Anti-Leukemic Effects of Idesia polycarpa Maxim Branch on Human B-Cell Acute Lymphoblastic Leukemia Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Plant Extracts
2.2. Cell Culture, Reagents, and Antibodies
2.3. Measurement of Cell Viability
2.4. Apoptosis Assays
2.5. Analysis of Caspase 3/7 Activity and Mitochondrial Membrane Potential
2.6. Western Blot Analysis
2.7. Cell Proliferation Assay
2.8. Colony Formation Assay
2.9. Giemsa Staining
2.10. Cell Cycle Analysis
2.11. Real-Time PCR
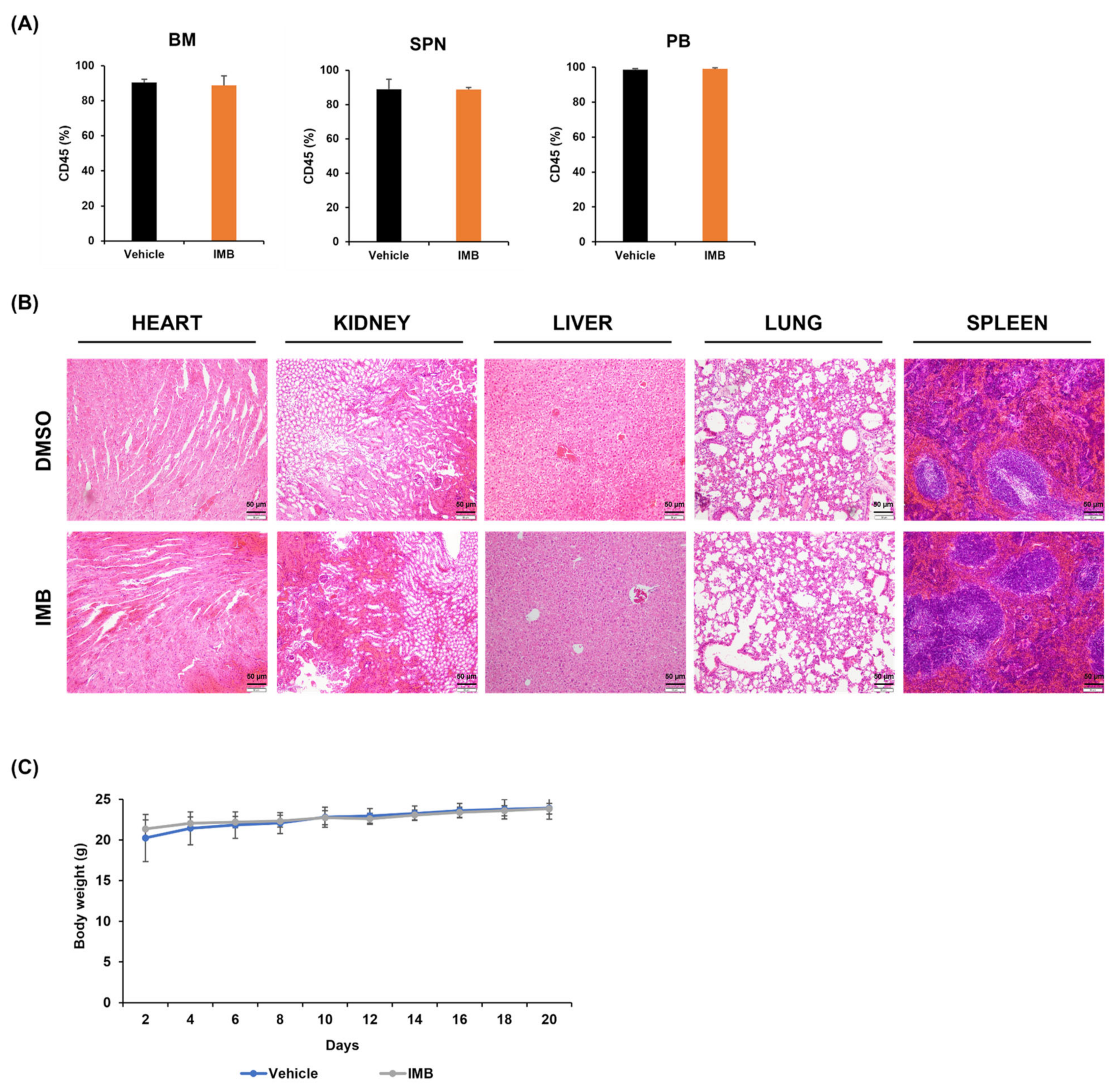
2.12. Animal Studies
2.13. Statistical Analysis
3. Results
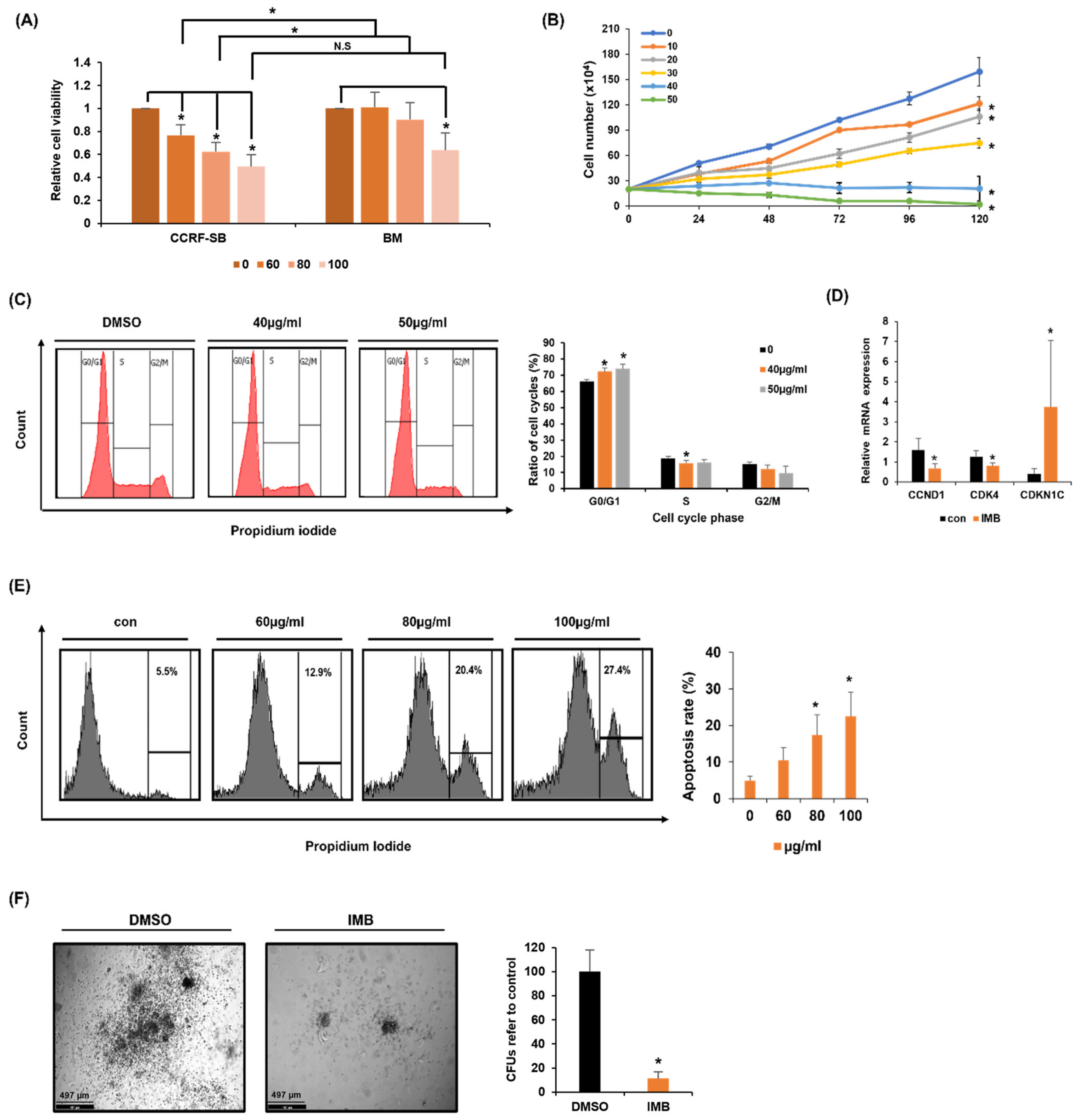
3.1. Screening of Plant Extracts as an Anti-Leukemic Agent
3.2. IMB Inhibited the Cell Growth of CCRF-SB Cells
3.3. IMB’s Antiproliferative Effect Is Cancer Cell-Specific
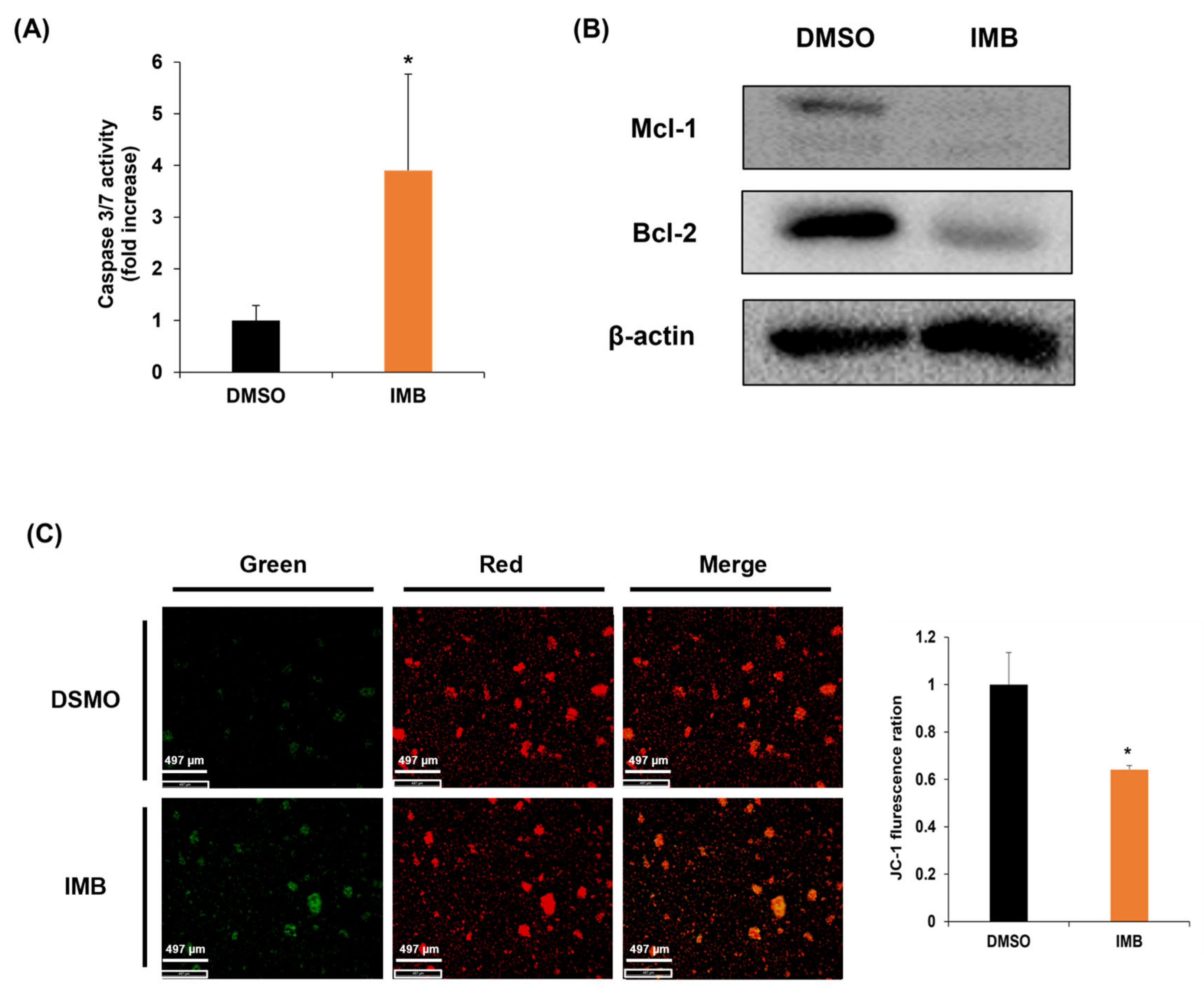
3.4. IMB Induces Apoptosis by Disrupting the Mitochondrial Membrane Potential Regulated by Bcl-2 Family Members
3.5. Upregulation of Differentiation-Related Genes by IMB Resulted in Phenotypic Differentiation of CCRF-SB Cells
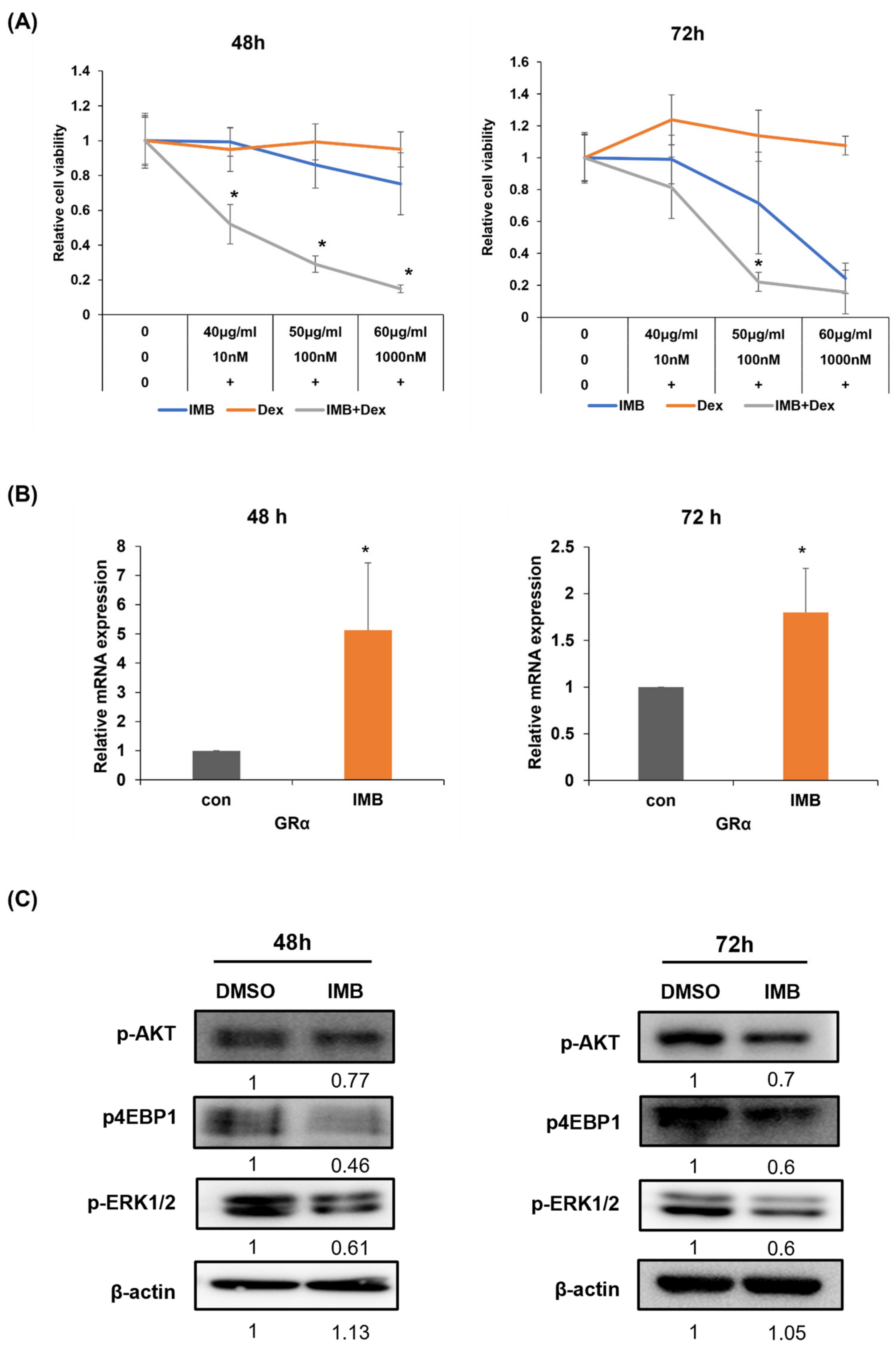
3.6. IMB Overcomes Glucocorticoid Resistance in CCRF-SB Cells by Increasing GR and Downregulating the mTOR and MAPK Signaling Pathways
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Samra, B.; Jabbour, E.; Ravandi, F.; Kantarjian, H.; Short, N.J. Evolving therapy of adult acute lymphoblastic leukemia: State-of-the-art treatment and future directions. J. Hematol. Oncol. 2020, 13, 70. [Google Scholar] [CrossRef] [PubMed]
- Ratti, S.; Lonetti, A.; Follo, M.Y.; Paganelli, F.; Martelli, A.M.; Chiarini, F.; Evangelisti, C. B-ALL Complexity: Is Targeted Therapy Still A Valuable Approach for Pediatric Patients? Cancers 2020, 12, 3498. [Google Scholar] [CrossRef] [PubMed]
- Schrappe, M.; Reiter, A.; Ludwig, W.D.; Harbott, J.; Zimmermann, M.; Hiddemann, W.; Niemeyer, C.; Henze, G.; Feldges, A.; Zintl, F.; et al. Improved outcome in childhood acute lymphoblastic leukemia despite reduced use of anthracyclines and cranial radiotherapy: Results of trial ALL-BFM 90. Blood 2000, 95, 3310–3322. [Google Scholar] [PubMed]
- Boissel, N.; Auclerc, M.F.; Lhéritier, V.; Perel, Y.; Thomas, X.; Leblanc, T.; Rousselot, P.; Cayuela, J.M.; Gabert, J.; Fegueux, N.; et al. Should adolescents with acute lymphoblastic leukemia be treated as old children or young adults? Comparison of the French FRALLE-93 and LALA-94 trials. J. Clin. Oncol. 2003, 21, 774–780. [Google Scholar] [CrossRef] [PubMed]
- Annino, L.; Vegna, M.L.; Camera, A.; Specchia, G.; Visani, G.; Fioritoni, G.; Ferrara, F.; Peta, A.; Ciolli, S.; Deplano, W.; et al. Treatment of adult acute lymphoblastic leukemia (ALL): Long-term follow-up of the GIMEMA ALL 0288 randomized study. Blood 2002, 99, 863–871. [Google Scholar] [CrossRef]
- Rowe, J.M.; Buck, G.; Burnett, A.K.; Chopra, R.; Wiernik, P.H.; Richards, S.M.; Lazarus, H.M.; Franklin, I.M.; Litzow, M.R.; Ciobanu, N.; et al. Induction therapy for adults with acute lymphoblastic leukemia: Results of more than 1500 patients from the international ALL trial: MRC UKALL XII/ECOG E2993. Blood 2005, 106, 3760–3767. [Google Scholar] [CrossRef]
- Olivas-Aguirre, M.; Torres-López, L.; Pottosin, I.; Dobrovinskaya, O. Overcoming Glucocorticoid Resistance in Acute Lymphoblastic Leukemia: Repurposed Drugs Can Improve the Protocol. Front. Oncol. 2021, 11, 617937. [Google Scholar] [CrossRef]
- Pufall, M.A. Glucocorticoids and Cancer. Adv. Exp. Med. Biol. 2015, 872, 315–333. [Google Scholar] [CrossRef]
- Chan, L.N.; Chen, Z.; Braas, D.; Lee, J.-W.; Xiao, G.; Geng, H.; Cosgun, K.N.; Hurtz, C.; Shojaee, S.; Cazzaniga, V.; et al. Metabolic gatekeeper function of B-lymphoid transcription factors. Nature 2017, 542, 479–483. [Google Scholar] [CrossRef]
- Jones, C.L.; Gearheart, C.M.; Fosmire, S.; Delgado-Martin, C.; Evensen, N.A.; Bride, K.; Waanders, A.J.; Pais, F.; Wang, J.; Bhatla, T.; et al. MAPK signaling cascades mediate distinct glucocorticoid resistance mechanisms in pediatric leukemia. Blood 2015, 126, 2202–2212. [Google Scholar] [CrossRef]
- Easton, J.B.; Houghton, P.J. mTOR and cancer therapy. Oncogene 2006, 25, 6436–6446. [Google Scholar] [CrossRef] [PubMed]
- Li, J.W.-H.; Vederas, J.C. Drug Discovery and Natural Products: End of an Era or an Endless Frontier? Science 2009, 325, 161–165. [Google Scholar] [CrossRef] [PubMed]
- Harvey, A.L. Medicines from nature: Are natural products still relevant to drug discovery? Trends Pharmacol. Sci. 1999, 20, 196–198. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.; Nam, J.; Chang, W.; Zulfugarov, I.S.; Okhlopkova, Z.M.; Olennikov, D.; Chirikova, N.K.; Kim, S.-W. Angelica gigas Nakai and Decursin Downregulate Myc Expression to Promote Cell Death in B-cell Lymphoma. Sci. Rep. 2018, 8, 10590. [Google Scholar] [CrossRef]
- Jeon, B.-E.; Kwon, C.-S.; Lee, J.-E.; Moon, K.; Cha, J.; Park, I.; Koh, S.; Yoon, M.; Kim, S.-W.; Kim, J.N. Anticancer Activity of Continentalic Acid in B-Cell Lymphoma. Molecules 2021, 26, 6845. [Google Scholar] [CrossRef]
- Huang, Q.; Wang, L.; Ran, Q.; Wang, J.; Wang, C.; He, H.; Li, L.; Qi, H. Notopterol-induced apoptosis and differentiation in human acute myeloid leukemia HL-60 cells. Drug Des. Dev. Ther. 2019, 13, 1927–1940. [Google Scholar] [CrossRef]
- Lee, J.-E.; Kwon, C.-S.; Jeon, B.-E.; Kim, W.R.; Lee, D.H.; Koh, S.; Kim, H.-S.; Kim, S.-W. Genome-Wide Gene Expression Profiling Defines the Mechanism of Anticancer Effect of Colorectal Cancer Cell-Derived Conditioned Medium on Acute Myeloid Leukemia. Genes 2022, 13, 883. [Google Scholar] [CrossRef]
- Ngan, L.T.M.; Jang, M.J.; Kwon, M.J.; Ahn, Y.J. Antiviral Activity and Possible Mechanism of Action of Constituents Identified in Paeonia lactiflora Root toward Human Rhinoviruses. PLoS ONE 2015, 10, e0121629. [Google Scholar] [CrossRef]
- Kim, J.; Kim, J.N.; Park, I.; Sivtseva, S.; Okhlopkova, Z.; Zulfugarov, I.S.; Kim, S. Dracocephalum palmatum Stephan extract induces caspase- and mitochondria-dependent apoptosis via Myc inhibition in diffuse large B cell lymphoma. Oncol. Rep. 2020, 44, 2746–2756. [Google Scholar] [CrossRef]
- Wei, G.; Twomey, D.; Lamb, J.; Schlis, K.; Agarwal, J.; Stam, R.W.; Opferman, J.T.; Sallan, S.E.; Den Boer, M.L.; Pieters, R.; et al. Gene expression-based chemical genomics identifies rapamycin as a modulator of MCL1 and glucocorticoid resistance. Cancer Cell 2006, 10, 331–342. [Google Scholar] [CrossRef]
- Zhang, C.; Ryu, Y.-K.; Chen, T.Z.; Hall, C.P.; Webster, D.R.; Kang, M.H. Synergistic activity of rapamycin and dexamethasone in vitro and in vivo in acute lymphoblastic leukemia via cell-cycle arrest and apoptosis. Leuk. Res. 2012, 36, 342–349. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Zhou, C.Y.; Li, Q.; Gao, J.; Zhu, Y.P.; Gu, L.; Ma, Z.G. Rapamycin sensitizes glucocorticoid resistant acute lymphoblastic leukemia CEM-C1 cells to dexamethasone induced apoptosis through both mTOR suppression and up-regulation and activation of glucocorticoid receptor. Biomed. Environ. Sci. 2013, 26, 371–381. [Google Scholar] [CrossRef] [PubMed]
- Yilmaz, M.; Kantarjian, H.; Ravandi, F. Acute promyelocytic leukemia current treatment algorithms. Blood Cancer J. 2021, 11, 123. [Google Scholar] [CrossRef] [PubMed]
- Lin, T.; Vala, M.S.; Barber, J.P.; Karp, J.E.; Smith, B.D.; Matsui, W.; Jones, R.J. Induction of acute lymphocytic leukemia differentiation by maintenance therapy. Leukemia 2007, 21, 1915–1920. [Google Scholar] [CrossRef]
- Takahashi, S. Current Understandings of Myeloid Differentiation Inducers in Leukemia Therapy. Acta Haematol. 2020, 144, 380–388. [Google Scholar] [CrossRef]
- Leszczyniecka, M.; Roberts, T.; Dent, P.; Grant, S.; Fisher, P.B. Differentiation therapy of human cancer: Basic science and clinical applications. Pharmacol. Ther. 2001, 90, 105–156. [Google Scholar] [CrossRef]
- Lejman, M.; Chałupnik, A.; Chilimoniuk, Z.; Dobosz, M. Genetic Biomarkers and Their Clinical Implications in B-Cell Acute Lymphoblastic Leukemia in Children. Int. J. Mol. Sci. 2022, 23, 2755. [Google Scholar] [CrossRef]
- Inaba, H.; Pui, C.-H. Glucocorticoid use in acute lymphoblastic leukaemia. Lancet Oncol. 2010, 11, 1096–1106. [Google Scholar] [CrossRef]
- Huang, F.; Liao, E.; Li, C.; Yen, C.; Yu, S. Pathogenesis of pediatric B-cell acute lymphoblastic leukemia: Molecular pathways and disease treatments (Review). Oncol. Lett. 2020, 20, 448–454. [Google Scholar] [CrossRef]
- Cooper, S.L.; Brown, P.A. Treatment of Pediatric Acute Lymphoblastic Leukemia. Pediatr. Clin. North Am. 2014, 62, 61–73. [Google Scholar] [CrossRef]
- Locatelli, F.; Schrappe, M.; Bernardo, M.E.; Rutella, S. How I treat relapsed childhood acute lymphoblastic leukemia. Blood 2012, 120, 2807–2816. [Google Scholar] [CrossRef] [PubMed]
- Lucafò, M.; Sicari, D.; Chicco, A.; Curci, D.; Bellazzo, A.; Di Silvestre, A.; Pegolo, C.; Autry, R.; Cecchin, E.; De Iudicibus, S.; et al. miR-331-3p is involved in glucocorticoid resistance reversion by rapamycin through suppression of the MAPK signaling pathway. Cancer Chemother. Pharmacol. 2020, 86, 361–374. [Google Scholar] [CrossRef] [PubMed]
- Lucafò, M.; Bravin, V.; Tommasini, A.; Martelossi, S.; Rabach, I.; Ventura, A.; Decorti, G.; De Iudicibus, S. Differential expression of GAS5 in rapamycin-induced reversion of glucocorticoid resistance. Clin. Exp. Pharmacol. Physiol. 2016, 43, 602–605. [Google Scholar] [CrossRef] [PubMed]

| NO | Scientific Name | CC50 (µg/mL) BM | IC50 (µg/mL) CCRF-SB | Selective Index (SI) |
|---|---|---|---|---|
| 57 | Fatsia japonica (Thunb.) Decne. & Planch. | 97.20 ± 8.05 | 35.82 ± 1.54 | 2.71 |
| 61 | Isodon inflexus (Thunb.) Kudo | 171.20 ± 6.75 | 87.84 ± 6.66 | 1.95 |
| 72 | Ribes fasciculatum var. chinense Maxim. | 83.99 ± 0.23 | 134.07 ± 5.31 | 0.63 |
| 84 | Idesia polycarpa Maxim. | 281.87 ± 4.05 | 85.58 ± 8.47 | 3.29 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kwon, C.-S.; Lee, J.-E.; Jeon, B.-E.; Woo, Y.-R.; Kim, Y.-S.; Kim, J.-W.; Park, C.-J.; Jang, S.-Y.; Kim, S.-W. Anti-Leukemic Effects of Idesia polycarpa Maxim Branch on Human B-Cell Acute Lymphoblastic Leukemia Cells. Curr. Issues Mol. Biol. 2023, 45, 4035-4049. https://doi.org/10.3390/cimb45050257
Kwon C-S, Lee J-E, Jeon B-E, Woo Y-R, Kim Y-S, Kim J-W, Park C-J, Jang S-Y, Kim S-W. Anti-Leukemic Effects of Idesia polycarpa Maxim Branch on Human B-Cell Acute Lymphoblastic Leukemia Cells. Current Issues in Molecular Biology. 2023; 45(5):4035-4049. https://doi.org/10.3390/cimb45050257
Chicago/Turabian StyleKwon, Chan-Seong, Ji-Eun Lee, Byeol-Eun Jeon, Ye-Rin Woo, Yun-Seo Kim, Jae-Woo Kim, Chae-Jin Park, Seo-Yun Jang, and Sang-Woo Kim. 2023. "Anti-Leukemic Effects of Idesia polycarpa Maxim Branch on Human B-Cell Acute Lymphoblastic Leukemia Cells" Current Issues in Molecular Biology 45, no. 5: 4035-4049. https://doi.org/10.3390/cimb45050257
APA StyleKwon, C.-S., Lee, J.-E., Jeon, B.-E., Woo, Y.-R., Kim, Y.-S., Kim, J.-W., Park, C.-J., Jang, S.-Y., & Kim, S.-W. (2023). Anti-Leukemic Effects of Idesia polycarpa Maxim Branch on Human B-Cell Acute Lymphoblastic Leukemia Cells. Current Issues in Molecular Biology, 45(5), 4035-4049. https://doi.org/10.3390/cimb45050257




