Abstract
Genomic alterations of CDKN2A and CDKN2B in astrocytomas have been an evolving area of study for decades. Most recently, there has been considerable interest in the effect of CDKN2A and/or CDKN2B (CDKN2A/B) homozygous deletions (HD) on the prognosis of isocitrate dehydrogenase (IDH)-mutant astrocytomas. This is highlighted by the adoption of CDKN2A/B HD as an essential criterion for astrocytoma and IDH-mutant central nervous system (CNS) WHO grade 4 in the fifth edition of the World Health Organisation (WHO) Classification of Central Nervous System Tumours (2021). The CDKN2A and CDKN2B genes are located on the short arm of chromosome 9. CDKN2A encodes for two proteins, p14 and p16, and CDKN2B encodes for p15. These proteins regulate cell growth and angiogenesis. Interpreting the impact of CDKN2A/B alterations on astrocytoma prognosis is complicated by recent changes in tumour classification and a lack of uniform standards for testing CDKN2A/B. While the prognostic impact of CDKN2A/B HD is established, the role of different CDKN2A/B alterations—heterozygous deletions (HeD), point mutations, and promoter methylation—is less clear. Consequently, how these alternations should be incorporated into patient management remains controversial. To this end, we reviewed the literature on different CDKN2A/B alterations in IDH-mutant astrocytomas and their impact on diagnosis and management. We also provided a historical review of the changing impact of CDKN2A/B alterations as glioma classification has evolved over time. Through this historical context, we demonstrate that CDKN2A/B HD is an important negative prognostic marker in IDH-mutant astrocytomas; however, the historical data is challenging to interpret given changes in tumour classification over time, variation in the quality of evidence, and variations in the techniques used to identify CDKN2A/B deletions. Therefore, future prospective studies using uniform classification and detection techniques are required to improve the clinical interpretation of this molecular marker.
1. Introduction
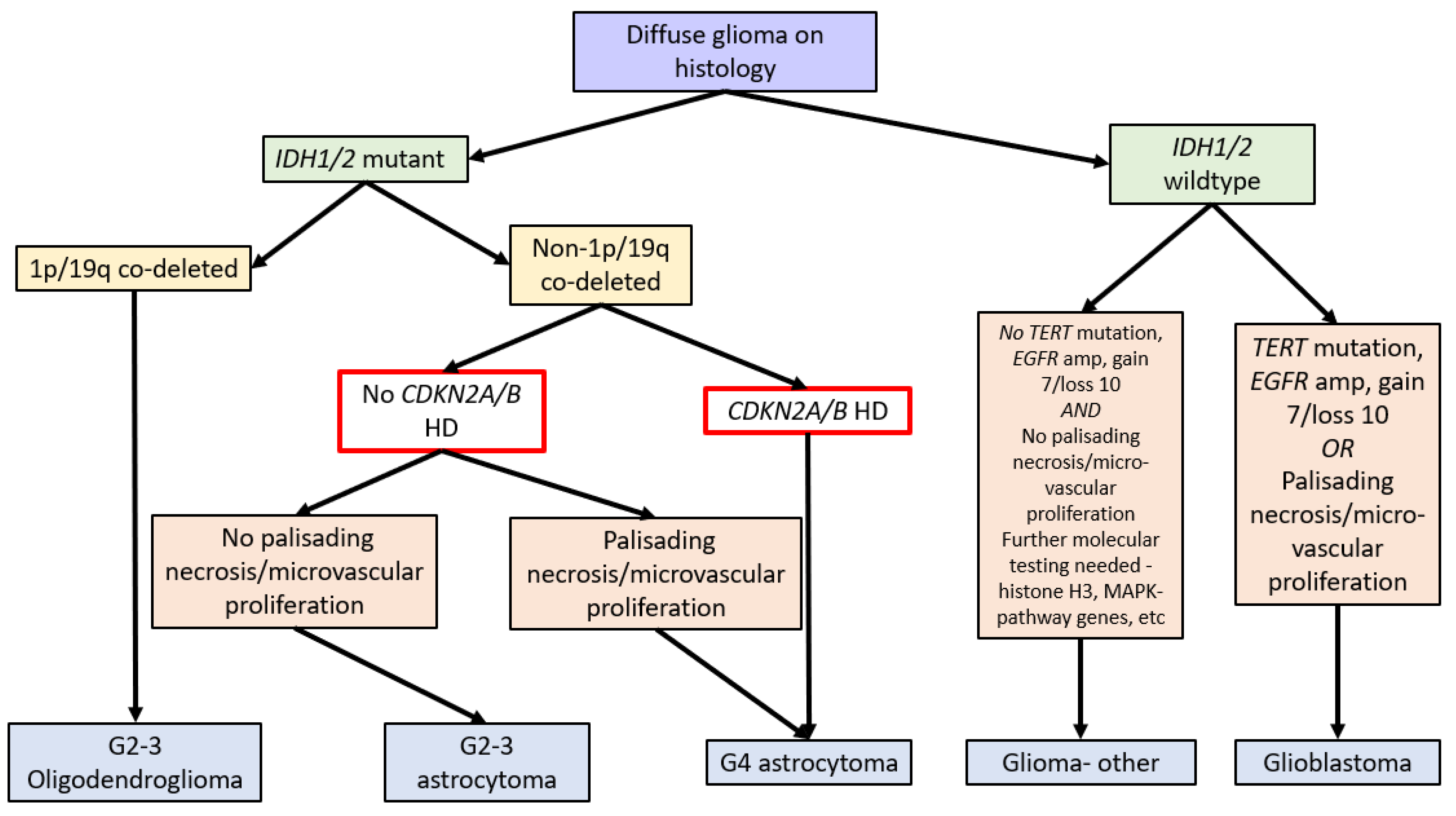
In 2016, the WHO Classification of Tumours of the Central Nervous System (revised 4th edition) incorporated isocitrate dehydrogenase 1 and 2 (IDH1/2) mutation status into the classification of diffuse gliomas [1]. IDH1/2 mutations (point mutations in either IDH1 codon R132 or IDH2 codon R172) lead to IDH dysfunction, which converts alpha-ketoglutarate to R-2-hydroxygluterate, driving oncogenesis and global epigenetic changes [1,2,3,4]. The WHO Classification in 2016 further stratified diffuse IDH-mutant gliomas into oligodendrogliomas and astrocytomas based on the respective presence or absence of chromosome 1p and 19q co-deletions (see Figure 1) [1].
IDH-mutant astrocytomas account for 80% of WHO grades 2 to 3 and 5% of high-grade astrocytomas [5,6]. When compared to IDH-wildtype glioblastomas, patients with IDH-mutant astrocytomas are younger at diagnosis (30–40 years vs. over 50 years). In addition, IDH-mutant astrocytomas have a more favourable prognosis compared to IDH-wildtype glioblastomas, even in high-grade cases, with grade 4 IDH-mutant astrocytomas having a median overall survival (OS) of 31 months, compared to IDH-wildtype glioblastomas with a median OS of 13 months [5,6]. Unfortunately, a proportion of IDH-mutant astrocytomas have poor outcomes similar to those of IDH-wildtype glioblastomas [7].
CDKN2A/B HD are identified in approximately 22% of IDH-mutant astrocytomas [8] and are thought to lead to the loss of cell cycle control and promote cell proliferation [9]. Several retrospective studies have shown CDKN2A/B HD is associated with decreased survival among IDH-mutant astrocytomas [10,11,12,13]. A timeline of discovery and key developments in the understanding of CDKN2A/B deletions is presented in Table 1.
Due to the improved prognosis of tumours previously classified as IDH-mutant glioblastomas compared to IDH-wildtype glioblastomas, these have been reclassified as astrocytoma, IDH-mutant, CNS WHO grade 4 in the fifth edition of the WHO Classification of Tumours of the Central Nervous System (WHO CNS5, 2021). A hallmark of WHO CNS5 is the integration of molecular markers into tumour grading. As such, IDH-mutant astrocytomas with CDKN2A/B HD are classified as grade 4 tumours independent of morphologic features. Therefore, a diagnosis of astrocytoma, IDH mutant, or CNS WHO grade 4 requires either morphologic features of a glioblastoma, namely necrosis or microvascular proliferation, or homozygous deletion of CDKN2A and/or CDKN2B (see Figure 1) [1,14].
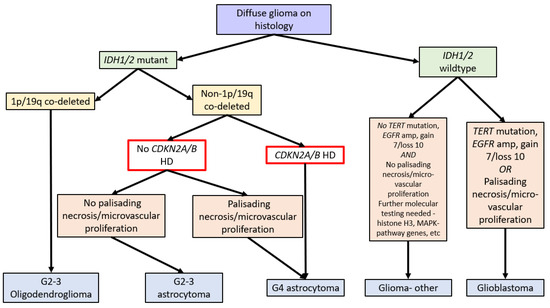
Figure 1.
Flow diagram summarising the WHO Classification of CNS Tumours (fifth edition, 2021) [14] and the role of CDKN2A/B HD (red box). Note: gain 7/loss 10 refers to the gain of chromosome 7 and the loss of chromosome 10.
Figure 1.
Flow diagram summarising the WHO Classification of CNS Tumours (fifth edition, 2021) [14] and the role of CDKN2A/B HD (red box). Note: gain 7/loss 10 refers to the gain of chromosome 7 and the loss of chromosome 10.
The significance of CDKN2A/B alterations in gliomas is difficult to assess in historical cohorts. Prior to 2016, many studies classified tumours based only on morphology. Consequently, tumours previously classified as astrocytomas on morphological grounds are likely to include tumours that are currently classified as astrocytoma, IDH-mutant, oligodendroglioma IDH-mutant, 1p/19q codeleted or glioblastoma, and IDH-wildtype [15,16,17]. Interpretation of the CDKN2A/B literature is further complicated by the multiple different techniques used to interrogate the CDKN2A and CDKN2B genes (see Table 1) and whether one or both genes are interrogated. In addition, there is ambiguity concerning the significance of isolated CDKN2A or CDKN2B loss compared to loss of both CDKN2A and CDKN2B [11,13,18,19,20].
Currently, there are no consensus treatment protocols for IDH-mutant CDKN2A/B HD astrocytomas. A significant proportion (6–20%) of grade 4 IDH-mutant astrocytomas with CDKN2A/B HD were previously classified as either grade 2 or grade 3 tumours [7,11,12,13]. While some centres have started to manage IDH-mutant CDKN2A/B HD astrocytomas using the EORTC-NCIC protocol for IDH-wildtype glioblastomas, historically most of these cases would have been treated as lower-grade (Grade 2 and 3) gliomas with radiation therapy and sequential chemotherapy.
To guide understanding and management of this newly defined group, we performed a literature review on CDKN2A/B HD in astrocytomas (see Table 1 and Table 2). We present our findings in the following categories: the normal role of CDKN2A/B and effect of their deletion in translational studies; identification of CDKN2A/B deletions; CDKN2A/B deletions in clinical studies; management of tumours with CDKN2A/B homozygous deletions; and putting CDKN2A/B deletions in perspective.

Table 2.
Summary of clinical studies addressing the prognostic impact of CDKN2A/B HD. The classification used is described as pre-molecular classification (pre-WHO 2016) or post-molecular classification (post-WHO 2016). Bias resulting from poor translatability of glioma classification to current classification is ranked as “Low”, “Medium”, or “High”. The bias ranking was performed qualitatively by author consensus.

Table 1.
Timeline of the evidence landscape for CDKN2A/deletions in gliomas, highlighting key findings from the respective studies.
Table 1.
Timeline of the evidence landscape for CDKN2A/deletions in gliomas, highlighting key findings from the respective studies.
| Study | Year | Importance/Findings |
|---|---|---|
| Rey et al. [21] | 1987 | Noted the glioma cell lines had an increased loss of the short arm of chromosome 9. |
| James et al. [22] | 1991 | Found that in gliomas, the loss of 9p most commonly involved the p21 region. |
| Serrano et al. [23] | 1993 | Serrano et al. then identified the gene as CDKN2A, which encoded the p16 protein (identified in 1993) and was located in 9p21. |
| Jen et al. [24] | 1994 | Found that CDKN2A deletions closely identified with CDKN2B |
| Zhang et al. [25] | 1996 | Mapped out the frequency of deletions occurring with CDKN2A deletions, including MTAP, IFNA1, and IFNB1. |
| Sonoda et al. [26] | 1995 | Demonstrated increased frequency of CDKN2A/B in the clinical setting. |
| Dehais et al. [15] | 2006 | One of the earliest reports of negative survival associated with CDKN2A homozygous deletion. |
| Idbaih et al. [27] | 2008 | Comparison of low-grade gliomas with progression to higher-grade counterparts, with loss of chromosome 9 and the CDKN2A locus found to be significantly associated with tumour progression. |
| Reis et al. [28] | 2015 | First to identify the prognostic role of CDKN2A in the setting of IDH1/2 mutations (though they found a weak association with poor OS). |
| Louis et al. (WHO 2016 CNS Tumour Classification) [1] | 2016 | IDH1/2 mutation status adopted into the WHO CNS Tumour Classification 2016. |
| Ceccarelli et al. [29] | 2016 | G-CIMP-low IDH-mutant astrocytomas are associated with abnormalities in CDKN2A. |
| Roy et al. [30] | 2016 | Investigated the impact of the loss of the 9p region in gliomas. Showed CDKN2A HD was not predictive in IDH-mutant 1p/19q non-codeleted astrocytomas but was for IDH-wildtype gliomas. They did demonstrate. Heterozygous loss was associated with poor OS, but mRNA expression was not altered. |
| Cimino et al. [19] | 2017 | Proposed a combined molecular model that included CDKN2A homozygous deletions to prognosticate in IDH-mutant astrocytomas. |
| Aoki et al. [31] | 2018 | Assessed institutional cases with validation against the TCGA database. CDKN2A HD alone did not correlate with survival; however, Rb pathway alterations as a group (including CDKN2A) were associated with poor OS. |
| Shirahata et al. [11] | 2018 | The first study to propose a grade 4 diagnosis for IDH-mutant astrocytoma in the presence of CDKN2A/B. |
| Appay et al. [12] | 2019 | Assessed the prognostic utility of CDKN2A HD, CDK4 amplification, and RB1 HD in IDH-mutant astrocytomas. Only CDKN2A HD predicted poor prognosis in univariate and multivariate analyses. It was also suggested that CDKN2A HD could define grade 4 astrocytomas. |
| Yoda et al. [13] | 2019 | Demonstrated CDKN2A as a strong predictor for OS in IDH-mutant astrocytomas. |
| Korshunov et al. [18] | 2019 | In a study of IDH-mutant ‘glioblastomas’ (now known as IDH-mutant grade 4 astrocytomas), CDKN2A/B HD was found to be a poor prognostic factor. |
| Yang et al. [10] | 2020 | Assessed PDGFRA and CDK4 amplification, CDKN2A deletion, TERT promoter mutation, ATRX loss, and p53 expression in IDH-mutant astrocytomas. Multivariate analysis showed correlation with all three markers, and a risk stratification model was suggested using these three alterations. |
| Brat et al. [7] | 2020 | Presented the findings of the working group for grading criteria and terminologies in IDH-mutant astrocytomas in the fifth update for cIMPACT-NOW. Suggested that if CDKN2A/B homozygous deletion, necrosis, or microvascular proliferation were present, a grade 4 designation was appropriate. The term IDH-mutant glioblastoma would no longer exist. |
| Lu et al. [8] | 2020 | Meta-analysis of nine studies (80% low-grade and 20% grade 4) concerning the impact of CDKN2A HD in astrocytomas. They found CDKN2A HD was predictive for OS. |
| Satomi et al. [32] | 2021 | Reported a negative survival impact in CKDN2A HD IDH-mutant grade 3 astrocytomas but not in IDH-mutant grade 4 astrocytomas (based on morphologic criteria). |
| Louis et al. (WHO 2021 CNS Tumour Classification) [14] | 2021 | CDKN2A/B HD was adopted as a criterion for grade 4 IDH-mutant astrocytomas in the WHO CNS Tumour Classification 2021 (in addition to morphologic features). |
| Tesileanu et al. [33] | 2021 | Demonstrated that G-CIMP’s low methylation profile and CDKN2A/B HD bared an extremely poor prognosis in IDH-mutant astrocytomas, similar to IDH-wildtype glioblastomas. |
2. Normal Role of CDKN2A/B and Effect of Their Deletion
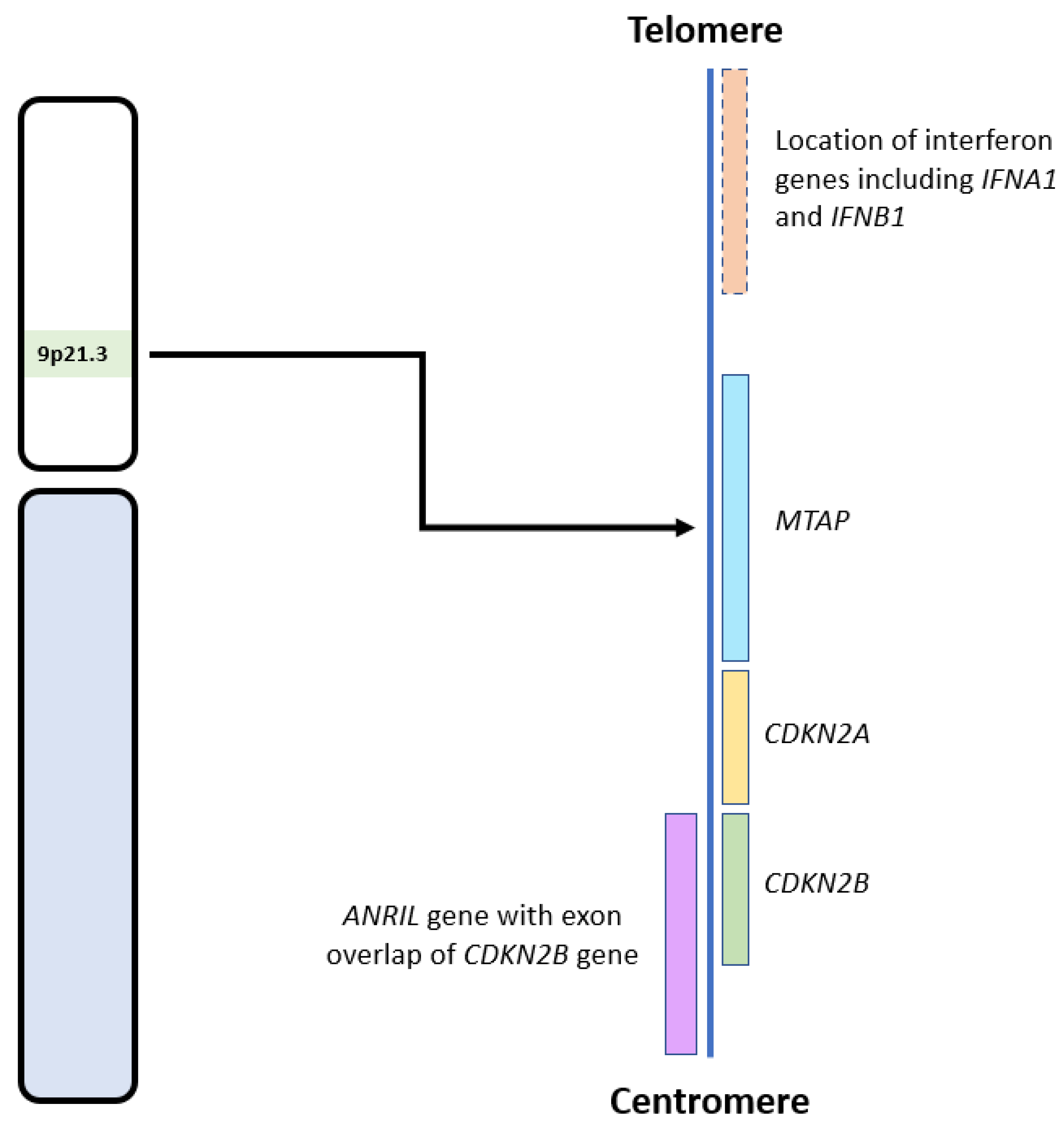
CDKN2A and CDKN2B are adjacent to each other on chromosome 9p21, with CDKN2B being 25 kilobases (kb) centromeric to CDKN2A (see Figure 2) [34]. These genes code for three proteins that suppress the oncogenic cyclin-dependent kinase (CDK) pathway. CDKN2A encodes for p14 and p16, and CDKN2B encodes for p15 [35]. Early cytogenetic studies identified recurrent loss of the short arm of chromosome 9 in glioma cell lines [21,22,23,36], many involving the 9p21 locus [22,37], which includes CDKN2A and CDKN2B [23,36].
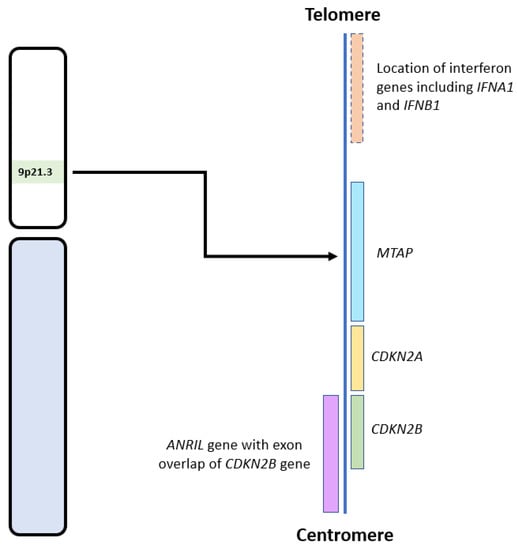
Figure 2.
Diagram showing the locations of CDKN2A/B and other relevant genes on the 9p21.3 locus. Adapted from the National Institute of Health, National Library of Medicine: Genome Data Viewer [38].
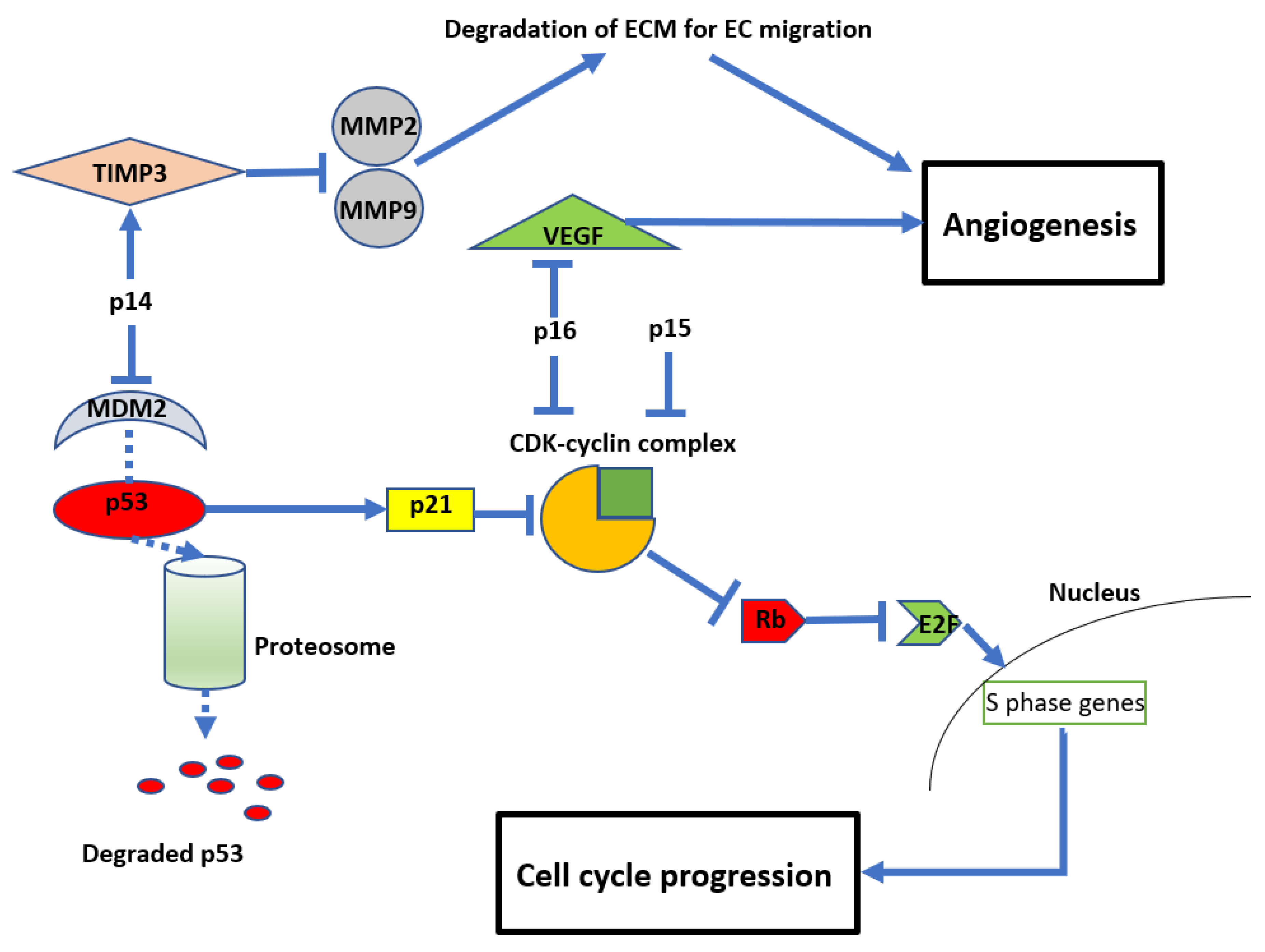
The resultant loss of p14, p15, and p16 proteins from CDKN2A/B HD leads to dysregulation of the cell cycle and other parallel oncogenic processes (see Figure 2). Reflecting this, inactivation of CDKN2A function has been reported in a variety of other malignancies, including breast cancer, lung cancer, head and neck cancer, melanoma, and bladder cancer [9]. In normal cells, the retinoblastoma (Rb) protein prevents cell growth by binding to the transcription factor E2F, preventing its translocation into the nucleus. A complex formed by cyclin D and CDK4/6 can phosphorylate Rb, thereby releasing E2F and allowing translocation into the nucleus, leading to cell growth. The products of CDKN2A/B, p15 and p16, can directly inhibit the formation of the CDK4/6-cyclin D complex, maintaining the association between E2F and Rb [39,40,41] and preventing cell cycle progression. Another product of CDKN2A is p14, which acts on cyclin-CDK complexes indirectly by inhibiting MDM2. MDM2 tags p53, targeting it for ubiquitination and subsequent proteasomal degradation. 14 prevents MDM2 tagging, resulting in p53 stabilisation. This in turn promotes the cellular accumulation of the inhibitory protein p21, which blocks several cyclin-CDK complexes and promotes cell cycle arrest [42,43]. Due to these important functions of the protein products of CDKN2A/B, their deletion enhances oncogenic potential and leads to unregulated cellular proliferation (see Figure 3) [42,43].
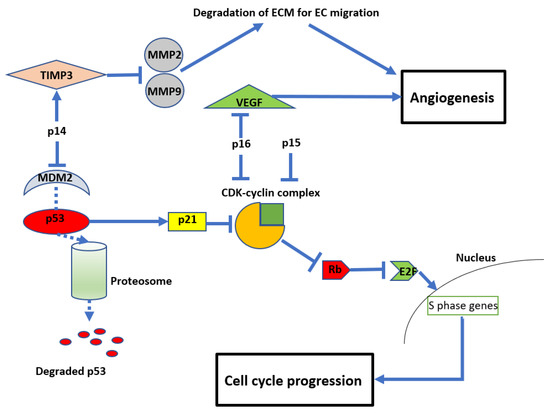
Figure 3.
Diagram showing the anti-proliferative and anti-angiogenic effects of CDKN2A/B Mouse double minute 2 homolog (MDM2), tissue inhibitor of metalloproteinase 3 (TIMP3), matrix metalloproteinases (MMP), retinoblastoma protein (Rb), vascular endothelial growth factor (VEGF), and cyclin-dependent kinases (CDK).
In addition to their role in regulating cell growth, CDKN2A/B also impact angiogenesis (see Figure 3). For example, p14 (unrelated to its inhibition of MDM2) can also inhibit endothelial cell migration required for angiogenesis by stimulating the expression of tissue inhibitor of metalloproteinase 3 (TIMP3), which inhibits matrix metalloproteinases (MMP) 2 and 9 [44]. MMPs are required to degrade the extracellular matrix to allow endothelial cell migration and subsequent vessel formation [45,46]. Similarly, p16 inhibits angiogenesis by regulating vascular endothelial growth factor (VEGF), a well-recognised and significant biomarker in glioma development and a current therapeutic target clinically [47,48,49].
The effects of CDKN2A/B deletion on tumour development may also be mediated, at least in part, by co-deletion of adjacent genes in the 9p21 region (see Figure 2), such as MTAP, IFNA1, IFNB1, and ANRIL [25,50]. MTAP encodes for the protein methylthioadenosine phosphorylase (MTAP) and is located approximately 100 kb telomeric to CDKN2A. MTAP is required for adenosine monophosphate and methionine salvage and has a tumour suppressive effect in multiple cancers [32,51]. IFNA1 and IFNB1, which encode for interferons, are also located telomeric to CDKN2A. Interferons are cytokines with anti-tumour effects due to their role in modulating the immune system [25,52]. Therefore, their loss also aids tumour survival and growth. ANRIL, an antisense long non-coding RNA (lncRNA), located centromeric to CDKN2A, contains CDKN2B within its first intron. ANRIL promotes pro-oncogenic gene expression and has been implicated in many malignancies, including gliomas [50]. Zhang and colleagues mapped the co-deletion of genes adjacent to CDKN2A in 14 cell lines, including gliomas. Only two had an isolated CDKN2A deletion, while the remaining ten had concurrent deletions of MTAP (12 cell lines), IFNA1 (8 cell lines), and IFNB1 (5 cell lines) (see Figure 2) [25]. Therefore, loss of the 9p21 region, inclusive of CDKN2A/B HD, can lead to multiple deleterious and oncogenic effects involving the loss of tumour suppressor genes and subsequent upregulation of multiple oncogenes and related pathways.
3. Identification of CDKN2A/B Deletions
A variety of methods can be used to evaluate CDKN2A/B HD. These include single-nucleotide polymorphism (SNP) microarrays [31,53], next-generation sequencing (NGS) [12,29], DNA-based methylation studies [11,13,18,19,20], and fluorescent in situ hybridisation (FISH) [10,28]. It should be noted that the accuracy of this variety of methods depends on the specific assay types used, as the genomic/cytogenetic resolution of each method differs. We are therefore unable to uniformly describe the technical parameters of each method, but have attempted to give an overview where possible.
Although SNP arrays, NGS, and methylation arrays possess greater resolution for individual gene-level detection, many studies combine CDKN2A and CDKN2B in the assessment of HD [11,18,29,31,53]. The accuracy of these methods is determined by the degree and depth of coverage of the genes of interest. NGS methods used in the literature to date include targeted gene panels [12] and whole exome sequencing (WES) [29], whereas methylation arrays include a combination of the HumanMethylation450 (450k) and MethylationEPIC (850k) arrays (Illumina, San Diego, CA, USA) [11,13,18,19,20]
Fluorescence in situ hybridization (FISH) can be used to detect deletions and has been validated against methods utilising polymerase chain reaction (PCR) [54]. Thresholds of detection for FISH need to be around 20% to 30% tumour cells with HD [10,55]. A commonly used FISH probe in clinical diagnostic practise, the Vysis CDKN2A/CEP 9 FISH Probe Kit (Abbott Laboratories, North Chicago, IL, USA), is large and spans CDKN2A, CDKN2B, and MTAP genes [56]. Therefore, smaller deletions not involving all three of these genes may be missed.
Immunohistochemistry (IHC) has been used to identify CDKN2A HD in gliomas with mixed results. Given the close proximity of the CDKN2A and MTAP genes (see Figure 2), loss of MTAP immunoreactivity has been suggested as a surrogate for CDKN2A HD [32] and has been demonstrated in mesothelioma [57,58]. In gliomas, Satomi et al. reported a sensitivity of 88% and a specificity of 98% for loss of MTAP immunoreactivity and CDKN2A deletion [32]. However, the authors could not demonstrate a correlation between the loss of MTAP immunoreactivity and OS in IDH-mutant astrocytomas [32].
However, Satomi et al. did show that loss of p16 immunoreactivity correlated with clinical outcome in IDH-mutant astrocytomas [32]. While this is supported by other studies that demonstrated p16-negative tumours on IHC had a high negative predictive value for CDKN2A HD in adult and paediatric morphologic glioblastomas [59], other studies reported p16/CDKN2A discordance with the IHC method [28]. Sensitivity and specificity for p16 immunoreactivity in detecting CDKN2A HD have been reported as 78–94% and 70–82%, respectively [32]. Furthermore, the full prognostic impact of CDKN2A/B deletions may be related to the additional loss of nearby genes, as noted above. Therefore, some tumours that are CDKN2A/B intact but have suppressed CDKN2A/B expression will have no immunoreactivity on p16 IHC but retain other genes that may confer a less aggressive phenotype. This means loss of CDKN2A/B in molecular studies may be more informative for prognosis than loss of p16 staining on IHC.
4. CDKN2A/B Deletions in Clinical Studies
The advent of molecular classification introduced by the WHO 2016 CNS classification creates a distinct change in categorising gliomas [1]. Therefore, previous studies involving CDKN2A/B deletions need to be interpreted in the context of the WHO classification used at that time (see Table 2). We reviewed CDKN2A/B clinical studies in three categories: initial clinical studies, clinical outcomes of CDKN2A/B deletion in the pre-molecular classification era, and clinical outcomes of CDKN2A/B deletion in the post-molecular classification era.
4.1. Initial Clinical Studies
Initial studies by Schmidt et al. [60] and Giani and Finocchiaro et al. [61] confirmed that CDKN2A HD was present in patients’ tumours and not just in glioma cell lines but did not assess CDKN2B. (see Table 1). Giani and Finocchiaro et al. demonstrated CDKN2A HD in over 30% of gliomas (not further defined) and CDKN2A HeD in 25% [61]. Moulton et al. analysed 27 glioblastomas (not further defined) and identified 9 with CDKN2A HD, 3 with a heterozygous deletion, and one with a point mutation [62].
4.2. Clinical Outcomes of CDKN2A/B Deletion in the Pre-Molecular Classification Era (Pre-2016 WHO CNS Tumour Classification)
Initial studies assessing the clinical impact of CDKN2A/B HD on prognosis in astrocytomas yielded conflicting results (see Table 2). This was likely due to tumour misclassification in the absence of routine assessment of IDH and 1p/19q status [63].
4.2.1. Correlation with High- and Low-Grade Gliomas
Initial studies described the relationship between CDKN2A/B and biologic markers of tumour aggressiveness (tumour grade and Ki-67 index). Sonoda et al. suggested CDKN2A/B deletions may have a role in gliomagenesis and therefore more aggressive tumour biology. Using single-strand conformation polymorphism (SSCP) and quantitative polymerase chain reaction (qPCR), they showed an increased incidence of CDKN2A/B HD in high-grade gliomas (44%, n = 12/27) compared to low-grade gliomas (10%, n = 1/10) [26]. Building on this concept, Ono et al. (1996) used multiplex PCR to assess CDKN2A/B HD in 50 astrocytomas and found a positive correlation between the Ki-67 index and CDKN2A HD (5/20 grade 3 astrocytomas and 6/13 glioblastomas had CDKN2A HD). CDKN2A HD was not identified in 17 grade 2 astrocytomas [64]. Using SSCP and qPCR, Barker et al. (1997) analysed 42 gliomas (16 glioblastomas [5 recurrent], 15 anaplastic astrocytomas [4 recurrent], and 11 astrocytomas, oligodendrogliomas, and mixed oligoastrocytomas [1 recurrent]) and found a higher incidence of CDKN2A HD in higher vs. lower grade tumours (80% vs. 20%, p = 0.001). They also showed a similar incidence of CDKN2A HD in the recurrent tumours (78%) [65]. These studies confirmed that CDKN2A HD was associated with higher-grade tumours.
4.2.2. Correlation with Survival
In 2006, Dehais et al. reported that CDKN2A HD was a negative prognostic factor in a heterogeneous group of gliomas that included anaplastic astrocytomas, oligoastrocytomas, and oligodendrogliomas. Although 1p/19q status was assessed, the authors did not identify which cases had CDKN2A HD and 1p/19q co-deletion [15]. However, other reports did not find an association between CKDN2A HD and clinical outcome [66,67]. This may reflect differences in methodology and/or patient selection for tumours classified by morphology alone. One of these studies (Rich et al.) used a DNA microarray to assess the prognostic impact of CDKN2A deletion in patients older than 50 years. Although IDH status was not reported in the study, this population was likely enriched for IDH-wildtype tumours, and it was later shown that CDKN2A deletions lack prognostic impact in these tumours [66]. The other study (Zolota et al.) used p16 IHC as a surrogate for CDKN2A loss [67]. However, as discussed above, p16 loss is less sensitive than direct methods for assessing CDKN2A deletion [15].
James et al. further supported CDKN2A HD as a negative prognostic factor by assessing 135 gliomas for PTEN and CDKN2A copy number status. They reported an increasing frequency of CDKN2A deletions with grade (0% in grade 2, 14.3% in grade 3, and 27.3 in grade 4 tumours). They observed that CDKN2A deletions were negative prognostic indicators of survival in all 135 gliomas, but this was not seen when stratifying for grade 3 or 4 separately [16]. This lack of stratification by grade is likely related to heterogeneity within the tumour grades designated at the time, in the pre-molecular classification era.
As interest in CDKN2A deletions increased, there was further focus on glioma subtypes, including oligodendrogliomas. Cairncross et al. explored the role of CDKN2A deletions in oligodendrogliomas using 1p/19q co-deletion instead of morphologic criteria alone. They analysed 39 morphologic oligodendrogliomas (2 of which were grade 2, the remainder grade 3). Losses involving both chromosomes 1p and 19q were strongly associated with longer overall survival, whereas CDKN2A deletions were independent poor prognostic factors. The authors were able to analyse 34 of the 39 samples and noted that 22 patients had 1p/19q co-deletion. It is therefore assumed that the remaining 12 cases would most likely be astrocytomas by current classification [14,17] The overall survival for CKDN2A-deleted gliomas was less than 2 years and occurred preferentially in gliomas without loss of 1p or 19q [17].
4.3. Clinical Outcomes in the Post-Molecular Classification Era (Post-2016 WHO CNS Tumour Classification)
4.3.1. Incorporation of CDKN2A/B Status into the fifth Edition of the WHO Classification (2021)
In 2020, the Consortium to Inform Molecular and Practical Approaches to CNS Tumour Taxonomy (cIMPACT-NOW), upgrade 5, published recommendations for grading criteria and terminologies in IDH-mutant astrocytomas. After reviewing the literature on multiple potential prognostic biomarkers, including CDKN2A/B HD, other Rb pathway genes, PIK3R1 and PIK3CA mutations, PDGFRA and MYCN amplification, reduced global DNA methylation, genomic instability (high copy number variants or somatic mutations), and mitotic activity and proliferation indices, they concluded that while “significant mitotic activity” should remain as a criterion for distinguishing grade 3 from grade 2 IDH-mutant astrocytomas, if CDKN2A/B HD, necrosis, or microvascular proliferation was present, a grade 4 designation was appropriate [7].
These recommendations were incorporated into the fifth edition of the WHO Classification of Tumours of the Central Nervous System 2021 [14]. While there is strong evidence to support the use of CDKN2A/B HD in grading IDH-mutant astrocytomas, several conflicting reports have been published.
4.3.2. Literature That Supports CDKN2A/B Stratification
In 2015, Reis et al. identified CDKN2A deletions as a prognostic marker specifically in IDH-mutant grade 2 and 3 gliomas. The authors analysed 270 gliomas and identified CDKN2A deletions via FISH in 57/108 grade 2 astrocytomas, 31/61 grade 3 astrocytomas, 23/96 oligodendrogliomas, and 19/49 oligoastrocytomas, inclusive of both homozygous and heterozygous CDKN2A deletions. The authors assessed tumours for 1p/19q deletion if they were not morphologic astrocytomas and assessed all tumours for IDH1/2 mutations by genome sequencing. They reported worse overall survival in grade 2 and 3 gliomas after adjusting for age, sex, and IDH mutation (HR 1.6, 95% CI = 1.0–2.4, p = 0.03). This significance was maintained in the astrocytoma subgroup (HR 2.0, 95% CI 1.1–3.5, p = 0.02) but not for oligodendrogliomas or oligoastrocytomas (HR 0.7, 95% CI 0.2–2.0, p = 0.5 and HR 0.8, 95% CI 0.3–2.4, p = 0.7, respectively). Again, a portion of these morphologic oligodendrogliomas in this cohort would no longer be classified as such without the corresponding molecularly confirmed 1p19q co-deletion. Interestingly, the presence of deletions in the IDH-mutant/ATRX expression loss astrocytoma group, without TP53 mutation, was non-prognostic (p = 0.2) [68]. Furthermore, as ATRX loss and TP53 mutations are strongly associated with IDH-mutant astrocytomas, it is unclear what this ATRX/TP53 discordance represents in IDH-mutant gliomas. Interestingly, given the FISH probe used covers a broad genomic region at 9p21, CDKN2B status can be said to be assessed by proxy. This study is therefore one of the few that demonstrates the prognostic role of CDKN2B [28].
In 2016, the WHO Classification of Tumours of the Central Nervous System officially recognised that the diagnosis of oligodendrogliomas now required 1p/19q co-deletion in addition to IDH-mutant status [1]. While this improved prognostic accuracy for oligodendrogliomas and gliomas now known as grade 4 IDH-mutant astrocytomas, clinical outcomes for grade 2 and 3 IDH-mutant diffuse astrocytomas remained heterogenous [53,63,68,69].
To resolve this, Cimino et al. used The Cancer Genome Atlas (TCGA) sequencing datasets for glioblastomas, astrocytomas, and oligodendrogliomas to identify prognostic molecular markers. They found that CDKN2A deletions, CDK4 amplification, and chromosome 14q loss were prognostic markers. Using these, they stratified IDH-mutant glioblastomas into 3 prognostically relevant molecular subgroups: M1 with chromosome 14q loss and either CDK4 amplification or CDKN2A deletion; M2 with either CDK4 amplification, CDKN2A deletion, or chromosome 14q loss; and M3 with no 14q loss, CDK amplification, or CDKN2A deletion. The median overall survivals for M1, 2, and 3 were 23.3, 63.0, and 94.5 months, respectively (p < 0.05) [19]. This was one of the first studies to suggest a combination of molecular factors for risk stratification and highlights that CDKN2A HD can interact with other genomic alterations [19].
These results were supported by Shirahata et al., who assessed prognostic features in 211 IDH-mutant astrocytoma samples. The findings were then validated using three independent cohorts of 108, 154, and 224 IDH-mutant astrocytomas. CDKN2A/B status was evaluated via the analysis of DNA-based methylation data. In the initial discovery cohort, they found 38 CDKN2A/B HD tumours. On univariate analysis, there was a significant negative correlation with OS and CDKN2A/B HD (p = 0.0001). They proposed three prognostic models for OS based on multivariate analysis of a discovery set and confirmation in three validation sets. In all models, tumours were classified as grade 4 if CDKN2A/B HD was present [11]. It is important to note that this study is the first to formally assess CDKN2B HD in addition to CDKN2A HD and is one of only three studies in the Post-Molecular Characterisation Era to include both CDKN2A and CDKN2B deletions (see Table 2).
To compare the utility of CDKN2A HD and the histology-based WHO grading criteria, Appay et al. analysed 428 IDH-mutant astrocytomas (1p/19q non-co-deletion) and 483 anaplastic oligodendrogliomas (1p/19q co-deleted) using a combination of SNP arrays, CGH arrays, and targeted gene panel NGS data. CDK4 amplification and RB1 homozygous deletion were assessed in a subset of tumours. CDKN2A HD was associated with a dismal outcome in IDH-mutant astrocytomas (p < 0.0001 for PFS and p = 0.004 for OS) in both univariate and multivariate analyses. They suggested that IDH-mutant astrocytomas with CDKN2A HD should be considered grade 4 tumours irrespective of the presence of microvascular proliferation or necrosis [12]. Interestingly, CDK4 and RB1 alterations did not correlate with clinical outcomes.
Further reports using methylation data and FISH later emerged, indicating CDKN2A could stratify IDH-mutant astrocytomas, either alone or in combination with other molecular alterations [13,68,69]. However, the most pertinent of these latter studies was analysed by a meta-analysis assessing the association between CDKN2A HD and survival in IDH-mutant glioma [8]. The meta-analysis comprised nine studies, including 1756 (80%) LGG and 437 (20%) glioblastomas. Oligodendrogliomas and astrocytomas were defined by 1p/19q co-deletion status. Multivariate analysis identified CDKN2A HD as a predictor of significantly shorter PFS and OS in both LGG and glioblastoma in all included studies. Although this analysis included both astrocytomas and oligodendrogliomas, the authors noted that of the three studies reporting 1p/19q co-deletions, when the co-deletion was excluded, CDKN2A retained its prognostic value [8].
4.3.3. Literature That Counters CDKN2A/B Stratification
Not all studies supported the use of CDKN2A/B in IDH-mutant astrocytomas. Roy et al. analysed the 9p region lost in malignancies by analysing two cohorts (the first group being 10,985 samples from 33 different cancer types and the second group being 540 low-grade gliomas from three databases) and reported that CDKN2A inactivation did not promote tumour aggressiveness. Even when accounting for IDH and 1p/19q status (IDH-mutant 1p/19q non-deleted astrocytoma), there was no survival impact of CKDN2A HD. While they did show that heterozygous loss was associated with poor OS, mRNA expression was not altered. It was therefore postulated that this survival impact was due to the loss of other 9p genes [30]. It is unclear why this report differs from the majority of other studies, but it highlights that not all studies support the role of CDKN2A/B HD as a prognostic marker in IDH-mutant astrocytomas.
Aoki et al. also failed to demonstrate the prognostic value of CDKN2A/B HD. They described a Japanese cohort of 308 low-grade gliomas that were comprehensively profiled for glioma-relevant genes via WES and CDKN2A/B via SNP array. The results were validated using a dataset of 414 LGG cases available from TCGA. Although alterations in retinoblastoma pathway genes (including RB1, CDKN2A, and CDK4) correlated with poor OS in general, CDKN2A HD alone did not correlate with OS in IDH-mutant astrocytomas (p = 0.19 for univariate and multivariate analysis) or IDH-wildtype astrocytomas (p = 0.57 for univariate analysis and p = 0.88 adjusted) [31].
Other studies reported a more mixed effect of CDKN2A/B on IDH-mutant astrocytoma prognosis. Satomi et al., using a combination of FISH and MLPA (multiplex ligation-dependent probe amplification), found a negative correlation between CDKN2A HD and OS in IDH-mutant grade 3 astrocytomas (n = 4/35 patients had a CDKN2A HD) (p < 0.001) but not in IDH-mutant high-grade gliomas (n = 13/27 had a CDKN2A HD) (p = 0.128). Astrocytoma was diagnosed with either the absence of 1p/19q co-deletion or loss of ATRX expression and strong diffuse p53 positivity. The non-significant finding in IDH-mutant glioblastoma and OS may be due to the small sample size [32]. Another study by Marker et al., which also employed FISH, analysed 151 IDH-mutant astrocytomas for CDKN2A HD. They reported CDKN2A/B as a predictor of survival in morphologically grade 4 IDH-astrocytomas but not for grade 2 and 3 IDH-mutant astrocytomas [55]. The authors of this paper suggest that this may be due to technical differences between detection methods.
5. Management of Tumours with CDKN2A/B Homozygous Deletions
There is no clear consensus on the treatment of IDH-mutant astrocytomas with CDKN2A/B HD, and reports related to their management are scarce. Reflecting this ambiguity, the current joint American Society of Clinical Oncology and Society of Neuro-Oncology guidelines recommend grade 4 astrocytomas be treated with concurrent temozolomide-radiotherapy with sequential temozolomide or radiotherapy alone with sequential temozolomide [70].
However, given the evidence that CDKN2A/B HD alters tumour biology (increased angiogenesis and cell growth), we cannot assume that these tumours will be as susceptible to temozolomide as their non-deleted counterparts. Unfortunately, the evidence for treatment specifically for CDKN2A/B HD astrocytomas is minimal. In 2000, Iwadate et al. investigated the relationship between CDKN2A deletion, p16 expression, and chemosensitivity to 30 different cytotoxic agents in vitro. They analysed 56 astrocytoma specimens (based on morphologic criteria, IDH status unknown) and found 17 specimens had p16 alterations (CDKN2A HD = 7, CDKN2A mutation = 5, p16 loss on IHC = 5). When looking at samples with p16 alterations, they found that deletions correlated with increased sensitivity to anti-metabolite agents but not to alkylating agents, antibiotics, topoisomerase inhibitors, or anti-microtubule agents [71].
Funakoshi et al. analysed OS in IDH-wildtype glioblastomas in patients before and after the use of bevacizumab. They were able to show that in the historical pre-bevacizumab group, there was a statistical difference in OS favouring the CDKN2A non-deleted group (OS 10.1 and 15.6 months, p = 0.0351). However, the significance is lost in the bevacizumab-treated group (OS 16.0 and 16.9 months, p = 0.1010, respectively). They proposed that the addition of bevacizumab should be considered in patients with CDKN2A HD glioblastoma [72]. While these studies serve as an indicator that the presence of a CDKN2A/B HD changes tumour response to treatment, given that they did not look at IDH-mutant tumours, it is hard to put them into the context of the current classification.
6. Putting CDKN2A/B Deletions into Perspective
Overall, the literature supports the use of CDKN2A/B homozygous deletions as a negative prognostic feature. As demonstrated in our literature summary in Table 2, most studies (n = 9/12) had a low risk of tumour classification bias, with 2 studies not showing a prognostic impact and one not describing it. However, due to the significant variance in tumour classifications used, study methodology, and techniques of deletion detection, it is difficult to appreciate the depth of this effect. It is also unclear how this molecular marker is best interpreted in the clinical context, such as the co-occurring molecular alterations or CDKN2B deletions in the absence of CDKN2A deletions.
The clinical studies in this review span five different CNS tumour grading classifications (WHO 1993, WHO 2000, WHO 2007, WHO 2016, and WHO 2021). This means that extrapolating most of the available evidence to the current clinical situation is difficult. Varying tumour classifications have been used to describe the effect of CDKN2A/B HD. For example, Cimino et al. proposed different grading systems based on a combination of CDKN2A/B HD and other molecular markers [10,19]. However, Shirahata et al., Appay et al., and Yoda et al. proposed using CDKN2A/B alone as a molecular marker for grade 4 IDH-mutant astrocytoma, determined by a number of methods, including methylation, SNP array, and NGS [11,12,13]. Authors such as Roy et al. and Aoki et al. found that CDKN2A/B had no impact on survival in IDH-mutant astrocytomas; however, both studies used FISH [30,31]. The results of the meta-analysis by Lu et al. provide reassurance that CDKN2A/B HD are prognostic in IDH-mutant astrocytomas, given the variety of testing methods used [8].
The literature has not shown that CDKN2A/B heterozygous deletions impact survival. Roy et al. demonstrated that CDKN2A/B HeD did not impact survival and that survival differences seen in this group are likely due to deletions of surrounding genes [30] Therefore, the survival impact of CDKN2A/B deletion may be related to the loss of the surrounding genes (MTAP, IFNA1, IFNB1).
It should be noted that point mutations were also highlighted by some of the presented research. However, the evidence for this particular alteration is very limited and does not show an impact on survival. Again, as these alterations do not result in the loss of genes neighbouring CDKN2A/B, this may also indicate the importance of these bystander genes in outcomes for astrocytomas with CDKN2A/B deletions [62,71].
There are also studies lacking a focus on CDKN2B deletions. In the post-molecular era, 13 studies examined CDKN2A deletion, with only three looking at CDKN2B. There is a general assumption that, due to its close proximity to and similar function to CDKN2A, the prognostic effect of CDKN2B is likely similar. Supporting this assumption are early translational studies that demonstrated that CDKN2B deletions, in addition to CDKN2A, are related to the aggressive phenotype [26]. However, this limitation still needs to be considered when interpreting the impact of CDKN2B HD in astrocytoma patients.
There is also a paucity of data on the management of CDKN2A/B HD IDH-mutant astrocytomas. The new entity of grade 4 astrocytoma with CDKN2A/B HD (WHO 2021) encompasses IDH-mutant astrocytomas previously treated with different regimens. Those previously classified as morphologic grade 3 astrocytomas would have been treated with radiotherapy alone followed by chemotherapy, while those previously characterised as IDH-mutant glioblastomas would have been treated with concurrent temozolomide-radiotherapy followed by sequential temozolomide. Therefore, which regimen should be used in IDH-mutant astrocytomas with CDKN2A/B HD is debatable.
7. Conclusions
CDKN2A/B HD have a direct oncogenic effect through loss of cell cycle inhibition and other parallel processes and are a molecular marker that influences grading and survival in IDH-mutant astrocytomas. Here, we review the evidence concerning CDKN2A/B deletions in a historical context. Overall, the evidence supports the use of CDKN2A/B HD as a negative prognostic marker in IDH-mutant astrocytomas. However, there is a significant variation in certainty, methods used for deletion detection, and the quality of the presented literature. There are also inaccuracies resulting from misclassification of tumours in older studies based on the revised WHO classification. These limitations hamper conclusions regarding the certainty and depth of impact CDKN2A/B HD has on prognosis and management and how this impact is affected by other co-occurring molecular alterations. Therefore, the strongest evidence for CDKN2A/B HD in IDH-mutant astrocytomas must come from prospective reports with the current WHO 2021 classification. Furthermore, clinical trials that use the current WHO 2021 classification are required to determine the optimal management of IDH-mutant astrocytomas with CDKN2A/B HD.
Author Contributions
Conceptualisation, A.Y. and A.H.; methodology, A.Y., J.Q.W., M.B., L.S., N.P. and A.L.; writing—original draft preparation, A.Y., J.Q.W. and L.S.; writing—review and editing, M.K., M.R., N.P. and A.L.; supervision, A.H., M.B., H.R.W., N.P. and A.L.; project administration, A.H., H.R.W. and A.L.; illustrations: A.Y. and J.Q.W. All authors have read and agreed to the published version of the manuscript.
Funding
A.Y. received scholarship support from the Brain Cancer Group.
Institutional Review Board Statement
No applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created or analysed in this study. Data sharing is not applicable to this article.
Conflicts of Interest
The funders had no role in the design of the study, in the collection, analysis, or interpretation of data, in the writing of the manuscript, or in the decision to publish the results.
References
- Louis, D.N.; Perry, A.; Reifenberger, G.; von Deimling, A.; Figarella-Branger, D.; Cavenee, W.K.; Ohgaki, H.; Wiestler, O.D.; Kleihues, P.; Ellison, D.W. The 2016 World Health Organization Classification of Tumors of the Central Nervous System: A summary. Acta Neuropathol. 2016, 131, 803–820. [Google Scholar] [CrossRef]
- Dang, L.; White, D.W.; Gross, S.; Bennett, B.D.; Bittinger, M.A.; Driggers, E.M.; Fantin, V.R.; Jang, H.G.; Jin, S.; Keenan, M.C.; et al. Cancer-associated IDH1 mutations produce 2-hydroxyglutarate. Nature 2009, 462, 739–744. [Google Scholar] [CrossRef]
- Huang, L.E. Friend or foe-IDH1 mutations in glioma 10 years on. Carcinogenesis 2019, 40, 1299–1307. [Google Scholar] [CrossRef]
- Yuile, A.; Satgunaseelan, L.; Wei, J.; Kastelan, M.; Back, M.F.; Lee, M.; Wei, H.; Buckland, M.E.; Lee, A.; Wheeler, H.R. Implications of Concurrent IDH1 and IDH2 Mutations on Survival in Glioma—A Case Report and Systematic Review. Curr. Issues Mol. Biol. 2022, 44, 5117–5125. [Google Scholar] [CrossRef]
- Eckel-Passow, J.E.; Lachance, D.H.; Molinaro, A.M.; Walsh, K.M.; Decker, P.A.; Sicotte, H.; Pekmezci, M.; Rice, T.; Kosel, M.L.; Smirnov, I.V.; et al. Glioma Groups Based on 1p/19q, IDH, and TERT Promoter Mutations in Tumors. N. Engl. J. Med. 2015, 372, 2499–2508. [Google Scholar] [CrossRef] [PubMed]
- Yan, H.; Parsons, D.W.; Jin, G.; McLendon, R.; Rasheed, B.A.; Yuan, W.; Kos, I.; Batinic-Haberle, I.; Jones, S.; Riggins, G.J.; et al. IDH1 and IDH2 Mutations in Gliomas. N. Engl. J. Med. 2009, 360, 765–773. [Google Scholar] [CrossRef] [PubMed]
- Brat, D.J.; Aldape, K.; Colman, H.; Figrarella-Branger, D.; Fuller, G.N.; Giannini, C.; Holland, E.C.; Jenkins, R.B.; Kleinschmidt-DeMasters, B.; Komori, T.; et al. cIMPACT-NOW update 5: Recommended grading criteria and terminologies for IDH-mutant astrocytomas. Acta Neuropathol. 2020, 139, 603–608. [Google Scholar] [CrossRef]
- Lu, V.M.; O’Connor, K.P.; Shah, A.H.; Eichberg, D.G.; Luther, E.M.; Komotar, R.J.; Ivan, M.E. The prognostic significance of CDKN2A homozygous deletion in IDH-mutant lower-grade glioma and glioblastoma: A systematic review of the contemporary literature. J. Neurooncol. 2020, 148, 221–229. [Google Scholar] [CrossRef] [PubMed]
- Sharpless, N.E. INK4a/ARF: A multifunctional tumor suppressor locus. Mutat. Res. 2005, 576, 22–38. [Google Scholar] [CrossRef]
- Yang, R.R.; Shi, Z.-F.; Zhang, Z.-Y.; Chan, A.K.-Y.; Aibaidula, A.; Wang, W.-W.; Kwan, J.S.H.; Poon, W.S.; Chen, H.; Li, W.-C.; et al. IDH mutant lower grade (WHO Grades II/III) astrocytomas can be stratified for risk by CDKN2A, CDK4 and PDGFRA copy number alterations. Brain Pathol. 2020, 30, 541–553. [Google Scholar] [CrossRef]
- Shirahata, M.; Ono, T.; Stichel, D.; Schrimpf, D.; Reuss, D.E.; Sahm, F.; Koelsche, C.; Wefers, A.; Reinhardt, A.; Huang, K.; et al. Novel, improved grading system(s) for IDH-mutant astrocytic gliomas. Acta Neuropathol. 2018, 136, 153–166. [Google Scholar] [CrossRef]
- Appay, R.; Dehais, C.; Maurage, C.-A.; Alentorn, A.; Carpentier, C.; Colin, C.; Ducray, F.; Escande, F.; Idbaih, A.; Kamoun, A.; et al. CDKN2A homozygous deletion is a strong adverse prognosis factor in diffuse malignant IDH-mutant gliomas. Neuro Oncol. 2019, 21, 1519–1528. [Google Scholar] [CrossRef]
- Yoda, R.A.; Marxen, T.; Longo, L.; Ene, C.; Wirsching, H.-G.; Keene, C.D.; Holland, E.C.; Cimino, P.J. Mitotic Index Thresholds Do Not Predict Clinical Outcome for IDH-Mutant Astrocytoma. J. Neuropathol. Exp. Neurol. 2019, 78, 1002–1010. [Google Scholar] [CrossRef] [PubMed]
- Louis, D.N.; Perry, A.; Wesseling, P.; Brat, D.J.; Cree, I.A.; Figarella-Branger, D.; Hawkins, C.; Ng, H.K.; Pfister, S.M.; Reifenberger, G.; et al. The 2021 WHO Classification of Tumors of the Central Nervous System: A summary. Neuro Oncol. 2021, 23, 1231–1251. [Google Scholar] [CrossRef] [PubMed]
- Dehais, C.; Laigle-Donadey, F.; Marie, Y.; Kujas, M.; Lejeune, J.; Benouaich-Amiel, A.; Pedretti, M.; Polivka, M.; Xuan, K.-H.; Thillet, J.; et al. Prognostic stratification of patients with anaplastic gliomas according to genetic profile. Cancer 2006, 107, 1891–1897. [Google Scholar] [CrossRef]
- James, C.D.; Galanis, E.; Frederick, L.; Kimmel, D.W.; Cunningham, J.M.; Atherton-Skaff, P.J.; O’Fallon, J.R.; Jenkins, R.B.; Buckner, J.C.; Hunter, S.B.; et al. Tumor suppressor gene alterations in malignant gliomas: Histopathological associations and prognostic evaluation. Int. J. Oncol. 1999, 15, 547–600. [Google Scholar] [CrossRef]
- Cairncross, J.G.; Ueki, K.; Zlatescu, M.C.; Lisle, D.K.; Finkelstein, D.M.; Hammond, R.R.; Silver, J.S.; Stark, P.C.; Macdonald, D.R.; Ino, Y.; et al. Specific genetic predictors of chemotherapeutic response and survival in patients with anaplastic oligodendrogliomas. J. Natl. Cancer Inst. 1998, 90, 1473–1479. [Google Scholar] [CrossRef] [PubMed]
- Korshunov, A.; Casalini, B.; Chavez, L.; Hielscher, T.; Sill, M.; Ryzhova, M.; Sharma, T.; Schrimpf, D.; Stichel, D.; Capper, D.; et al. Integrated molecular characterization of IDH-mutant glioblastomas. Neuropathol. Appl. Neurobiol. 2019, 45, 108–118. [Google Scholar] [CrossRef]
- Cimino, P.J.; Zager, M.; McFerrin, L.; Wirsching, H.-G.; Bolouri, H.; Hentschel, B.; von Deimling, A.; Jones, D.; Reifenberger, G.; Weller, M.; et al. Multidimensional scaling of diffuse gliomas: Application to the 2016 World Health Organization classification system with prognostically relevant molecular subtype discovery. Acta Neuropathol. Commun. 2017, 5, 39. [Google Scholar] [CrossRef] [PubMed]
- Cimino, P.J.; Holland, E.C. Targeted copy number analysis outperforms histologic grading in predicting patient survival for WHO grades II/III IDH-mutant astrocytomas. Neuro Oncol. 2019, 21, 819–821. [Google Scholar] [CrossRef]
- Rey, J.A.; Bello, M.J.; de Campos, J.M.; Kusak, M.E.; Ramos, C.; Benitez, J. Chromosomal patterns in human malignant astrocytomas. Cancer Genet. Cytogenet. 1987, 29, 201–221. [Google Scholar] [CrossRef]
- James, C.D.; He, J.; Carlbom, E.; Nordenskjold, M.; Cavenee, W.K.; Collins, V.P. Chromosome 9 Deletion Mapping Reveals Interferon α and Interferon β-1 Gene Deletions in Human Glial Tumors. Cancer Res. 1991, 51, 1684–1688. [Google Scholar] [PubMed]
- Serrano, M.; Hannon, G.J.; Beach, D. A new regulatory motif in cell-cycle control causing specific inhibition of cyclin D/CDK4. Nature 1993, 366, 704–707. [Google Scholar] [CrossRef] [PubMed]
- Jen, J.; Harper, J.W.; Bigner, S.H.; Bigner, D.D.; Papadopoulos, N.; Markowitz, S.; Willson, J.K.; Kinzler, K.W.; Vogelstein, B. Deletion of p16 and p15 genes in brain tumors. Cancer Res. 1994, 54, 6353–6358. [Google Scholar]
- Zhang, H.; Chen, Z.-H.; Savarese, T.M. Codeletion of the genes for p16INK4, methylthioadenosine phosphorylase, interferon-α1, interferon-β1, and other 9p21 markers in human malignant cell lines. Cancer Genet. Cytogenet. 1996, 86, 22–28. [Google Scholar] [CrossRef]
- Sonoda, Y.; Yoshimoto, T.; Sekiya, T. Homozygous deletion of the MTS1/p16 and MTS2/p15 genes and amplification of the CDK4 gene in glioma. Oncogene 1995, 11, 2145–2149. [Google Scholar]
- Idbaih, A.; Carvalho Silva, R.; Crinière, E.; Marie, Y.; Carpentier, C.; Boisselier, B.; Taillibert, S.; Rousseau, A.; Mokhtari, K.; Ducray, F.; et al. Genomic changes in progression of low-grade gliomas. J. Neurooncol. 2008, 90, 133–140. [Google Scholar] [CrossRef]
- Reis, G.F.; Pekmezci, M.; Hansen, H.M.; Rice, T.; Marshall, R.E.; Molinaro, A.M.; Phillips, J.J.; Vogel, H.; Wiencke, J.K.; Wrensch, M.R.; et al. CDKN2A Loss Is Associated with Shortened Overall Survival in Lower Grade (World Health Organization II-III) Astrocytomas. J. Neuropathol. Exp. Neurol. 2015, 74, 442–452. [Google Scholar] [CrossRef] [PubMed]
- Ceccarelli, M.; Barthel, F.P.; Malta, T.M.; Sabedot, T.S.; Salama, S.R.; Murray, B.A.; Morozova, O.; Newton, Y.; Radenbaugh, A.; Pagnotta, S.M.; et al. Molecular Profiling Reveals Biologically Discrete Subsets and Pathways of Progression in Diffuse Glioma. Cell 2016, 164, 550–563. [Google Scholar] [CrossRef]
- Roy, D.M.; Walsh, L.A.; Desrichard, A.; Huse, J.T.; Wu, W.; Gao, J.; Bose, P.; Lee, W.; Chan, T.A. Integrated genomics for pinpointing survival loci within arm-level somatic copy number alterations. Cancer Cell 2016, 29, 737–750. [Google Scholar] [CrossRef]
- Aoki, K.; Nakamura, H.; Suzuki, H.; Matsuo, K.; Kataoka, K.; Shimamura, T.; Motomura, K.; Ohka, F.; Shiina, S.; Yamamoto, T.; et al. Prognostic relevance of genetic alterations in diffuse lower-grade gliomas. Neuro Oncol. 2018, 20, 66–77. [Google Scholar] [CrossRef]
- Satomi, K.; Ohno, M.; Matsushita, Y.; Takahashi, M.; Miyakita, Y.; Narita, Y.; Ichimura, K.; Yoshida, A. Utility of methylthioadenosine phosphorylase immunohistochemical deficiency as a surrogate for CDKN2A homozygous deletion in the assessment of adult-type infiltrating astrocytoma. Mod. Pathol. 2021, 34, 688–700. [Google Scholar] [CrossRef]
- Tesileanu, C.M.S.; Vallentgoed, W.R.; Sanson, M.; Taal, W.; Clement, P.M.; Wick, W.; Brandes, A.A.; Baurain, J.F.; Chinot, O.L.; Wheeler, H.; et al. Non-IDH1-R132H IDH1/2 mutations are associated with increased DNA methylation and improved survival in astrocytomas, compared to IDH1-R132H mutations. Acta Neuropathol. 2021, 141, 945–957. [Google Scholar] [CrossRef]
- Worsham, M.J.; Chen, K.M.; Tiwari, N.; Pals, G.; Schouten, J.P.; Sethi, S.; Benninger, M.S. Fine-mapping loss of gene architecture at the CDKN2B (p15INK4b), CDKN2A (p14ARF, p16INK4a), and MTAP genes in head and neck squamous cell carcinoma. Arch. Otolaryngol. Head. Neck Surg. 2006, 132, 409–415. [Google Scholar] [CrossRef] [PubMed]
- Cánepa, E.T.; Scassa, M.E.; Ceruti, J.M.; Marazita, M.C.; Carcagno, A.L.; Sirkin, P.F.; Ogara, M.F. INK4 proteins, a family of mammalian CDK inhibitors with novel biological functions. IUBMB Life 2007, 59, 419–426. [Google Scholar] [CrossRef] [PubMed]
- Nobori, T.; Miura, K.; Wu, D.J.; Lois, A.; Takabayashi, K.; Carson, D.A. Deletions of the cyclin-dependent kinase-4 inhibitor gene in multiple human cancers. Nature 1994, 368, 753–756. [Google Scholar] [CrossRef] [PubMed]
- Miyakoshi, J.; Dobler, K.D.; Allalunis-Turner, J.; McKean, J.D.; Petruk, K.; Allen, P.B.; Aronyk, K.N.; Weir, B.; Huyser-Wierenga, D.; Fulton, D. Absence of IFNA and IFNB genes from human malignant glioma cell lines and lack of correlation with cellular sensitivity to interferons. Cancer Res. 1990, 50, 278–283. [Google Scholar] [PubMed]
- CDKN2A: Chr9-Genome Data Viewer-NCBI. Available online: https://www.ncbi.nlm.nih.gov/genome/gdv/browser/gene/?id=1029 (accessed on 18 April 2023).
- Arap, W.; Nishikawa, R.; Furnari, F.B.; Cavenee, W.K.; Huang, H.-J.S. Replacement of the pl6/CDKN2 Gene Suppresses Human Glioma Cell Growth. Cancer Res. 1995, 55, 1351–1354. [Google Scholar]
- Nishikawa, R.; Furnari, F.B.; Lin, H.; Arap, W.; Berger, M.S.; Cavenee, W.K.; Su Huang, H.J. Loss of P16INK4 expression is frequent in high grade gliomas. Cancer Res. 1995, 55, 1941–1945. [Google Scholar]
- Liban, T.J.; Thwaites, M.J.; Dick, F.A.; Rubin, S.M. Structural Conservation and E2F Binding Specificity within the Retinoblastoma Pocket Protein Family. J. Mol. Biol. 2016, 428, 3960–3971. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Prives, C. Cyclin A-CDK Phosphorylation Regulates MDM2 Protein Interactions*. J. Biol. Chem. 2001, 276, 29702–29710. [Google Scholar] [CrossRef] [PubMed]
- Giono, L.E.; Manfredi, J.J. Mdm2 Is Required for Inhibition of Cdk2 Activity by p21, Thereby Contributing to p53-Dependent Cell Cycle Arrest. Mol. Cell Biol. 2007, 27, 4166–4178. [Google Scholar] [CrossRef] [PubMed]
- Zerrouqi, A.; Pyrzynska, B.; Febbraio, M.; Brat, D.J.; Van Meir, E.G. P14ARF inhibits human glioblastoma-induced angiogenesis by upregulating the expression of TIMP3. J. Clin. Investig. 2012, 122, 1283–1295. [Google Scholar] [CrossRef] [PubMed]
- Yassini, P.R.; Stickler, D.L.; Bloomfield, S.M.; Wiggins, R.C.; Konat, G.W. Glioma-stimulated chemoattraction of endothelial cells and fibroblasts in vitro: A model for the study of glioma-induced angiogenesis. Metab. Brain Dis. 1994, 9, 391–399. [Google Scholar] [CrossRef]
- Cornelius, L.A.; Nehring, L.C.; Roby, J.D.; Parks, W.C.; Welgus, H.G. Human dermal microvascular endothelial cells produce matrix metalloproteinases in response to angiogenic factors and migration. J. Investig. Dermatol. 1995, 105, 170–176. [Google Scholar] [CrossRef]
- Harada, H.; Nakagawa, K.; Iwata, S.; Saito, M.; Kumon, Y.; Sakaki, S.; Sato, K.; Hamada, K. Restoration of Wild-Type p16 Down-Regulates Vascular Endothelial Growth Factor Expression and Inhibits Angiogenesis in Human Gliomas1. Cancer Res. 1999, 59, 3783–3789. [Google Scholar]
- Nalabothula, N.; Lakka, S.S.; Dinh, D.H.; Gujrati, M.; Olivero, W.C.; Rao, J.S. Sense p16 and antisense uPAR bicistronic construct inhibits angiogenesis and induces glioma cell death. Int. J. Oncol. 2007, 30, 669–678. [Google Scholar] [CrossRef][Green Version]
- Khasraw, M.; Ameratunga, M.S.; Grant, R.; Wheeler, H.; Pavlakis, N. Antiangiogenic therapy for high-grade glioma. Cochrane Database Syst. Rev. 2014, 9, CD008218. [Google Scholar] [CrossRef]
- Kong, Y.; Hsieh, C.-H.; Alonso, L.C. ANRIL: A lncRNA at the CDKN2A/B Locus With Roles in Cancer and Metabolic Disease. Front. Endocrinol. 2018, 9, 405. [Google Scholar] [CrossRef]
- Tang, B.; Kadariya, Y.; Chen, Y.; Slifker, M.; Kruger, W.D. Expression of MTAP Inhibits Tumor-Related Phenotypes in HT1080 Cells via a Mechanism Unrelated to Its Enzymatic Function. G3 Genes Genomes Genet. 2015, 5, 35–44. [Google Scholar] [CrossRef]
- Delaunay, T.; Achard, C.; Boisgerault, N.; Grard, M.; Petithomme, T.; Chatelain, C.; Dutoit, S.; Blanquart, C.; Royer, P.-J.; Minvielle, S.; et al. Frequent Homozygous Deletions of Type I Interferon Genes in Pleural Mesothelioma Confer Sensitivity to Oncolytic Measles Virus. J. Thorac. Oncol. 2020, 15, 827–842. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, H.; Aoki, K.; Chiba, K.; Sato, Y.; Shiozawa, Y.; Shiraishi, Y.; Shimamura, T.; Niida, A.; Motomura, K.; Ohka, F.; et al. Mutational landscape and clonal architecture in grade II and III gliomas. Nat. Genet. 2015, 47, 458–468. [Google Scholar] [CrossRef] [PubMed]
- Perry, A.; Nobori, T.; Ru, N.; Anderl, K.; Borell, T.J.; Mohapatra, G.; Feuerstein, B.G.; Jenkins, R.B.; Carson, D.A. Detection of p16 gene deletions in gliomas: A comparison of fluorescence in situ hybridization (FISH) versus quantitative PCR. J. Neuropathol. Exp. Neurol. 1997, 56, 999–1008. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Marker, D.F.; Pearce, T.M. Homozygous deletion of CDKN2A by fluorescence in situ hybridization is prognostic in grade 4, but not grade 2 or 3, IDH-mutant astrocytomas. Acta Neuropathol. Commun. 2020, 8, 169. [Google Scholar] [CrossRef]
- Vysis CDKN2A/CEP 9 FISH Probe Kit. Available online: https://www.molecularcatalog.abbott/int/en/Vysis-CDKN2A-CEP-9-FISH-Probe-Kit (accessed on 18 January 2023).
- Hida, T.; Hamasaki, M.; Matsumoto, S.; Sato, A.; Tsujimura, T.; Kawahara, K.; Iwasaki, A.; Okamoto, T.; Oda, Y.; Honda, H.; et al. Immunohistochemical detection of MTAP and BAP1 protein loss for mesothelioma diagnosis: Comparison with 9p21 FISH and BAP1 immunohistochemistry. Lung Cancer 2017, 104, 98–105. [Google Scholar] [CrossRef]
- Chapel, D.B.; Schulte, J.J.; Berg, K.; Churg, A.; Dacic, S.; Fitzpatrick, C.; Galateau-Salle, F.; Hiroshima, K.; Krausz, T.; Le Stang, N.; et al. MTAP immunohistochemistry is an accurate and reproducible surrogate for CDKN2A fluorescence in situ hybridization in diagnosis of malignant pleural mesothelioma. Mod. Pathol. 2020, 33, 245–254. [Google Scholar] [CrossRef]
- Purkait, S.; Jha, P.; Sharma, M.C.; Suri, V.; Sharma, M.; Kale, S.S.; Sarkar, C. CDKN2A deletion in pediatric versus adult glioblastomas and predictive value of p16 immunohistochemistry. Neuropathology 2013, 33, 405–412. [Google Scholar] [CrossRef]
- Schmidt, E.E.; Ichimura, K.; Reifenberger, G.; Collins, V.P. CDKN2 (p16/MTS1) gene deletion or CDK4 amplification occurs in the majority of glioblastomas. Cancer Res. 1994, 54, 6321–6324. [Google Scholar]
- Giani, C.; Finocchiaro, G. Mutation rate of the CDKN2 gene in malignant gliomas. Cancer Res. 1994, 54, 6338–6339. [Google Scholar]
- Moulton, T.; Samara, G.; Chung, W.Y.; Yuan, L.; Desai, R.; Sisti, M.; Bruce, J.; Tycko, B. MTS1/p16/CDKN2 lesions in primary glioblastoma multiforme. Am. J. Pathol. 1995, 146, 613–619. [Google Scholar]
- Pekmezci, M.; Rice, T.; Molinaro, A.M.; Walsh, K.M.; Decker, P.A.; Hansen, H.; Sicotte, H.; Kollmeyer, T.M.; McCoy, L.S.; Sarkar, G.; et al. Adult infiltrating gliomas with WHO 2016 integrated diagnosis: Additional prognostic roles of ATRX and TERT. Acta Neuropathol. 2017, 133, 1001–1016. [Google Scholar] [CrossRef] [PubMed]
- Ono, Y.; Tamiya, T.; Ichikawa, T.; Kunishio, K.; Matsumoto, K.; Furuta, T.; Ohmoto, T.; Ueki, K.; Louis, D.N. Malignant astrocytomas with homozygous CDKN2/p16 gene deletions have higher Ki-67 proliferation indices. J. Neuropathol. Exp. Neurol. 1996, 55, 1026–1031. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Barker, F.G.; Chen, P.; Furman, F.; Aldape, K.D.; Edwards, M.S.; Israel, M.A. P16 deletion and mutation analysis in human brain tumors. J. Neurooncol. 1997, 31, 17–23. [Google Scholar] [CrossRef]
- Rich, J.N.; Hans, C.; Jones, B.; Iversen, E.S.; McLendon, R.E.; Rasheed, B.K.A.; Dobra, A.; Dressman, H.K.; Bigner, D.D.; Nevins, J.R.; et al. Gene expression profiling and genetic markers in glioblastoma survival. Cancer Res. 2005, 65, 4051–4058. [Google Scholar] [CrossRef]
- Zolota, V.; Tsamandas, A.C.; Aroukatos, P.; Panagiotopoulos, V.; Maraziotis, T.; Poulos, C.; Scopa, C.D. Expression of cell cycle inhibitors p21, p27, p14 and p16 in gliomas. Correlation with classic prognostic factors and patients’ outcome. Neuropathology 2008, 28, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Cancer Genome Atlas Research Network; Brat, D.J.; Verhaak, R.G.W.; Aldape, K.D.; Yung, W.K.A.; Salama, S.R.; Cooper, L.A.D.; Rheinbay, E.; Miller, C.R.; Vitucci, M.; et al. Comprehensive, Integrative Genomic Analysis of Diffuse Lower-Grade Gliomas. N. Engl. J. Med. 2015, 372, 2481–2498. [Google Scholar] [CrossRef]
- Olar, A.; Wani, K.M.; Alfaro-Munoz, K.D.; Heathcock, L.E.; van Thuijl, H.F.; Gilbert, M.R.; Armstrong, T.S.; Sulman, E.P.; Cahill, D.P.; Vera-Bolanos, E.; et al. IDH mutation status and role of WHO grade and mitotic index in overall survival in grade II-III difuse gliomas. Acta Neuropathol. 2015, 129, 585–596. [Google Scholar] [CrossRef]
- Mohile, N.A.; Messersmith, H.; Gatson, N.T.; Hottinger, A.F.; Lassman, A.; Morton, J.; Ney, D.; Nghiemphu, P.L.; Olar, A.; Olson, J.; et al. Therapy for Diffuse Astrocytic and Oligodendroglial Tumors in Adults: ASCO-SNO Guideline. J. Clin. Oncol. 2022, 40, 403–426. [Google Scholar] [CrossRef]
- Iwadate, Y. Epithelial-mesenchymal transition in glioblastoma progression. Oncol. Lett. 2016, 11, 1615–1620. [Google Scholar] [CrossRef]
- Funakoshi, Y.; Hata, N.; Takigawa, K.; Arita, H.; Kuga, D.; Hatae, R.; Sangatsuda, Y.; Fujioka, Y.; Sako, A.; Umehara, T.; et al. Clinical significance of CDKN2A homozygous deletion in combination with methylated MGMT status for IDH-wildtype glioblastoma. Cancer Med. 2021, 10, 3177–3187. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).