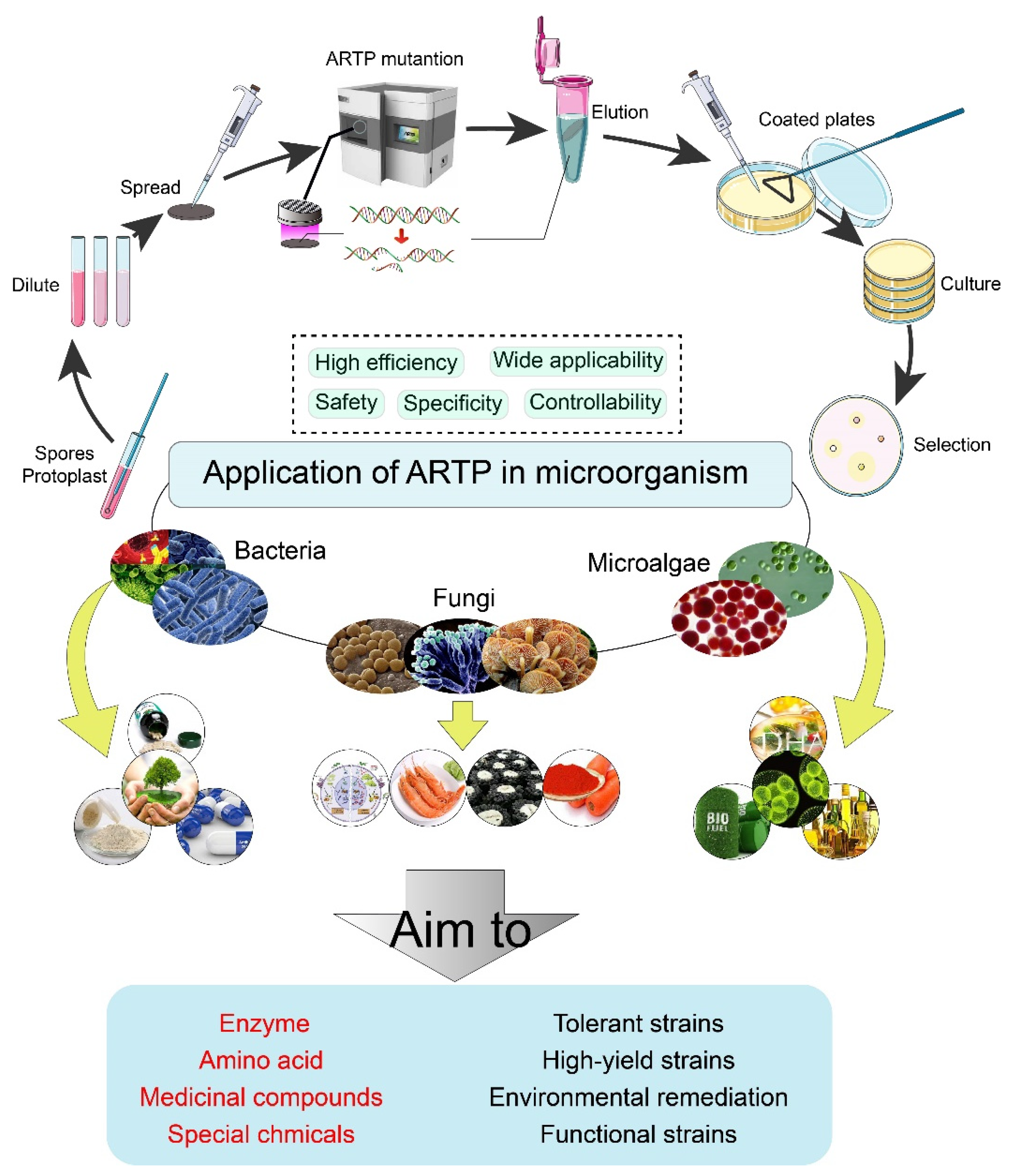
Application of Atmospheric and Room-Temperature Plasma (ARTP) to Microbial Breeding
Abstract
1. Introduction
1.1. Principle of ARTP
1.2. Technical Parameters
1.3. Mutagenesis Targets
2. Application of ARTP to Bacteria
2.1. Enzyme Overproducers
2.2. Amino Acid Overproducers
2.3. Antibiotics Overproducers
2.4. Environmental Remediation
2.5. Others
| Bacteria Species | Compounds Property | Mutant Method | Time | Ability | Refs |
|---|---|---|---|---|---|
| B. cereus | Chitosanase | ARTP | 60 s | Increase in chitosanase productivity of 3.66 times | [25] |
| B. licheniformis | Thermostable protease | ARTP | 60 s | Increase in thermostable protease activity of 1.56 times | [27] |
| R. sphaeroides | CoQ10 | ARTP | 30 s | Increase in CoQ10 productivity of 22.1% | [28] |
| R. sphaeroides | CoQ10 | ARTP | 20 s | Increase in CoQ10 productivity of 25.5% | [29] |
| R. sphaeroides | CoQ10 | ARTP | 50 s | Increase in CoQ10 productivity of 26.9% | [30] |
| R. sphaeroides | CoQ10 | ARTP | 25 s | Increase in CoQ10 productivity of 18% | [31] |
| R. sphaeroides | CoQ10 | ARTP | 120 s | Increase in CoQ10 productivity of 16.1% | [32] |
| B. amyloliquefaciens | Alkaline phosphatase | ARTP | Increase in alkaline phosphatase activity of 4.67-fold | [33] | |
| E. profundum | Protease | ARTP | 120 s | Increase in protease activity of more than 20% | [34] |
| B. licheniformis | Protease and amylase | ARTP | 60 s | Increase in protease and amylase activity of 143.10% | [35] |
| P. algicola | Alginate lyase | ARTP | 50S | Increase in alginate lyase activity of 32.6% and 21.6% | [36] |
| B. amyloliquefaciens | α-Amylase | ARTP | 30 s | Increase in α-amylase of 86.92% | [37] |
| B. subtilis | Alkaline protease | ARTP | 50 s | Increase in alkaline protease activity of 23.38% | [38] |
| B. subtilis | γ-PGA | ARTP | 30–180 s | Increase in γ-PGA producing of 86.8% | [39] |
| C. glutamicum | L-serine | ARTP | 30 s | Increase in yield of L-serine of 66.7% | [40] |
| C. glutamicum | L-Glutamic Acid | ARTP | 40 s | Increase in L-glutamic acid producing of 12.9% | [41] |
| C. glutamicum | L-glutamine | ARTP; gene editing | 20 s | Increase in L-glutamine producing of 3500% | [42] |
| C. glutamicum | L-histidine | ARTP | 210 s | Increase in L-histidine producing at 0.561 ± 0.016 g/L | [43] |
| C. glutamicum | L-isoleucine | ARTP | 180S | Increase in L-isoleucine producing of 62.03% | [44] |
| L. plantarum | Bacteriocin | ARTP; NTG; genome shuffling | 10S | Increase in bacteriocin activity of 2.35 times | [48] |
| L. plantarum | Bacteriocin | Microwave; NTG; ARTP; UV | 6 s | Increase in relative bacteriostatic titers of 5.51-fold | [50] |
| L. plantarum | Bacteriocin | ARTP; MNNG; gene editing | 40 s | Increase in bacteriocin yield of 103.48% | [49] |
| Nonomuria spp. | Dalbavancin precursor | ARTP; UV | 30 s | Increase in dalbavancin precursor yield of 68.7% | [52] |
| B. amyloliquefaciens | Remove petroleum hydrocarbons | ARTP | 30 s | Removal of petroleum hydrocarbons of 45.44% | [53] |
| B. velezensis | Remove Cr | ARTP | 60 s | Increased cadmium tolerance of 400 mg/L | [22] |
| P. fluorescein | EPS | ARTP | 60 s | Increase in flocculating activity of 106.48% | [54] |
| Pantoea sp. | Plant growth promoting | ARTP | 50–125 s | Enhanced plant growth and antioxidative activities. | [55] |
| Franconibacter sp. | Plant growth promoting | ARTP | 50–125 s | Enhanced plant growth and antioxidative activities. | [56] |
| L. reuteri | Antibacterial activity | ARTP | 30 s | Showed higher antibacterial activity by 7% | [57] |
| B. subtilis | Surfactin | ARTP | 24 s | Increase in surfactin yield of 334.2% | [61] |
| Notoacmeibacter sp. | HPG | ARTP | 60 s | Increase in HPG yield of 94.9% | [62] |
| B. mucilaginosus | Acid | ARTP | 50–70 s | Increase in acid production of about twofold | [58] |
| L. acidophilus | Acid tolerance | ARTP | 60 s | 75.67% and 25.78% survival rates with pH 3.0 and 2.5 | [60] |
| B. coagulans | Acid/salt tolerance | ARTP | 15 s | 22.4% survival rate with pH 2.5 and 0.3% bile salt | [59] |
3. Application of ARTP to Fungi
3.1. Application to Yeast Mutation
3.2. Application to Mold Mutation
3.3. Application to Edible Fungi
4. Application of ARTP in Microalgae
| Strain | Compound/Property | Mutant Method | Time | Ability | Refs |
|---|---|---|---|---|---|
| P. kessleri | Biodiesel | ARTP | 40 s | Increases in biomass and lipid productivity of 75% and 44%, respectively. | [117] |
| S. platensis | Astaxanthin | ARTP | 70 s | Increase in astaxanthin productivity of 196%. | [118] |
| H. pluvialis | Astaxanthin | ARTP | 40 s | Increase in astaxanthin yield of 61.73%. | [119] |
| C. pyrenoidosa | High yield; lipid | ARTP | 40–60 s | Increases in dry weight and lipid productivity of 22.07% and 16.85%, respectively. | [120] |
| Aurantiochytrium sp. | DHA | ARTP | 25 s | Increases in biomass, lipid and DHA yield of 5.77%, 16.9% and 83.2%, respectiviely. | [121] |
| S. limacinum | DHA | ARTP | 20 s | Increase in DHA yield of 25.51%. | [124] |
| Schizochytrium | DHA | ARTP | 60 s | Increases in DHA concentration and productivity at 41.4 g/L and 430.7 mg/L/h. | [125] |
| Schizochytrium | DHA | ARTP | 40 s | Increase in DHA content of 54.1%. | [126] |
| Desmodesmus sp. | Lipid | ARTP | 60–65 s | Increase in triglyceride (TAG) production of 234%. | [127] |
| Desmodesmus sp. | Lipid | ARTP | 90 s | Increases in triglyceride and total lipid content of 48.98% and 114.99%, respectively. | [128] |
| Desmodesmus sp. | Lipid | ARTP | 60 s | Increases in lipid production and biomass of >100% and >15%. | [129] |
5. Future Perspectives
6. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Magocha, T.A.; Zabed, H.; Yang, M.; Yun, J.; Zhang, H.; Qi, X. Improvement of industrially important microbial strains by genome shuffling: Current status and future prospects. Bioresour. Technol. 2018, 257, 281–289. [Google Scholar] [CrossRef]
- Naumov, G.I.; Naumova, E.S.; Kondratieva, V.I. The use of hybridization in breeding of eukaryotic microorganisms. Russ. J. Genet. 2006, 42, 1324–1328. [Google Scholar] [CrossRef]
- Qiu, J.; Xu, Y.; Ruan, W.; Qun, Y. The Metabolic Control Breeding of L-Lactic Acid Fermentation and Optimization of Media and Cultivation Conditions. Microbiol. China 2007, 34, 929–933. [Google Scholar] [CrossRef]
- Urban, D.; Pfenning, U. Attitudes towards genetic engineering between change and stability: Results of a panel study. New Genet. Soc. 2000, 19, 251–268. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, X.F.; Li, H.P.; Wang, L.Y.; Zhang, C.; Xing, X.H.; Bao, C.Y. Atmospheric and room temperature plasma (ARTP) as a new powerful mutagenesis tool. Appl. Microbiol. Biotechnol. 2014, 98, 5387–5396. [Google Scholar] [CrossRef]
- Malook, S.; Qaisrani, S.; Shabaz, M.; Ahmed, H.; Nawaz, M.; Iqbal, M.; Mustafa, G.; Ali, Q. Mutation breeding approach to breed drought tolerant maize hybrids. Int. J. Biosci. (IJB) 2015, 6, 427–436. [Google Scholar] [CrossRef]
- Kodym, A.; Afza, R. Physical and chemical mutagenesis. Methods Mol. Biol. 2003, 236, 189–204. [Google Scholar] [CrossRef] [PubMed]
- Ren, F.; Chen, L.; Tong, Q. Highly improved acarbose production of Actinomyces through the combination of ARTP and penicillin susceptible mutant screening. World J. Microbiol. Biotechnol. 2017, 33, 16. [Google Scholar] [CrossRef]
- Khan, S.A.; Rahman, L.U.; Verma, R.; Shanker, K. Physical and chemical mutagenesis in Stevia rebaudiana: Variant generation with higher UGT expression and glycosidic profile but with low photosynthetic capabilities. Acta Physiol. Plant. 2015, 38, 4. [Google Scholar] [CrossRef]
- Wang, L.; Zhao, H.; He, D.; Wu, Y.; Jin, L.; Li, G.; Su, N.; Li, H.; Xing, X.H. Insights into the molecular-level effects of atmospheric and room-temperature plasma on mononucleotides and single-stranded homo- and hetero-oligonucleotides. Sci. Rep. 2020, 10, 14298. [Google Scholar] [CrossRef]
- Wang, L.Y.; Huang, Z.L.; Li, G.; Zhao, H.X.; Xing, X.H.; Sun, W.T.; Li, H.P.; Gou, Z.X.; Bao, C.Y. Novel mutation breeding method for Streptomyces avermitilis using an atmospheric pressure glow discharge plasma. J. Appl. Microbiol. 2010, 108, 851–858. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Wang, L.; Zhang, X.; Su, N.; Li, H.; Oda, Y.; Xing, X. Quantitative evaluation of DNA damage caused by atmospheric and room-temperature plasma (ARTP) and other mutagenesis methods using a rapid umu-microplate test protocol for microbial mutation breeding. Chin. J. Chem. Eng. 2021, 39, 205–210. [Google Scholar] [CrossRef]
- Ottenheim, C.; Nawrath, M.; Wu, J.C. Microbial mutagenesis by atmospheric and room-temperature plasma (ARTP): The latest development. Bioresour. Bioprocess. 2018, 5, 12. [Google Scholar] [CrossRef]
- Arjunan, K.P.; Sharma, V.K.; Ptasinska, S. Effects of atmospheric pressure plasmas on isolated and cellular DNA—A review. Int. J. Mol. Sci. 2015, 16, 2971–3016. [Google Scholar] [CrossRef]
- Maslowska, K.H.; Makiela-Dzbenska, K.; Fijalkowska, I.J. The SOS system: A complex and tightly regulated response to DNA damage. Environ. Mol. Mutagen. 2019, 60, 368–384. [Google Scholar] [CrossRef]
- Janion, C. Inducible SOS response system of DNA repair and mutagenesis in Escherichia coli. Int. J. Biol. Sci. 2008, 4, 338–344. [Google Scholar] [CrossRef]
- Su, X.-L.; Zhao, S.-S.; Xu, W.-J.; Shuang, L.; Zheng, G.-D.; Zou, S.-M. Efficiently whole-genomic mutagenesis approach by ARTP in blunt snout bream (Megalobrama amblycephala). Aquaculture 2022, 555, 738241. [Google Scholar] [CrossRef]
- Jay, J.M.; Loessner, M.J.; Golden, D.A. Modern Food Microbiologi; Springer: New York, NY, USA, 2005. [Google Scholar]
- Peng, H. Breeding and Fermentation Optimization of Avilamycin High-Producing Strains. Master’s Thesis, Henan University of Technology, Zhengzhou, China, 2022. [Google Scholar]
- Yu, F.; Zhang, M.; Sun, J.; Wang, F.; Li, X.; Liu, Y.; Wang, Z.; Zhao, X.; Li, J.; Chen, J.; et al. Improved Neomycin Sulfate Potency in Streptomyces fradiae Using Atmospheric and Room Temperature Plasma (ARTP) Mutagenesis and Fermentation Medium Optimization. Microorganisms 2022, 10, 94. [Google Scholar] [CrossRef]
- Yao, Z.; Fan, J.; Dai, J.; Yu, C.; Zeng, H.; Li, Q.; Hu, W.; Yan, C.; Hao, M.; Li, H.; et al. A High-Throughput Method Based on Microculture Technology for Screening of High-Yield Strains of Tylosin-Producing Streptomyces fradiae. J. Microbiol. Biotechnol. 2023, 33, 831–839. [Google Scholar] [CrossRef]
- Bao, Z.; Wang, X.; Wang, Q.; Zou, L.; Peng, L.; Li, L.; Tu, W.; Li, Q. A novel method of domestication combined with ARTP to improve the reduction ability of Bacillus velezensis to Cr(VI). J. Environ. Chem. Eng. 2023, 11, 109091. [Google Scholar] [CrossRef]
- Xiao, B.; Hu, Y.; Feng, X.; Sui, Z. Breeding of New Strains of Gracilariopsis lemaneiformis with High Agar Content by ARTP Mutagenesis and High Osmotic Pressure Screening. Mar. Biotechnol. 2022, 25, 100–108. [Google Scholar] [CrossRef]
- Chen, X.; Wang, B.; Wei, D. Breeding of Chlorella mutants deficient in chlorophyll synthesis and evaluation of its protein yield and quality. Chin. J. Biotechnol. 2023, 39, 1247–1259. [Google Scholar] [CrossRef]
- Zhang, C.; Li, Y.; Zhang, T.; Zhao, H. Increasing chitosanase production in Bacillus cereus by a novel mutagenesis and screen method. Bioengineered 2021, 12, 266–277. [Google Scholar] [CrossRef] [PubMed]
- Thadathil, N.; Velappan, S.P. Recent developments in chitosanase research and its biotechnological applications: A review. Food Chem. 2014, 150, 392–399. [Google Scholar] [CrossRef]
- Xue, G.; Chen, L.; Wu, B.; He, B. Selection of high-yield thermostable protease producing strain by ARTP and the study on its enzymological properties. Sci. Technol. Food Ind. 2015, 36, 177–180, 206. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, S.; Huo, K.; Wang, B.; Liu, J.; Zhao, G.; Liu, J. Iterative mutagenesis induced by atmospheric and room temperature plasma treatment under multiple selection pressures for the improvement of coenzyme Q10 production by Rhodobacter sphaeroides. FEMS Microbiol. Lett. 2021, 368, fnab154. [Google Scholar] [CrossRef]
- Zou, R.S.; Li, S.; Zhang, L.L.; Zhang, C.; Han, Y.J.; Gao, G.; Sun, X.; Gong, X. Mutagenesis of Rhodobacter sphaeroides using atmospheric and room temperature plasma treatment for efficient production of coenzyme Q10. J. Biosci. Bioeng. 2019, 127, 698–702. [Google Scholar] [CrossRef]
- Ding, Y.; Li, C.; Niu, C.; Zhang, P. Breeding and study on fermentation technology of high-yield CoQ10 in Rhodobacter sphaeroides. Chin. J. Antibiot. 2020, 45, 33–37. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, X.; Wang, Z.; Chen, B.; Li, D.; Guo, M.; Chu, J.; Zhuang, Y. Screening of High-Yield Coenzyme Q10 Producing Strain by Using Atmospheric and Room Temperature Plasma and Oxygen-Limited Model. J. East China Univ. Sci. Technol. 2021, 47, 308–315. [Google Scholar] [CrossRef]
- Li, W.; Zeng, W.; Zhou, J. Breeding and fermentation optimization of Rhodobacter sphaeroides with high yield of coenzyme Q10. Food Ferment. Ind. 2022, 48, 34–41, 56. [Google Scholar] [CrossRef]
- Bo, L.; Kang, X.; Chen, Z.; Zhao, Y.; Wu, S.; Li, J.; Bao, S. Isolation and identification of high-yielding alkaline phosphatase strain: A novel mutagenesis technique and optimization of fermentation conditions. Prep. Biochem. Biotechnol. 2023, 1–12. [Google Scholar] [CrossRef]
- Xin, R.; Xie, W.; Xu, Z.; Che, H.; Zheng, Z.; Yang, X. Efficient extraction of chitin from shrimp waste by mutagenized strain fermentation using atmospheric and room-temperature plasma. Int. J. Biol. Macromol. 2020, 155, 1561–1568. [Google Scholar] [CrossRef] [PubMed]
- Zhang, A.; Ma, Y.; Deng, Y.; Zhou, Z.; Cao, Y.; Yang, B.; Bai, J.; Sun, Q. Enhancing Protease and Amylase Activities in Bacillus licheniformis XS-4 for Traditional Soy Sauce Fermentation Using ARTP Mutagenesis. Foods 2023, 12, 2381. [Google Scholar] [CrossRef]
- Gao, R.; Zheng, Z.; Bao, S.; Mo, K.; Huang, H. Screening of alginate lyase-producing bacteria and breeding of high enzyme-producing strain by ARTP mutagenesis. China Brew. 2022, 41, 64–69. [Google Scholar] [CrossRef]
- Xu, T.-L.; Jing Peng, J.P.; Zhu, Y.-L.; Su, L.; Zhou, K.-Y.; Cheng, H.-N.; Tang, S.-Z.; Zhou, H.-B. Yield Enhancement of Recombinant α-Amylases in Bacillus amyloliquefaciens by ARTP Mutagenesis-Screening and Medium Optimization. Sains Malays. 2019, 48, 965–974. [Google Scholar] [CrossRef]
- Liu, Y.; Li, S. Breeding of High-yield Alkaline Protease Producing Strain by Atmospheric and Room Temperature Plasma Mutagenesis. IOP Conf. Ser. Earth Environ. Sci. 2020, 453, 012089. [Google Scholar] [CrossRef]
- Shen, F.; Li, X.; Xu, W.; Zhu, X.; Wang, F.; Yan, H. Mutagenesis of poly-γ-Glumatic Acid High-Producing Bacillus subtilis Strain and Optimization of Fermentation Condition. Amino Acids Biot. Resour. 2015, 37, 61–66. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, X.; Xu, G.; Zhang, X.; Shi, J.; Xu, Z. Integration of ARTP mutagenesis with biosensor-mediated high-throughput screening to improve L-serine yield in Corynebacterium glutamicum. Appl. Microbiol. Biotechnol. 2018, 102, 5939–5951. [Google Scholar] [CrossRef]
- Liang, L.; Huang, Q.; Weng, X.; Wu, S.; Huang, J. Breeding L-Glutamic Acid Producing Engineering Strain by Mutagenesis and Its Fermentation Efficiency. Biotechnol. Bull. 2020, 36, 143–149. [Google Scholar] [CrossRef]
- Lv, Q.; Hu, M.; Tian, L.; Liu, F.; Wang, Q.; Xu, M.; Rao, Z. Enhancing l-glutamine production in Corynebacterium glutamicum by rational metabolic engineering combined with a two-stage pH control strategy. Bioresour. Technol. 2021, 341, 125799. [Google Scholar] [CrossRef]
- Deng, L.; Zhang, H.; Zhen, H. Screening of L-histidine producing strain based on ARTP mutagenesis combined with microbial microdroplet culture system. China Brew. 2021, 40, 53–58. [Google Scholar] [CrossRef]
- Kong, S.; Chen, M.; Zhen, M.; Xu, P.; Lv, Y.; Xie, F.; Zhou, Y.; Gong, D. Mutation breeding of high-yield L-isoleucine Corynebacterium glutamicum by atmospheric and room temperature plasmas mutagenesis. China Brew. 2019, 38, 76–79. [Google Scholar] [CrossRef]
- Inglis, R.F.; Bayramoglu, B.; Gillor, O.; Ackermann, M. The role of bacteriocins as selfish genetic elements. Biol. Lett. 2013, 9, 20121173. [Google Scholar] [CrossRef]
- Miao, J.; Guo, H.; Ou, Y.; Liu, G.; Fang, X.; Liao, Z.; Ke, C.; Chen, Y.; Zhao, L.; Cao, Y. Purification and characterization of bacteriocin F1, a novel bacteriocin produced by Lactobacillus paracasei subsp. tolerans FX-6 from Tibetan kefir, a traditional fermented milk from Tibet, China. Food Control 2014, 42, 48–53. [Google Scholar] [CrossRef]
- Lü, X.; Yi, L.; Dang, J.; Dang, Y.; Liu, B. Purification of novel bacteriocin produced by Lactobacillus coryniformis MXJ 32 for inhibiting bacterial foodborne pathogens including antibiotic-resistant microorganisms. Food Control 2014, 46, 264–271. [Google Scholar] [CrossRef]
- Wang, F.; Lu, W.; Yang, J.; Bie, X. Study on screeging of high-yield bacteriocin producing Lactobacillus plantarum stains induced by mutations. Sci. Technol. Food Ind. 2017, 38, 191–195. [Google Scholar]
- Chen, R.; Huang, Y.Z.; He, B.; Qing, S.; Hu, L.; Lu, Z.; Bie, X. Screeging of High-yield Plantaricin Producing Stains Induced by Mutations and the Fresh-keeping Effect of Plantaricin on the Preservation of Meatballs. Sci. Technol. Food Ind. 2018, 39, 121–127. [Google Scholar]
- An, Y.; Wang, Y.; Zuo, Z.; Yi, H.; Zhang, D. Breeding of new high bacteriocin-producing Lactobacillus plantarum by complex mutagenesis. China J. Biol. 2019, 32, 265–271, 285. [Google Scholar] [CrossRef]
- Roecker, A.M.; Pope, S.D. Dalbavancin: A lipoglycopeptide antibacterial for Gram-positive infections. Expert Opin. Pharmacother. 2008, 9, 1745–1754. [Google Scholar] [CrossRef]
- Wang, X.; Qie, L.; Ma, J.; Dai, M.; Deng, P. Breeding of Dalbavancin Precursor High-Yield Strain by UV-ARTP Composite Mutagenesis. Chem. Bioeng. 2017, 34, 59–62. [Google Scholar] [CrossRef]
- Wang, Y.; Wu, S.; Wang, H.; Dong, Y.; Li, X.; Wang, S.; Fan, H.; Zhuang, X. Optimization of conditions for a surfactant-producing strain and application to petroleum hydrocarbon-contaminated soil bioremediation. Colloids Surf. B Biointerfaces 2022, 213, 112428. [Google Scholar] [CrossRef]
- Yang, L.; Chen, Z.; Zhang, Y.; Lu, F.; Liu, Y.; Cao, M.; He, N. Hyperproduction of extracellular polymeric substance in Pseudomonas fluorescens for efficient chromium (VI) absorption. Bioresour. Bioprocess. 2023, 10, 17. [Google Scholar] [CrossRef]
- Wang, S.; Wang, J.; Zhou, Y.; Huang, Y.; Tang, X. Isolation, Classification, and Growth-Promoting Effects of Pantoea sp. YSD J2 from the Aboveground Leaves of Cyperus esculentus L. var. sativus. Curr. Microbiol. 2022, 79, 66. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Wang, J.; Zhou, Y.; Huang, Y.; Tang, X. Prospecting the plant growth–promoting activities of endophytic bacteria Franconibacter sp. YSD YN2 isolated from Cyperus esculentus L. var. sativus leaves. Ann. Microbiol. 2022, 72, 1. [Google Scholar] [CrossRef]
- Ma, E.; An, Y.; Zhang, G.; Zhao, M.; Iqbal, M.W.; Zabed, H.M.; Qi, X. Enhancing the antibacterial activity of Lactobacillus reuteri against Escherichia coli by random mutagenesis and delineating its mechanism. Food Biosci. 2023, 51, 102209. [Google Scholar] [CrossRef]
- Dong, Y.-B.; Liu, Y.; Lin, H.; Liu, C.-J. Improving vanadium extraction from stone coal via combination of blank roasting and bioleaching by ARTP-mutated Bacillus mucilaginosus. Trans. Nonferrous Met. Soc. China 2019, 29, 849–858. [Google Scholar] [CrossRef]
- Liu, K.; Fang, H.; Cui, F.; Nyabako, B.A.; Tao, T.; Zan, X.; Chen, H.; Sun, W. ARTP mutation and adaptive laboratory evolution improve probiotic performance of Bacillus coagulans. Appl. Microbiol. Biotechnol. 2020, 104, 6363–6373. [Google Scholar] [CrossRef]
- Nyabako, B.A.; Fang, H.; Cui, F.; Liu, K.; Tao, T.; Zan, X.; Sun, W. Enhanced Acid Tolerance in Lactobacillus acidophilus by Atmospheric and Room Temperature Plasma (ARTP) Coupled with Adaptive Laboratory Evolution (ALE). Appl. Biochem. Biotechnol. 2020, 191, 1499–1514. [Google Scholar] [CrossRef]
- Xu, H.; Dai, C.; Tang, Y.; Xu, X.; Umego, E.C.; He, R.; Ma, H. The selective breeding and mutagenesis mechanism of high-yielding surfactin Bacillus subtilis strains with atmospheric and room temperature plasma. J. Sci. Food Agric. 2022, 102, 1851–1861. [Google Scholar] [CrossRef]
- Su, z.; Lu, T.; Zhao, M.; Li, Y.; Liu, Y.; Gao, C.; Liu, Y. Screening of High-production-heptylprodigiosin Strain from Notoacmeibacter sp. BGMRC2072 by ARTP. Guangxi Sci. 2020, 27, 503–508. [Google Scholar] [CrossRef]
- Jiang, G.; Yang, Z.; Wang, Y.; Yao, M.; Chen, Y.; Xiao, W.; Yuan, Y. Enhanced astaxanthin production in yeast via combined mutagenesis and evolution. Biochem. Eng. J. 2020, 156, 107519. [Google Scholar] [CrossRef]
- Jin, J.; Jia, B.; Yuan, Y.J. Combining nucleotide variations and structure variations for improving astaxanthin biosynthesis. Microb. Cell Factories 2022, 21, 79. [Google Scholar] [CrossRef] [PubMed]
- Zhou, K.; Yu, C.; Liang, N.; Xiao, W.; Wang, Y.; Yao, M.; Yuan, Y. Adaptive Evolution and Metabolic Engineering Boost Lycopene Production in Saccharomyces cerevisiae via Enhanced Precursors Supply and Utilization. J. Agric. Food Chem. 2023, 71, 3821–3831. [Google Scholar] [CrossRef] [PubMed]
- Xia, L.; Lv, Y.; Liu, S.; Yu, S.; Zeng, W.; Zhou, J. Enhancing Squalene Production in Saccharomyces cerevisiae by Metabolic Engineering and Random Mutagenesis. Front. Chem. Eng. 2022, 3, 790261. [Google Scholar] [CrossRef]
- Cai, M.; Wu, Y.; Qi, H.; He, J.; Wu, Z.; Xu, H.; Qiao, M. Improving the Level of the Tyrosine Biosynthesis Pathway in Saccharomyces cerevisiae through HTZ1 Knockout and Atmospheric and Room Temperature Plasma (ARTP) Mutagenesis. ACS Synth. Biol. 2021, 10, 49–62. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Niu, C.; Liu, C.; Wang, J.; Zheng, F.; Li, Q. A Novel Approach to Develop Lager Yeast with Higher NADH Availability to Improve the Flavor Stability of Industrial Beer. Foods 2021, 10, 3057. [Google Scholar] [CrossRef]
- Chen, H.; Wang, J.; Li, Q.; Xu, X.; Niu, C.; Zheng, F.; Liu, C. Fed-Batch Fermentation of Saccharomyces pastorianus with High Ribonucleic Acid Yield. Foods 2022, 11, 2742. [Google Scholar] [CrossRef]
- Nie, X.; Xing, Y.; Li, Q.; Gao, F.; Wang, S.; Liu, P.; Li, X.; Tan, Z.; Wang, P.; Shi, H. ARTP mutagenesis promotes selenium accumulation in Saccharomyces boulardii. LWT 2022, 168, 113916. [Google Scholar] [CrossRef]
- Zhang, C.; Qin, J.; Dai, Y.; Mu, W.; Zhang, T. Atmospheric and room temperature plasma (ARTP) mutagenesis enables xylitol over-production with yeast Candida tropicalis. J. Biotechnol. 2019, 296, 7–13. [Google Scholar] [CrossRef]
- Yuan, W.; Lin, X.; Zhong, S.; Chen, J.; Wang, Z.; Sun, J. Enhanced pyruvic acid yield in an osmotic stress-resistant mutant of Yarrowia lipolytica. Electron. J. Biotechnol. 2020, 44, 19–24. [Google Scholar] [CrossRef]
- Zheng, S.; Jiang, B.; Zhang, T.; Chen, J. Combined mutagenesis and metabolic regulation to enhance D-arabitol production from Candida parapsilosis. J. Ind. Microbiol. Biotechnol. 2020, 47, 425–435. [Google Scholar] [CrossRef]
- Huang, R.; Ding, R.; Liu, Y.; Li, F.; Zhang, Z.; Wang, S. GATA transcription factor WC2 regulates the biosynthesis of astaxanthin in yeast Xanthophyllomyces dendrorhous. Microb. Biotechnol. 2022, 15, 2578–2593. [Google Scholar] [CrossRef] [PubMed]
- Wei, F.; Li, M.; Wang, M.; Li, H.; Li, Z.; Qin, W.; Wei, T.; Zhao, J.; Bao, X. A C6/C5 co-fermenting Saccharomyces cerevisiae strain with the alleviation of antagonism between xylose utilization and robustness. GCB Bioenergy 2020, 13, 83–97. [Google Scholar] [CrossRef]
- Qiu, W.; Ren, T.; Chen, C.; Yang, J.; Wu, H.; Dong, L. Research on Mutation of High Lactase Activity Kluyveromyces lactis by ARTP. J. Chin. Inst. Food Sci. Technol. 2014, 14, 132–137. [Google Scholar] [CrossRef]
- Li, Y.C.; Rao, J.W.; Meng, F.B.; Wang, Z.W.; Liu, D.Y.; Yu, H. Combination of mutagenesis and adaptive evolution to engineer salt-tolerant and aroma-producing yeast for soy sauce fermentation. J. Sci. Food Agric. 2021, 101, 4288–4297. [Google Scholar] [CrossRef]
- Guo, J.; Luo, W.; Wu, X.M.; Fan, J.; Zhang, W.X.; Suyama, T. Improving RNA content of salt-tolerant Zygosaccharomyces rouxii by atmospheric and room temperature plasma (ARTP) mutagenesis and its application in soy sauce brewing. World J. Microbiol. Biotechnol. 2019, 35, 180. [Google Scholar] [CrossRef]
- Tian, T.; Wu, D.; Ng, C.T.; Yang, H.; Sun, J.; Liu, J.; Lu, J. A multiple-step strategy for screening Saccharomyces cerevisiae strains with improved acid tolerance and aroma profiles. Appl. Microbiol. Biotechnol. 2020, 104, 3097–3107. [Google Scholar] [CrossRef]
- Stewart, G.G. SACCHAROMYCES|Saccharomyces cerevisiae. In Encyclopedia of Food Microbiology, 2nd ed.; Batt, C.A., Tortorello, M.L., Eds.; Academic Press: Oxford, UK, 2014; pp. 309–315. [Google Scholar]
- Liu, C.; Li, Q.; Niu, C.; Tian, Y.; Zhao, Y.; Yin, X. The use of atmospheric and room temperature plasma mutagenesis to create a brewing yeast with reduced acetaldehyde production. J. Inst. Brew. 2018, 124, 236–243. [Google Scholar] [CrossRef]
- Wang, X.; Tian, X.; Wu, Y.; Shen, X.; Yang, S.; Chen, S. Enhanced doxorubicin production by Streptomyces peucetius using a combination of classical strain mutation and medium optimization. Prep. Biochem. Biotechnol. 2018, 48, 514–521. [Google Scholar] [CrossRef]
- Sun, J.; Li, J.; Yao, L.; Zheng, Y.; Yuan, J.; Wang, D. UV-ARTP-DES compound mutagenesis breeding improves natamycin production of Streptomyces natalensis HW-2 and reveals transcriptional changes by RNA-seq. Food Sci. Biotechnol. 2023, 32, 341–352. [Google Scholar] [CrossRef]
- Yu, G.; Peng, H.; Cao, J.; Liao, A.; Long, P.; Huang, J.; Hui, M. Avilamycin production enhancement by mutagenesis and fermentation optimization in Streptomyces viridochromogenes. World J. Microbiol. Biotechnol. 2022, 38, 50. [Google Scholar] [CrossRef]
- Zhang, K.; Mohsin, A.; Dai, Y.; Chen, Z.; Zhuang, Y.; Chu, J.; Guo, M. Combinatorial Effect of ARTP Mutagenesis and Ribosome Engineering on an Industrial Strain of Streptomyces albus S12 for Enhanced Biosynthesis of Salinomycin. Front. Bioeng. Biotechnol. 2019, 7, 212. [Google Scholar] [CrossRef]
- Thein, Y.W.; Shi, L.; Liu, B.; Wei, Q.; Zhang, K.; Ge, B. Enhancing wuyiencin productivity of Streptomyces albulus (CK15) by mutagenesis breeding with atmospheric and room temperature plasma. World J. Microbiol. Biotechnol. 2023, 39, 202. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Guo, S.; Hua, Q.; Hu, F. Improved AP-3 production through combined ARTP mutagenesis, fermentation optimization, and subsequent genome shuffling. Biotechnol. Lett. 2021, 43, 1143–1154. [Google Scholar] [CrossRef]
- Xia, X.K.; Zhang, Y.E.; Lei, S.J.; Hu, B.; Fu, C.X. Identification and iterative combinatorial mutagenesis of a new naringinase-producing strain, Aspergillus tubingensis MN589840. Lett. Appl. Microbiol. 2021, 72, 141–148. [Google Scholar] [CrossRef] [PubMed]
- Dong, X.; Wan, Y.; Chen, Y.; Wu, X.; Zhang, Y.; Deng, M.; Cai, W.; Wu, X.; Fu, G. Molecular mechanism of high-production tannase of Aspergillus carbonarius NCUF M8 after ARTP mutagenesis: Revealed by RNA-seq and molecular docking. J. Sci. Food Agric. 2022, 102, 4054–4064. [Google Scholar] [CrossRef] [PubMed]
- Dong, X.; Wu, X.; Zhang, Y.; Wu, X.; Wan, Y.; Liu, C.; Fu, G. Breeding of tannase-producing Aspergillus carbonarius using ARTP mutagenesis and fermentation optimization. Food Ferment. Ind. 2021, 47, 15–21. [Google Scholar] [CrossRef]
- Gu, L.S.; Tan, M.Z.; Li, S.H.; Zhang, T.; Zhang, Q.Q.; Li, C.X.; Luo, X.M.; Feng, J.X.; Zhao, S. ARTP/EMS-combined multiple mutagenesis efficiently improved production of raw starch-degrading enzymes in Penicillium oxalicum and characterization of the enzyme-hyperproducing mutant. Biotechnol. Biofuels 2020, 13, 187. [Google Scholar] [CrossRef]
- Zhang, N.; Jiang, Y.; Sun, Y.J.; Jiang, J.C.; Tong, Y.J. Breeding of a thermostable xylanase-producing strain of Myceliophthora thermophila by atmospheric room temperature plasma (ARTP) mutagenesis. Front. Bioeng. Biotechnol. 2022, 10, 1095323. [Google Scholar] [CrossRef]
- He, R.; Ding, R.; Heyman, J.A.; Zhang, D.; Tu, R. Ultra-high-throughput picoliter-droplet microfluidics screening of the industrial cellulase-producing filamentous fungus Trichoderma reesei. J. Ind. Microbiol. Biotechnol. 2019, 46, 1603–1610. [Google Scholar] [CrossRef]
- Peng, Z.Q.; Li, C.; Lin, Y.; Wu, S.S.; Gan, L.H.; Liu, J.; Yang, S.L.; Zeng, X.H.; Lin, L. Cellulase production and efficient saccharification of biomass by a new mutant Trichoderma afroharzianum MEA-12. Biotechnol. Biofuels 2021, 14, 219. [Google Scholar] [CrossRef] [PubMed]
- Asitok, A.; Ekpenyong, M.; Akwagiobe, E.; Asuquo, M.; Rao, A.; Ubi, D.; Iheanacho, J.; Ikharia, E.; Antai, A.; Essien, J.; et al. Interspecific protoplast fusion of atmospheric and room-temperature plasma mutants of Aspergillus generates an L-asparaginase hyper-producing hybrid with techno-economic benefits. Prep. Biochem. Biotechnol. 2023, 53, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Gou, Z.C.; Lu, M.J.; Cui, X.Y.; Wang, X.Q.; Jiang, M.Y.; Wang, Y.S.; Wang, Z.Q.; Yu, X.X.; Tang, S.S.; Chen, G.; et al. Enhanced laccase production by mutagenized Myrothecium verrucaria using corn stover as a carbon source and its potential in the degradation of 2-chlorophen. Bioprocess. Biosyst. Eng. 2022, 45, 1581–1593. [Google Scholar] [CrossRef] [PubMed]
- Gou, Z.; Hopla, G.A.; Yao, M.; Cui, B.; Su, Y.; Rinklebe, J.; Sun, C.; Chen, G.; Ma, N.L.; Sun, Y. Removal of dye pollution by an oxidase derived from mutagenesis of the Deuteromycete Myrothecium with high potential in industrial applications. Environ. Pollut. 2022, 310, 119726. [Google Scholar] [CrossRef]
- Qiu, L.; Nie, S.X.; Hu, S.J.; Wang, S.J.; Wang, J.J.; Guo, K. Screening of Beauveria bassiana with high biocontrol potential based on ARTP mutagenesis and high-throughput FACS. Pestic. Biochem. Physiol. 2021, 171, 104732. [Google Scholar] [CrossRef]
- Huang, W.W.; Ge, X.Y.; Huang, Y.; Chai, X.T.; Zhang, L.; Zhang, Y.X.; Deng, L.N.; Liu, C.Q.; Xu, H.; Gao, J. High-yield strain of fusidic acid obtained by atmospheric and room temperature plasma mutagenesis and the transcriptional changes involved in improving its production in fungus Fusidium coccineum. J. Appl. Microbiol. 2021, 130, 405–415. [Google Scholar] [CrossRef]
- Peng, Q.; Xiao, Y.; Zhang, S.; Zhou, C.; Xie, A.; Li, Z.; Tan, A.; Zhou, L.; Xie, Y.; Zhao, J.; et al. Mutation breeding of Aspergillus niger by atmospheric room temperature plasma to enhance phosphorus solubilization ability. PeerJ 2022, 10, e13076. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Ding, L.; Lu, J.; Wang, N.; Cai, M. Combinatorial strategies for production improvement of red pigments from Antarctic fungus Geomyces sp. J. Food Sci. 2020, 85, 3061–3071. [Google Scholar] [CrossRef]
- Liu, J.; Guo, T.; Luo, Y.; Chai, X.; Wu, J.; Zhao, W.; Jiao, P.; Luo, F.; Lin, Q. Enhancement of Monascus pigment productivity via a simultaneous fermentation process and separation system using immobilized-cell fermentation. Bioresour. Technol. 2019, 272, 552–560. [Google Scholar] [CrossRef]
- Wang, Q.; Chen, Y.; Fu, J.; Yang, Q.; Feng, L. High-throughput screening of lycopene-overproducing mutants of Blakeslea trispora by combining ARTP mutation with microtiter plate cultivation and transcriptional changes revealed by RNA-seq. Biochem. Eng. J. 2020, 161, 107664. [Google Scholar] [CrossRef]
- Zhang, H.; Jiang, F.; Qu, D.; Wang, W.; Dong, Y.; Zhang, J.; Wu, D.; Yang, Y. Employment of ARTP to Generate Phellinus baumii (Agaricomycetes) Strain with High Flavonoids Production and Validation by Liquid Fermentation. Int. J. Med. Mushrooms 2019, 21, 1207–1221. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Shah, A.M.; Mohamed, H.; Zhang, Y.; Sadaqat, B.; Tsiklauri, N.; Sadunishvili, T.; Song, Y. Improved laccase production in Pleurotus djamor RP by atmospheric and room temperature plasma (ARTP) mutagenesis. Electron. J. Biotechnol. 2022, 58, 1–9. [Google Scholar] [CrossRef]
- Gong, M.; Zhang, H.; Wu, D.; Zhang, Z.; Zhang, J.; Bao, D.; Yang, Y. Key metabolism pathways and regulatory mechanisms of high polysaccharide yielding in Hericium erinaceus. BMC Genom. 2021, 22, 160. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Yang, Y.; Li, Q.; Wu, D.; Yang, R.; Wang, W.; Zhang, H. Screening of high-yield polysaccharide Hericium erinareus by atmospheric and room temperature plasma mutagenesis. Acta Agric. Shanghai 2019, 35, 6–11. [Google Scholar] [CrossRef]
- Li, T.; Chen, L.; Wu, D.; Dong, G.; Chen, W.; Zhang, H.; Yang, Y.; Wu, W. The Structural Characteristics and Biological Activities of Intracellular Polysaccharide Derived from Mutagenic Sanghuangporous sanghuang Strain. Molecules 2020, 25, 3693. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, H.; Tan, Y.; Liu, Y.; Feng, J.; Zhang, Y.; Tang, C.; Zhang, J. Screening of a high polysaccharide content Ganoderma lucidum strain by ARTP. Acta Edulis Fungi 2021, 28, 36–41. [Google Scholar] [CrossRef]
- Liu, W.; Yang, W.; Wu, J.; Cheng, Y.; Wei, Z.; Wang, T.; Ampofo, K.A.; Ma, H.; Cui, F.; Yang, X.; et al. ARTP Mutagenesis to Improve Mycelial Polysaccharide Production of Grifola frondosa Using a Mixture of Wheat Bran and Rice Bran as Substrate. J. Food Qual. 2021, 2021, 6110743. [Google Scholar] [CrossRef]
- Zhang, H.; Chen, R.; Zhang, J.; Bu, Q.; Wang, W.; Liu, Y.; Li, Q.; Guo, Y.; Zhang, L.; Yang, Y. The integration of metabolome and proteome reveals bioactive polyphenols and hispidin in ARTP mutagenized Phellinus baumii. Sci. Rep. 2019, 9, 16172. [Google Scholar] [CrossRef]
- Li, Y.; Meng, N.; Wen, Z.; Li, X.; Li, S.; Du, X. Breeding of High Yield Strains of Cordyeps militaris by Combined Mutation of 60Co-γ and ARTP. North. Hortic. 2019, 43, 112–116. [Google Scholar]
- Wang, C.; Ma, X.; Ping, L.; Wang, Q. Preliminary Study on Breeding Excellent Fermentation Strains of Auricularia auriculae by ARTP Mutation Technology. Edible Fungi China 2021, 40, 17–22, 26. [Google Scholar] [CrossRef]
- Zhang, G.; Geng, B.; Wu, G.; Tian, X. Breeding of new strains of Lepista sordida by atmospheric and room temperature plasma mutagenesis. Mycosystema 2021, 40, 30096–33108. [Google Scholar] [CrossRef]
- Ma, N.; Che, S.; Zhang, X.; Wu, W.; Fu, Y.; Li, C.; Yang, D.; Liu, H. Rejuvenation and atmospheric room temperature plasma mutagenesis in breeding of Grifola frondosa. Microbiol. China 2020, 47, 2526–2535. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, H.; Tan, Y.; Feng, J.; Tang, Q.; Tang, C.; Zhang, J. Screening of Ganoderma lucidum Mutant Strains by ARTP for High Antioxidant Capacity. Acta Edulis Fungi 2021, 28, 47–55. [Google Scholar] [CrossRef]
- Elshobary, M.E.; Zabed, H.M.; Qi, X.; El-Shenody, R.A. Enhancing biomass and lipid productivity of a green microalga Parachlorella kessleri for biodiesel production using rapid mutation of atmospheric and room temperature plasma. Biotechnol. Biofuels Bioprod. 2022, 15, 122. [Google Scholar] [CrossRef] [PubMed]
- An, J.; Gao, F.; Ma, Q.; Xiang, Y.; Ren, D.; Lu, J. Screening for enhanced astaxanthin accumulation among Spirulina platensis mutants generated by atmospheric and room temperature plasmas. Algal Res. 2017, 25, 464–472. [Google Scholar] [CrossRef]
- Wu, X.; Liu, Z.; Jiang, Y. Mutation breeding of Haematococcus pluvialis by atmospheric and room temperature plasma and isolation of high-producing mutants. J. Food Saf. Qual. 2016, 7, 3781–3787. [Google Scholar] [CrossRef]
- Cao, S.; Zhou, X.; Jin, W.; Wang, F.; Tu, R.; Han, S.; Chen, H.; Chen, C.; Xie, G.J.; Ma, F. Improving of lipid productivity of the oleaginous microalgae Chlorella pyrenoidosa via atmospheric and room temperature plasma (ARTP). Bioresour. Technol. 2017, 244, 1400–1406. [Google Scholar] [CrossRef]
- Wang, Q.; Jin, W.; Han, W.; Song, K.; Chen, Y.; Chen, C.; Jiang, G.; Zhou, X. Enhancement of DHA production from Aurantiochytrium sp. by atmospheric and room temperature plasma mutagenesis aided with microbial microdroplet culture screening. Biomass Convers. Biorefin. 2022. [Google Scholar] [CrossRef]
- Li, J.; Liu, X.; He, Y.; Wang, G. Mutagenesis Breeding in DHA Production by Oleaginous Microorganisms. Biotechnol. Bull. 2020, 36, 110–115. [Google Scholar] [CrossRef]
- Fang, M.; Jin, L.; Zhang, C.; Tan, Y.; Jiang, P.; Ge, N.; Heping, L.; Xing, X. Rapid mutation of Spirulina platensis by a new mutagenesis system of atmospheric and room temperature plasmas (ARTP) and generation of a mutant library with diverse phenotypes. PLoS ONE 2013, 8, e77046. [Google Scholar] [CrossRef]
- Chen, L.; Liu, X.; Li, C.; Li, H.; Chen, W.; Li, D. Transcriptome analyses reveal the DHA enhancement mechanism in Schizochytrium limacinum LD11 mutant. Algal Res. 2022, 67, 102868. [Google Scholar] [CrossRef]
- Zeng, L.; Bi, Y.; Guo, P.; Bi, Y.; Wang, T.; Dong, L.; Wang, F.; Chen, L.; Zhang, W. Metabolic Analysis of Schizochytrium Mutants with High DHA Content Achieved with ARTP Mutagenesis Combined with Iodoacetic Acid and Dehydroepiandrosterone Screening. Front. Bioeng. Biotechnol. 2021, 9, 738052. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Bai, M.; Zhang, S.; Li, J.; Liu, X.; Sen, B.; Wang, G. ARTP Mutagenesis of Schizochytrium sp. PKU#Mn4 and Clethodim-Based Mutant Screening for Enhanced Docosahexaenoic Acid Accumulation. Mar. Drugs 2021, 19, 564. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Li, P.; Liu, X.; Wang, X.; Liu, Y.; Turaib, A.; Cheng, Z. Strategies for enhanced lipid production of Desmodesmus sp. mutated by atmospheric and room temperature plasma with a new efficient screening method. J. Clean. Prod. 2020, 250, 119509. [Google Scholar] [CrossRef]
- Sun, X.; Meng, L.; Li, P.; Su, Z.; Wang, X.; Lian, Y.; Liu, Z. Increasing lipid production of Desmodesmus sp. through atmospheric and room temperature plasma orientated with malonic acid: Performance and biochemical mechanism. J. Clean. Prod. 2022, 342, 130911. [Google Scholar] [CrossRef]
- Li, P.; Sun, X.; Sun, Z.; Huang, F.; Wei, W.; Liu, X.; Liu, Y.; Deng, L.; Cheng, Z. Biochemical and genetic changes revealing the enhanced lipid accumulation in Desmodesmus sp. mutated by atmospheric and room temperature plasma. Renew. Energy 2021, 172, 368–381. [Google Scholar] [CrossRef]
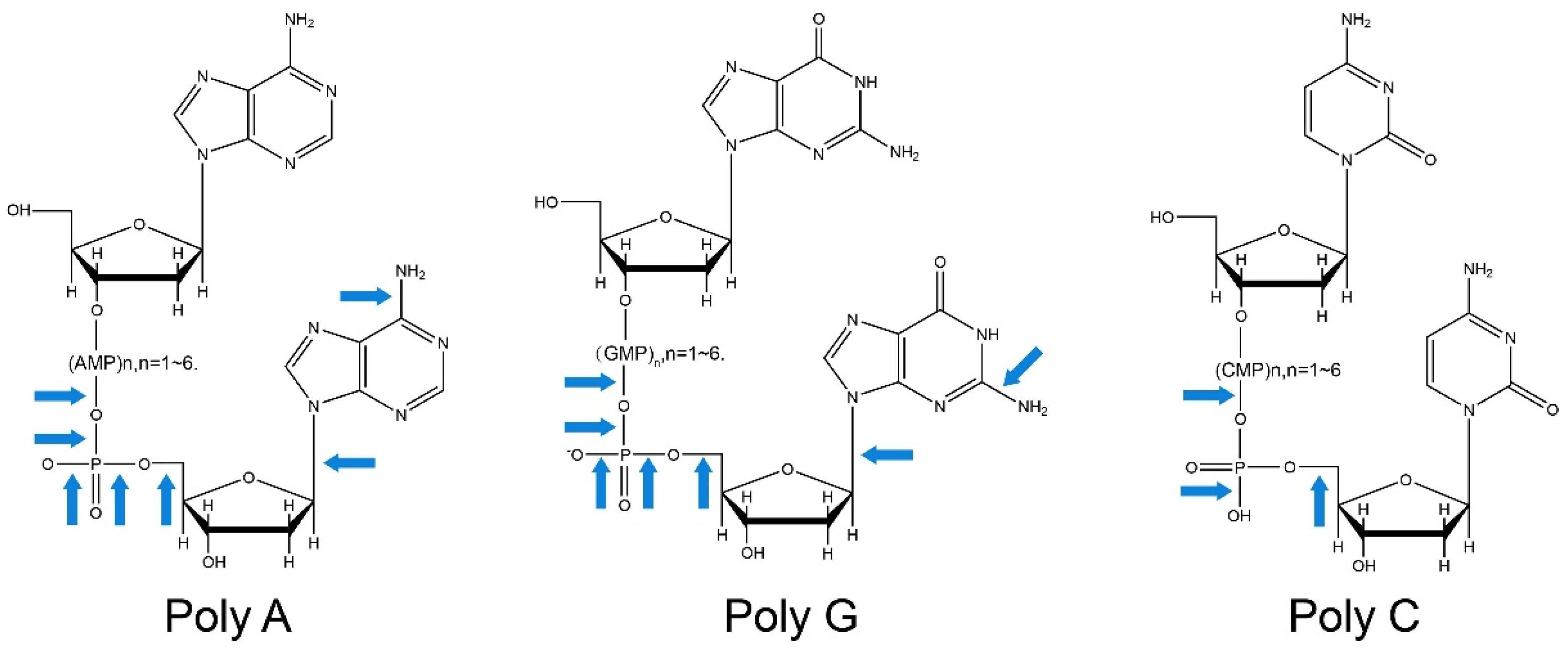
| Strain | Compound/Property | Mutant Method | Time | Ability | Refs. |
|---|---|---|---|---|---|
| S. cerevisiae | Astaxanthin | ARTP | 20 s | Increase in astaxanthin yield of 4-fold. | [63] |
| S. cerevisiae | Astaxanthin | ARTP; SCRM | 35 s | Increase in astaxanthin yield of 2.2- and 7.0-fold. | [64] |
| X. dendrorhous | Astaxanthin | ARTP | 100–175 s | High astaxanthin production. | [74] |
| S. cerevisiae | Lycopene | ARTP | 35 s | Increase in lycopene yield of 60% to 703 mg/L at shake flask. | [65] |
| S. cerevisiae | Squalene | ARTP | 75 s | Increase in squalene yield of 18.4%. | [66] |
| S. cerevisiae | p-coumaric acid | ARTP | 90 s | Increase in p-CA yield of 7.6-fold. | [67] |
| S. cerevisiae | Fermentation robustness | ARTP | 15 s | Enhanced fermentation robustness and highest ethanol yield. | [75] |
| S. cerevisiae | Low producing of acetaldehyde | ARTP | 45 s | LAL-8a produces 2.2 mg/L acetaldehyde, 88.2% less than M14. | [81] |
| S. pastorianus | NADH | ARTP; DNP | 45 s | The flavor stability of beer has been enhanced. | [68] |
| S. pastorianus | RNA | ARTP | 80 s | G03H8 increased RNA by 40% vs. G03. | [69] |
| S. boulardii | Selenium | ARTP | 40 s | Increase in selenium yield of 56.77%. | [70] |
| C. tropicalis | Xylitol | ARTP | 30 s | Increase in xylitol yield of 22%. | [71] |
| Y. lipolytica | Pyruvic acid | ARTP | 420 s | Increase in PA yield of 28.9%. | [72] |
| C. parapsilosis | D-Arabitol | ARTP | 140 s | The d-Arabitol yield increased by 53.98%. | [73] |
| W. anomalus | Salt tolerance | ARTP | 120–150 s | Enhance resistance to a sodium chloride concentration of 18%. | [77] |
| Z. rouxii | Salt tolerance | ARTP | 60 s | The RNA content increased by 160.54%. | [78] |
| S. cerevisiae | Acid tolerance | ARTP | 150–210 s | The survival rate increased by 10-fold under low pH conditions. | [79] |
| S. peucetiu | Doxorubicin | UV; ARTP | — | Increase in doxorubicin production of 379%. | [82] |
| S. natalensis | Natamycin | UV; ARTP; DES | 40 s | Increase in natamycin yield of 86.36%. | [83] |
| S. viridochromogenes | Avilamycin | UV; ARTP | 70 s | Increase in avilamycin yield of 57.92–146.39%. | [84] |
| S. fradiae | Neomycin | ARTP | 180 s | Increase in neomycin yield of 100%. | [20] |
| S. albus | Salinomycin | ARTP | 360 s | Increase in salinomycin yield of twofold. | [85] |
| S. albulus | Wuyiencin | ARTP | 180 s | Increase in wuyiencin production of 13.6–18.5%. | [86] |
| A. pretiosum | Ansamitocin | ARTP | — | Increase in ansamitocin production of 22.5%. | [87] |
| A. tubingensis | Naringinase | ARTP | 240 s | Increase in naringinase productivity of 79.08–206%. | [88] |
| A. carbonarius | Tannase | ARTP | 180 s | Enhanced the yield and properties of A. carbonarius tannase. | [89,90] |
| P. oxalicum | Raw starch-degrading enzyme | ARTP; EMS | 500 s | Increase in RSDEs activity of 61.6%. | [91] |
| M. thermophila | Thermostable xylanase | ARTP | 150–250 s | Increase in xylanase activity of 21.71%. | [92] |
| T. reesei | Cellulase | ARTP | 90 s | Increase in cellulase activity of 27–46%. | [93] |
| T. afroharzianum | Cellulase | ARTP; MNNG; EMS | 240 s | Increases in four different enzyme activity of 4.15- to 6.37-fold. | [94] |
| A.s candidus | L-asparaginase | ARTP | 180 s | Increase in L-asparaginase activities of 2.3-folds. | [95] |
| M. verrucaria | Environmental remediation | ARTP | 75 s | Increase in laccase activity of 19.04-fold. | [96] |
| M. verrucaria | Environmental remediation | UV; ARTP | 85 s | Increase in oxidase producing of 106.15%. | [97] |
| B. bassiana | Biocontrol agent | ARTP | 90 s | Increases in FSC and virulence of 37.4% and 32.6%. | [98] |
| F. coccineum | Fusidic acid | ARTP | 120–140 s | High yield of fusidic acid in mutant strain. | [99] |
| A. niger | Phosphate-solubilizing ability | ARTP | 120 | The ability to efficiently degrade P compounds in soils. | [100] |
| Geomyces sp. | Red pigments | ARTP | 90 s | Increase in red pigments yield of 24.4%. | [101] |
| M. purpureus | Monascus pigments | ARTP | 180 s | Increase in monascus pigments production of 150% | [102] |
| B. trispora | Lycopene | ARTP | 120 s | Increase in lycopene yield of 54.27%. | [103] |
| P. baumii | Flavonoids | ARTP | — | Increase in flavonoids yield of 86.67%. | [104] |
| P. djamor | Laccase | ARTP | 120 s | Increase in laccase activity of 86.36%. | [105] |
| H. erinaceus | Polysaccharide | ARTP | 30 s | Increase in polysaccharide yield of 23.25–47.45%. | [106,107] |
| S. sanghuang | Polysaccharide | ARTP | — | Polysaccharide yields from A130 mutants increased significantly. | [108] |
| G. lucidum | Polysaccharide | ARTP | — | Increase in mycelial polysaccharide yield of 46.14–268.57%. | [109] |
| G. frondosa | Polysaccharide | ARTP | 60 | Increase in mycelial polysaccharide yield of 5.90 g/L. | [110] |
| P. baumii | Hispidin | ARTP | — | Enhanced antioxidant activity. | [111] |
| C. militaris | High yield | 60Co-γ; ARTP | 150 s | Increase in fruit body yield of 32.27–36%. | [112] |
| A. auriculae | High yield | ARTP | 45 s | Showed increased yield and improved quality. | [113] |
| L. sordida | High yield | ARTP | 50 s | Showed improved yield and quality by 10.27% and 14.75%. | [114] |
| G. frondosa | High yield | ARTP | 80–90 s | Increases in dry weight and polysaccharide content of 40.15% and 39.33%. | [115] |
| G. lucidum | High antioxidant capacity | ARTP | — | Showed significantly higher antioxidant capacity than wild type. | [116] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Q.; Miao, R.; Feng, R.; Yan, J.; Wang, T.; Gan, Y.; Zhao, J.; Lin, J.; Gan, B. Application of Atmospheric and Room-Temperature Plasma (ARTP) to Microbial Breeding. Curr. Issues Mol. Biol. 2023, 45, 6466-6484. https://doi.org/10.3390/cimb45080408
Zhang Q, Miao R, Feng R, Yan J, Wang T, Gan Y, Zhao J, Lin J, Gan B. Application of Atmospheric and Room-Temperature Plasma (ARTP) to Microbial Breeding. Current Issues in Molecular Biology. 2023; 45(8):6466-6484. https://doi.org/10.3390/cimb45080408
Chicago/Turabian StyleZhang, Qin, Renyun Miao, Rencai Feng, Junjie Yan, Tao Wang, Ying Gan, Jin Zhao, Junbin Lin, and Bingcheng Gan. 2023. "Application of Atmospheric and Room-Temperature Plasma (ARTP) to Microbial Breeding" Current Issues in Molecular Biology 45, no. 8: 6466-6484. https://doi.org/10.3390/cimb45080408
APA StyleZhang, Q., Miao, R., Feng, R., Yan, J., Wang, T., Gan, Y., Zhao, J., Lin, J., & Gan, B. (2023). Application of Atmospheric and Room-Temperature Plasma (ARTP) to Microbial Breeding. Current Issues in Molecular Biology, 45(8), 6466-6484. https://doi.org/10.3390/cimb45080408





