Anti-Inflammatory and Anti-Quorum Sensing Effect of Camellia sinensis Callus Lysate for Treatment of Acne
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Total Terpene Quantification
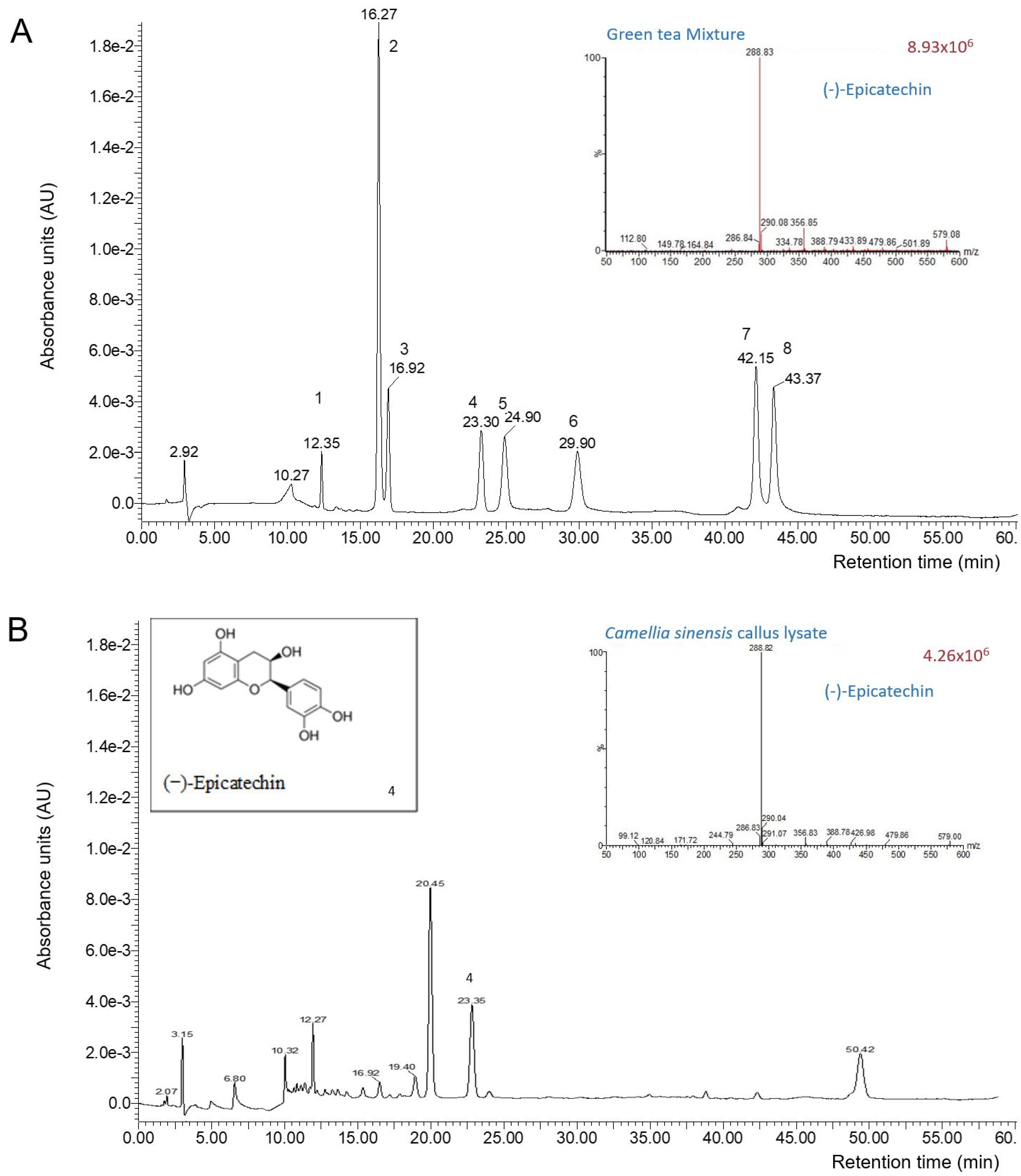
2.2.2. Catechin Quantification by Liquid Chromatography-Mass Spectrometry (LC-MS)
2.2.3. HaCaT Cells and C. acnes Stimuli
2.2.4. In Vitro Cytotoxicity Evaluation
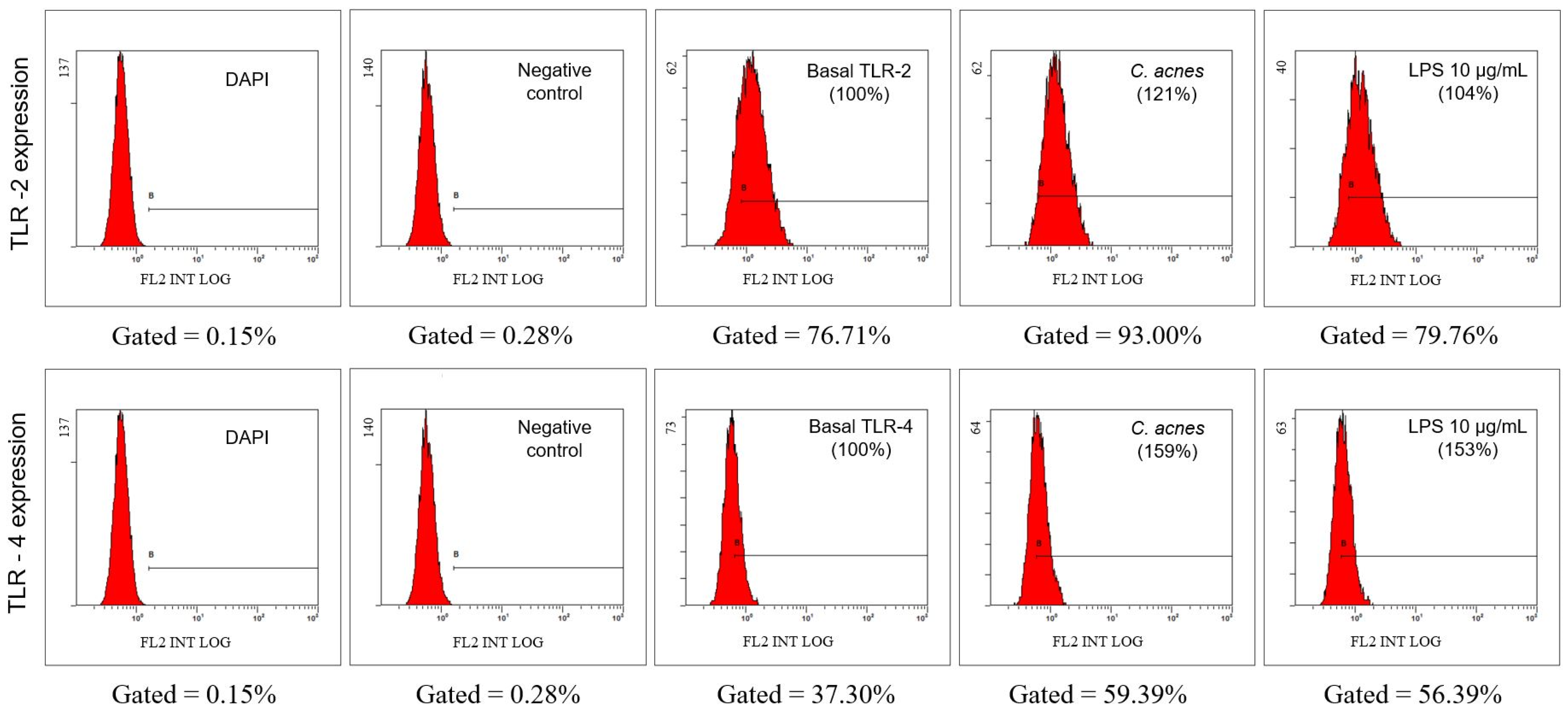
2.2.5. Evaluation of the Expression of Toll-Like Receptors (TLR) TLR2 and TLR4 in the Cell Membrane by Flow Cytometry
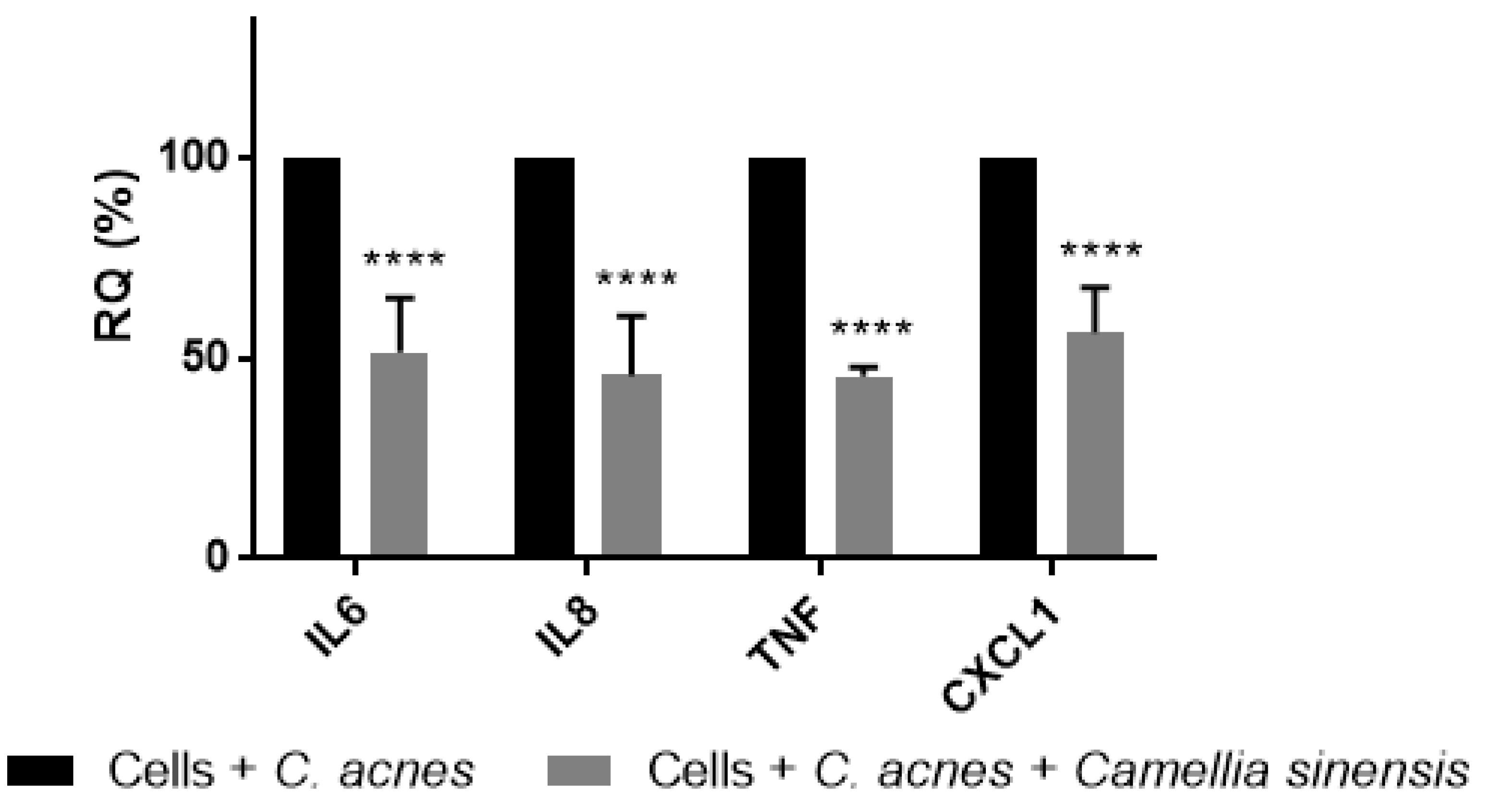
2.2.6. Camellia sinensis Anti-Inflammatory Activity Evaluation
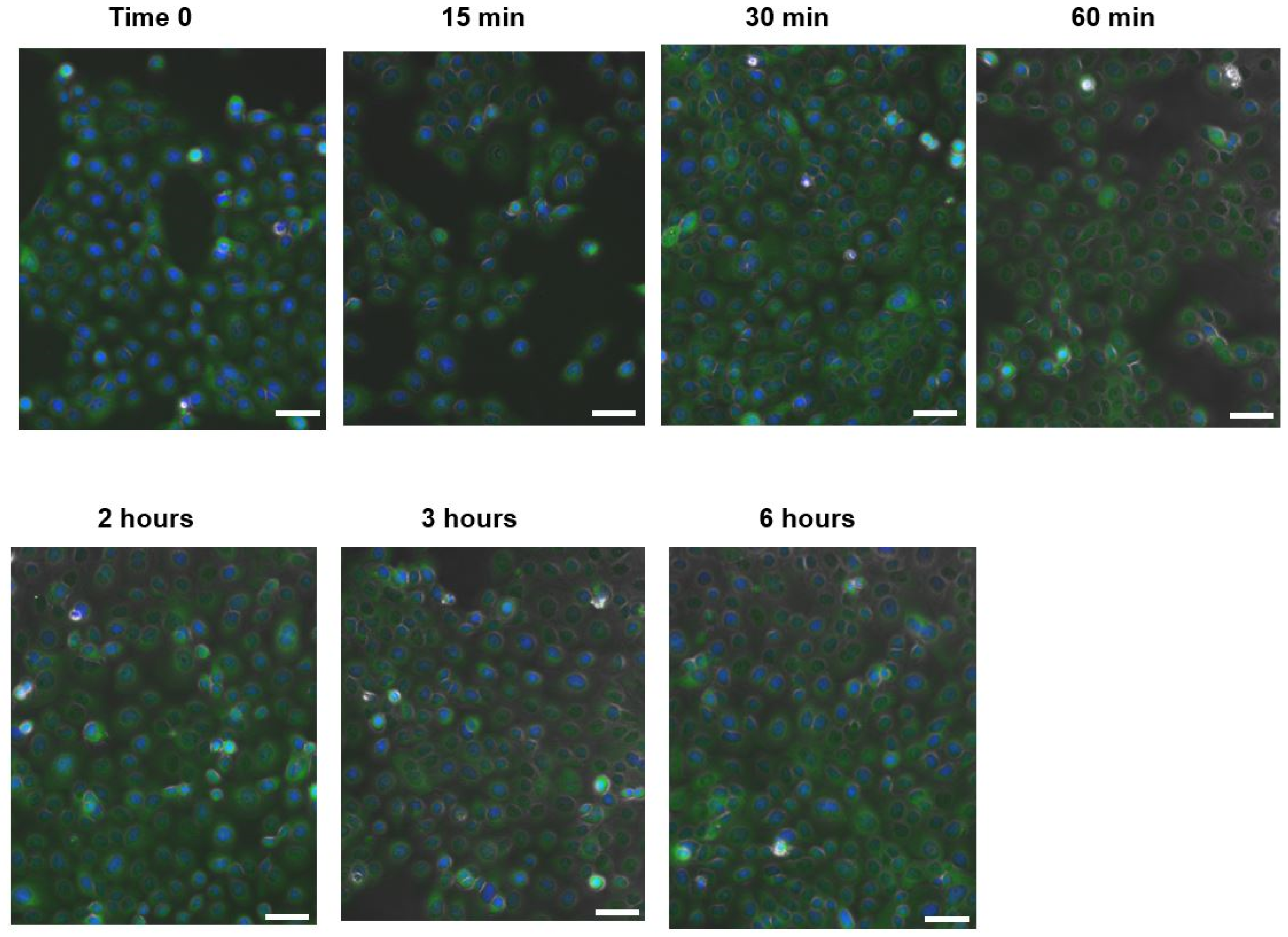
2.2.7. NF-κB Nuclear Translocation Evaluation
2.2.8. C. acnes Anti-Biofilm Activity of Camellia sinensis Callus Lysate
2.2.9. Antilipase Activity
2.2.10. Quantification of AI-2
2.2.11. Statistical Analysis of the Results
3. Results
3.1. Chemical Characterization of the Camellia sinensis Callus Extract
3.2. In Vitro Anti-Inflammatory Effect of Camellia sinensis Callus Cells Extract
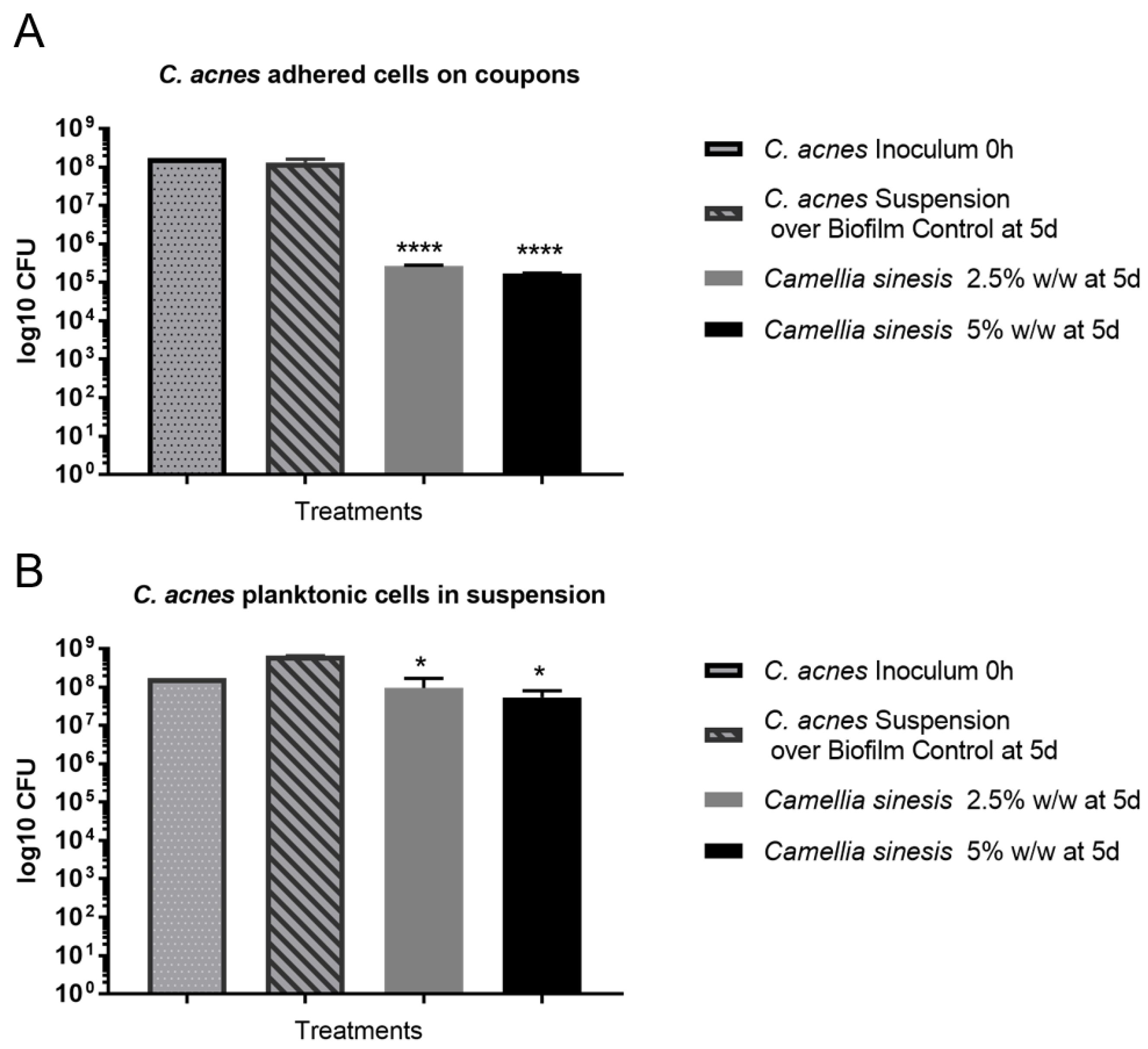
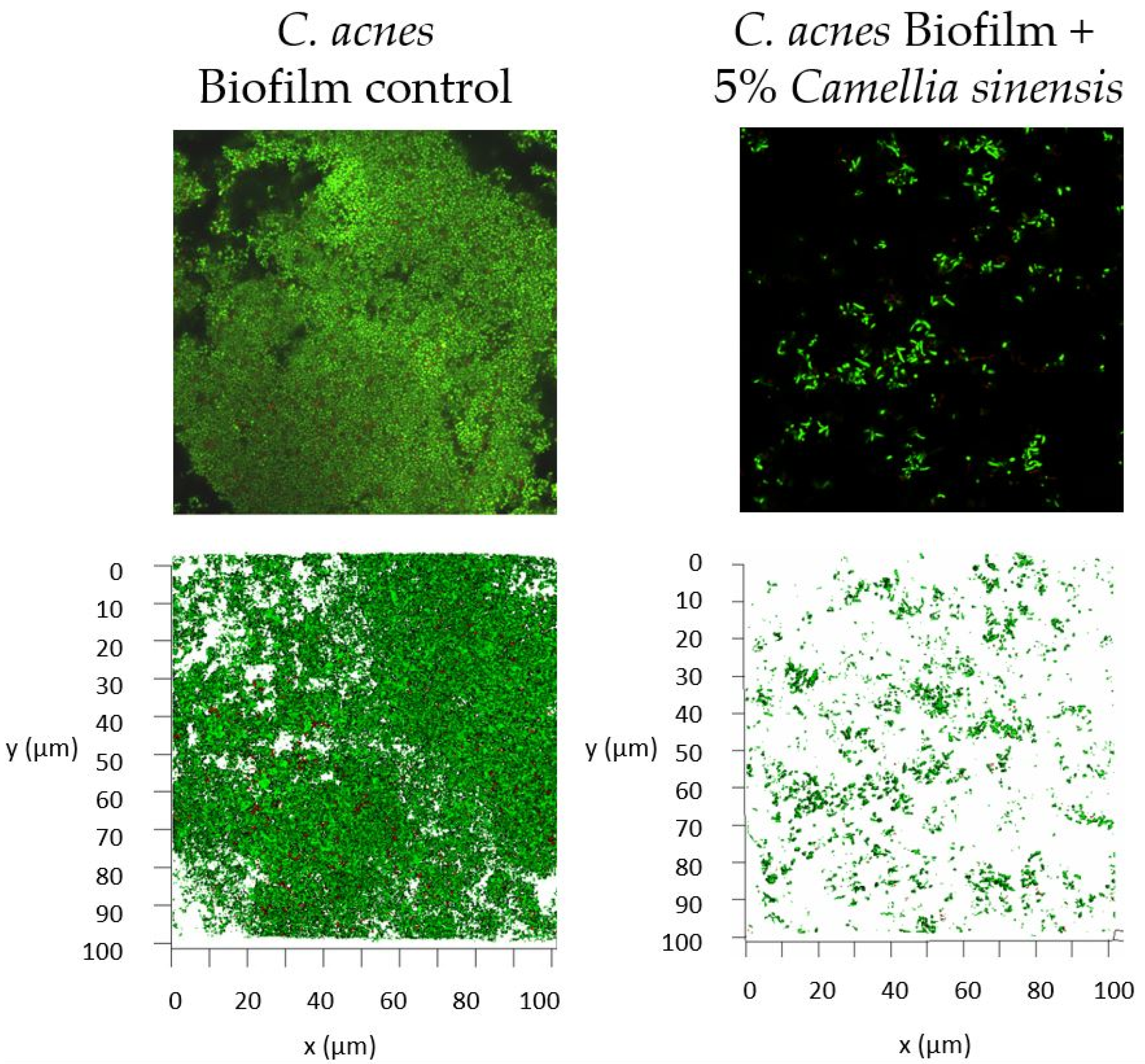
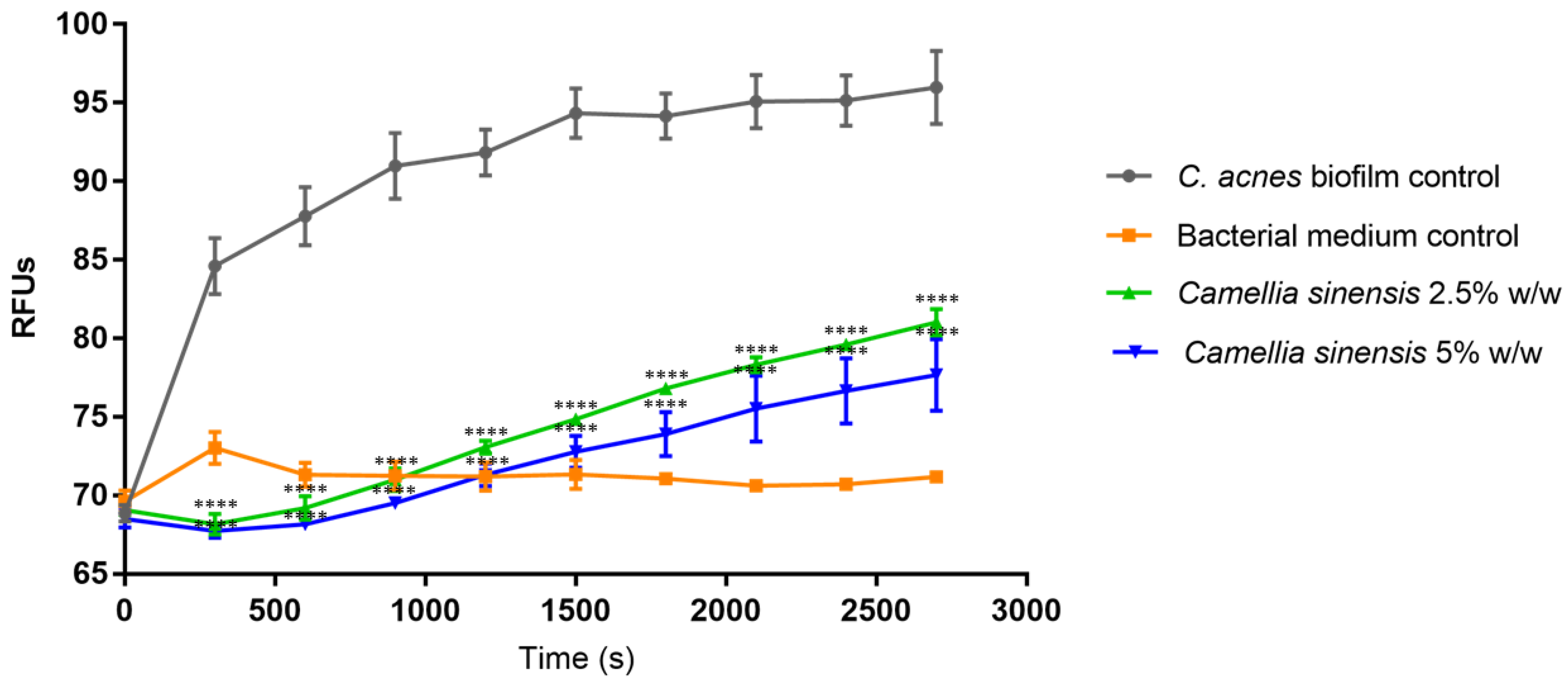
3.3. In Vitro Anti-Biofilm Activity
3.4. In Vitro Anti-Lipase Activity
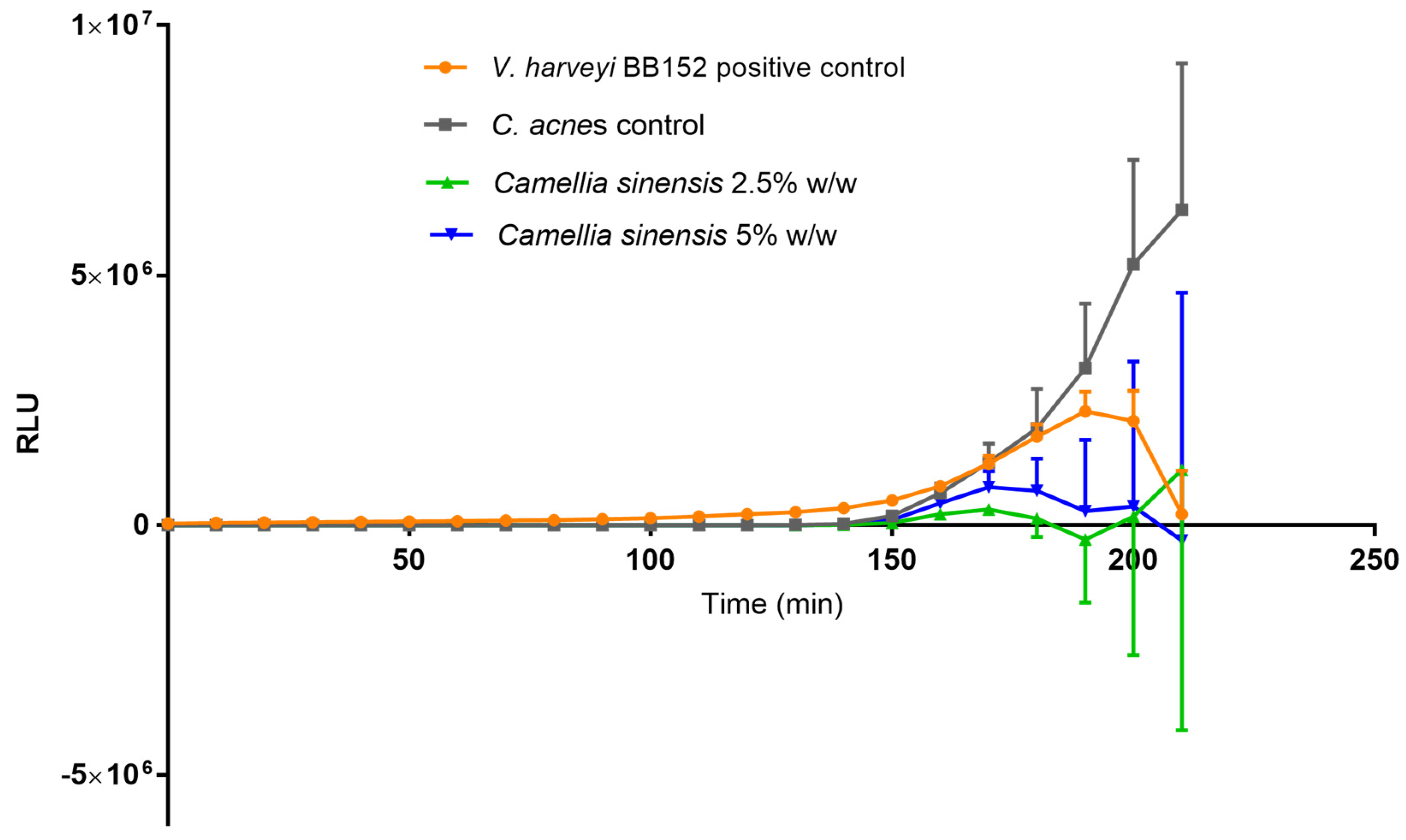
3.5. Quantification of Autoinducer 2 (AI-2)
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Greydanus, D.E.; Azmeh, R.; Cabral, M.D.; Dickson, C.A.; Patel, D.R. Acne in the first three decades of life: An update of a disorder with profound implications for all decades of life. Dis. Mon. 2021, 67, 101103. [Google Scholar] [CrossRef] [PubMed]
- Lynn, D.; Umari, T.; Dellavalle, R.; Dunnick, C. The Epidemiology of Acne Vulgaris in Late Adolescence. Adolesc. Health Med. Ther. 2016, 7, 13. [Google Scholar] [CrossRef]
- Gannesen, A.V.; Zdorovenko, E.L.; Botchkova, E.A.; Hardouin, J.; Massier, S.; Kopitsyn, D.S.; Gorbachevskii, M.V.; Kadykova, A.A.; Shashkov, A.S.; Zhurina, M.V.; et al. Composition of the Biofilm Matrix of Cutibacterium acnes Acneic Strain RT5. Front. Microbiol. 2019, 10, 1284. [Google Scholar] [CrossRef]
- Parsek, M.R.; Greenberg, E.P. Sociomicrobiology: The Connections between Quorum Sensing and Biofilms. Trends Microbiol. 2005, 13, 27–33. [Google Scholar] [CrossRef]
- Bandara, H.M.H.N.; Lam, O.L.T.; Jin, L.J.; Samaranayake, L. Microbial Chemical Signaling: A Current Perspective. Crit. Rev. Microbiol. 2012, 38, 217–249. [Google Scholar] [CrossRef] [PubMed]
- Lwin, S.M.; Kimber, I.; McFadden, J.P. Acne, Quorum Sensing and Danger. Clin. Exp. Dermatol. 2014, 39, 162–167. [Google Scholar] [CrossRef]
- Zaenglein, A.L.; Pathy, A.L.; Schlosser, B.J.; Alikhan, A.; Baldwin, H.E.; Berson, D.S.; Bowe, W.P.; Graber, E.M.; Harper, J.C.; Kang, S.; et al. Guidelines of Care for the Management of Acne Vulgaris. J. Am. Acad. Dermatol. 2016, 74, 945–973.e33. [Google Scholar] [CrossRef] [PubMed]
- Leyden, J.; Stein-Gold, L.; Weiss, J. Why Topical Retinoids Are Mainstay of Therapy for Acne. Dermatol. Ther. 2017, 7, 293–304. [Google Scholar] [CrossRef] [PubMed]
- Zasada, M.; Budzisz, E. Retinoids: Active Molecules Influencing Skin Structure Formation in Cosmetic and Dermatological Treatments. Postep. Dermatol. I Alergol. 2019, 36, 392–397. [Google Scholar] [CrossRef] [PubMed]
- Sagransky, M.; Yentzer, B.A.; Feldman, S.R. Benzoyl Peroxide: A Review of Its Current Use in the Treatment of Acne Vulgaris. Expert. Opin. Pharmacother. 2009, 10, 2555–2562. [Google Scholar] [CrossRef]
- Nast, A.; Dreno, B.; Bettoli, V.; Degitz, K.; Erdmann, R.; Finlay, A.Y.; Ganceviciene, R.; Haedersdal, M.; Layton, A.; López-Estebaranz, J.L.; et al. European Evidence-Based (S3) Guidelines for the Treatment of Acne. J. Eur. Acad. Dermatol. Venereol. 2012, 26, 1–29. [Google Scholar] [CrossRef]
- Azimi, H.; Fallah-Tafti, M.; Khakshur, A.A.; Abdollahi, M. A review of phytotherapy of acne vulgaris: Perspective of new pharmacological treatments. Fitoterapia 2012, 83, 1306–1317. [Google Scholar] [CrossRef]
- Fisk, W.A.; Lev-Tov, H.A.; Sivamani, R.K. Botanical and phytochemical therapy of acne: A systematic review. Phytother. Res. 2014, 28, 1137–1152. [Google Scholar] [CrossRef]
- Feuillolay, C.; Pecastaings, S.; Le Gac, C.; Fiorini-Puybaret, C.; Luc, J.; Joulia, P.; Roques, C. A Myrtus Communis Extract Enriched in Myrtucummulones and Ursolic Acid Reduces Resistance of Propionibacterium acnes Biofilms to Antibiotics Used in Acne Vulgaris. Phytomedicine 2016, 23, 307–315. [Google Scholar] [CrossRef] [PubMed]
- de Canha, M.N.; Komarnytsky, S.; Langhansova, L.; Lall, N. Exploring the Anti-Acne Potential of Impepho [Helichrysum odoratissimum (L.) Sweet] to Combat Cutibacterium acnes Virulence. Front. Pharmacol. 2020, 10, 1559. [Google Scholar] [CrossRef]
- Abozeid, D.; Fawzy, G.; Issa, M.; Abdeltawab, N.; Soliman, F. Medicinal Plants and Their Constituents in the Treatment of Acne Vulgaris. Biointerface Res. Appl. Chem. 2023, 13, 189. [Google Scholar] [CrossRef]
- Vuong, Q.V. Epidemiological evidence linking tea consumption to human health: A review. Crit. Rev. Food Sci. Nutr. 2014, 54, 523–536. [Google Scholar] [CrossRef] [PubMed]
- Hagiu, A.; Attin, T.; Schmidlin, P.R.; Ramenzoni, L.L. Dose-Dependent Green Tea Effect on Decrease of Inflammation in Human Oral Gingival Epithelial Keratinocytes: In Vitro Study. Clin. Oral. Investig. 2020, 24, 2375–2383. [Google Scholar] [CrossRef]
- Abe, K.; Ijiri, M.; Suzuki, T.; Taguchi, K.; Koyama, Y.; Isemura, M. Green tea with a high catechin content suppresses inflammatory cytokine expression in the galactosamine-injured rat liver. Biomed. Res. 2005, 26, 187–192. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, Y.; Miyoshi, N.; Isemura, M. Health-promoting effects of green tea. Proc. Jpn. Acad. Ser. B 2012, 88, 88–101. [Google Scholar] [CrossRef]
- Hamilton-Miller, J.M.T. Antimicrobial properties of tea (Camellia sinensis L.). Antimicrob. Agents Chemother. 1995, 39, 2375–2377. [Google Scholar] [CrossRef]
- Cox-Georgian, D.; Ramadoss, N.; Dona, C.; Basu, C. Therapeutic and Medicinal Uses of Terpenes. Med. Plants 2019, 12, 333–359. [Google Scholar] [CrossRef]
- Afzal, M.; Safer, A.M.; Menon, M. Green tea polyphenols and their potential role in health and disease. Inflammopharmacology 2015, 23, 151–161. [Google Scholar] [CrossRef]
- Wawrosch, C.; Zotchev, S.B. Production of bioactive plan secondary metabolites through in vitro technologies-status and outlook. Appl. Microbiol. Biotechnol. 2021, 18, 6649–6668. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, A.; Chiruvella, K.K.; Rao, Y.K.; Geethangili, M.; Raghavan, S.C.; Ghanta, R.G. In Vitro Production of Echioidinin, 7-O-Methywogonin from Callus Cultures of Andrographis lineata and Their Cytotoxicity on Cancer Cells. PLoS ONE 2015, 10, e0141154. [Google Scholar] [CrossRef]
- Georgiev, V.; Slavov, A.; Vasileva, I.; Pavlov, A. Plant Cell Culture as Emerging Technology for Production of Active Cosmetic Ingredients. Eng. Life Sci. 2018, 18, 779–798. [Google Scholar] [CrossRef] [PubMed]
- Efferth, T. Biotechnology Applications of Plant Callus Cultures. Engineering 2019, 5, 50–59. [Google Scholar] [CrossRef]
- Ghorai, N.; Ghorai, N.; Chakraborty, S.; Gucchait, S.; Saha, S.K.; Biswas, S. Estimation of Total Terpenoids Concentration in Plant Tissues Using a Monoterpene, Linalool as Standard Reagent. Protoc. Exch. 2012, 1–7. [Google Scholar] [CrossRef]
- Fischer, K.; Tschismarov, R.; Pilz, A.; Straubinger, S.; Carotta, S.; McDowell, A.; Decker, T. Cutibacterium acnes Infection Induces Type I Interferon Synthesis Through the CGAS-STING Pathway. Front. Immunol. 2020, 11, 2630. [Google Scholar] [CrossRef]
- Barnard, E.; Liu, J.; Yankova, E.; Cavalcanti, S.M.; Magalhães, M.; Li, H.; Patrick, S.; McDowell, A. Strains of the Propionibacterium acnes Type III Lineage Are Associated with the Skin Condition Progressive Macular Hypomelanosis. Sci. Rep. 2016, 6, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Champer, J.; Kim, J. Analysis of the Surface, Secreted, and Intracellular Proteome of Propionibacterium acnes. EuPA Open Proteom. 2015, 9, 1–7. [Google Scholar] [CrossRef]
- Denhez, B.; Rousseau, M.; Spino, C.; Dancosst, D.A.; Dumas, M.È.; Guay, A.; Lizotte, F.; Geraldes, P. Saturated fatty acids induce Insulin resistance in podocytes through inhibition of IRS1 via activation of both IKKβ and mTORC1. Sci. Rep. 2020, 10, 21628. [Google Scholar] [CrossRef]
- Coenye, T.; Peeters, E.; Nelis, H.J. Biofilm Formation by Propionibacterium acnes Is Associated with Increased Resistance to Antimicrobial Agents and Increased Production of Putative Virulence Factors. Res. Microbiol. 2007, 158, 386–392. [Google Scholar] [CrossRef]
- Humpton, T.J.; Nomura, K.; Weber, J.; Magnussen, H.M.; Hock, A.K.; Nixon, C.; Dhayade, S.; Stevenson, D.; Huang, D.T.; Strathdee, D.; et al. Differential requirements for MDM2 E3 activity during embryogenesis and in adult mice. Genes Dev. 2021, 35, 117–132. [Google Scholar] [CrossRef] [PubMed]
- Taga, M.E.; Xavier, K.B. Methods for Analysis of Bacterial Autoinducer-2 Production. Curr. Protoc. Microbiol. 2011, 23, 1C.1.1–1C.1.15. [Google Scholar] [CrossRef]
- Bae, J.; Kim, N.; Shin, Y.; Kim, S.-Y.; Kim, Y.-J. Activity of Catechins and Their Applications. Biomed. Dermatol. 2020, 4, 8. [Google Scholar] [CrossRef]
- Zhao, T.; Li, C.; Wang, S.; Song, X. Green Tea (Camellia sinensis): A Review of Its Phytochemistry, Pharmacology, and Toxicology. Molecules 2022, 27, 3909. [Google Scholar] [CrossRef]
- Dréno, B.; Dagnelie, M.A.; Khammari, A.; Corvec, S. The Skin Microbiome: A New Actor in Inflammatory Acne. Am. J. Clin. Dermatol. 2020, 21, 18–24. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Ochoa, M.T.; Krutzik, S.R.; Takeuchi, O.; Uematsu, S.; Legaspi, A.J.; Brightbill, H.D.; Holland, D.; Cunliffe, W.J.; Akira, S.; et al. Activation of Toll-Like receptor 2 in acne triggers inflammatory cytokine responses. J. Immunol. 2002, 169, 1535–1541. [Google Scholar] [CrossRef]
- Pivarcsi, A.; Bodai, L.; Réthi, B.; Kenderessy-Szabó, A.; Koreck, A.; Széll, M.; Beer, Z.; Bata-Csörgoő, Z.; Magócsi, M.; Rajnavölgyi, É.; et al. Expression and function of Toll-like receptors 2 and 4 in human keratinocytes. Int. Immunol. 2003, 15, 721–730. [Google Scholar] [CrossRef] [PubMed]
- Olaru, F.; Jensen, L.E. Chemokine expression by human keratinocyte cell lines after activation of Toll-like receptors. Exp. Dermatol. 2010, 19, e314–e316. [Google Scholar] [CrossRef]
- Takeuchi, O.; Hoshino, K.; Kawai, T.; Sanjo, H.; Takada, H.; Ogawa, T.; Takeda, K.; Akira, S. Differential Roles of TLR2 and TLR4 in Recognition of Gram-Negative and Gram-Positive Bacterial Cell Wall Components. Immunity 1999, 11, 443–451. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.C.; Shamala, L.F.; Yi, X.K.; Yan, Z.; Wei, S. Analysis of Terpene Synthase Family Genes in Camellia sinensis with an Emphasis on Abiotic Stress Conditions. Sci. Rep. 2020, 10, 933. [Google Scholar] [CrossRef] [PubMed]
- Jiang, C.; Moon, D.G.; Ma, J.; Chen, L. Characteristics of non-volatile metabolites in fresh shoots from tea plant (Camellia sinensis) and its closely related species and varieties. Beverage Plant Res. 2022, 2, 9. [Google Scholar] [CrossRef]
- Menezes, J.C.; Borges, G.B.; Gomes, F.D.; Vieira, M.D.; Marques, A.R.; Machado, A.M. Volatile compounds and quality analysis in commercial medicinal plants of Camellia sinensis. Cienc. Rural 2019, 49, 12. [Google Scholar] [CrossRef]
- Kawai, T.; Akira, S. Signaling to NF-kB by Toll-like Receptors. Trends Mol. Med. 2007, 13, 460–469. [Google Scholar] [CrossRef] [PubMed]
- Firlej, E.; Kowalska, W.; Szymaszek, K.; Roliński, J.; Bartosińska, J. The Role of Skin Immune System in Acne. J. Clin. Med. 2022, 11, 1579. [Google Scholar] [CrossRef]
- Kelhälä, H.L.; Palatsi, R.; Fyhrquist, N.; Lehtimäki, S.; Väyrynen, J.P.; Kallioinen, M.; Kubin, M.E.; Greco, D.; Tasanen, K.; Alenius, H.; et al. IL-17/Th17 Pathway Is Activated in Acne Lesions. PLoS ONE 2014, 9, e105238. [Google Scholar] [CrossRef]
- Laclaverie, M.; Rouaud-Tinguely, P.; Grimaldi, C.; Jugé, R.; Marchand, L.; Aymard, E.; Closs, B. Development and Characterization of a 3D in Vitro Model Mimicking Acneic Skin. Exp. Dermatol. 2021, 30, 347–357. [Google Scholar] [CrossRef]
- Nguyen, A.T.; Kim, K.Y. Inhibition of Proinflammatory Cytokines in Cutibacterium acnes-Induced Inflammation in HaCaT Cells by Using Buddleja Davidii Aqueous Extract. Int. J. Inflam. 2020. [Google Scholar] [CrossRef]
- Hwang, D.H.; Lee, D.Y.; Koh, P.O.; Yang, H.R.; Kang, C.; Kim, E. Rosa davurica Pall. improves Propionibacterium acnes-Induced Inflammatory Responses in Mouse Ear Edema Model and Suppresses pro-Inflammatory Chemokine Production via MAPK and NF-kB Pathways in HaCaT Cells. Int. J. Mol. Sci. 2020, 21, 1717. [Google Scholar] [CrossRef]
- Bandyopadhyay, D.; Chatterjee, T.K.; Dasgupta, A.; Lourduraja, J.; Dastidar, S.G. In Vitro and in Vivo Antimicrobial Action of Tea: The Commonest Beverage of Asia. Biol. Pharm. Bull. 2005, 28, 2125–2127. [Google Scholar] [CrossRef]
- Sowjanya, N.S.; Ramya, S.R.; Kanungo, R. In Vitro Antibacterial Activity of Green Tea (Camellia sinensis) Extract against Staphylococcus Aureus and MRSA. IP Int. J. Med. Microbiol. Trop. Dis. 2020, 4, 214–217. [Google Scholar] [CrossRef]
- Anita, P.; Sivasamy, S.; Kumar, P.M.; Balan, I.N.; Ethiraj, S. In Vitro Antibacterial Activity of Camellia sinensis Extract against Cariogenic Microorganisms. J. Basic Clin. Pharm. 2015, 6, 35. [Google Scholar] [CrossRef] [PubMed]
- Fournière, M.; Latire, T.; Souak, D.; Feuilloley, M.G.J.; Bedoux, G. Staphylococcus epidermidis and cutibacterium Acnes: Two Major Sentinels of Skin Microbiota and the Influence of Cosmetics. Microorganisms 2020, 8, 1752. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Cheng, J.W.; Zhang, Q.; Hua, Z.X.; Miao, X. Comparison of the Skin Microbiota of Patients with Acne Vulgaris and Healthy Controls. Ann. Palliat. Med. 2021, 10, 7933–7941. [Google Scholar] [CrossRef] [PubMed]
- Coenye, T.; Spittaels, K.J.; Achermann, Y. The Role of Biofilm Formation in the Pathogenesis and Antimicrobial Susceptibility of Cutibacterium acnes. Biofilm 2022, 4, 100063. [Google Scholar] [CrossRef]
- Pena, R.T.; Blasco, L.; Ambroa, A.; González-Pedrajo, B.; Fernández-García, L.; López, M.; Bleriot, I.; Bou, G.; García-Contreras, R.; Wood, T.K.; et al. Relationship between Quorum Sensing and Secretion Systems. Front. Microbiol. 2019, 10, 1100. [Google Scholar] [CrossRef]
- Unno, M.; Cho, O.; Sugita, T. Inhibition of Propionibacterium acnes Lipase Activity by the Antifungal Agent Ketoconazole. Microbiol. Immunol. 2017, 61, 42–44. [Google Scholar] [CrossRef]
- Nakase, K.; Tashiro, A.; Yamada, T.; Ikoshi, H.; Noguchi, N. Shiunko and Chuoko, topical Kampo medicines, inhibit the expression of gehA encoding the extracellular lipase in Cutibacterium acnes. J. Dermatol. 2019, 46, 308–313. [Google Scholar] [CrossRef]
- Wunnoo, S.; Saising, J.; Voravuthikunchai, S.P. Rhodomyrtone inhibits lipase production, biofilm formation, and disorganizes established biofilm in Propionibacterium acnes. Anaerobe 2017, 43, 61–68. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cañellas-Santos, M.; Rosell-Vives, E.; Montell, L.; Bilbao, A.; Goñi-de-Cerio, F.; Fernandez-Campos, F. Anti-Inflammatory and Anti-Quorum Sensing Effect of Camellia sinensis Callus Lysate for Treatment of Acne. Curr. Issues Mol. Biol. 2023, 45, 3997-4016. https://doi.org/10.3390/cimb45050255
Cañellas-Santos M, Rosell-Vives E, Montell L, Bilbao A, Goñi-de-Cerio F, Fernandez-Campos F. Anti-Inflammatory and Anti-Quorum Sensing Effect of Camellia sinensis Callus Lysate for Treatment of Acne. Current Issues in Molecular Biology. 2023; 45(5):3997-4016. https://doi.org/10.3390/cimb45050255
Chicago/Turabian StyleCañellas-Santos, Mariona, Elisabet Rosell-Vives, Laia Montell, Ainhoa Bilbao, Felipe Goñi-de-Cerio, and Francisco Fernandez-Campos. 2023. "Anti-Inflammatory and Anti-Quorum Sensing Effect of Camellia sinensis Callus Lysate for Treatment of Acne" Current Issues in Molecular Biology 45, no. 5: 3997-4016. https://doi.org/10.3390/cimb45050255
APA StyleCañellas-Santos, M., Rosell-Vives, E., Montell, L., Bilbao, A., Goñi-de-Cerio, F., & Fernandez-Campos, F. (2023). Anti-Inflammatory and Anti-Quorum Sensing Effect of Camellia sinensis Callus Lysate for Treatment of Acne. Current Issues in Molecular Biology, 45(5), 3997-4016. https://doi.org/10.3390/cimb45050255







