Protocatechuic Acid and Syringin from Saussurea neoserrata Nakai Attenuate Prostaglandin Production in Human Keratinocytes Exposed to Airborne Particulate Matter
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Extraction and Isolation
2.3. Cell Culture
2.4. Treatment of Cells with PM10
2.5. Cell Viability Assay
2.6. Enzyme-Linked Immunosorbent Assay (ELISA)
2.7. Assay for Cellular ROS Production
2.8. Analysis of Quantitative Reverse Transcription Polymerase Chain Reaction (qRT-PCR)
2.9. Statistical Analysis
3. Results
3.1. Isolation of Protocatechuic Acid from S. neoserrata Nakai
3.2. Isolation of Syringin from S. neoserrata Nakai
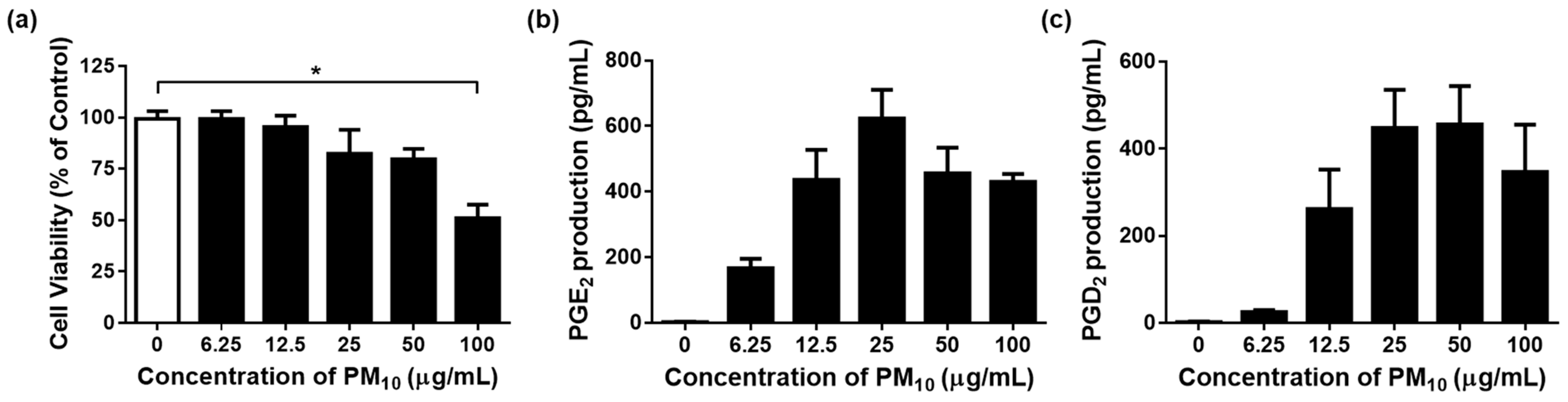
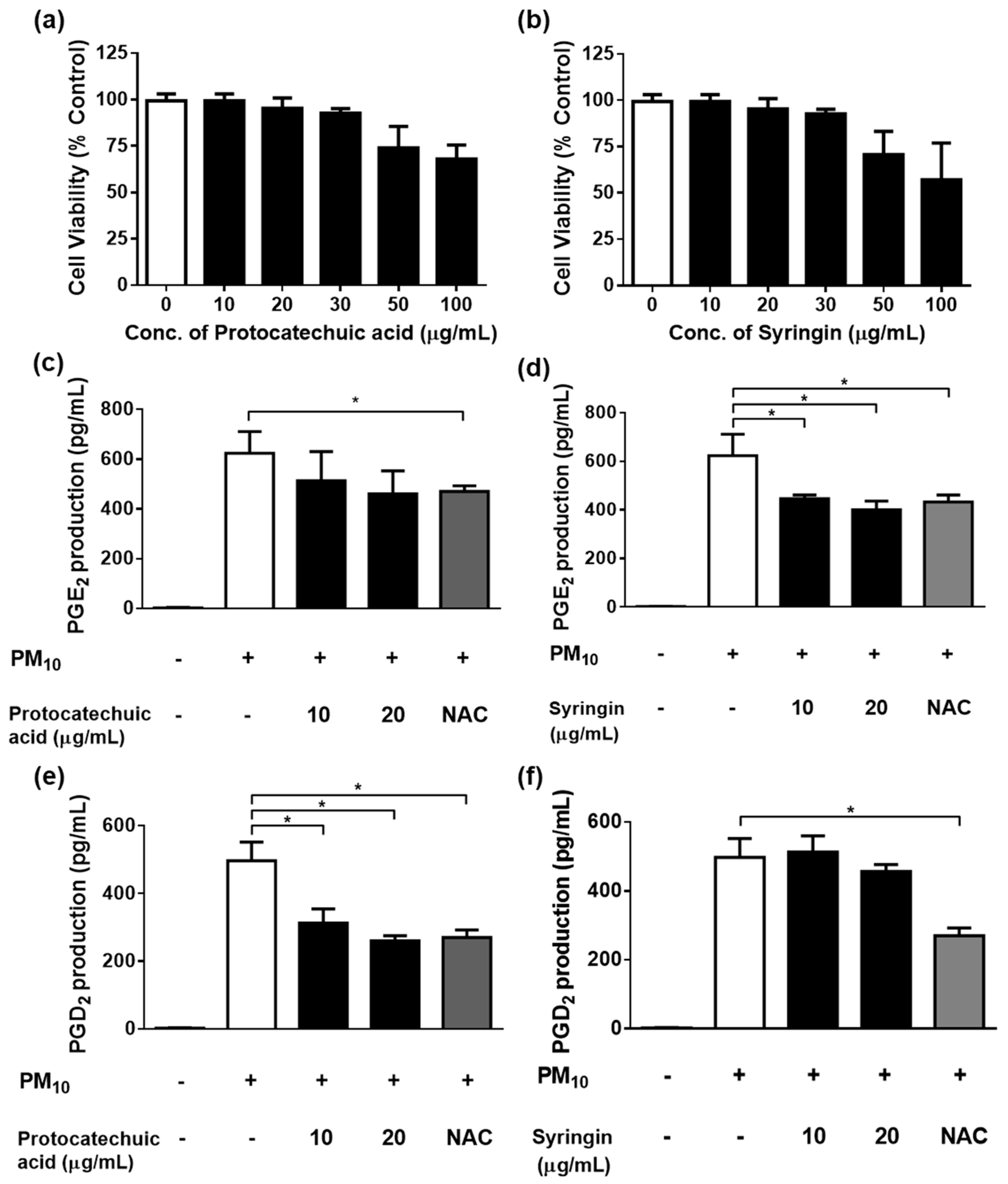
3.3. PM10 Induces Cytotoxicity in PGE2 and PGD2 Release from Keratinocytes
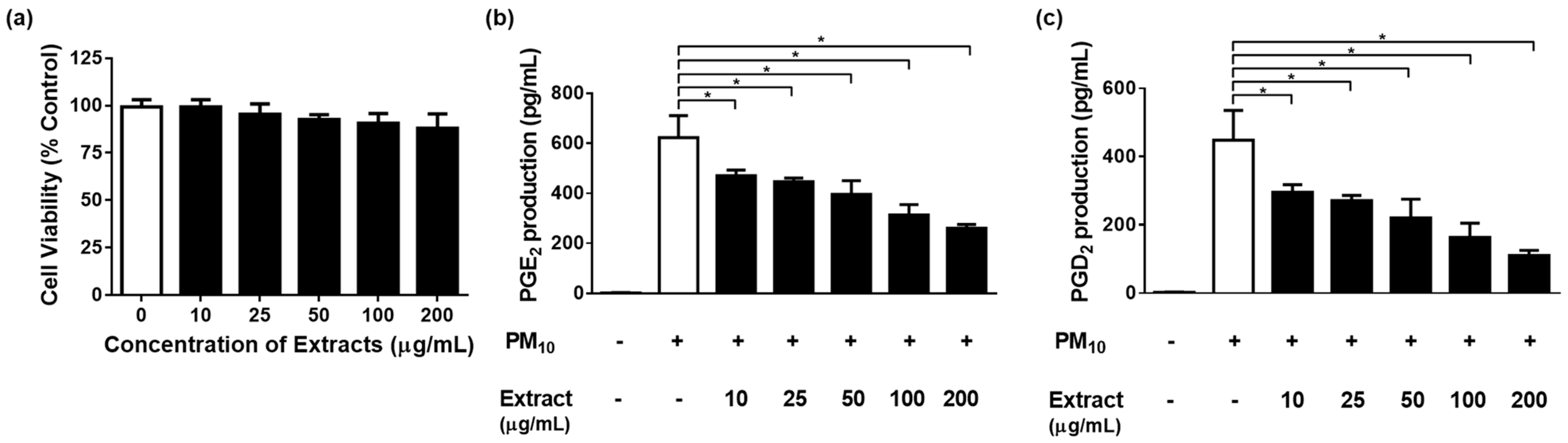
3.4. Effects of Various Concentrations of S. neoserrata Nakai Extracts on 12.5 µg/mL PM10-Induced Cyto-Toxicity and PGE2 and PGD2 Release
3.5. Effects of Protocatechuic Acid and Syringin on PM10-Induced Keratinocyte Cytotoxicity and PGE2 and PGD2 Release
3.6. Effects of Protocatechuic Acid and Syringin on PM10-Induced ROS Production
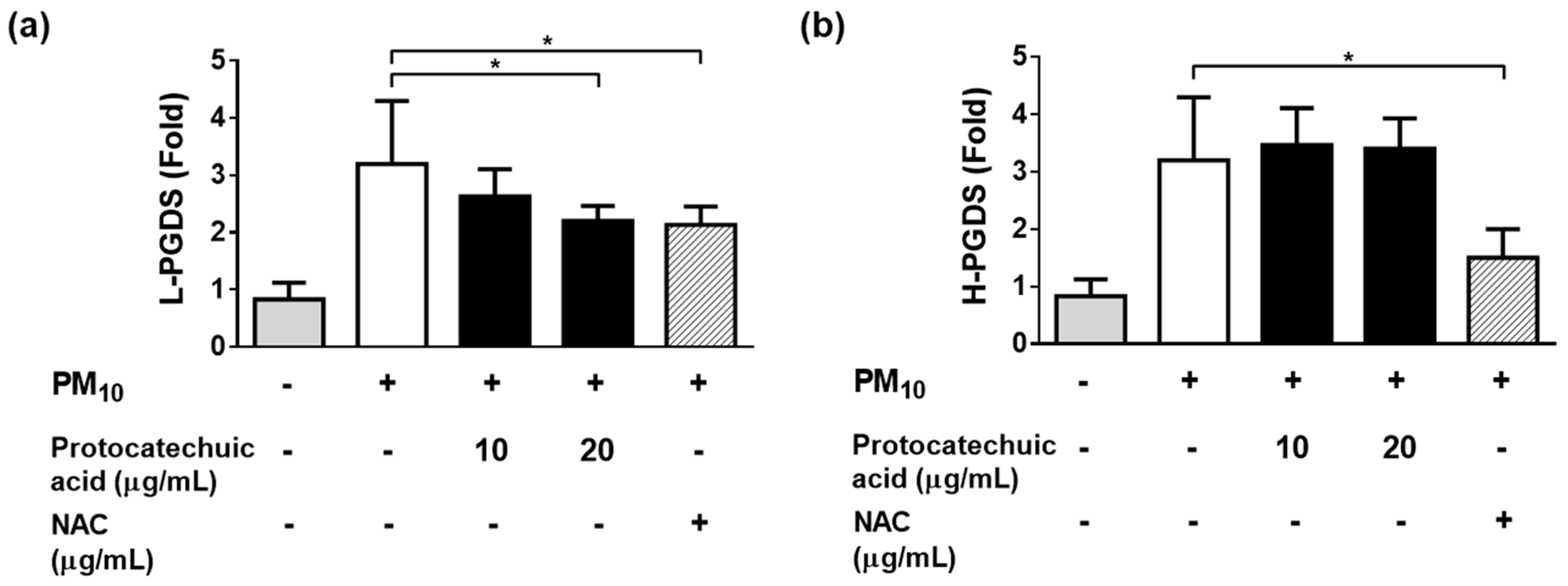
3.7. Effects of Protocatechuic Acid and Syringin on the PM10-Induced Gene Expression of the Enzymes Involved in the PGE2 and PGD2 Synthesis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Krzyzanowski, M. WHO air quality guidelines for Europe. J. Toxicol. Environ. Health Part A 2008, 71, 47–50. [Google Scholar] [CrossRef] [PubMed]
- Jia, Y.-Y.; Wang, Q.; Liu, T. Toxicity research of PM2. 5 compositions in vitro. Int. J. Environ. Res. Public Health 2017, 14, 232. [Google Scholar] [CrossRef] [PubMed]
- Momtazan, M.; Geravandi, S.; Rastegarimehr, B.; Valipour, A.; Ranjbarzadeh, A.; Yari, A.R.; Dobaradaran, S.; Bostan, H.; Farhadi, M.; Darabi, F. An investigation of particulate matter and relevant cardiovascular risks in Abadan and Khorramshahr in 2014–2016. Toxin Rev. 2019, 38, 290–297. [Google Scholar] [CrossRef]
- Shahriyari, H.A.; Nikmanesh, Y.; Jalali, S.; Tahery, N.; Zhiani Fard, A.; Hatamzadeh, N.; Zarea, K.; Cheraghi, M.; Mohammadi, M.J. Air pollution and human health risks: Mechanisms and clinical manifestations of cardiovascular and respiratory diseases. Toxin Rev. 2022, 41, 606–617. [Google Scholar] [CrossRef]
- Dobaradaran, S.; Geravandi, S.; Goudarzi, G.; Idani, E.; Salmanzadeh, S.; Soltani, F.; Yari, A.R.; Mohammadi, M.J. Determination of cardiovascular and respiratory diseases caused by PM10 exposure in Bushehr, 2013. J. Maz. Univ. Med. Sci. 2016, 26, 42–52. [Google Scholar]
- Bakke, J.V.; Wieslander, G.; Norback, D.; Moen, B.E. Eczema increases susceptibility to PM10 in office indoor environments. Arch. Environ. Occup. Health 2012, 67, 15–21. [Google Scholar] [CrossRef]
- Jin, S.-P.; Li, Z.; Choi, E.K.; Lee, S.; Kim, Y.K.; Seo, E.Y.; Chung, J.H.; Cho, S. Urban particulate matter in air pollution penetrates into the barrier-disrupted skin and produces ROS-dependent cutaneous inflammatory response in vivo. J. Dermatol. Sci. 2018, 91, 175–183. [Google Scholar] [CrossRef]
- Pan, T.-L.; Wang, P.-W.; Aljuffali, I.A.; Huang, C.-T.; Lee, C.-W.; Fang, J.-Y. The impact of urban particulate pollution on skin barrier function and the subsequent drug absorption. J. Dermatol. Sci. 2015, 78, 51–60. [Google Scholar] [CrossRef]
- Ahn, K. The role of air pollutants in atopic dermatitis. J. Allergy Clin. Immunol. 2014, 134, 993–999. [Google Scholar] [CrossRef]
- Vierkötter, A.; Schikowski, T.; Ranft, U.; Sugiri, D.; Matsui, M.; Krämer, U.; Krutmann, J. Airborne particle exposure and extrinsic skin aging. J. Investig. Dermatol. 2010, 130, 2719–2726. [Google Scholar] [CrossRef]
- Roberts, W.E. Pollution as a risk factor for the development of melasma and other skin disorders of facial hyperpigmentation-is there a case to be made? J. Drugs Dermatol. JDD 2015, 14, 337–341. [Google Scholar] [PubMed]
- Martic, I.; Jansen-Dürr, P.; Cavinato, M. Effects of air pollution on cellular senescence and skin aging. Cells 2022, 11, 2220. [Google Scholar] [CrossRef]
- Soeur, J.; Belaïdi, J.-P.; Chollet, C.; Denat, L.; Dimitrov, A.; Jones, C.; Perez, P.; Zanini, M.; Zobiri, O.; Mezzache, S. Photo-pollution stress in skin: Traces of pollutants (PAH and particulate matter) impair redox homeostasis in keratinocytes exposed to UVA1. J. Dermatol. Sci. 2017, 86, 162–169. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Huang, J.; Wang, L.; Chen, C.; Yang, D.; Jin, M.; Bai, C.; Song, Y. Urban particulate matter triggers lung inflammation via the ROS-MAPK-NF-κB signaling pathway. J. Thorac. Dis. 2017, 9, 4398. [Google Scholar] [CrossRef] [PubMed]
- Xia, T.; Kovochich, M.; Nel, A. The role of reactive oxygen species and oxidative stress in mediating particulate matter injury. Clin. Occup. Env. Med. 2006, 5, 817–836. [Google Scholar]
- Park, S.-Y.; Byun, E.J.; Lee, J.D.; Kim, S.; Kim, H.S. Air pollution, autophagy, and skin aging: Impact of particulate matter (PM10) on human dermal fibroblasts. Int. J. Mol. Sci. 2018, 19, 2727. [Google Scholar] [CrossRef]
- Romani, A.; Cervellati, C.; Muresan, X.M.; Belmonte, G.; Pecorelli, A.; Cervellati, F.; Benedusi, M.; Evelson, P.; Valacchi, G. Keratinocytes oxidative damage mechanisms related to airbone particle matter exposure. Mech. Ageing Dev. 2018, 172, 86–95. [Google Scholar] [CrossRef]
- Donaldson, K.; Stone, V. Current hypotheses on the mechanisms of toxicity of ultrafine particles. Ann. Dell’istituto Super. Di Sanitã 2003, 39, 405–410. [Google Scholar]
- Araujo, J.A.; Nel, A.E. Particulate matter and atherosclerosis: Role of particle size, composition and oxidative stress. Part. Fibre Toxicol. 2009, 6, 1–19. [Google Scholar] [CrossRef]
- Brook, R.D.; Rajagopalan, S. Particulate matter, air pollution, and blood pressure. J. Am. Soc. Hypertens. 2009, 3, 332–350. [Google Scholar] [CrossRef]
- Lee, C.-W.; Lin, Z.-C.; Hu, S.C.-S.; Chiang, Y.-C.; Hsu, L.-F.; Lin, Y.-C.; Lee, I.-T.; Tsai, M.-H.; Fang, J.-Y. Urban particulate matter down-regulates filaggrin via COX2 expression/PGE2 production leading to skin barrier dysfunction. Sci. Rep. 2016, 6, 27995. [Google Scholar] [CrossRef]
- Lee, I.-T.; Yang, C.-M. Role of NADPH oxidase/ROS in pro-inflammatory mediators-induced airway and pulmonary diseases. Biochem. Pharmacol. 2012, 84, 581–590. [Google Scholar] [CrossRef]
- Bedard, K.; Krause, K.-H. The NOX family of ROS-generating NADPH oxidases: Physiology and pathophysiology. Physiol. Rev. 2007, 87, 245–313. [Google Scholar] [CrossRef] [PubMed]
- Lassègue, B.; Griendling, K.K. NADPH oxidases: Functions and pathologies in the vasculature. Arterioscler. Thromb. Vasc. Biol. 2010, 30, 653–661. [Google Scholar] [CrossRef]
- Seok, J.K.; Choi, M.A.; Ha, J.W.; Boo, Y.C. Role of dual oxidase 2 in reactive oxygen species production induced by airborne particulate matter PM10 in human epidermal keratinocytes. J. Soc. Cosmet. Sci. Korea 2019, 45, 57–67. [Google Scholar]
- Gong, W.; Zhao, N.; Zhang, Z.; Zhang, Y.; Yan, L.; Li, J. The inhibitory effect of resveratrol on COX-2 expression in human colorectal cancer: A promising therapeutic strategy. Eur. Rev. Med. Pharm. Sci. 2017, 21, 1136–1143. [Google Scholar]
- Cianciulli, A.; Calvello, R.; Cavallo, P.; Dragone, T.; Carofiglio, V.; Panaro, M.A. Modulation of NF-κB activation by resveratrol in LPS treated human intestinal cells results in downregulation of PGE2 production and COX-2 expression. Toxicol. Vitr. 2012, 26, 1122–1128. [Google Scholar] [CrossRef] [PubMed]
- Tsai, M.-H.; Hsu, L.-F.; Lee, C.-W.; Chiang, Y.-C.; Lee, M.-H.; How, J.-M.; Wu, C.-M.; Huang, C.-L.; Lee, I.-T. Resveratrol inhibits urban particulate matter-induced COX-2/PGE2 release in human fibroblast-like synoviocytes via the inhibition of activation of NADPH oxidase/ROS/NF-κB. Int. J. Biochem. Cell Biol. 2017, 88, 113–123. [Google Scholar] [CrossRef]
- Lee, C.-W.; Lin, Z.-C.; Hsu, L.-F.; Fang, J.-Y.; Chiang, Y.-C.; Tsai, M.-H.; Lee, M.-H.; Li, S.-Y.; Hu, S.C.-S.; Lee, I.-T. Eupafolin ameliorates COX-2 expression and PGE2 production in particulate pollutants-exposed human keratinocytes through ROS/MAPKs pathways. J. Ethnopharmacol. 2016, 189, 300–309. [Google Scholar] [CrossRef]
- Boo, Y.C. Can plant phenolic compounds protect the skin from airborne particulate matter? Antioxidants 2019, 8, 379. [Google Scholar] [CrossRef]
- Juliano, C.; Magrini, G.A. Cosmetic functional ingredients from botanical sources for anti-pollution skincare products. Cosmetics 2018, 5, 19. [Google Scholar] [CrossRef]
- Michalak, M. Plant-derived antioxidants: Significance in skin health and the ageing process. Int. J. Mol. Sci. 2022, 23, 585. [Google Scholar] [CrossRef] [PubMed]
- Shakya, A.K. Medicinal plants: Future source of new drugs. Int. J. Herb. Med. 2016, 4, 59–64. [Google Scholar]
- Süntar, I. Importance of ethnopharmacological studies in drug discovery: Role of medicinal plants. Phytochem. Rev. 2020, 19, 1199–1209. [Google Scholar] [CrossRef]
- Zhao, T.; Li, S.-J.; Zhang, Z.-X.; Zhang, M.-L.; Shi, Q.-W.; Gu, Y.-C.; Dong, M.; Kiyota, H. Chemical constituents from the genus Saussurea and their biological activities. Heterocycl. Commun. 2017, 23, 331–358. [Google Scholar] [CrossRef]
- Mishra, A.P.; Saklani, S.; Sharifi-Rad, M.; Iriti, M.; Salehi, B.; Maurya, V.K.; Rauf, A.; Milella, L.; Rajabi, S.; Baghalpour, N. Antibacterial potential of Saussurea obvallata petroleum ether extract: A spiritually revered medicinal plant. Cell. Mol. Biol. 2018, 64, 65–70. [Google Scholar] [CrossRef]
- Fan, C.-Q.; Yue, J.-M. Biologically active phenols from Saussurea medusa. Bioorganic Med. Chem. 2003, 11, 703–708. [Google Scholar] [CrossRef]
- Lee, W.T.; Im, H.T. Saussurea grandicapitula W. Lee et HT Im (Compositae), a new species from the Taebaek mountains, Korea. Korean J. Plant Taxon. 2007, 37, 387–393. [Google Scholar] [CrossRef]
- Krishnan, S.S.C.; Subramanian, I.P.; Subramanian, S.P. Isolation, characterization of syringin, phenylpropanoid glycoside from Musa paradisiaca tepal extract and evaluation of its antidiabetic effect in streptozotocin-induced diabetic rats. Biomed. Prev. Nutr. 2014, 4, 105–111. [Google Scholar] [CrossRef]
- Lee, C.-H.; Huang, C.-W.; Chang, P.-C.; Shiau, J.-P.; Lin, I.-P.; Lin, M.-Y.; Lai, C.-C.; Chen, C.-Y. Reactive oxygen species mediate the chemopreventive effects of syringin in breast cancer cells. Phytomedicine 2019, 61, 152844. [Google Scholar] [CrossRef]
- Lall, N.; Kishore, N.; Binneman, B.; Twilley, D.; Van de Venter, M.; Plessis-Stoman, D.d.; Boukes, G.; Hussein, A. Cytotoxicity of syringin and 4-methoxycinnamyl alcohol isolated from Foeniculum vulgare on selected human cell lines. Nat. Prod. Res. 2015, 29, 1752–1756. [Google Scholar] [CrossRef] [PubMed]
- Tan, J.; Luo, J.; Meng, C.; Jiang, N.; Cao, J.; Zhao, J. Syringin exerts neuroprotective effects in a rat model of cerebral ischemia through the FOXO3a/NF-κB pathway. Int. Immunopharmacol. 2021, 90, 107268. [Google Scholar] [CrossRef] [PubMed]
- Misiuk, W.; Zalewska, M. Investigation of inclusion complex of trazodone hydrochloride with hydroxypropyl-β-cyclodextrin. Carbohydr. Polym. 2009, 77, 482–488. [Google Scholar] [CrossRef]
- Zhou, R.-r.; Liu, X.-H.; Chen, L.; Huang, J.-H.; Liang, X.-J.; Wan, D.; Zhang, S.-H.; Huang, L.-Q. Comparison of the antioxidant activities and phenolic content of five Lonicera flowers by HPLC-DAD/MS-DPPH and chemometrics. Int. J. Anal. Chem. 2020, 2020, 2348903. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.-H.; Yu, J.; Li, Q.-W.; Zhao, J.-P.; Wedge, D.E.; Duke, S.O.; Liao, D.-F.; Wang, Y.-H.; Fronczek, F.R.; Khan, I.A. 7α-Hydroxyfriedelan-3-one-26-ol-29-oic acid and other Constituents from Pileostegia viburnoides var. glabrescens. Nat. Prod. Commun. 2016, 11, 1934578X1601100716. [Google Scholar] [CrossRef]
- Lee, S.-S.; Baek, Y.-S.; Eun, C.-S.; Yu, M.-H.; Baek, N.-I.; Chung, D.-k.; Bang, M.-H.; Yang, S.-A. Tricin derivatives as anti-inflammatory and anti-allergic constituents from the aerial part of Zizania latifolia. Biosci. Biotechnol. Biochem. 2015, 79, 700–706. [Google Scholar] [CrossRef]
- Wang, B.; McHugh, B.J.; Qureshi, A.; Campopiano, D.J.; Clarke, D.J.; Fitzgerald, J.R.; Dorin, J.R.; Weller, R.; Davidson, D.J. IL-1β–induced protection of keratinocytes against Staphylococcus aureus-secreted proteases is mediated by human β-defensin 2. J. Investig. Dermatol. 2017, 137, 95–105. [Google Scholar] [CrossRef]
- Ha, J.W.; Song, H.; Hong, S.S.; Boo, Y.C. Marine alga ecklonia cava extract and dieckol attenuate prostaglandin E2 production in HaCaT keratinocytes exposed to airborne particulate matter. Antioxidants 2019, 8, 190. [Google Scholar] [CrossRef]
- Kim, J.-Y.; Choi, G.-E.; Yoo, H.J.; Kim, H.S. Interferon potentiates toll-like receptor-induced prostaglandin D2 production through positive feedback regulation between signal transducer and activators of transcription 1 and reactive oxygen species. Front. Immunol. 2017, 8, 1720. [Google Scholar] [CrossRef]
- Lin, W.; Li, Z. Blueberries inhibit cyclooxygenase-1 and cyclooxygenase-2 activity in human epithelial ovarian cancer. Oncol. Lett. 2017, 13, 4897–4904. [Google Scholar] [CrossRef]
- Xu, J.; Wu, W.; Zhang, H.; Yang, L. Berberine alleviates amyloid β25-35-induced inflammatory response in human neuroblastoma cells by inhibiting proinflammatory factors. Exp. Ther. Med. 2018, 16, 4865–4872. [Google Scholar] [CrossRef] [PubMed]
- Molloy, E.; Morgan, M.; Doherty, G.; McDonnell, B.; O’Byrne, J.; Fitzgerald, D.; McCarthy, G. Microsomal prostaglandin E2 synthase 1 expression in basic calcium phosphate crystal-stimulated fibroblasts: Role of prostaglandin E2 and the EP4 receptor. Osteoarthr. Cartil. 2009, 17, 686–692. [Google Scholar] [CrossRef] [PubMed]
- Zayed, N.; Li, X.; Chabane, N.; Benderdour, M.; Martel-Pelletier, J.; Pelletier, J.-P.; Duval, N.; Fahmi, H. Increased expression of lipocalin-type prostaglandin D 2 synthase in osteoarthritic cartilage. Arthritis Res. Ther. 2008, 10, 1–12. [Google Scholar] [CrossRef]
- Lee, J.-w.; Seok, J.K.; Boo, Y.C. Ecklonia cava extract and dieckol attenuate cellular lipid peroxidation in keratinocytes exposed to PM10. Evid.-Based Complement. Altern. Med. 2018, 2018, 8248323. [Google Scholar] [CrossRef]
- Kakkar, S.; Bais, S. A review on protocatechuic acid and its pharmacological potential. Int. Sch. Res. Not. 2014, 2014, 952943. [Google Scholar] [CrossRef]
- Yang, C.-Y.; Pan, C.-C.; Tseng, C.-H.; Yen, F.-L. Antioxidant, Anti-Inflammation and Antiaging Activities of Artocarpus altilis Methanolic Extract on Urban Particulate Matter-Induced HaCaT Keratinocytes Damage. Antioxidants 2022, 11, 2304. [Google Scholar] [CrossRef]
- Liu, R.H. Potential synergy of phytochemicals in cancer prevention: Mechanism of action. J. Nutr. 2004, 134, 3479S–3485S. [Google Scholar] [CrossRef] [PubMed]
- Winter, A.N.; Brenner, M.C.; Punessen, N.; Snodgrass, M.; Byars, C.; Arora, Y.; Linseman, D.A. Comparison of the neuroprotective and anti-inflammatory effects of the anthocyanin metabolites, protocatechuic acid and 4-hydroxybenzoic acid. Oxidative Med. Cell. Longev. 2017, 2017, 6297080. [Google Scholar] [CrossRef]
- Flower, D.R. The lipocalin protein family: Structure and function. Biochem. J. 1996, 318, 1–14. [Google Scholar] [CrossRef]
- Yun, G.; Yufeng, H.; Xinyi, X.; Baikun, M. Secretory Exprossion of Human Testis Prostaglandin D Synthase in Pichia pastoris. Zhongguo Sheng Wu Hua Xue Yu Fen Zi Sheng Wu Xue Bao = Chin. J. Biochem. Mol. Biol. 2003, 19, 757–762. [Google Scholar]
- Moyo, R.; Chimponda, T.; Mukanganyama, S. Inhibition of hematopoietic prostaglandin D2 Synthase (H-PGDS) by an alkaloid extract from Combretum molle. BMC Complement. Altern. Med. 2014, 14, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, K.; Ogawa, K.; Sugamura, K.; Nakamura, M.; Takano, S.; Nagata, K. Cutting edge: Differential production of prostaglandin D2 by human helper T cell subsets. J. Immunol. 2000, 164, 2277–2280. [Google Scholar] [CrossRef] [PubMed]
- Boyce, J.A. Mast cells and eicosanoid mediators: A system of reciprocal paracrine and autocrine regulation. Immunol. Rev. 2007, 217, 168–185. [Google Scholar] [CrossRef] [PubMed]
- Kanaoka, Y.; Urade, Y. Hematopoietic prostaglandin D synthase. Prostaglandins Leukot. Essent. Fat. Acids 2003, 69, 163–167. [Google Scholar] [CrossRef] [PubMed]
- Pettipher, R. The roles of the prostaglandin D2 receptors DP1 and CRTH2 in promoting allergic responses. Br. J. Pharmacol. 2008, 153, S191–S199. [Google Scholar] [CrossRef] [PubMed]
- Satoh, T.; Moroi, R.; Aritake, K.; Urade, Y.; Kanai, Y.; Sumi, K.; Yokozeki, H.; Hirai, H.; Nagata, K.; Hara, T. Prostaglandin D2 plays an essential role in chronic allergic inflammation of the skin via CRTH2 receptor. J. Immunol. 2006, 177, 2621–2629. [Google Scholar] [CrossRef]
- Ueno, N.; Murakami, M.; Tanioka, T.; Fujimori, K.; Tanabe, T.; Urade, Y.; Kudo, I. Coupling between cyclooxygenase, terminal prostanoid synthase, and phospholipase A2. J. Biol. Chem. 2001, 276, 34918–34927. [Google Scholar] [CrossRef]
- Zidar, N.; Odar, K.; Glavac, D.; Jerse, M.; Zupanc, T.; Stajer, D. Cyclooxygenase in normal human tissues–is COX-1 really a constitutive isoform, and COX-2 an inducible isoform? J. Cell. Mol. Med. 2009, 13, 3753–3763. [Google Scholar] [CrossRef]
- Mattila, S.; Tuominen, H.; Koivukangas, J.; Stenbäck, F. The terminal prostaglandin synthases mPGES-1, mPGES-2, and cPGES are all overexpressed in human gliomas. Neuropathology 2009, 29, 156–165. [Google Scholar] [CrossRef]
- Camacho, M.; Gerboles, E.; Escudero, J.R.; Anton, R.; Garcia-Moll, X.; Vila, L. Microsomal prostaglandin E synthase-1, which is not coupled to a particular cyclooxygenase isoenzyme, is essential for prostaglandin E2 biosynthesis in vascular smooth muscle cells. J. Thromb. Haemost. 2007, 5, 1411–1419. [Google Scholar] [CrossRef]
- Choi, S.-M.; Lee, P.-H.; An, M.-H.; Yun-Gi, L.; Park, S.; Baek, A.R.; Jang, A.-S. N-acetylcysteine decreases lung inflammation and fibrosis by modulating ROS and Nrf2 in mice model exposed to particulate matter. Immunopharmacol. Immunotoxicol. 2022, 44, 832–837. [Google Scholar] [CrossRef] [PubMed]
- Jin, L.; Ni, J.; Tao, Y.; Weng, X.; Zhu, Y.; Yan, J.; Hu, B. N-acetylcysteine attenuates PM2. 5-induced apoptosis by ROS-mediated Nrf2 pathway in human embryonic stem cells. Sci. Total Environ. 2019, 666, 713–720. [Google Scholar] [CrossRef] [PubMed]
- Choi, M.-K.; Kim, J.; Park, H.-M.; Lim, C.-M.; Pham, T.-H.; Shin, H.Y.; Kim, S.-E.; Oh, D.-K.; Yoon, D.-Y. The DPA-derivative 11S, 17S-dihydroxy 7, 9, 13, 15, 19 (Z, E, Z, E, Z)-docosapentaenoic acid inhibits IL-6 production by inhibiting ROS production and ERK/NF-κB pathway in keratinocytes HaCaT stimulated with a fine dust PM10. Ecotoxicol. Environ. Saf. 2022, 232, 113252. [Google Scholar] [CrossRef]
- Ain, N.U.; Qamar, S.U.R. Particulate matter-induced cardiovascular dysfunction: A mechanistic insight. Cardiovasc. Toxicol. 2021, 21, 505–516. [Google Scholar] [CrossRef]
- Klein, T.; Shephard, P.; Kleinert, H.; Kömhoff, M. Regulation of cyclooxygenase-2 expression by cyclic AMP. Biochim. Biophys. Acta (BBA)-Mol. Cell Res. 2007, 1773, 1605–1618. [Google Scholar] [CrossRef] [PubMed]
- Teng, W.-L.; Huang, P.-H.; Wang, H.-C.; Tseng, C.-H.; Yen, F.-L. Pterostilbene attenuates particulate matter-induced oxidative stress, inflammation and aging in keratinocytes. Antioxidants 2021, 10, 1552. [Google Scholar] [CrossRef] [PubMed]

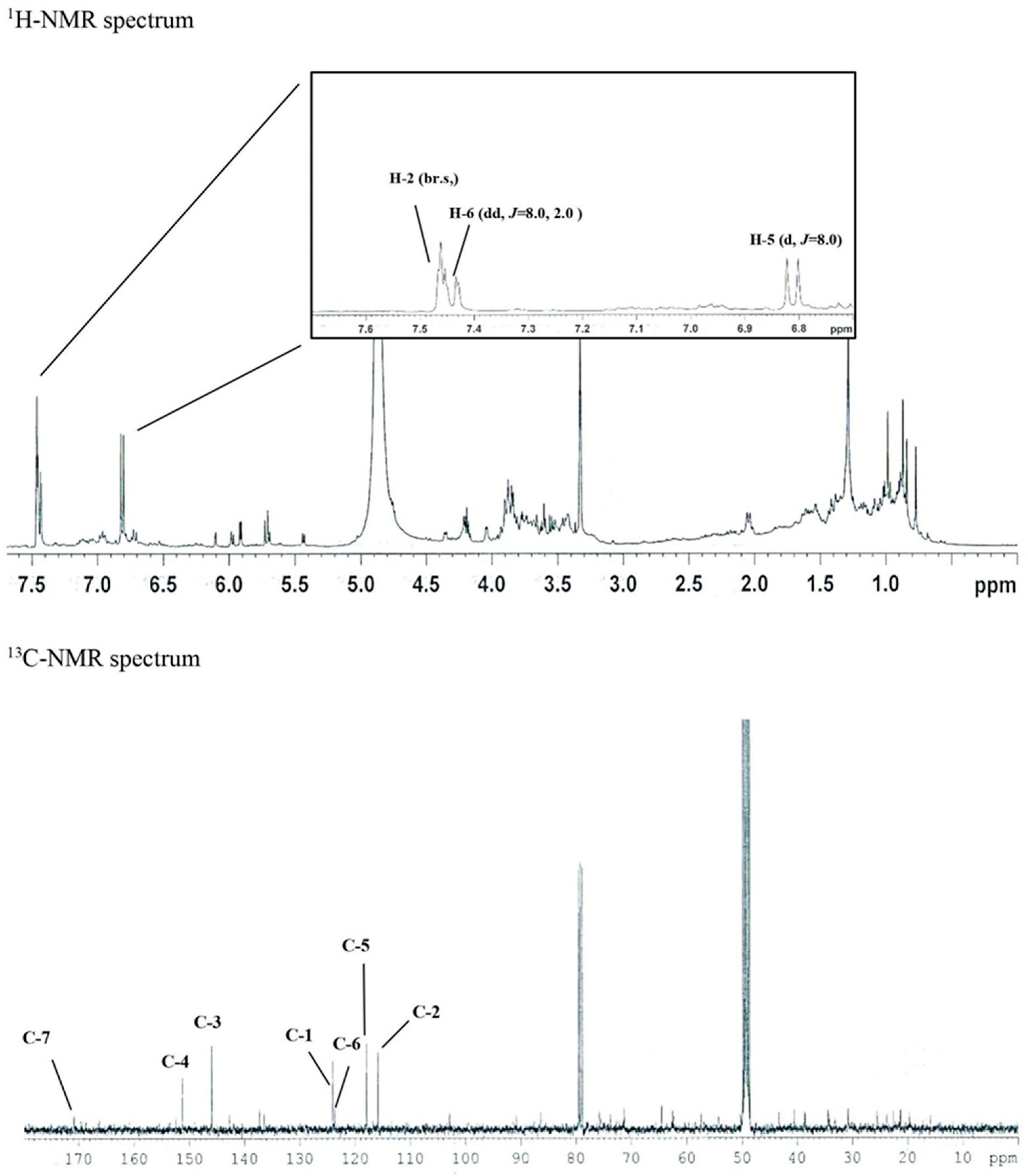
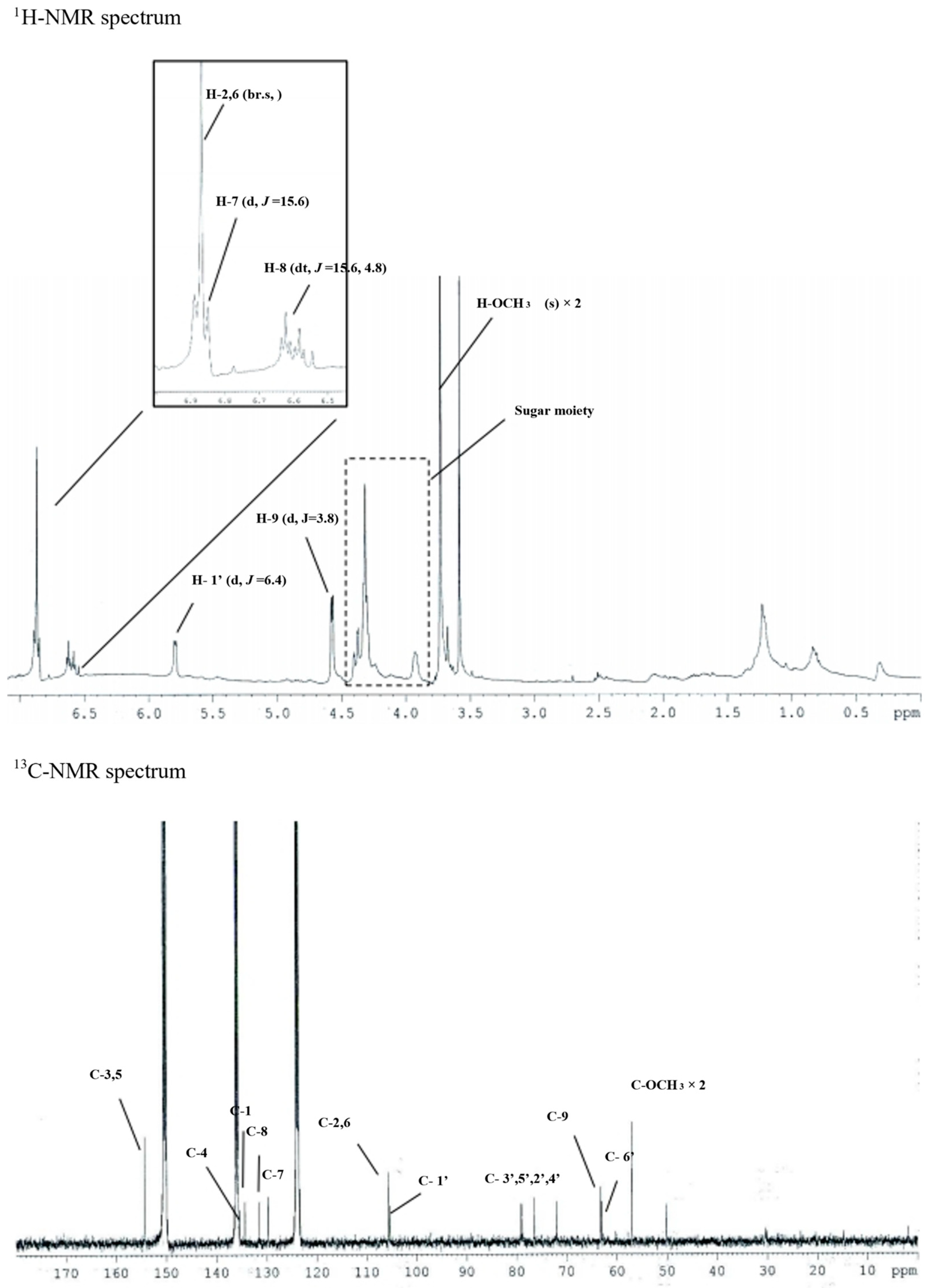


| Gene Name | GenBank Accession Number | Primer Sequences | Ref. |
|---|---|---|---|
| Cyclooxygenase 1 (COX-1)/Prostaglandin-endoperoxide synthase 1 (PTGS1) | NM_000962.4 | Forward: 5′-CAGAGCCAGATGGCTGTGGG-3′ Reverse: 5′-AAGCTGCTCATCGCCCCAGG-3′ | [50] |
| Cyclooxygenase 2 (COX-2)/Prostaglandin-endoperoxide synthase 2 (PTGS2) | NM_000963.3 | Forward: 5′-CTGCGCCTTTTCAAGGATGG-3′ Reverse: 5′-CCCCACAGCAAACCGTAGAT-3′ | [51] |
| Microsomal prostaglandin E synthase 1 (mPGES-1)/Prostaglandin E synthase (PTGES) | NM_004878.5 | Forward: 5′-AACCCTTTTGTCGCCTG-3′ Reverse: 5′-GTAGGCCACGGTGTGT-3′ | [52] |
| Microsomal prostaglandin E synthase 2 (mPGES-2); Prostaglandin E synthase 2 (PTGES2) | NM_025072.7 | Forward: 5′-GAAAGCTCGCAACAACTAAAT-3′ Reverse: 5′-CTTCATGGCTGGGTAGTAG-3′ | [52] |
| Cytosolic prostaglandin E synthase (cPGES)/ Prostaglandin E synthase 3 (PTGES3) | NM_006601.6 | Forward: 5′-ATAAAAGAACGGACAGATCAA-3′ Reverse: 5′-CACTAAGCCAATTAAGCTTTG-3′ | [52] |
| L-PGDS (PTGDS) | NM000954 | Forward: 5′-AACCAGTGTGAGACCCGAAC-3′ Reverse: 5′-AGGCGGTGAATTTCTCCTTT-3′ | [53] |
| H-PGDS (HPGDS) | NM014485 | Forward: 5′-CCCCATTTTGGAAGTTGATG-3′ Reverse: 5′-TGAGGCGCATTATACGTGAG-3 | [53] |
| GAPDH (glyceraldehyde 3-phosphate dehydrogenase) | NM_002046.3 | Forward: 5′-ATGGGGAAGGTGAAGGTCG-3′ Reverse: 5′-GGGGTCATTGATGGCAACAA-3′ | [54] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jeong, M.; Ju, Y.; Kwon, H.; Kim, Y.; Hyun, K.-Y.; Choi, G.-E. Protocatechuic Acid and Syringin from Saussurea neoserrata Nakai Attenuate Prostaglandin Production in Human Keratinocytes Exposed to Airborne Particulate Matter. Curr. Issues Mol. Biol. 2023, 45, 5950-5966. https://doi.org/10.3390/cimb45070376
Jeong M, Ju Y, Kwon H, Kim Y, Hyun K-Y, Choi G-E. Protocatechuic Acid and Syringin from Saussurea neoserrata Nakai Attenuate Prostaglandin Production in Human Keratinocytes Exposed to Airborne Particulate Matter. Current Issues in Molecular Biology. 2023; 45(7):5950-5966. https://doi.org/10.3390/cimb45070376
Chicago/Turabian StyleJeong, Myeongguk, Yeongdon Ju, Hyeokjin Kwon, Yeeun Kim, Kyung-Yae Hyun, and Go-Eun Choi. 2023. "Protocatechuic Acid and Syringin from Saussurea neoserrata Nakai Attenuate Prostaglandin Production in Human Keratinocytes Exposed to Airborne Particulate Matter" Current Issues in Molecular Biology 45, no. 7: 5950-5966. https://doi.org/10.3390/cimb45070376
APA StyleJeong, M., Ju, Y., Kwon, H., Kim, Y., Hyun, K.-Y., & Choi, G.-E. (2023). Protocatechuic Acid and Syringin from Saussurea neoserrata Nakai Attenuate Prostaglandin Production in Human Keratinocytes Exposed to Airborne Particulate Matter. Current Issues in Molecular Biology, 45(7), 5950-5966. https://doi.org/10.3390/cimb45070376






