancer proliferation and metastasis [
5]. FAK survival signals, triggered by integrins and other extracellular stimuli, play a crucial role in hindering anoikis and various types of cell death [
9]. In simplistic terms, the enhancement of survival signals due to increased FAK activity in vitro parallels with enhanced tumor growth. In human breast cancer cell lines, FAK depletion has been demonstrated to obstruct tumor growth, which was primarily induced by oncogenic mutations in the PI3K and RAS signaling pathways [
10]. Essentially, these studies emphasize the role of FAK as a key regulator of intrinsic cellular signals that promote proliferation. Canonical FAK signaling is also associated with the formation and disassembly of focal adhesions [
11]. FAK plays a key role in dynamically reorganizing the actin cytoskeleton, which is a fundamental aspect of cellular motility and protrusion [
5]. FAK activity is linked to the upregulation of MMP9 expression and spontaneous metastasis in syngeneic and orthotopic mouse models of breast carcinoma [
12]. FAK is the protein product of the
PTK2 gene. Contrary to typical oncogenes such as PI3K or RAS, only a limited number of missense mutations within PTK2 have been identified in tumors [
13]. While mutations in the PTK2 gene are uncommon, amplification of the gene and increased FAK expression are frequently observed in a variety of cancers [
4,
5]. Data from The Cancer Genome Atlas illustrate that FAK mRNA levels are elevated in about 26% of invasive breast cancers, a situation that is strongly associated with reduced overall patient survival rates [
14].
Proline-rich tyrosine kinase 2 (PYK2), which shares 45% of its amino acid sequence with its orthologue FAK, has been found to replace certain FAK functions in knockout mouse models after FAK loss. Additionally, in fibroblasts where FAK has been knocked out, elevated levels of PYK2 activate an intrinsic survival mechanism, further promoting cellular survival [
15,
16,
17]. Given these roles, the activity of PYK2 has the potential to negatively affect the outcomes of therapeutic strategies aimed at targeting FAK.
FAK inhibitors, which primarily consist of small molecules, exert their therapeutic action by hindering the kinase-dependent activity of FAK. These molecules predominantly function as ATP competitive inhibitors, blocking the ATP-binding site to inhibit FAK kinase catalytic activity, or as allosteric inhibitors, inducing conformational changes that suppress kinase activity [
18]. BI-853520 is a highly selective FAK inhibitor that demonstrates considerable potency, effectively reducing FAK autophosphorylation in preclinical models of prostate cancer. This molecule has shown potential in hindering primary tumor growth and metastasis, leading to its current evaluation in a Phase I clinical trial aimed at assessing safety and tolerability and defining the maximum tolerated dose [
19]. GSK2256098 distinguishes itself by its highly selective, ATP-competitive FAK inhibitory activity. Currently in Phase I clinical trials, it has exhibited potential in reducing cell viability and growth, as well as inhibiting FAK-mediated AKT and ERK activation [
20]. VS-4718 and VS-6062, potent FAK or dual FAK/PYK2 inhibitors, respectively, have demonstrated potential in preclinical models, limiting tumor progression and inhibiting tumor growth. They are now under investigation in Phase I clinical trials [
21,
22]. Finally, Defactinib (VS-6063), a potent dual FAK/PYK2 inhibitor, has successfully transitioned to Phase I and II trials after demonstrating promising preclinical results such as reducing tumor weight and enhancing chemosensitivity [
23,
24]. While all these inhibitors have demonstrated potential in preclinical studies and early phase clinical trials, further rigorous evaluations are crucial in ongoing and future clinical trials to conclusively establish their therapeutic efficacy.
However, despite the progress made in the development of FAK inhibitors, none have yet achieved clinical success in the treatment of triple-negative breast cancer (TNBC). It is noteworthy that FAK is frequently found to be upregulated in TNBC compared to normal breast tissue, suggesting the potential therapeutic value of FAK inhibitors in this specific subgroup [
25]. The high expression of FAK in breast tumors holds significant prognostic value as it has been associated with more aggressive tumor characteristics, including invasion and the triple-negative phenotype. This correlation reinforces the necessity to explore FAK inhibitors as a therapeutic strategy, especially for treating more aggressive and difficult-to-manage subtypes of breast cancer, such as TNBC [
26].
This study explores the potential of SJP1602, a novel dual inhibitor of FAK and PYK2, as a promising therapeutic option for TNBC. Current therapies for TNBC are inadequate, necessitating the pursuit of new, effective therapeutics. SJP1602 exhibits potent inhibitory activity against both FAK and PYK2, outperforming existing inhibitors such as VS-6063 in in vitro studies with TNBC cell lines. Notably, SJP1602 demonstrates superior inhibitory performance in 3D cultures, representing the tumor microenvironment. It also hinders migration and invasion of TNBC cells, indicating its potential in suppressing metastatic progression. In vivo pharmacokinetic studies in Sprague Dawley (SD) rats confirm the high bioavailability and sustained plasma concentrations of SJP1602, supporting its therapeutic promise. In xenograft models of TNBC, SJP1602 administration leads to significant and dose-dependent reductions in tumor growth across various TNBC cell lines.
In conclusion, the research presented in this study highlights the potential of SJP1602 as a potent dual inhibitor of FAK and PYK2, offering promising implications for TNBC treatment. These findings contribute to the advancing field of targeted therapeutics in oncology, positioning SJP1602 as a promising candidate for further clinical evaluation.
2. Materials and Methods
2.1. Materials
SJP1602 (99% purity) was synthesized by Samjin Pharmaceutical Co. (Seoul, Republic of Korea). For in vitro studies, VS-6063 was sourced from Selleckchem (Houston, TX, USA) and for in vivo experiments, it was obtained from DAEJUNG Chemicals & Metals (Seoul, Republic of Korea). GSK2256098 was procured from MedKoo Biosciences (Morrisville, NC, USA). The cell culture media, HEPES buffer solution, 1X DPBS, and antibiotics were purchased from Gibco (Waltham, MA, USA). Fetal bovine serum (FBS) was acquired from Hyclone (Logan, MA, USA). Trypsin-EDTA solution and Dimethyl sulfoxide (DMSO) were both purchased from Sigma, with the latter specifically from Sigma-Aldrich (St. Louis, MO, USA). The culture dishes (100 mm), 96-well clear round-bottom ultra-low attachment microplates, 96-well white flat-bottom microplates, and Matrigel growth factor-reduced, phenol red-free were sourced from Corning (Corning, NY, USA). Lastly, the 3D CellTiter-Glo assay kit was bought from Promega (Madison, WI, USA).
2.2. Modeling of FAK/SJP1602 Complex
The X-ray crystal structure of the FAK kinase domain (PDB ID: 2JKK) was downloaded from the Protein Data Bank in PDB format. For the molecular docking analysis of SJP1602 with the FAK kinase domain (PDB ID: 2JKK), Discovery Studio® 2020 (BIOVIA; San Diego, CA, USA) was utilized. In the docking studies, the structure of SJP1602 was superimposed onto the FAK kinase domain. The initial complex was optimized using 1000 steps of steepest descent and 3000 steps of conjugate gradient while restraining the FAK heavy atoms to their initial positions by means of a harmonic force constant of 1 kcal·mol−1·Å−2.
2.3. In Vitro FAK, PYK2, and Insulin Receptor (InsR) Activity
The kinase assays were performed and quantified according to the manufacturer’s instructions using the ADP-Glo™ Kinase Assay Kit for FAK (Promega, V1971), PYK2 (Promega, V4083) and InsR (Promega, V3901). Each kinase reaction mixture was prepared to contain the compounds of interest. After thorough pipetting, 25 μL of the mixture was added to each well and incubated for 1 h at room temperature. Subsequently, 25 μL of ADP-Glo reagent was added, followed by a 40 m incubation at room temperature. Next, 50 μL of the kinase detection reagent was added, and the mixture was incubated for an additional 30 m at room temperature. Luminescence was measured using the Tecan Spark 10M (Integration time: 1 s) (Männedorf, Switzerland).
The kinase profiling analysis was performed at Eurofins (Poitiers, France). A total of 110 kinase assays were carried out according to Eurofins’ standard protocols. For protein kinase assays, the kinase activity was measured using Eurofins’ KinaseProfiler radiometric assay. In this assay, a panel of kinases was incubated with the test compound SJP1602 at a concentration of 1 µM, and the kinase activity was determined using radiolabeled phosphate as a substrate. Lipid and atypical kinase assays were conducted using Eurofins’ homogeneous time-resolved fluorescence assay. The same panel of kinases was incubated with SJP1602 at a concentration of 1 µM, and the activity of lipid and atypical kinases was measured using this assay.
2.4. Cell Culture
The breast cancer cell lines used were MDA-MB-231, MDA-MB-453, HCC70, BT-20, and Hs578T cells, which were purchased from the Korean Cell Line Bank (Seoul, Republic of Korea), and BT-549 cells, which were purchased from ATCC (Manassas, VA, USA). The cells were grown in a humidified atmosphere with 5% CO2 at 37 °C in either Dulbecco’s Modified Eagle Medium (DMEM) or RPMI Medium 1640 (RPMI), supplemented with 10% FBS and penicillin.
2.5. FAK [pY397] Enzyme-Linked Immunosorbent Assay
TNBC cells were seeded into 60 mm dishes at a density of 1 × 106 cells/dish in 5 mL of culture media. After 24 h, cells were treated with concentrations ranging from 1 nM to 10 μM of SJP1602 for 1 h, and cell lysates were prepared to detect phospho-FAK levels. The FAK [pY397] enzyme-linked immunosorbent assay was performed according to the manufacturer’s instructions. The absorbance at 450 nm was measured to quantify the results.
2.6. Colony Formation Assay
MDA-MB-231 cells were seeded at 2.5 × 102 cells/well in 12-well plates. FAK inhibitors (SJP1602, VS-6063, GSK2256098) were added 24 h post seeding without changing the media. After 7 days, the cells were replaced with fresh media containing 10% FBS and the same initial concentration of the FAK inhibitor. After 11 days, the cells were fixed with 4% PFA for 10 m at room temperature and stained with 0.5% crystal violet dissolved in 25% methanol for 20 min at room temperature. The cells were then washed with distilled water, dried, and images of the entire plate were obtained.
2.7. Two-Dimensional Cell Invasion Assay
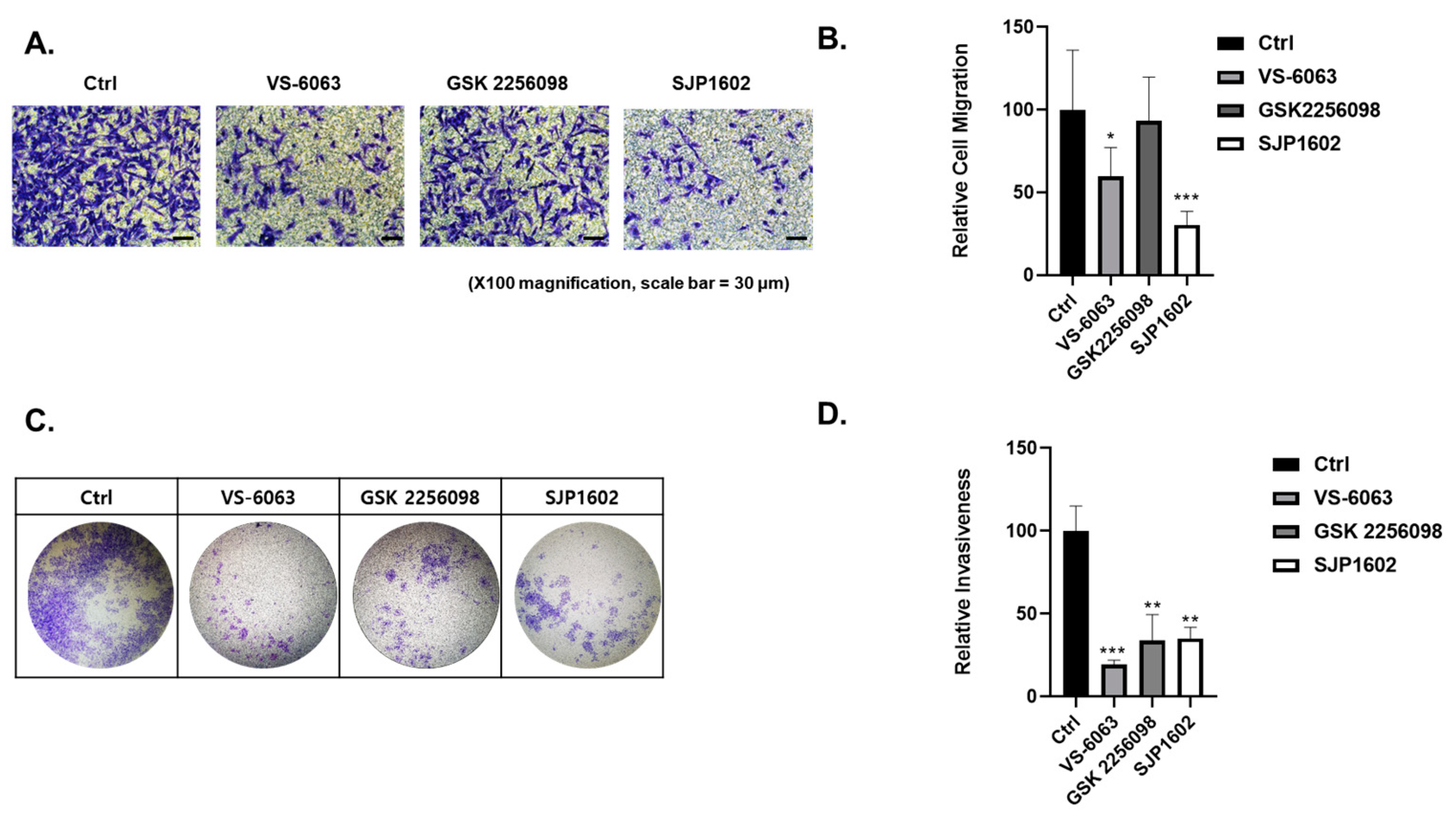
Cell invasion capacity was analyzed using the Boyden chamber assay. The 24-well Boyden chambers with Matrigel-coated filters (8 μm pore size) were obtained from Becton-Dickinson (San Diego, CA, USA). Cells treated with vehicle or FAK inhibitors (SJP1602, VS-6063, GSK2256098) at a concentration of 5 μM were resuspended in serum-free media and 5 × 105 cells were added to the Matrigel-coated upper compartment of the invasion chambers. Fresh culture medium with 5% FBS was added to the lower compartment. After 48 h, cells on the upper side of the filter were removed using cotton swabs. The underside of the filter was fixed in 100% methanol, washed in PBS, and stained using crystal violet. Cells that invaded the Matrigel were analyzed using a microscope (Nikon, TE2000). Image analysis was performed using the color analysis function in Adobe Photoshop 2021 (Version 22.4.2, Adobe, Adobe (San Jose, CA, USA)). Quantitative data were obtained from color measurement records in Adobe Photoshop 2021.
2.8. Cell Migration Assay
Cell migration capacity was analyzed using the transwell migration assay. The 24-well chambers (8 μm pore size) were obtained from Corning (Corning, NY, USA). Cells treated with vehicle or FAK inhibitors (SJP1602, VS-6063, GSK2256098) at a concentration of 5 μM were resuspended in serum-free media and 5 × 104 cells were added to the upper compartment of the transwell chambers. Fresh culture medium with 5% FBS was added to the lower compartment. After 16 h, cells on the upper side of the filter were removed using cotton swabs. The underside of the filter was fixed in 100% methanol, washed in PBS, and stained using crystal violet. Migrated cells were analyzed using a microscope (Nikon, TE2000). Image analysis was performed using the color analysis function in Adobe Photoshop 2021 (Version 22.4.2, Adobe (San Jose, CA, USA)). Quantitative data were obtained from color measurement records in Adobe Photoshop 2021.
2.9. Three-Dimensional Spheroid Assay
Breast cancer cell lines were cultured in medium with or without 2% Matrigel (Corning, NY, USA) for 3 days in each 96-well ultra-low attachment round plate to form a single spheroid at a density of 3 × 103 cells/well. SJP1602 was dissolved in DMSO and treated for 4 days at concentrations ranging from 200 nM to 20 μM. Cell viability was quantified according to the manufacturer’s instructions using a CellTiter-Glo 3D cell viability assay (Promega, Madison, WI, USA). Luminescence was measured using the Tecan Spark 10M.
2.10. Three-Dimensional Cell Invasion Assay
Cells were added to an ultra-low 96-well round-bottom plate at a density of 5 × 10
3 cells/well and cultured for 3 days to form spheroids. The medium was removed, and a mixture of neutralized Type I Collagen and Matrigel was added to the wells. After 1 h of incubation, compound concentrations (2×) in 100 μL of solution were added per well. The plate was then incubated for 72 h. Relative invasion was quantified based on the following formula using the microscope and imaging software ImageJ (Version 2.1.0/1.54e, National Institutes of Health, Bethesda, MD, USA):
2.11. Animals
Six-week-old female BALB/c nude mice were procured from Orient Bio (Seongnam, Republic of Korea). The mice were individually housed in a controlled environment with a temperature of 22 ± 3 °C, relative humidity of 50 ± 20%, and a 12 h light–dark cycle. The mice were provided sterilized feed and distilled water. All animal handling and experimental procedures complied with the relevant Standard Operating Procedures (SOPs). Ethics Approval: All animal experiments were carried out in accordance with the ethical guidelines and approved by the Institutional Animal Care and Use Committee (IACUC) at the following institutions:
- (1)
Samjin Pharmaceutical Co., Seoul, Republic of Korea (Approval Code: SJ-2018-004). Approval Period: 1 December 2018–30 November 2019.
- (2)
Daegu-Gyeongbuk Medical Innovation Foundation (DGMIF), Daegu, Republic of Korea (Approval Code: DGMIF-18041702-01). Approval Period: Starting from 20 January 2015.
- (3)
Chaon, Seongnam, Republic of Korea (Approval Code: CE2019094). Approval Period: 3 October 2019–31 January 2020.
2.12. Pharmacokinetics
The pharmacokinetics (PK) study was conducted in male SD rats. Three rats received a single-dose intravenous injection of SJP1602 at 5 mg/kg, and another set of three rats received a single-dose oral administration of SJP1602 at 20 mg/kg. The vehicle composition used was DMA 3% and Cremophor ELP 4%. Blood drug concentrations over time were determined by drawing blood after 0.17, 0.5, 1, 2, 4, 6, and 24 h. Plasma was isolated using K3EDTA anticoagulation tubes, and the drug concentration in the plasma was analyzed by LC/MS/MS (Waters, MA, USA). To analyze the concentration of pretreated rat plasma samples, a Waters Acquity ultra-performance liquid chromatography system (UPLC, Waters) was employed, in tandem with a C18 column (Acquity UPLC® BEH C18 1.7 μm, 2.1 × 50 mm). The mass spectrometry analysis was conducted using the Waters Xevo-TQ-S micro. For the mobile phases, phase A consisted of distilled water with 0.1% formic acid, while phase B comprised methanol, also with 0.1% formic acid. PK parameters were subsequently derived using WinNonlin software (version 2.1), which enabled the determination of AUC (ng×h/mL), Cmax (ng/mL), and bioavailability (BA). The area under the drug concentration curve (AUC) was calculated utilizing the trapezoidal rule, focusing on the time interval AUC0–24h.
2.13. Xenograft Mouse Model
Triple-negative breast cancer cell lines derived from humans, specifically MDA-MB-231 (1 × 10
6/mouse), BT-549 (1 × 10
7/mouse), and MDA-MB-453 (2 × 10
7/mouse), were heterologously transplanted subcutaneously into the tissue of 6-week-old female BALB/c nude mice to initiate tumor growth. Upon establishment of tumors, with an average tumor volume reaching approximately 100 mm
3, mice were grouped and subjected to daily oral dosing. Treatments included: vehicle control, low concentration of SJP1602 (20 mg/kg), medium concentration of SJP1602 (40 mg/kg), high concentration of SJP1602 (80 mg/kg), and a reference compound, Defactinib (80 mg/kg). The vehicle composition used was DMSO:PEG 400:tween 80:DW = 8%:50%:10%:32%. The duration of drug administration was defined for 2 weeks based on the growth rate of the vehicle group of each xenografted tumor. The tumor volume was measured three times a week for potency measurement, and the body weight of mice was also measured three times a week for toxicity evaluation in vivo. Tumor volume was calculated based on Equation (2) and the tumor growth inhibition rate (% TGI) was calculated using Equation (3).
2.14. Western Blotting
TNBC cells were seeded into ultra-low attachment microplates and incubated at 37 °C under a 5% CO2 atmosphere for 72 h. After the incubation period, the cells were treated with the compound SJP1602 at its IC50 concentration. Following this treatment, cell lysates were prepared, and the protein levels of FAK, p-FAK (Tyr397), PYK2, and p-PYK2 (Y402) were analyzed. Equal amounts of these proteins were denatured by boiling in Laemmli sample buffer for 5 min, followed by electrophoresis on sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) gels. The separated proteins were then electrotransferred onto nitrocellulose (NC) membranes. To prevent non-specific binding, the membranes were blocked using 5% skim milk in Tris-buffered saline containing 0.01% Tween-20 (TBS/T) for one hour. After rinsing the blots three times with TBS/T, they were incubated overnight at 4 °C with primary antibodies: Anti-FAK (Cell Signaling, Danvers, MA, USA #3285S), p-FAK (Cell Signaling, Danvers, MA, USA #3283S), PYK2 (Abcam, Cambridge, UK #ab32571), p-PYK2 (Abcam, Cambridge, UK #ab4800), and β-actin (Sigma Aldrich, St. Louis, MO, USA #A5316), all prepared in TBS/T. The blots underwent another set of three washes in TBS/T and were then exposed to secondary antibodies: Anti-rabbit HRP-conjugated (Invitrogen, Carlsbad, CA, USA #G21234) and Anti-mouse HRP-conjugated (Invitrogen, Carlsbad, CA, USA #G21040) at room temperature for an hour. After three additional washes in TBS/T, the protein bands were detected using the ECL™ Western blotting reagent (GE Healthcare, Chicago, IL, USA #RPN2106).
4. Discussion
TNBC is a subtype of breast cancer known for its aggressive nature and limited targeted treatment options [
1,
3]. Focal adhesion kinase (FAK) is upregulated in TNBC and plays a pivotal role in promoting tumor growth, invasion, and metastasis [
26]. The effect of SJP1602 was observed to effectively inhibit both FAK and PYK2. In vitro studies revealed that SJP1602 outperforms currently used FAK inhibitors such as VS-6063 and exhibits robust efficacy in TNBC cell lines. Specifically, the inhibitory effect of SJP1602 was more pronounced in 3D cultures compared to 2D cultures, highlighting the significance of FAK signaling in a 3D environment where cell survival is more reliant on FAK due to spatial constraints and limited adhesion. The preference for FAK inhibitors in 3D culture arises from the increased reliance of cancer cells on FAK signaling in a 3D environment [
29]. In 2D cultures, cells can spread out and adhere to the culture dish surface, providing ample surface area for interaction and survival without relying heavily on FAK signaling. Conversely, in 3D environments, cells are surrounded by other cells and the extracellular matrix, limiting their ability to spread out and increasing their dependence on FAK signaling for survival, migration, and invasion [
4].
The effect of SJP1602 extended to TNBC cell growth, migration, and invasion, indicating its potential to impede metastatic progression. In vivo pharmacokinetic studies in rats demonstrated the high bioavailability of SJP1602 and its ability to reach and maintain therapeutically relevant plasma concentrations. Furthermore, the effect of SJP1602 was validated in xenograft models of TNBC, where it led to a significant and dose-dependent reduction in tumor growth across various TNBC cell lines. A limitation of this study is the absence of mechanism-of-action studies for SJP-1602 in animal efficacy experiments. To address this limitation, we are currently conducting further investigations to determine whether any of the kinases identified in the kinase profiling analysis of SJP-1602 are involved in xenograft efficacy. These additional studies aim to provide insights into the specific molecular pathways and targets through which SJP-1602 exerts its inhibitory effects on tumor growth and metastasis in vivo.
Despite promising results, it is essential to acknowledge that, to date, no FAK inhibitors have achieved clinical success in TNBC treatment. However, the high expression of FAK in TNBC and its association with aggressive tumor characteristics highlight the importance of exploring FAK inhibitors as potential therapeutic options for this specific subtype of breast cancer. Comparatively, SJP1602 and VS-6063 demonstrated superior efficacy in inhibiting proliferation and metastasis in TNBC cells compared to the selective FAK inhibitor GSK 2256098, which does not target PYK2. The dual inhibition of both FAK and PYK2 by SJP1602 contributed to its potent effects, whereas the selective targeting of FAK alone by GSK 2256098 showed limited effectiveness in blocking cancer cell proliferation and metastasis. The higher potency of SJP1602 in inhibiting the kinase activity of both FAK and PYK2 translated to enhanced effectiveness in TNBC cell lines and xenograft models. This highlights the importance of simultaneous inhibition of both FAK and PYK2 for achieving optimal therapeutic outcomes in TNBC treatment. These findings underscore the potential of SJP1602 as a promising candidate for further preclinical and clinical evaluation in the context of TNBC treatment. The potent inhibitory effect of SJP1602 on the kinase activity of both FAK and PYK2 offers promising prospects for advancing targeted therapies against TNBC.
Ongoing investigations are currently assessing the efficacy of SJP1602 in tumor models in conjunction with established drug classes [
6]. Moreover, the research is being extended to encompass other types of cancer, such as malignant pleural mesothelioma [
33]. Preliminary observations indicate that certain mesothelioma cell lines demonstrate sensitivity to the effects of SJP1602. These findings suggest potential broader applications for SJP1602 in targeting other cancer types beyond TNBC.