Advances in Molecular Regulation of Prostate Cancer Cells by Top Natural Products of Malaysia
Abstract
1. Introduction
2. Aims and Methods
3. Molecular and Genetic Factors of Prostate Cancer
3.1. Chromosomal Abnormalities and Oncogenes
3.2. Androgen Receptor
3.3. Metastasis
3.4. Arachidonic Acid Metabolism
3.5. Angiogenesis
4. Chemoprevention of Prostate Cancer
5. Clinical Management of Prostate Cancer
6. Possible Therapeutic Targets for Prostate Cancer Based on the Major Hallmarks of Cancer
6.1. Resisting Cell Death: Role of Smac/DIABLO in Cancer Progression
6.2. Activation of Invasion and Metastasis: Role of CXCL12/CXCR4 Axis in Cancer Metastasis
6.3. Induction of Angiogenesis: Role of VEGF in Angiogenesis
7. Opportunities for Phytochemicals: Food or Drug?
7.1. Research and Development of Anticancer Drugs from Natural Products
7.2. Research and Development of Nutraceuticals for Prostate Health
8. Research and Development of Natural Anticancer Products from Malay Biodiversity
8.1. Andrographis paniculata (Burm.f.) Nees
8.2. Centella asiatica (L.) Urb
8.3. Clinacanthus nutans (Burm.f.) Lindau
8.4. Eurycoma longifolia Jack
8.5. Ficus deltoidea L.
8.6. Hibiscus sabdariffa L.
8.7. Marantodes pumilum (Blume) Kuntze (syn. Labisia pumila (Blume) Mez)
8.8. Morinda citrifolia L.
8.9. Orthosiphon aristatus (Blume) Miq.
8.10. Phyllanthus niruri L.
9. Concluding Remarks
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef]
- Wang, L.; Lu, B.; He, M.; Wang, Y.; Wang, Z.; Du, L. Prostate Cancer Incidence and Mortality: Global Status and Temporal Trends in 89 Countries From 2000 to 2019. Front. Public Health 2022, 10, 811044. [Google Scholar] [CrossRef]
- Ferlay, J.; Soerjomataram, I.; Ervik, M.; Dikshit, R.; Eser, S.; Mathers, C.; Rebelo, M.; Parkin, D.M.; Forman, D.; Bray, F. GLOBOCAN 2012 v1.0, Cancer Incidence and Mortality Worldwide: IARC CancerBase No. 11; International Agency for Research on Cancer: Lyon, France, 2013. [Google Scholar]
- Cancer Research UK. U.K. Prostate Cancer Statistics. Available online: http://www.cancerresearchuk.org/health-professional/cancer-statistics/statistics-by-cancer-type/prostate-cancer#heading-Zero (accessed on 16 February 2017).
- Institut Kanser Negara. Malaysia National Cancer Registry Report 2007; National Cancer Registry, M.o.H.M.: Kuala Lampur, Malaysia, 2011.
- Wang, M.; Takahashi, A.; Liu, F.; Ye, D.; Ding, Q.; Qin, C.; Yin, C.; Zhang, Z.; Matsuda, K.; Kubo, M.; et al. Large-scale association analysis in Asians identifies new susceptibility loci for prostate cancer. Nat. Commun. 2015, 6, 8469. [Google Scholar] [CrossRef]
- Nelson, W.G.; De Marzo, A.M.; Isaacs, W.B. Prostate cancer. N. Engl. J. Med. 2003, 349, 366–381. [Google Scholar] [CrossRef]
- Liu, M.; Hai, A.; Huang, A.T. Cancer epidemiology in the Far East--contrast with the United States. Oncology 1993, 6, 99–110. [Google Scholar]
- Wynder, E.L.; Fujita, Y.; Harris, R.E.; Hirayama, T.; Hiyama, T. Comparative epidemiology of cancer between the united states and japan. A second look. Cancer 1991, 67, 746–763. [Google Scholar] [CrossRef]
- Nelson, W.G.; Demarzo, A.M.; Yegnasubramanian, S. The diet as a cause of human prostate cancer. Cancer Treat. Res. 2014, 159, 51–68. [Google Scholar] [CrossRef]
- Sugimura, T. Food and cancer. Toxicology 2002, 181–182, 17–21. [Google Scholar] [CrossRef]
- Coffey, D.S. Similarities of prostate and breast cancer: Evolution, diet, and estrogens. Urology 2001, 57, 31–38. [Google Scholar] [CrossRef]
- Seethalakshmi, L.; Bala, R.S.; Malhotra, R.K.; Austin-Ritchie, T.; Miller-Graziano, C.; Menon, M.; Luber-Narod, J. 17 beta-estradiol induced prostatitis in the rat is an autoimmune disease. J. Urol. 1996, 156, 1838–1842. [Google Scholar] [CrossRef]
- Pescatori, S.; Berardinelli, F.; Albanesi, J.; Ascenzi, P.; Marino, M.; Antoccia, A.; di Masi, A.; Acconcia, F. A Tale of Ice and Fire: The Dual Role for 17β-Estradiol in Balancing DNA Damage and Genome Integrity. Cancers 2021, 13, 1583. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Zhang, L.; Na, R.; Xu, J.; Xiong, Z.; Zhang, N.; Dai, W.; Jiang, H.; Ding, Q. Plasma genistein and risk of prostate cancer in Chinese population. Int. Urol. Nephrol. 2015, 47, 965–970. [Google Scholar] [CrossRef] [PubMed]
- Puangsombat, K.; Jirapakkul, W.; Smith, J.S. Inhibitory Activity of Asian Spices on Heterocyclic Amines Formation in Cooked Beef Patties. J. Food Sci. 2011, 76, T174–T180. [Google Scholar] [CrossRef] [PubMed]
- Morrissey, C.; Watson, R.W. Phytoestrogens and prostate cancer. Curr. Drug Targets 2003, 4, 231–241. [Google Scholar] [CrossRef] [PubMed]
- Christensen, M.J.; Quiner, T.E.; Nakken, H.L.; Lephart, E.D.; Eggett, D.L.; Urie, P.M. Combination effects of dietary soy and methylselenocysteine in a mouse model of prostate cancer. Prostate 2013, 73, 986–995. [Google Scholar] [CrossRef]
- Hwang, Y.W.; Kim, S.Y.; Jee, S.H.; Kim, Y.N.; Nam, C.M. Soy food consumption and risk of prostate cancer: A meta-analysis of observational studies. Nutr. Cancer 2009, 61, 598–606. [Google Scholar] [CrossRef]
- Sohel, M.; Sultana, H.; Sultana, T.; Mamun, A.A.; Amin, M.N.; Hossain, M.A.; Ali, M.C.; Aktar, S.; Sultana, A.; Rahim, Z.B.; et al. Chemotherapeutic Activities of Dietary Phytoestrogens against Prostate Cancer: From Observational to Clinical Studies. Curr. Pharm. Des. 2022, 28, 1561–1580. [Google Scholar] [CrossRef]
- Russo, M.; Russo, G.L.; Daglia, M.; Kasi, P.D.; Ravi, S.; Nabavi, S.F.; Nabavi, S.M. Understanding genistein in cancer: The "good" and the "bad" effects: A review. Food Chem. 2016, 196, 589–600. [Google Scholar] [CrossRef]
- Vastag, B. Soy and Prostate Cancer Study Results Mixed. JNCI J. Natl. Cancer Inst. 2007, 99, 1364–1365. [Google Scholar] [CrossRef]
- Nupponen, N.; Visakorpi, T. Molecular biology of progression of prostate cancer. Eur. Urol. 1999, 35, 351–354. [Google Scholar] [CrossRef]
- Hughes, C.; Murphy, A.; Martin, C.; Sheils, O.; O’Leary, J. Molecular pathology of prostate cancer. J. Clin. Pathol. 2005, 58, 673. [Google Scholar] [CrossRef]
- Shi, X.; Sun, M.; Liu, H.; Yao, Y.; Song, Y. Long non-coding RNAs: A new frontier in the study of human diseases. Cancer Lett. 2013, 339, 159–166. [Google Scholar] [CrossRef]
- Rubin, C. The Genetic Basis of Human Cancer. Ann. Intern. Med. 1998, 129, 759. [Google Scholar] [CrossRef]
- Shen, M.M.; Abate-Shen, C. Molecular genetics of prostate cancer: New prospects for old challenges. Genes Dev. 2010, 24, 1967–2000. [Google Scholar] [CrossRef]
- Fraser, M.; Sabelnykova, V.Y.; Yamaguchi, T.N.; Heisler, L.E.; Livingstone, J.; Huang, V.; Shiah, Y.-J.; Yousif, F.; Lin, X.; Masella, A.P.; et al. Genomic hallmarks of localized, non-indolent prostate cancer. Nature 2017, 541, 359–364. Available online: http://www.nature.com/nature/journal/v541/n7637/abs/nature20788.html#supplementary-information (accessed on 30 July 2017). [CrossRef]
- Cher, M.L.; Bova, G.S.; Moore, D.H.; Small, E.J.; Carroll, P.R.; Pin, S.S.; Epstein, J.I.; Isaacs, W.B.; Jensen, R.H. Genetic Alterations in Untreated Metastases and Androgen-independent Prostate Cancer Detected by Comparative Genomic Hybridization and Allelotyping. Cancer Res. 1996, 56, 3091–3102. [Google Scholar]
- Nupponen, N.N.; Kakkola, L.; Koivisto, P.; Visakorpi, T. Genetic Alterations in Hormone-Refractory Recurrent Prostate Carcinomas. Am. J. Pathol. 1998, 153, 141–148. [Google Scholar] [CrossRef]
- Visakorpi, T.; Kallioniemi, A.H.; Syvänen, A.-C.; Hyytinen, E.R.; Karhu, R.; Tammela, T.; Isola, J.J.; Kallioniemi, O.-P. Genetic Changes in Primary and Recurrent Prostate Cancer by Comparative Genomic Hybridization. Cancer Res. 1995, 55, 342–347. [Google Scholar]
- Taylor, B.S.; Schultz, N.; Hieronymus, H.; Gopalan, A.; Xiao, Y.; Carver, B.S.; Arora, V.K.; Kaushik, P.; Cerami, E.; Reva, B. Integrative genomic profiling of human prostate cancer. Cancer Cell 2010, 18, 11–22. [Google Scholar] [CrossRef]
- Bubendorf, L.; Kononen, J.; Koivisto, P.; Schraml, P.; Moch, H.; Gasser, T.C.; Willi, N.; Mihatsch, M.J.; Sauter, G.; Kallioniemi, O.-P. Survey of Gene Amplifications during Prostate Cancer Progression by High-Throughput Fluorescence in Situ Hybridization on Tissue Microarrays. Cancer Res. 1999, 59, 803–806. [Google Scholar]
- Rickman, D.S.; Beltran, H.; Demichelis, F.; Rubin, M.A. Biology and evolution of poorly differentiated neuroendocrine tumors. Nat. Med. 2017, 23, 664–673. [Google Scholar] [CrossRef] [PubMed]
- Min, J.; Zaslavsky, A.; Fedele, G.; McLaughlin, S.K.; Reczek, E.E.; De Raedt, T.; Guney, I.; Strochlic, D.E.; MacConaill, L.E.; Beroukhim, R. An oncogene-tumor suppressor cascade drives metastatic prostate cancer by coordinately activating Ras and nuclear factor-[kappa] B. Nat. Med. 2010, 16, 286–294. [Google Scholar] [CrossRef] [PubMed]
- Zeng, X.; Hu, Z.; Wang, Z.; Tao, J.; Lu, T.; Yang, C.; Lee, B.; Ye, Z. Upregulation of RASGRP3 expression in prostate cancer correlates with aggressive capabilities and predicts biochemical recurrence after radical prostatectomy. Prostate Cancer Prostatic Dis. 2014, 17, 119–125. [Google Scholar] [CrossRef] [PubMed]
- Morote, J.; de Torres, I.; Caceres, C.; Vallejo, C.; Schwartz, S.; Reventos, J. Prognostic value of immunohistochemical expression of the c-erbB-2 oncoprotein in metastasic prostate cancer. Int. J. Cancer 1999, 84, 421–425. [Google Scholar] [CrossRef]
- Pignon, J.-C.; Koopmansch, B.; Nolens, G.; Delacroix, L.; Waltregny, D.; Winkler, R. Androgen receptor controls EGFR and ERBB2 gene expression at different levels in prostate cancer cell lines. Cancer Res. 2009, 69, 2941–2949. [Google Scholar] [CrossRef]
- Yan, M.; Parker, B.A.; Schwab, R.; Kurzrock, R. HER2 aberrations in cancer: Implications for therapy. Cancer Treat. Rev. 2014, 40, 770–780. [Google Scholar] [CrossRef]
- Colombel, M.; Symmans, F.; Gil, S.; O’Toole, K.M.; Chopin, D.; Benson, M.; Olsson, C.A.; Korsmeyer, S.; Buttyan, R. Detection of the apoptosis-suppressing oncoprotein bc1-2 in hormone-refractory human prostate cancers. Am. J. Pathol. 1993, 143, 390–400. [Google Scholar]
- Krajewska, M.; Krajewski, S.; Epstein, J.I.; Shabaik, A.; Sauvageot, J.; Song, K.; Kitada, S.; Reed, J.C. Immunohistochemical analysis of bcl-2, bax, bcl-X, and mcl-1 expression in prostate cancers. Am. J. Pathol. 1996, 148, 1567–1576. [Google Scholar]
- McDonnell, T.J.; Troncoso, P.; Brisbay, S.M.; Logothetis, C.; Chung, L.W.K.; Hsieh, J.-T.; Tu, S.-M.; Campbell, M.L. Expression of the Protooncogene bcl-2 in the Prostate and Its Association with Emergence of Androgen-independent Prostate Cancer. Cancer Res. 1992, 52, 6940–6944. [Google Scholar]
- Pienta, K.J.; Bradley, D. Mechanisms underlying the development of androgen-independent prostate cancer. Clin. Cancer Res. 2006, 12, 1665–1671. [Google Scholar] [CrossRef]
- Delbridge, A.R.D.; Grabow, S.; Strasser, A.; Vaux, D.L. Thirty years of BCL-2: Translating cell death discoveries into novel cancer therapies. Nat. Rev. Cancer 2016, 16, 99–109. [Google Scholar] [CrossRef]
- Velcheti, V.; Karnik, S.; Bardot, S.F.; Prakash, O. Pathogenesis of Prostate Cancer: Lessons from Basic Research. Ochsner J. 2008, 8, 213–218. [Google Scholar]
- Edwards, J.; Bartlett, J. The androgen receptor and signal-transduction pathways in hormone-refractory prostate cancer. Part 2: Androgen-receptor cofactors and bypass pathways. BJU Int. 2005, 95, 1327–1335. [Google Scholar] [CrossRef]
- Chmelar, R.; Buchanan, G.; Need, E.F.; Tilley, W.; Greenberg, N.M. Androgen receptor coregulators and their involvement in the development and progression of prostate cancer. Int. J. Cancer 2007, 120, 719–733. [Google Scholar] [CrossRef]
- Denmeade, S.R.; Lin, X.S.; Isaacs, J.T. Role of programmed (apoptotic) cell death during the progression and therapy for prostate cancer. Prostate 1996, 28, 251–265. [Google Scholar] [CrossRef]
- Tan, M.H.E.; Li, J.; Xu, H.E.; Melcher, K.; Yong, E.-l. Androgen receptor: Structure, role in prostate cancer and drug discovery. Acta Pharmacol. Sin. 2015, 36, 3–23. [Google Scholar] [CrossRef]
- Scher, H.I.; Sawyers, C.L. Biology of progressive, castration-resistant prostate cancer: Directed therapies targeting the androgen-receptor signaling axis. J. Clin. Oncol. 2005, 23, 8253–8261. [Google Scholar] [CrossRef]
- Roudier, M.P.; Morrissey, C.; True, L.D.; Higano, C.S.; Vessella, R.L.; Ott, S.M. Histopathological assessment of prostate cancer bone osteoblastic metastases. J. Urol. 2008, 180, 1154–1160. [Google Scholar] [CrossRef]
- Rubens, R. Bone metastases—The clinical problem. Eur. J. Cancer 1998, 34, 210–213. [Google Scholar] [CrossRef]
- Fizazi, K.; Carducci, M.; Smith, M.; Damião, R.; Brown, J.; Karsh, L.; Milecki, P.; Shore, N.; Rader, M.; Wang, H.; et al. Denosumab versus zoledronic acid for treatment of bone metastases in men with castration-resistant prostate cancer: A randomised, double-blind study. Lancet 2011, 377, 813–822. [Google Scholar] [CrossRef]
- Geldof, A. Models for cancer skeletal metastasis: A reappraisal of Batson’s plexus. Anticancer Res. 1996, 17, 1535–1539. [Google Scholar]
- Diel, I. Historical remarks on metastasis and metastatic bone disease. In Metastatic Bone Disease; Springer: Berlin/Heidelberg, Germany, 1994; pp. 1–11. [Google Scholar]
- Kim, C.H.; Broxmeyer, H.E. SLC/exodus2/6Ckine/TCA4 induces chemotaxis of hematopoietic progenitor cells: Differential activity of ligands of CCR7, CXCR3, or CXCR4 in chemotaxis vs. suppression of progenitor proliferation. J. Leukoc. Biol. 1999, 66, 455–461. [Google Scholar] [CrossRef] [PubMed]
- Aiuti, A.; Tavian, M.; Cipponi, A.; Ficara, F.; Zappone, E.; Hoxie, J.; Peault, B.; Bordignon, C. Expression of CXCR4, the receptor for stromal cell-derived factor-1 on fetal and adult human lymphohematopoietic progenitors. Eur. J. Immunol. 1999, 29, 1823–1831. [Google Scholar] [CrossRef]
- Taichman, R.S.; Cooper, C.; Keller, E.T.; Pienta, K.J.; Taichman, N.S.; McCauley, L.K. Use of the Stromal Cell-derived Factor-1/CXCR4 Pathway in Prostate Cancer Metastasis to Bone. Cancer Res. 2002, 62, 1832. [Google Scholar] [PubMed]
- Sun, Y.X.; Wang, J.; Shelburne, C.E.; Lopatin, D.E.; Chinnaiyan, A.M.; Rubin, M.A.; Pienta, K.J.; Taichman, R.S. Expression of CXCR4 and CXCL12 (SDF-1) in human prostate cancers (PCa) in vivo. J. Cell. Biochem. 2003, 89, 462–473. [Google Scholar] [CrossRef]
- Müller, A.; Homey, B.; Soto, H.; Ge, N.; Catron, D.; Buchanan, M.E.; McClanahan, T.; Murphy, E.; Yuan, W.; Wagner, S.N. Involvement of chemokine receptors in breast cancer metastasis. Nature 2001, 410, 50–56. [Google Scholar] [CrossRef]
- Morton, R.A.; Ewing, C.M.; Nagafuchi, A.; Tsukita, S.; Isaacs, W.B. Reduction of E-Cadherin Levels and Deletion of the α-Catenin Gene in Human Prostate Cancer Cells. Cancer Res. 1993, 53, 3585–3590. [Google Scholar]
- Umbas, R.; Schalken, J.A.; Aalders, T.W.; Carter, B.S.; Karthaus, H.F.M.; Schaafsma, H.K.; Debruyne, F.M.J.; Isaacs, W.B. Expression of the Cellular Adhesion Molecule E-Cadherin Is Reduced or Absent in High-Grade Prostate Cancer. Cancer Res. 1992, 52, 5104–5109. [Google Scholar]
- Mashimo, T.; Watabe, M.; Hirota, S.; Hosobe, S.; Miura, K.; Tegtmeyer, P.J.; Rinker-Shaeffer, C.W.; Watabe, K. The expression of the KAI1 gene, a tumor metastasis suppressor, is directly activated by p53. Proc. Natl. Acad. Sci. USA 1998, 95, 11307–11311. [Google Scholar] [CrossRef]
- Hida, T.; Yatabe, Y.; Achiwa, H.; Muramatsu, H.; Kozaki, K.-i.; Nakamura, S.; Ogawa, M.; Mitsudomi, T.; Sugiura, T.; Takahashi, T. Increased expression of cyclooxygenase 2 occurs frequently in human lung cancers, specifically in adenocarcinomas. Cancer Res. 1998, 58, 3761–3764. [Google Scholar]
- Tucker, O.N.; Dannenberg, A.J.; Yang, E.K.; Zhang, F.; Teng, L.; Daly, J.M.; Soslow, R.A.; Masferrer, J.L.; Woerner, B.M.; Koki, A.T. Cyclooxygenase-2 expression is up-regulated in human pancreatic cancer. Cancer Res. 1999, 59, 987–990. [Google Scholar]
- Aggarwal, B.B.; Shishodia, S. Molecular targets of dietary agents for prevention and therapy of cancer. Biochem. Pharmacol. 2006, 71, 1397–1421. [Google Scholar] [CrossRef]
- Wolfesberger, B.; Walter, I.; Hoelzl, C.; Thalhammer, J.G.; Egerbacher, M. Antineoplastic effect of the cyclooxygenase inhibitor meloxicam on canine osteosarcoma cells. Res. Vet. Sci. 2006, 80, 308–316. [Google Scholar] [CrossRef]
- Eberhart, C.E.; Coffey, R.J.; Radhika, A.; Giardiello, F.M.; Ferrenbach, S.; Dubois, R.N. Up-regulation of cyclooxygenase 2 gene expression in human colorectal adenomas and adenocarcinomas. Gastroenterology 1994, 107, 1183–1188. [Google Scholar] [CrossRef]
- Koga, H.; Sakisaka, S.; Ohishi, M.; Kawaguchi, T.; Taniguchi, E.; Sasatomi, K.; Harada, M.; Kusaba, T.; Tanaka, M.; Kimura, R. Expression of cyclooxygenase-2 in human hepatocellular carcinoma: Relevance to tumor dedifferentiation. Hepatology 1999, 29, 688–696. [Google Scholar] [CrossRef]
- Hwang, D.; Byrne, J.; Scollard, D.; Levine, E. Expression of cyclooxygenase-1 and cyclooxygenase-2 in human breast cancer. J. Natl. Cancer Inst. 1998, 90, 455–460. [Google Scholar] [CrossRef]
- Mohammed, S.I.; Knapp, D.W.; Bostwick, D.G.; Foster, R.S.; Khan, K.N.M.; Masferrer, J.L.; Woerner, B.M.; Snyder, P.W.; Koki, A.T. Expression of cyclooxygenase-2 (COX-2) in human invasive transitional cell carcinoma (TCC) of the urinary bladder. Cancer Res. 1999, 59, 5647–5650. [Google Scholar]
- Ghosh, J.; Myers, C.E. Arachidonic Acid Stimulates Prostate Cancer Cell Growth: Critical Role of 5-Lipoxygenase. Biochem. Biophys. Res. Commun. 1997, 235, 418–423. [Google Scholar] [CrossRef]
- Yang, P.; Cartwright, C.A.; Li, J.I.N.; Wen, S.; Prokhorova, I.N.; Shureiqi, I.; Troncoso, P.; Navone, N.M.; Newman, R.A.; Kim, J. Arachidonic acid metabolism in human prostate cancer. Int. J. Oncol. 2012, 41, 1495–1503. [Google Scholar] [CrossRef]
- Werz, O.; Steinhilber, D. Therapeutic options for 5-lipoxygenase inhibitors. Pharmacol. Ther. 2006, 112, 701–718. [Google Scholar] [CrossRef]
- Rådmark, O.; Werz, O.; Steinhilber, D.; Samuelsson, B. 5-Lipoxygenase: Regulation of expression and enzyme activity. Trends Biochem. Sci. 2007, 32, 332–341. [Google Scholar] [CrossRef] [PubMed]
- Fürstenberger, G.; Krieg, P.; Müller-Decker, K.; Habenicht, A.J.R. What are cyclooxygenases and lipoxygenases doing in the driver’s seat of carcinogenesis? Int. J. Cancer 2006, 119, 2247–2254. [Google Scholar] [CrossRef]
- Ghosh, J. Targeting 5-lipoxygenase for prevention and treatment of cancer. Curr. Enzym. Inhib. 2008, 4, 18–28. [Google Scholar] [CrossRef]
- Holmgren, L.; O’Reilly, M.S.; Folkman, J. Dormancy of micrometastases: Balanced proliferation and apoptosis in the presence of angiogenesis suppression. Nat. Med. 1995, 1, 149–153. [Google Scholar] [CrossRef] [PubMed]
- Carmeliet, P. Angiogenesis in health and disease. Nat. Med. 2003, 9, 653–660. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Younes, M.; Wheeler, T.M.; Scardino, P.; Ohori, M.; Frolov, A.; Ayala, G. Expression of vascular endothelial growth factor receptor-3 (VEGFR-3) in human prostate. Prostate 2004, 58, 193–199. [Google Scholar] [CrossRef]
- Ferrara, N.; Gerber, H.-P.; LeCouter, J. The biology of VEGF and its receptors. Nat. Med. 2003, 9, 669–676. [Google Scholar] [CrossRef]
- Friesel, R.E.; Maciag, T. Molecular mechanisms of angiogenesis: Fibroblast growth factor signal transduction. FASEB J. 1995, 9, 919–925. [Google Scholar] [CrossRef]
- Battegay, E.J.; Rupp, J.; Iruela-Arispe, L.; Sage, E.H.; Pech, M. PDGF-BB modulates endothelial proliferation and angiogenesis in vitro via PDGF beta-receptors. J. Cell Biol. 1994, 125, 917–928. [Google Scholar] [CrossRef]
- Wang, B.; Hendricks, D.T.; Wamunyokoli, F.; Parker, M.I. A Growth-Related Oncogene/CXC Chemokine Receptor 2 Autocrine Loop Contributes to Cellular Proliferation in Esophageal Cancer. Cancer Res. 2006, 66, 3071–3077. [Google Scholar] [CrossRef]
- Sankar, S.; Mahooti-Brooks, N.; Bensen, L.; McCarthy, T.L.; Centrella, M.; Madri, J.A. Modulation of transforming growth factor beta receptor levels on microvascular endothelial cells during in vitro angiogenesis. J. Clin. Investig. 1996, 97, 1436–1446. [Google Scholar] [CrossRef]
- Egeblad, M.; Werb, Z. New functions for the matrix metalloproteinases in cancer progression. Nat. Rev. Cancer 2002, 2, 161–174. [Google Scholar] [CrossRef]
- He, Y.; Kozaki, K.-i.; Karpanen, T.; Koshikawa, K.; Yla-Herttuala, S.; Takahashi, T.; Alitalo, K. Suppression of Tumor Lymphangiogenesis and Lymph Node Metastasis by Blocking Vascular Endothelial Growth Factor Receptor 3 Signaling. J. Natl. Cancer Inst. 2002, 94, 819–825. [Google Scholar] [CrossRef]
- Murphy, G.; Ragde, H.; Kenny, G.; Barren, R., 3rd; Erickson, S.; Tjoa, B.; Boynton, A.; Holmes, E.; Gilbaugh, J.; Douglas, T. Comparison of prostate specific membrane antigen, and prostate specific antigen levels in prostatic cancer patients. Anticancer Res. 1995, 15, 1473–1479. [Google Scholar]
- Chang, S.S.; Heston, W.D. The clinical role of prostate-specific membrane antigen (PSMA). Urol. Oncol. 2002, 7, 7–12. [Google Scholar] [CrossRef]
- Conway, R.E.; Petrovic, N.; Li, Z.; Heston, W.; Wu, D.; Shapiro, L.H. Prostate-specific membrane antigen regulates angiogenesis by modulating integrin signal transduction. Mol. Cell Biol. 2006, 26, 5310–5324. [Google Scholar] [CrossRef]
- Gao, Y.; Zheng, H.; Li, L.; Feng, M.; Chen, X.; Hao, B.; Lv, Z.; Zhou, X.; Cao, Y. Prostate-Specific Membrane Antigen (PSMA) Promotes Angiogenesis of Glioblastoma Through Interacting With ITGB4 and Regulating NF-κB Signaling Pathway. Front. Cell Dev. Biol. 2021, 9, 598377. [Google Scholar] [CrossRef]
- Bradbury, R.; Jiang, W.G.; Cui, Y.-X. The clinical and therapeutic uses of MDM2 and PSMA and their potential interaction in aggressive cancers. Biomark. Med. 2015, 9, 1353–1370. [Google Scholar] [CrossRef]
- Watanabe, R.; Maekawa, M.; Kiyoi, T.; Kurata, M.; Miura, N.; Kikugawa, T.; Higashiyama, S.; Saika, T. PSMA-positive membranes secreted from prostate cancer cells have potency to transform vascular endothelial cells into an angiogenic state. Prostate 2021, 81, 1390–1401. [Google Scholar] [CrossRef]
- Machado, C.M.L.; Skubal, M.; Haedicke, K.; Silva, F.P.; Stater, E.P.; de Oliveira Silva, T.L.A.; Costa, E.T.; Masotti, C.; Otake, A.H.; Andrade, L.N.S.; et al. PSMA-bearing extracellular vesicles secreted from prostate cancer convert the microenvironment to a tumor-supporting, pro-angiogenic state. bioRxiv 2022, 2022-02. [Google Scholar] [CrossRef]
- Brawley, O.W. The Potential for Prostate Cancer Chemoprevention. Rev. Urol. 2002, 4, S11–S17. [Google Scholar] [CrossRef] [PubMed]
- Sporn, M.B.; Newton, D.L. Chemoprevention of cancer with retinoids. In Federation Proceedings; Federation of American Societies for Experimental Biology: Rockville, MD, USA, 1979; pp. 2528–2534. [Google Scholar]
- Kelloff, G.J.; Lieberman, R.; Steele, V.E.; Boone, C.W.; Lubet, R.A.; Kopelovitch, L.; Malone, W.A.; Crowell, J.A.; Sigman, C.C. Chemoprevention of prostate cancer: Concepts and strategies. Eur. Urol. 1999, 35, 342–350. [Google Scholar] [CrossRef] [PubMed]
- Sandhu, G.S.; Nepple, K.G.; Tanagho, Y.S.; Andriole, G.L. Prostate cancer chemoprevention. In Seminars in Oncology; WB Saunders: Philadelphia, PA, USA, 2013; pp. 276–285. [Google Scholar]
- Gonzalgo, M.L.; Isaacs, W.B. Molecular pathways to prostate cancer. J. Urol. 2003, 170, 2444–2452. [Google Scholar] [CrossRef] [PubMed]
- Epstein, J.I.; Herawi, M. Prostate needle biopsies containing prostatic intraepithelial neoplasia or atypical foci suspicious for carcinoma: Implications for patient care. J. Urol. 2006, 175, 820–834. [Google Scholar] [CrossRef] [PubMed]
- Stamatiou, K.; Alevizos, A.; Agapitos, E.; Sofras, F. Incidence of impalpable carcinoma of the prostate and of non-malignant and precarcinomatous lesions in Greek male population: An autopsy study. Prostate 2006, 66, 1319–1328. [Google Scholar] [CrossRef]
- Hong, W.K.; Lippman, S.M. Cancer chemoprevention. J. Natl. Cancer Inst. Monogr. 1994, 12, 49–53. [Google Scholar]
- Walsh, P.C. Chemoprevention of prostate cancer. N. Engl. J. Med. 2010, 362, 1237–1238. [Google Scholar] [CrossRef]
- Tsukamoto, S.; Akaza, H.; Onozawa, M.; Shirai, T.; Ideyama, Y. A five-alpha reductase inhibitor or an antiandrogen prevents the progression of microscopic prostate carcinoma to macroscopic carcinoma in rats. Cancer 1998, 82, 531–537. [Google Scholar] [CrossRef]
- Roehrborn, C.G.; Boyle, P.; Bergner, D.; Gray, T.; Gittelman, M.; Shown, T.; Melman, A.; Bracken, R.B.; deVere White, R.; Taylor, A. Serum prostate-specific antigen and prostate volume predict long-term changes in symptoms and flow rate: Results of a four-year, randomized trial comparing finasteride versus placebo. Urology 1999, 54, 662–669. [Google Scholar] [CrossRef]
- Stoner, E. Three-year safety and efficacy data on the use of finasteride in the treatment of benign prostatic hyperplasia. Urology 1994, 43, 284–294. [Google Scholar] [CrossRef]
- Bramson, H.N.; Hermann, D.; Batchelor, K.W.; Lee, F.W.; James, M.K.; Frye, S.V. Unique preclinical characteristics of GG745, a potent dual inhibitor of 5AR. J. Pharmacol. Exp. Ther. 1997, 282, 1496–1502. [Google Scholar]
- Andriole, G.L.; Bostwick, D.G.; Brawley, O.W.; Gomella, L.G.; Marberger, M.; Montorsi, F.; Pettaway, C.A.; Tammela, T.L.; Teloken, C.; Tindall, D.J.; et al. Effect of Dutasteride on the Risk of Prostate Cancer. N. Engl. J. Med. 2010, 362, 1192–1202. [Google Scholar] [CrossRef]
- Lu-Yao, G.L.; Albertsen, P.C.; Moore, D.F.; Shih, W.; Lin, Y.; DiPaola, R.S.; Barry, M.J.; Zietman, A.; O’Leary, M.; Walker-Corkery, E.; et al. Outcomes of Localized Prostate Cancer Following Conservative Management. JAMA J. Am. Med. Assoc. 2009, 302, 1202–1209. [Google Scholar] [CrossRef]
- Mongiat-Artus, P.; Peyromaure, M.; Richaud, P.; Droz, J.P.; Rainfray, M.; Jeandel, C.; Rebillard, X.; Moreau, J.L.; Davin, J.L.; Salomon, L.; et al. Recommandations pour la prise en charge du cancer de la prostate chez l’homme âgé: Un travail du comité de cancérologie de l’association française d’urologie. Progrès En Urol. 2009, 19, 810–817. [Google Scholar] [CrossRef]
- Picard, J.C.; Golshayan, A.-R.; Marshall, D.T.; Opfermann, K.J.; Keane, T.E. The multi-disciplinary management of high-risk prostate cancer. Urol. Oncol. Semin. Orig. Investig. 2012, 30, 3–15. [Google Scholar] [CrossRef]
- Pound, C. Natural History of Progression After PSA Elevation Following Radical Prostatectomy. JAMA J. Am. Med. Assoc. 1999, 281, 1591. [Google Scholar] [CrossRef]
- Uchio, E. Impact of Biochemical Recurrence in Prostate Cancer Among US Veterans. Arch. Intern. Med. 2010, 170, 1390. [Google Scholar] [CrossRef]
- Antonarakis, E.; Trock, B.; Feng, Z.; Humphreys, E.; Carducci, M.; Partin, A.; Walsh, P.; Eisenberger, M. The natural history of metastatic progression in men with PSA-recurrent prostate cancer after radical prostatectomy: 25-year follow-up. J. Clin. Oncol. 2009, 27 (Suppl. S15), 5008. [Google Scholar] [CrossRef]
- D’Amico, A.; Cote, K.; Loffredo, M.; Renshaw, A.; Schultz, D. Determinants of prostate cancer-specific survival after radiation therapy for patients with clinically localized prostate cancer. J. Clin. Oncol. 2002, 20, 4567–4573. [Google Scholar] [CrossRef]
- Paller, C.J.; Antonarakis, E.S. Cabazitaxel: A novel second-line treatment for metastatic castration-resistant prostate cancer. Drug Des. Dev. Ther. 2011, 5, 117–124. [Google Scholar] [CrossRef]
- Tannock, I.F.; Osoba, D.; Stockler, M.R.; Ernst, D.S.; Neville, A.J.; Moore, M.J.; Armitage, G.R.; Wilson, J.J.; Venner, P.M.; Coppin, C.M.; et al. Chemotherapy with mitoxantrone plus prednisone or prednisone alone for symptomatic hormone-resistant prostate cancer: A Canadian randomized trial with palliative end points. J. Clin. Oncol. 1996, 14, 1756–1764. [Google Scholar] [CrossRef] [PubMed]
- Petrylak, D.; Petrylak, C.; Tangen, M.H.A.; Hussain, P.; Lara, J.; Jones, M.; Taplin, P.; Burch, D.; Berry, C.; Moinpour, M.; et al. Docetaxel and Estramustine Compared with Mitoxantrone and Prednisone for Advanced Refractory Prostate Cancer. N. Engl. J. Med. 2004, 351, 1513–1520. [Google Scholar] [CrossRef] [PubMed]
- Fiorica, F.; Buttigliero, C.; Grigolato, D.; Muraro, M.; Turco, F.; Munoz, F.; Tucci, M. Addition of New Androgen Receptor Pathway Inhibitors to Docetaxel and Androgen Deprivation Therapy in Metastatic Hormone-Sensitive Prostate Cancer: A Systematic Review and Metanalysis. Curr. Oncol. 2022, 29, 9511–9524. [Google Scholar] [CrossRef] [PubMed]
- D’Aniello, C.; Cavaliere, C.; Foglia, C.; Facchini, S.; Uricchio, F.; Balsamo, R.; Franzese, E.; De Falco, S.; Izzo, M.; Laterza, M.; et al. Management of systemic prostate cancer: Current algorithm from castration sensitive to castration resistant setting. Eur. Rev. Med. Pharmacol. Sci. 2022, 26, 8481–8501. [Google Scholar] [CrossRef] [PubMed]
- Pozas, J.; Álvarez Rodríguez, S.; Fernández, V.A.; Burgos, J.; Santoni, M.; Manneh Kopp, R.; Molina-Cerrillo, J.; Alonso-Gordoa, T. Androgen Receptor Signaling Inhibition in Advanced Castration Resistance Prostate Cancer: What Is Expected for the Near Future? Cancers 2022, 14, 6071. [Google Scholar] [CrossRef]
- Chen, K.; O’Brien, J.; McVey, A.; Jenjitranant, P.; Kelly, B.D.; Kasivisvanathan, V.; Lawrentschuk, N.; Murphy, D.G.; Azad, A.A. Combination treatment in metastatic prostate cancer: Is the bar too high or have we fallen short? Nat. Rev. Urol. 2022, 20, 116–123. [Google Scholar] [CrossRef]
- Higano, C.S.; Hafron, J. Adherence with Oral Anticancer Therapies: Clinical Trial vs Real-world Experiences with a Focus on Prostate Cancer. J. Urol. 2022, 209, 10–1097. [Google Scholar] [CrossRef]
- Klose, P.; Werner, M.; Saha, F.; Voiß, P. Mind-body medicine in integrative uro-oncology: Studies and areas of application. Urologie 2022, 62, 27–33. [Google Scholar] [CrossRef]
- Kumar, R.; Sena, L.A.; Denmeade, S.R.; Kachhap, S. The testosterone paradox of advanced prostate cancer: Mechanistic insights and clinical implications. Nat. Rev. Urol. 2022, 1–14. [Google Scholar] [CrossRef]
- Hanahan, D.; Weinberg, R.A. The hallmarks of cancer. Cell 2000, 100, 57–70. [Google Scholar] [CrossRef]
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef]
- Blandin, A.-F.; Renner, G.; Lehmann, M.; Lelong-Rebel, I.; Martin, S.; Dontenwill, M. β1 Integrins as Therapeutic Targets to Disrupt Hallmarks of Cancer. Front. Pharmacol. 2015, 6, 279. [Google Scholar] [CrossRef]
- Vaux, D.L.; Korsmeyer, S.J. Cell death in development. Cell 1999, 96, 245–254. [Google Scholar] [CrossRef]
- Martinez-Ruiz, G.; Maldonado, V.; Ceballos-Cancino, G.; Grajeda, J.P.R.; Melendez-Zajgla, J. Role of Smac/DIABLO in cancer progression. J. Exp. Clin. Cancer Res. CR 2008, 27, 48. [Google Scholar] [CrossRef]
- Walczak, H.; Krammer, P.H. The CD95 (APO-1/Fas) and the TRAIL (APO-2L) Apoptosis Systems. Exp. Cell Res. 2000, 256, 58–66. [Google Scholar] [CrossRef]
- Fulda, S.; Debatin, K. Extrinsic versus intrinsic apoptosis pathways in anticancer chemotherapy. Oncogene 2006, 25, 4798–4811. [Google Scholar] [CrossRef]
- Green, D.R.; Kroemer, G. The Pathophysiology of Mitochondrial Cell Death. Science 2004, 305, 626. [Google Scholar] [CrossRef]
- Candé, C.; Cecconi, F.; Dessen, P.; Kroemer, G. Apoptosis-inducing factor (AIF): Key to the conserved caspase-independent pathways of cell death? J. Cell Sci. 2002, 115, 4727. [Google Scholar] [CrossRef]
- Saelens, X.; Festjens, N.; Walle, L.V.; Van Gurp, M.; van Loo, G.; Vandenabeele, P. Toxic proteins released from mitochondria in cell death. Oncogene 2004, 23, 2861–2874. [Google Scholar] [CrossRef]
- Du, C.; Fang, M.; Li, Y.; Li, L.; Wang, X. Smac, a mitochondrial protein that promotes cytochrome c–dependent caspase activation by eliminating IAP inhibition. Cell 2000, 102, 33–42. [Google Scholar] [CrossRef]
- Verhagen, A.M.; Ekert, P.G.; Pakusch, M.; Silke, J.; Connolly, L.M.; Reid, G.E.; Moritz, R.L.; Simpson, R.J.; Vaux, D.L. Identification of DIABLO, a mammalian protein that promotes apoptosis by binding to and antagonizing IAP proteins. Cell 2000, 102, 43–53. [Google Scholar] [CrossRef] [PubMed]
- Jia, L.; Patwari, Y.; Kelsey, S.M.; Srinivasula, S.M.; Agrawal, S.G.; Alnemri, E.S.; Newland, A.C. Role of Smac in human leukaemic cell apoptosis and proliferation. Oncogene 2003, 22, 1589–1599. [Google Scholar] [CrossRef] [PubMed]
- Deveraux, Q.L.; Roy, N.; Stennicke, H.R.; Van Arsdale, T.; Zhou, Q.; Srinivasula, S.M.; Alnemri, E.S.; Salvesen, G.S.; Reed, J.C. IAPs block apoptotic events induced by caspase-8 and cytochrome c by direct inhibition of distinct caspases. EMBO J. 1998, 17, 2215–2223. [Google Scholar] [CrossRef] [PubMed]
- Srinivasula, S.M.; Hegde, R.; Saleh, A.; Datta, P.; Shiozaki, E.; Chai, J.; Lee, R.-A.; Robbins, P.D.; Fernandes-Alnemri, T.; Shi, Y. A conserved XIAP-interaction motif in caspase-9 and Smac/DIABLO regulates caspase activity and apoptosis. Nature 2001, 410, 112–116. [Google Scholar] [CrossRef] [PubMed]
- Carson, J.P.; Behnam, M.; Sutton, J.N.; Du, C.; Wang, X.; Hunt, D.F.; Weber, M.J.; Kulik, G. Smac is required for cytochrome c-induced apoptosis in prostate cancer LNCaP cells. Cancer Res. 2002, 62, 18–23. [Google Scholar]
- Guo, F.; Nimmanapalli, R.; Paranawithana, S.; Wittman, S.; Griffin, D.; Bali, P.; O’Bryan, E.; Fumero, C.; Wang, H.G.; Bhalla, K. Ectopic overexpression of second mitochondria-derived activator of caspases (Smac/DIABLO) or cotreatment with N-terminus of Smac/DIABLO peptide potentiates epothilone B derivative–(BMS 247550) and Apo-2L/TRAIL–induced apoptosis. Blood 2002, 99, 3419–3426. [Google Scholar] [CrossRef]
- Fulda, S.; Wick, W.; Weller, M.; Debatin, K.-M. Smac agonists sensitize for Apo2L/TRAIL-or anticancer drug-induced apoptosis and induce regression of malignant glioma in vivo. Nat. Med. 2002, 8, 808–815. [Google Scholar] [CrossRef]
- Perimenis, P.; Galaris, A.; Voulgari, A.; Prassa, M.; Pintzas, A. IAP antagonists Birinapant and AT-406 efficiently synergise with either TRAIL, BRAF, or BCL-2 inhibitors to sensitise BRAFV600E colorectal tumour cells to apoptosis. BMC Cancer 2016, 16, 624. [Google Scholar] [CrossRef]
- Chinni, S.R.; Sivalogan, S.; Dong, Z.; Deng, X.; Bonfil, R.D.; Cher, M.L. CXCL12/CXCR4 signaling activates Akt-1 and MMP-9 expression in prostate cancer cells: The role of bone microenvironment-associated CXCL12. Prostate 2006, 66, 32–48. [Google Scholar] [CrossRef]
- Singareddy, R.; Semaan, L.; Conley-LaComb, M.K.; John, J.S.; Powell, K.; Iyer, M.; Smith, D.; Heilbrun, L.K.; Shi, D.; Sakr, W. Transcriptional regulation of CXCR4 in prostate cancer: Significance of TMPRSS2-ERG fusions. Mol. Cancer Res. 2013, 11, 1349–1361. [Google Scholar] [CrossRef]
- Conley-LaComb, M.K.; Saliganan, A.; Kandagatla, P.; Chen, Y.Q.; Cher, M.L.; Chinni, S.R. PTEN loss mediated Akt activation promotes prostate tumor growth and metastasis via CXCL12/CXCR4 signaling. Mol. Cancer 2013, 12, 85. [Google Scholar] [CrossRef]
- Sun, X.; Cheng, G.; Hao, M.; Zheng, J.; Zhou, X.; Zhang, J.; Taichman, R.S.; Pienta, K.J.; Wang, J. CXCL12/CXCR4/CXCR7 Chemokine Axis and Cancer Progression. Cancer Metastasis Rev. 2010, 29, 709–722. [Google Scholar] [CrossRef]
- Vindrieux, D.; Escobar, P.; Lazennec, G. Emerging roles of chemokines in prostate cancer. Endocr.-Relat. Cancer 2009, 16, 663–673. [Google Scholar] [CrossRef]
- Thelen, M. Dancing to the tune of chemokines. Nat. Immunol. 2001, 2, 129–134. [Google Scholar] [CrossRef]
- Gillette, J.M.; Larochelle, A.; Dunbar, C.E.; Lippincott-Schwartz, J. Intercellular transfer to signalling endosomes regulates an ex vivo bone marrow niche. Nat. Cell Biol. 2009, 11, 303–311. [Google Scholar] [CrossRef]
- Kyriakou, C.; Rabin, N.; Pizzey, A.; Nathwani, A.; Yong, K. Factors that influence short-term homing of human bone marrow-derived mesenchymal stem cells in a xenogeneic animal model. Haematologica 2008, 93, 1457–1465. [Google Scholar] [CrossRef]
- Sun, Y.X.; Schneider, A.; Jung, Y.; Wang, J.; Dai, J.; Wang, J.; Cook, K.; Osman, N.I.; Koh-Paige, A.J.; Shim, H. Skeletal localization and neutralization of the SDF-1 (CXCL12)/CXCR4 axis blocks prostate cancer metastasis and growth in osseous sites in vivo. J. Bone Miner. Res. 2005, 20, 318–329. [Google Scholar] [CrossRef]
- Engl, T.; Relja, B.; Marian, D.; Blumenberg, C.; Müller, I.; Beecken, W.-D.; Jones, J.; Ringel, E.M.; Bereiter-Hahn, J.; Jonas, D. CXCR4 chemokine receptor mediates prostate tumor cell adhesion through α5 and β3 integrins. Neoplasia 2006, 8, 290–301. [Google Scholar] [CrossRef]
- Lee, J.Y.; Kang, D.H.; Chung, D.Y.; Kwon, J.K.; Lee, H.; Cho, N.H.; Choi, Y.D.; Hong, S.J.; Cho, K.S. Meta-analysis of the relationship between CXCR4 expression and metastasis in prostate cancer. World J. Men’s Health 2014, 32, 167–175. [Google Scholar] [CrossRef]
- Folkman, J. What is the evidence that tumors are angiogenesis dependent? CancerSpectrum Knowl. Environ. 1990, 82, 4–6. [Google Scholar] [CrossRef]
- Ferrara, N.; Hillan, K.J.; Gerber, H.-P.; Novotny, W. Discovery and development of bevacizumab, an anti-VEGF antibody for treating cancer. Nat. Rev. Drug Discov. 2004, 3, 391–400. [Google Scholar] [CrossRef] [PubMed]
- Ide, A.G.; Baker, N.H.; Warren, S.L. Vascularization of the Brown Pearce rabbit epithelioma transplant as seen in the transparent ear chamber. Am. J. Roentgenol. 1939, 42, 891–899. [Google Scholar]
- Algire, G.H.; Chalkley, H.W.; Legallais, F.Y.; Park, H.D. Vascular reactions of normal and malignant tissues in vivo. I. Vascular reactions of mice to wounds and to normal and neoplastic transplants. J. Natl. Cancer Inst. 1945, 6, 73–85. [Google Scholar] [CrossRef]
- Algire, G.H. An adaptation of the transparent-chamber technique to the mouse. J. Natl. Cancer Inst. 1943, 4, 1–11. [Google Scholar]
- Folkman, J. Tumor angiogenesis: Therapeutic implications. N. Engl. J. Med. 1971, 285, 1182–1186. [Google Scholar]
- Folkman, J.; Klagsbrun, M. Angiogenic factors. Science 1987, 235, 442–448. [Google Scholar] [CrossRef]
- Klagsbrun, M.; D’Amore, P.A. Regulators of angiogenesis. Annu. Rev. Physiol. 1991, 53, 217–239. [Google Scholar] [CrossRef]
- Senger, D.R.; Galli, S.J.; Dvorak, A.M.; Perruzzi, C.A.; Harvey, V.S.; Dvorak, H.F. Tumor cells secrete a vascular permeability factor that promotes accumulation of ascites fluid. Science 1983, 219, 983–985. [Google Scholar] [CrossRef]
- Ferrara, N.; Henzel, W.J. Pituitary follicular cells secrete a novel heparin-binding growth factor specific for vascular endothelial cells. Biochem. Biophys. Res. Commun. 1989, 161, 851–858. [Google Scholar] [CrossRef]
- Carmeliet, P. VEGF as a Key Mediator of Angiogenesis in Cancer. Oncology 2005, 69 (Suppl. S3), 4–10. [Google Scholar] [CrossRef]
- Hicklin, D.J.; Ellis, L.M. Role of the Vascular Endothelial Growth Factor Pathway in Tumor Growth and Angiogenesis. J. Clin. Oncol. 2005, 23, 1011–1027. [Google Scholar] [CrossRef]
- Ferrara, N.; Carver-Moore, K.; Chen, H.; Dowd, M. Heterozygous embryonic lethality induced by targeted inactivation of the VEGF gene. Nature 1996, 380, 439. [Google Scholar] [CrossRef]
- Ferrara, N. Molecular and biological properties of vascular endothelial growth factor. J. Mol. Med. 1999, 77, 527–543. [Google Scholar] [CrossRef]
- Ferrara, N. Role of vascular endothelial growth factor in regulation of physiological angiogenesis. Am. J. Physiol.-Cell Physiol. 2001, 280, C1358–C1366. [Google Scholar] [CrossRef]
- Ferrara, N.; Davis-Smyth, T. The biology of vascular endothelial growth factor. Endocr. Rev. 1997, 18, 4–25. [Google Scholar] [CrossRef]
- Shibuya, M.; Yamaguchi, S.; Yamane, A.; Ikeda, T.; Tojo, A.; Matsushime, H.; Sato, M. Nucleotide sequence and expression of a novel human receptor-type tyrosine kinase gene (flt) closely related to the fms family. Oncogene 1990, 5, 519–524. [Google Scholar]
- Terman, B.I.; Dougher-Vermazen, M.; Carrion, M.E.; Dimitrov, D.; Armellino, D.C.; Gospodarowicz, D.; Böhlen, P. Identification of the KDR tyrosine kinase as a receptor for vascular endothelial cell growth factor. Biochem. Biophys. Res. Commun. 1992, 187, 1579–1586. [Google Scholar] [CrossRef]
- Paavonen, K.; Puolakkainen, P.; Jussila, L.; Jahkola, T.; Alitalo, K. Vascular endothelial growth factor receptor-3 in lymphangiogenesis in wound healing. Am. J. Pathol. 2000, 156, 1499–1504. [Google Scholar] [CrossRef]
- Kaipainen, A.; Korhonen, J.; Mustonen, T.; Van Hinsbergh, V.; Fang, G.-H.; Dumont, D.; Breitman, M.; Alitalo, K. Expression of the fms-like tyrosine kinase 4 gene becomes restricted to lymphatic endothelium during development. Proc. Natl. Acad. Sci. USA 1995, 92, 3566–3570. [Google Scholar] [CrossRef]
- Cao, Y.; Chen, H.; Zhou, L.; Chiang, M.-K.; Anand-Apte, B.; Weatherbee, J.A.; Wang, Y.; Fang, F.; Flanagan, J.G.; Tsang, M.L.-S. Heterodimers of Placenta growth factor/vascular endothelial growth factor endothelial activity, tumor cell expression, and high affinity binding to Flk-1/KDR. J. Biol. Chem. 1996, 271, 3154–3162. [Google Scholar] [CrossRef]
- DiSalvo, J.; Bayne, M.L.; Conn, G.; Kwok, P.W.; Trivedi, P.G.; Soderman, D.D.; Palisi, T.M.; Sullivan, K.A.; Thomas, K.A. Purification and characterization of a naturally occurring vascular endothelial growth factor· placenta growth factor heterodimer. J. Biol. Chem. 1995, 270, 7717–7723. [Google Scholar] [CrossRef] [PubMed]
- Ferrara, N. Vascular endothelial growth factor: Basic science and clinical progress. Endocr. Rev. 2004, 25, 581–611. [Google Scholar] [CrossRef] [PubMed]
- Park, J.E.; Chen, H.H.; Winer, J.; Houck, K.A.; Ferrara, N. Placenta growth factor. Potentiation of vascular endothelial growth factor bioactivity, in vitro and in vivo, and high affinity binding to Flt-1 but not to Flk-1/KDR. J. Biol. Chem. 1994, 269, 25646–25654. [Google Scholar] [CrossRef] [PubMed]
- Ferrara, N. Role of vascular endothelial growth factor in physiologic and pathologic angiogenesis: Therapeutic implications. Semin. Oncol. 2002, 29, 10–14. [Google Scholar] [CrossRef] [PubMed]
- Yoshiji, H.; Gomez, D.E.; Shibuya, M.; Thorgeirsson, U.P. Expression of vascular endothelial growth factor, its receptor, and other angiogenic factors in human breast cancer. Cancer Res. 1996, 56, 2013–2016. [Google Scholar]
- Volm, M.; Koomägi, R.; Mattern, J. Prognostic value of vascular endothelial growth factor and its receptor Flt-1 in squamous cell lung cancer. Int. J. Cancer 1997, 74, 64–68. [Google Scholar] [CrossRef]
- Ellis, L.; Takahashi, Y.; Fenoglio, C.; Cleary, K.; Bucana, C.; Evans, D. Vessel counts and vascular endothelial growth factor expression in pancreatic adenocarcinoma. Eur. J. Cancer 1998, 34, 337–340. [Google Scholar] [CrossRef]
- Tomisawa, M.; Tokunaga, T.; Oshika, Y.; Tsuchida, T.; Fukushima, Y.; Sato, H.; Kijima, H.; Yamazaki, H.; Ueyama, Y.; Tamaoki, N. Expression pattern of vascular endothelial growth factor isoform is closely correlated with tumour stage and vascularisation in renal cell carcinoma. Eur. J. Cancer 1999, 35, 133–137. [Google Scholar] [CrossRef]
- Sowter, H.; Corps, A.; Evans, A.; Clark, D.; Charnock-Jones, D.; Smith, S. Expression and localization of the vascular endothelial growth factor family in ovarian epithelial tumors. Lab. Investig. A J. Tech. Methods Pathol. 1997, 77, 607–614. [Google Scholar]
- Ferrer, F.A.; Miller, L.J.; Andrawis, R.I.; Kurtzman, S.H.; Albertsen, P.C.; Laudone, V.P.; Kreutzer, D.L. Vascular Endothelial Growth Factor (VEGF) Expression in Human Prostate Cancer: In Situ and in Vitro Expression of VEGF by Human Prostate Cancer Cells. J. Urol. 1997, 157, 2329–2333. [Google Scholar] [CrossRef]
- Warren, R.S.; Yuan, H.; Matli, M.R.; Gillett, N.A.; Ferrara, N. Regulation by vascular endothelial growth factor of human colon cancer tumorigenesis in a mouse model of experimental liver metastasis. J. Clin. Investig. 1995, 95, 1789. [Google Scholar] [CrossRef] [PubMed]
- Dias, S.; Hattori, K.; Heissig, B.; Zhu, Z.; Wu, Y.; Witte, L.; Hicklin, D.J.; Tateno, M.; Bohlen, P.; Moore, M.A. Inhibition of both paracrine and autocrine VEGF/VEGFR-2 signaling pathways is essential to induce long-term remission of xenotransplanted human leukemias. Proc. Natl. Acad. Sci. USA 2001, 98, 10857–10862. [Google Scholar] [CrossRef] [PubMed]
- Mazurakova, A.; Samec, M.; Koklesova, L.; Biringer, K.; Kudela, E.; Al-Ishaq, R.K.; Pec, M.; Giordano, F.A.; Büsselberg, D.; Kubatka, P.; et al. Anti-prostate cancer protection and therapy in the framework of predictive, preventive and personalised medicine-comprehensive effects of phytochemicals in primary, secondary and tertiary care. EPMA J. 2022, 13, 461–486. [Google Scholar] [CrossRef] [PubMed]
- Bostwick, D.G.; Cooner, W.H.; Denis, L.; Jones, G.W.; Scardino, P.T.; Murphy, G.P. The association of benign prostatic hyperplasia and cancer of the prostate. Cancer 1992, 70, 291–301. [Google Scholar] [CrossRef]
- Chokkalingam, A.P.; Nyrén, O.; Johansson, J.E.; Gridley, G.; McLaughlin, J.K.; Adami, H.O.; Hsing, A.W. Prostate carcinoma risk subsequent to diagnosis of benign prostatic hyperplasia. Cancer 2003, 98, 1727–1734. [Google Scholar] [CrossRef]
- Schantz, M.M.; Bedner, M.; Long, S.E.; Molloy, J.L.; Murphy, K.E.; Porter, B.J.; Putzbach, K.; Rimmer, C.A.; Sander, L.C.; Sharpless, K.E. Development of saw palmetto (Serenoa repens) fruit and extract standard reference materials. Anal. Bioanal. Chem. 2008, 392, 427–438. [Google Scholar] [CrossRef]
- Gerber, G.S.; Fitzpatrick, J.M. The role of a lipido-sterolic extract of Serenoa repens in the management of lower urinary tract symptoms associated with benign prostatic hyperplasia. BJU Int. 2004, 94, 338–344. [Google Scholar] [CrossRef]
- Bonnar-Pizzorno, R.M.; Littman, A.J.; Kestin, M.; White, E. Saw palmetto supplement use and prostate cancer risk. Nutr. Cancer 2006, 55, 21–27. [Google Scholar] [CrossRef]
- Bent, S.; Ko, R. Commonly used herbal medicines in the United States: A review. Am. J. Med. 2004, 116, 478–485. [Google Scholar] [CrossRef]
- Barnes, P.M.; Powell-Griner, E.; McFann, K.; Nahin, R.L. Complementary and alternative medicine use among adults: United States, 2002. In Seminars in Integrative Medicine; WB Saunders: Philadelphia, PA, USA, 2004; pp. 54–71. [Google Scholar]
- Bent, S.; Kane, C.; Shinohara, K.; Neuhaus, J.; Hudes, E.S.; Goldberg, H.; Avins, A.L. Saw Palmetto for Benign Prostatic Hyperplasia. N. Engl. J. Med. 2006, 354, 557–566. [Google Scholar] [CrossRef]
- Lowe, F.C.; Ku, J.C. Phytotherapy in treatment of benign prostatic hyperplasia: A critical review. Urology 1996, 48, 12–20. [Google Scholar] [CrossRef]
- Goldmann, W.H.; Sharma, A.L.; Currier, S.J.; Johnston, P.D.; Rana, A.; Sharma, C.P. Saw palmetto berry extract inhibits cell growth and Cox-2 expression in prostatic cancer cells. Cell Biol. Int. 2001, 25, 1117–1124. [Google Scholar] [CrossRef]
- Iguchi, K.; Okumura, N.; Usui, S.; Sajiki, H.; Hirota, K.; Hirano, K. Myristoleic acid, a cytotoxic component in the extract from Serenoa repens, induces apoptosis and necrosis in human prostatic LNCaP cells. Prostate 2001, 47, 59–65. [Google Scholar] [CrossRef]
- Hill, B.; Kyprianou, N. Effect of permixon on human prostate cell growth: Lack of apoptotic action. Prostate 2004, 61, 73–80. [Google Scholar] [CrossRef]
- Mann, J. Natural products in cancer chemotherapy: Past, present and future. Nat Rev Cancer 2002, 2, 143–148. [Google Scholar] [CrossRef]
- Katiyar, C.; Gupta, A.; Kanjilal, S.; Katiyar, S. Drug discovery from plant sources: An integrated approach. Ayu 2012, 33, 10–19. [Google Scholar] [CrossRef]
- Monks, A.; Scudiero, D.; Skehan, P.; Shoemaker, R.; Paull, K.; Vistica, D.; Hose, C.; Langley, J.; Cronise, P.; Vaigro-Wolff, A. Feasibility of a high-flux anticancer drug screen using a diverse panel of cultured human tumor cell lines. J. Natl. Cancer Inst. 1991, 83, 757–766. [Google Scholar] [CrossRef]
- Ganesan, A. The impact of natural products upon modern drug discovery. Curr. Opin. Chem. Biol. 2008, 12, 306–317. [Google Scholar] [CrossRef]
- Bohn, T.; McDougall, G.J.; Alegría, A.; Alminger, M.; Arrigoni, E.; Aura, A.-M.; Brito, C.; Cilla, A.; El, S.N.; Karakaya, S.; et al. Mind the gap—Deficits in our knowledge of aspects impacting the bioavailability of phytochemicals and their metabolites—A position paper focusing on carotenoids and polyphenols. Mol. Nutr. Food Res. 2015, 59, 1307–1323. [Google Scholar] [CrossRef]
- Cordes, E.H.; Cordes, E.H. 103C7The discovery of taxol: Wall, Horwitz, Holton. In Hallelujah Moments: Tales of Drug Discovery; Oxford University Press: Oxford, UK, 2020. [Google Scholar] [CrossRef]
- Barani, M.; Sangiovanni, E.; Angarano, M.; Rajizadeh, M.A.; Mehrabani, M.; Piazza, S.; Gangadharappa, H.V.; Pardakhty, A.; Mehrbani, M.; Dell’Agli, M.; et al. Phytosomes as Innovative Delivery Systems for Phytochemicals: A Comprehensive Review of Literature. Int. J. Nanomed. 2021, 16, 6983–7022. [Google Scholar] [CrossRef]
- Suvarna, V.; Gujar, P.; Murahari, M. Complexation of phytochemicals with cyclodextrin derivatives—An insight. Biomed. Pharmacother. 2017, 88, 1122–1144. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Wang, Y.; Yang, C.; Wang, P.; Oelschlager, D.K.; Zheng, Y.; Tian, D.A.; Grizzle, W.E.; Buchsbaum, D.J.; Wan, M. Polyethylene glycosylated curcumin conjugate inhibits pancreatic cancer cell growth through inactivation of Jab1. Mol. Pharmacol. 2009, 76, 81–90. [Google Scholar] [CrossRef] [PubMed]
- Rijo, P.; Matias, D.; Fernandes, A.; Simões, M.; Nicolai, M.; Reis, C. Antimicrobial Plant Extracts Encapsulated into Polymeric Beads for Potential Application on the Skin. Polymers 2014, 6, 479–490. [Google Scholar] [CrossRef]
- Date, A.A.; Hanes, J.; Ensign, L.M. Nanoparticles for oral delivery: Design, evaluation and state-of-the-art. J. Control Release 2016, 240, 504–526. [Google Scholar] [CrossRef]
- Xie, J.; Yang, Z.; Zhou, C.; Zhu, J.; Lee, R.J.; Teng, L. Nanotechnology for the delivery of phytochemicals in cancer therapy. Biotechnol. Adv. 2016, 34, 343–353. [Google Scholar] [CrossRef]
- Huang, Q.; Yu, H.; Ru, Q. Bioavailability and Delivery of Nutraceuticals Using Nanotechnology. J. Food Sci. 2010, 75, R50–R57. [Google Scholar] [CrossRef]
- Chan, J.; Elkin, E.; Silva, S.; Broering, J.; Latini, D.; Carroll, P. Total and specific complementary and alternative medicine use in a large cohort of men with prostate cancer. Urology 2005, 66, 1223–1228. [Google Scholar] [CrossRef]
- Lee, M.M.; Chang, J.S.; Jacobs, B.; Wrensch, M.R. Complementary and alternative medicine use among men with prostate cancer in 4 ethnic populations. Am. J. Public Health 2002, 92, 1606–1609. [Google Scholar] [CrossRef]
- Trottier, G.; Bostrom, P.J.; Lawrentschuk, N.; Fleshner, N.E. Nutraceuticals and prostate cancer prevention: A current review. Nat Rev Urol 2010, 7, 21–30. [Google Scholar] [CrossRef]
- Curtis Nickel, J.; Shoskes, D.; Roehrborn, C.G.; Moyad, M. Nutraceuticals in Prostate Disease: The Urologist’s Role. Rev. Urol. 2008, 10, 192–206. [Google Scholar]
- Coppens, P.; Da Silva, M.F.; Pettman, S. European regulations on nutraceuticals, dietary supplements and functional foods: A framework based on safety. Toxicology 2006, 221, 59–74. [Google Scholar] [CrossRef]
- Arora, M.; Sharma, S.; Baldi, A. Comparative insight of regulatory guidelines for probiotics in USA, India and Malaysia: A critical review. Int. J. Biotechnol. Wellness Ind. 2013, 2, 51–64. [Google Scholar]
- Nelson, V.K.; Pullaiah, C.P.; Saleem Ts, M.; Roychoudhury, S.; Chinnappan, S.; Vishnusai, B.; Ram Mani, R.; Birudala, G.; Bottu, K.S. Natural Products as the Modulators of Oxidative Stress: An Herbal Approach in the Management of Prostate Cancer. Adv. Exp. Med. Biol. 2022, 1391, 161–179. [Google Scholar] [CrossRef]
- Singh, K.; Bhori, M.; Kasu, Y.A.; Bhat, G.; Marar, T. Antioxidants as precision weapons in war against cancer chemotherapy induced toxicity—Exploring the armoury of obscurity. Saudi Pharm. J. 2018, 26, 177–190. [Google Scholar] [CrossRef]
- Anon. National Agrofood Policy 2011–2020. Industry, M.o.A.a.A., Ed.; Malaysia. 2016. Available online: https://ap.fftc.org.tw/article/1368 (accessed on 30 December 2022).
- Akarasereenont, P.; Datiles, M.J.R.; Lumlerdkij, N.; Yaakob, H.; Prieto, J.M.; Heinrich, M. A South-East Asian Perspective on Ethnopharmacology. Ethnopharmacology 2015, 317–332. [Google Scholar] [CrossRef]
- Idris, M.K.H.; Hasham, R.; Ismail, H.F. Bioassay-Guided extraction of andrographis paniculata for intervention of in-vitro prostate cancer progression in metabolic syndrome environment. Daru 2022, 30, 253–272. [Google Scholar] [CrossRef]
- Vetvicka, V.; Vannucci, L. Biological properties of andrographolide, an active ingredient of Andrographis Paniculata: A narrative review. Ann. Transl. Med. 2021, 9, 1186. [Google Scholar] [CrossRef]
- Mir, H.; Kapur, N.; Singh, R.; Sonpavde, G.; Lillard, J.W., Jr.; Singh, S. Andrographolide inhibits prostate cancer by targeting cell cycle regulators, CXCR3 and CXCR7 chemokine receptors. Cell Cycle 2016, 15, 819–826. [Google Scholar] [CrossRef]
- Zhao, F.; He, E.Q.; Wang, L.; Liu, K. Anti-tumor activities of andrographolide, a diterpene from Andrographis paniculata, by inducing apoptosis and inhibiting VEGF level. J. Asian Nat. Prod. Res. 2008, 10, 467–473. [Google Scholar] [CrossRef]
- Chun, J.Y.; Tummala, R.; Nadiminty, N.; Lou, W.; Liu, C.; Yang, J.; Evans, C.P.; Zhou, Q.; Gao, A.C. Andrographolide, an herbal medicine, inhibits interleukin-6 expression and suppresses prostate cancer cell growth. Genes Cancer 2010, 1, 868–876. [Google Scholar] [CrossRef]
- Forestier-Román, I.S.; López-Rivas, A.; Sánchez-Vázquez, M.M.; Rohena-Rivera, K.; Nieves-Burgos, G.; Ortiz-Zuazaga, H.; Torres-Ramos, C.A.; Martínez-Ferrer, M. Andrographolide induces DNA damage in prostate cancer cells. Oncotarget 2019, 10, 1085–1101. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.-J.; Fan, M.; Wedamulla, N.E.; Tang, Y.; Bae, S.M.; Hwang, J.-Y.; Kim, E.-K. Inhibitory effects of Centella asiatica (L.) Urban on enlarged prostate through androgen receptor and PI3K/Akt signaling pathways. Food Funct. 2022, 13, 10235–10247. [Google Scholar] [CrossRef] [PubMed]
- Alafnan, A.; Hussain, T.; Rizvi, S.M.D.; Moin, A.; Alamri, A. Prostate Apoptotic Induction and NFκB Suppression by Dammarolic Acid: Mechanistic Insight into Onco-Therapeutic Action of an Aglycone Asiaticoside. Curr. Issues Mol. Biol. 2021, 43, 932–940. [Google Scholar] [CrossRef] [PubMed]
- Hsu, Y.L.; Kuo, P.L.; Lin, L.T.; Lin, C.C. Asiatic acid, a triterpene, induces apoptosis and cell cycle arrest through activation of extracellular signal-regulated kinase and p38 mitogen-activated protein kinase pathways in human breast cancer cells. J. Pharmacol. Exp. Ther. 2005, 313, 333–344. [Google Scholar] [CrossRef]
- Adtani, P.N.; Narasimhan, M.; Punnoose, A.M.; Kambalachenu, H.R. Antifibrotic effect of Centella asiatica Linn and asiatic acid on arecoline-induced fibrosis in human buccal fibroblasts. J. Investig. Clin. Dent. 2017, 8, e12208. [Google Scholar] [CrossRef]
- Hao, Y.; Huang, J.; Ma, Y.; Chen, W.; Fan, Q.; Sun, X.; Shao, M.; Cai, H. Asiatic acid inhibits proliferation, migration and induces apoptosis by regulating Pdcd4 via the PI3K/Akt/mTOR/p70S6K signaling pathway in human colon carcinoma cells. Oncol. Lett. 2018, 15, 8223–8230. [Google Scholar] [CrossRef]
- Zulkipli, I.N.; Rajabalaya, R.; Idris, A.; Sulaiman, N.A.; David, S.R. Clinacanthus nutans: A review on ethnomedicinal uses, chemical constituents and pharmacological properties. Pharm. Biol. 2017, 55, 1093–1113. [Google Scholar] [CrossRef]
- Teoh, P.L.; Cheng, A.Y.; Liau, M.; Lem, F.F.; Kaling, G.P.; Chua, F.N.; Cheong, B.E. Chemical composition and cytotoxic properties of Clinacanthus nutans root extracts. Pharm. Biol. 2017, 55, 394–401. [Google Scholar] [CrossRef]
- Ng, P.Y.; Chye, S.M.; Ng Ch, H.; Koh, R.Y.; Tiong, Y.L.; Pui, L.P.; Tan, Y.H.; Lim, C.S.; Ng Kh, Y. Clinacanthus Nutans Hexane Extracts Induce Apoptosis Through a Caspase-Dependent Pathway in Human Cancer Cell Lines. Asian Pac. J. Cancer Prev. 2017, 18, 917–926. [Google Scholar] [CrossRef]
- Md Toha, Z.; Haron, N.H.; Kamal, N.N.S.N.M.; Khairuddean, M.; Arsad, H. Exploring Clinacanthus nutans leaf different solvent extracts on antiproliferative effects induced metastasis through apoptosis and cell cycle against MCF-7 human breast cancer cell lines. Future J. Pharm. Sci. 2022, 8, 49. [Google Scholar] [CrossRef]
- Fazil, F.N.; Azzimi, N.S.; Yahaya, B.H.; Kamalaldin, N.A.; Zubairi, S.I. Kinetics Extraction Modelling and Antiproliferative Activity of Clinacanthus nutans Water Extract. Sci. World J. 2016, 2016, 7370536. [Google Scholar] [CrossRef]
- George, A.; Henkel, R. Phytoandrogenic properties of Eurycoma longifolia as natural alternative to testosterone replacement therapy. Andrologia 2014, 46, 708–721. [Google Scholar] [CrossRef]
- Tong, K.L.; Chan, K.L.; AbuBakar, S.; Low, B.S.; Ma, H.Q.; Wong, P.F. The in vitro and in vivo anti-cancer activities of a standardized quassinoids composition from Eurycoma longifolia on LNCaP human prostate cancer cells. PloS ONE 2015, 10, e0121752. [Google Scholar] [CrossRef]
- Berg, C. Flora Malesiana precursor for the treatment of Moraceae 3: Ficus subgenus Ficus. Blumea-Biodivers. Evol. Biogeogr. Plants 2003, 48, 529–550. [Google Scholar] [CrossRef]
- Burkill, I.H.; Haniff, M. Malay Village Medicine: Prescriptions Collected by; University Press: Oxford, UK, 1930. [Google Scholar]
- Abdullah, N.; Karsani, S.; Aminudin, N. Effects of Ficus Deltoidea Extract on the Serum Protein Profile of Simultaneously Hypertensive Rats (SHR). J. Proteom. Bioinform. (JPB) 2008, 2, 143. [Google Scholar] [CrossRef]
- Hadijah, H.; Normah, A.; Ahmad Tarmizi, S.; Aida, M. Cholesterol lowering effect of mas cotek tea in hypercholesterolemic rats. In Proceedings of the 2nd International Conference of East-West Perspective of Functional Food Science, Kuala Lumpur, Malaysia, 5–7 November 2007. [Google Scholar]
- Bunawan, H.; Amin, N.M.; Bunawan, S.N.; Baharum, S.N.; Mohd Noor, N. Ficus deltoidea Jack: A Review on Its Phytochemical and Pharmacological Importance. Evid.-Based Complement. Altern. Med. 2014, 2014, 902734. [Google Scholar] [CrossRef]
- Zakaria, Z.A.; Hussain, M.K.; Mohamad, A.S.; Abdullah, F.C.; Sulaiman, M.R. Anti-Inflammatory Activity of the Aqueous Extract of Ficus Deltoidea. Biol. Res. Nurs. 2012, 14, 90–97. [Google Scholar] [CrossRef]
- Abdullah, Z.; Hussain, K.; Ismail, Z.; Ali, R.M. Anti-inflammatory activity of standardised extracts of leaves of three varieties of Ficus deltoidea. Asian J. Pharm. Clin. Res. 2009, 1, 100–105. [Google Scholar]
- Uyub, A.M.; Nwachukwu, I.N.; Azlan, A.A.; Fariza, S.S. In-vitro antibacterial activity and cytotoxicity of selected medicinal plant extracts from Penang Island Malaysia on metronidazole-resistant-Helicobacter pylori and some pathogenic bacteria. Ethnobot. Res. Appl. 2010, 8, 95–106. [Google Scholar] [CrossRef]
- Akhir, N.A.M.; Chua, L.S.; Majid, F.A.A.; Sarmidi, M.R. Cytotoxicity of aqueous and ethanolic extracts of Ficus deltoidea on human ovarian carcinoma cell line. Br. J. Med. Med. Res. 2011, 1, 397–409. [Google Scholar] [CrossRef]
- Zhou, Y.; Liu, Y.E.; Cao, J.; Zeng, G.; Shen, C.; Li, Y.; Zhou, M.; Chen, Y.; Pu, W.; Potters, L. Vitexins, nature-derived lignan compounds, induce apoptosis and suppress tumor growth. Clin. Cancer Res. 2009, 15, 5161–5169. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.P.; Yang, J.S.; Lin, J.J.; Lai, K.C.; Lu, H.F.; Ma, C.Y.; Sai-Chuen Wu, R.; Wu, K.C.; Chueh, F.S.; Gibson Wood, W. Rutin inhibits human leukemia tumor growth in a murine xenograft model in vivo. Environ. Toxicol. 2012, 27, 480–484. [Google Scholar] [CrossRef]
- Zheng, S.-Y.; Li, Y.; Jiang, D.; Zhao, J.; Ge, J.-F. Anticancer effect and apoptosis induction by quercetin in the human lung cancer cell line A-549. Mol. Med. Rep. 2012, 5, 822–826. [Google Scholar] [CrossRef] [PubMed]
- Guo, Q.; Tian, X.; Yang, A.; Zhou, Y.; Wu, D.; Wang, Z. Orientin in Trollius chinensis Bunge inhibits proliferation of HeLa human cervical carcinoma cells by induction of apoptosis. Mon. Für Chem.-Chem. Mon. 2014, 145, 229–233. [Google Scholar] [CrossRef]
- Chiang, Y.-M.; Kuo, Y.-H. Novel Triterpenoids from the Aerial Roots of Ficus m icrocarpa. J. Org. Chem. 2002, 67, 7656–7661. [Google Scholar] [CrossRef] [PubMed]
- Chiang, Y.-M.; Chang, J.-Y.; Kuo, C.-C.; Chang, C.-Y.; Kuo, Y.-H. Cytotoxic triterpenes from the aerial roots of Ficus microcarpa. Phytochemistry 2005, 66, 495–501. [Google Scholar] [CrossRef]
- Hanafi, M.M.M.; Afzan, A.; Yaakob, H.; Aziz, R.; Sarmidi, M.R.; Wolfender, J.L.; Prieto, J.M. In Vitro Pro-apoptotic and Anti-migratory Effects of Ficus deltoidea L. Plant Extracts on the Human Prostate Cancer Cell Lines PC3. Front. Pharmacol. 2017, 8, 895. [Google Scholar] [CrossRef]
- Worawattananutai, P.; Itharat, A.; Ruangnoo, S. In vitro antioxidant, anti-inflammatory, cytotoxic activities against prostate cancer of extracts from Hibiscus sabdariffa leaves. J. Med. Assoc. Thail. 2014, 97 (Suppl. S8), S81–S87. [Google Scholar]
- Chiu, C.T.; Chen, J.H.; Chou, F.P.; Lin, H.H. Hibiscus sabdariffa Leaf Extract Inhibits Human Prostate Cancer Cell Invasion via Down-Regulation of Akt/NF-kB/MMP-9 Pathway. Nutrients 2015, 7, 5065–5087. [Google Scholar] [CrossRef]
- Song, B.; Shen, X.; Tong, C.; Zhang, S.; Chen, Q.; Li, Y.; Li, S. Gossypin: A flavonoid with diverse pharmacological effects. Chem. Biol. Drug Des. 2023, 101, 131–137. [Google Scholar] [CrossRef]
- Chen, D.L.; Ma, G.X.; Yang, E.L.; Yang, Y.; Wang, C.H.; Sun, Z.C.; Liang, H.Q.; Xu, X.D.; Wei, J.H. Cadinane-type sesquiterpenoid dimeric diastereomers hibisceusones A-C from infected stems of Hibiscus tiliaceus with cytotoxic activity against triple-negative breast cancer cells. Bioorganic Chem. 2022, 127, 105982. [Google Scholar] [CrossRef]
- Rasul, H.O.; Aziz, B.K.; Ghafour, D.D.; Kivrak, A. Screening the possible anti-cancer constituents of Hibiscus rosa-sinensis flower to address mammalian target of rapamycin: An in silico molecular docking, HYDE scoring, dynamic studies, and pharmacokinetic prediction. Mol. Divers. 2022, 1–24. [Google Scholar] [CrossRef]
- Laskar, Y.B.; Mazumder, P.B.; Talukdar, A.D. Hibiscus sabdariffa anthocyanins are potential modulators of estrogen receptor alpha activity with favourable toxicology: A computational analysis using molecular docking, ADME/Tox prediction, 2D/3D QSAR and molecular dynamics simulation. J. Biomol. Struct. Dyn. 2023, 41, 611–633. [Google Scholar] [CrossRef]
- Jamal, J.A.; Houghton, P.; Milligan, S.; Jantan, I. The Oestrogenis and cytotoxic effects of the extracts of Labisia pumila var. alata and Labisia pumila var. pumila in vitro. Malays. J. Health Sci. 2003, 1, 53–60. [Google Scholar]
- Ibrahim, M.H.; Jaafar, H.Z. Photosynthetic capacity, photochemical efficiency and chlorophyll content of three varieties of Labisia pumila Benth. exposed to open field and greenhouse growing conditions. Acta Physiol. Plant. 2011, 33, 2179–2185. [Google Scholar] [CrossRef]
- Al-Mekhlafi, N.A.; Shaari, K.; Abas, F.; Kneer, R.; Jeyaraj, E.J.; Stanslas, J.; Yamamoto, N.; Honda, T.; Lajis, N.H. Alkenylresorcinols and cytotoxic activity of the constituents isolated from Labisia pumila. Phytochemistry 2012, 80, 42–49. [Google Scholar] [CrossRef]
- Chua, L.S.; Lee, S.Y.; Abdullah, N.; Sarmidi, M.R. Review on Labisia pumila (Kacip Fatimah): Bioactive phytochemicals and skin collagen synthesis promoting herb. Fitoterapia 2012, 83, 1322–1335. [Google Scholar] [CrossRef]
- Chen, H.M.; Wu, Y.C.; Chia, Y.C.; Chang, F.R.; Hsu, H.K.; Hsieh, Y.C.; Chen, C.C.; Yuan, S.S. Gallic acid, a major component of Toona sinensis leaf extracts, contains a ROS-mediated anti-cancer activity in human prostate cancer cells. Cancer Lett. 2009, 286, 161–171. [Google Scholar] [CrossRef]
- Albrecht, D.S.; Clubbs, E.A.; Ferruzzi, M.; Bomser, J.A. Epigallocatechin-3-gallate (EGCG) inhibits PC-3 prostate cancer cell proliferation via MEK-independent ERK1/2 activation. Chem.-Biol. Interact. 2008, 171, 89–95. [Google Scholar] [CrossRef]
- Pravettoni, A.; Mornati, O.; Martini, P.; Marino, M.; Colciago, A.; Celotti, F.; Motta, M.; Negri-Cesi, P. Estrogen receptor beta (ERbeta) and inhibition of prostate cancer cell proliferation: Studies on the possible mechanism of action in DU145 cells. Mol. Cell. Endocrinol. 2007, 263, 46–54. [Google Scholar] [CrossRef]
- Adlercreutz, H. Phyto-oestrogens and cancer. Lancet Oncol. 2002, 3, 364–373. [Google Scholar] [CrossRef] [PubMed]
- Hussain, M.; Banerjee, M.; Sarkar, F.H.; Djuric, Z.; Pollak, M.N.; Doerge, D.; Fontana, J.; Chinni, S.; Davis, J.; Forman, J. Soy isoflavones in the treatment of prostate cancer. Nutr. Cancer 2003, 47, 111–117. [Google Scholar] [CrossRef] [PubMed]
- Mohd Hanafi, M.M.; Yaakob, H.; Sarmidi, M.R.; Aziz, R.; Prieto, J.M. Marantodes pumilum L. plant extracts induce apoptosis, cell cycle arrest and inhibit cell migration and invasion on prostate cancer cell lines. Planta Med. 2016, 82, P381. [Google Scholar] [CrossRef]
- Hirasawa, Y.; Pagano, I.; Huang, J.; Sasaki, Y.; Murakami, K.; Rosser, C.J.; Furuya, H. Case Study of Noni Extract in Men with Very Low-Risk or Low-Risk Prostate Cancer. Hawaii J. Health Soc. Welf. 2021, 80, 242–250. [Google Scholar]
- Akihisa, T.; Matsumoto, K.; Tokuda, H.; Yasukawa, K.; Seino, K.; Nakamoto, K.; Kuninaga, H.; Suzuki, T.; Kimura, Y. Anti-inflammatory and potential cancer chemopreventive constituents of the fruits of Morinda citrifolia (Noni). J. Nat. Prod. 2007, 70, 754–757. [Google Scholar] [CrossRef]
- Liu, G.; Bode, A.; Ma, W.Y.; Sang, S.; Ho, C.T.; Dong, Z. Two novel glycosides from the fruits of Morinda citrifolia (noni) inhibit AP-1 transactivation and cell transformation in the mouse epidermal JB6 cell line. Cancer Res. 2001, 61, 5749–5756. [Google Scholar]
- Hiwasa, T.; Arase, Y.; Chen, Z.; Kita, K.; Umezawa, K.; Ito, H.; Suzuki, N. Stimulation of ultraviolet-induced apoptosis of human fibroblast UVr-1 cells by tyrosine kinase inhibitors. FEBS Lett. 1999, 444, 173–176. [Google Scholar] [CrossRef]
- Liu, X.L.; Zhang, L.; Fu, X.L.; Chen, K.; Qian, B.C. Effect of scopoletin on PC3 cell proliferation and apoptosis. Acta Pharmacol. Sin. 2001, 22, 929–933. [Google Scholar]
- Carastro, L.M.; Vallebuona, E.J.; Cordova, R.; Gannon, A.N.; Kim, S.J.; Costello, C.M.; Declet-Bauzo, R.A.; Kumar, N.; Park, J.Y. Polyphenon E Effects on Gene Expression in PC-3 Prostate Cancer Cells. Int. J. Mol. Sci. 2022, 23, 14328. [Google Scholar] [CrossRef]
- Patel, K.; Patel, D.K. Therapeutic effectiveness of sinensetin against cancer and other human complications: A review of biological potential and pharmacological activities. Cardiovasc. Hematol. Disord. Drug Targets 2022, 22, 144–154. [Google Scholar] [CrossRef]
- Suhaimi, S.H.; Hasham, R.; Hafiz Idris, M.K.; Ismail, H.F.; Mohd Ariffin, N.H.; Abdul Majid, F.A. Optimization of Ultrasound-Assisted Extraction Conditions Followed by Solid Phase Extraction Fractionation from Orthosiphon stamineus Benth (Lamiace) Leaves for Antiproliferative Effect on Prostate Cancer Cells. Molecules 2019, 24, 4183. [Google Scholar] [CrossRef]
- Al-Suede, F.S.; Khadeer Ahamed, M.B.; Abdul Majid, A.S.; Baharetha, H.M.; Hassan, L.E.; Kadir, M.O.; Nassar, Z.D.; Abdul Majid, A.M. Optimization of Cat’s Whiskers Tea (Orthosiphon stamineus) Using Supercritical Carbon Dioxide and Selective Chemotherapeutic Potential against Prostate Cancer Cells. Evid.-Based Complement. Altern. Med. Ecam 2014, 2014, 396016. [Google Scholar] [CrossRef]
- Manthey, J.A.; Guthrie, N. Antiproliferative activities of citrus flavonoids against six human cancer cell lines. J. Agric. Food Chem. 2002, 50, 5837–5843. [Google Scholar] [CrossRef]
- Tang, Y.Q.; Jaganath, I.; Manikam, R.; Sekaran, S.D. Phyllanthus Suppresses Prostate Cancer Cell, PC-3, Proliferation and Induces Apoptosis through Multiple Signalling Pathways (MAPKs, PI3K/Akt, NFκB, and Hypoxia). Evid.-Based Complement. Altern. Med. Ecam 2013, 2013, 609581. [Google Scholar] [CrossRef]
- Tang, Y.Q.; Jaganath, I.B.; Sekaran, S.D. Phyllanthus spp. induces selective growth inhibition of PC-3 and MeWo human cancer cells through modulation of cell cycle and induction of apoptosis. PloS ONE 2010, 5, e12644. [Google Scholar] [CrossRef]
- Mohd Hanafi, M. In Vitro Pro-Apoptotic and Anti-Migratory Effects of Marantodes Pumilum (Blume) Kuntze and Ficus Deltoidea L. Extracts on Prostate Cancer Cell Lines. Ph.D. Thesis, University College London, London, UK, 2017. Available online: https://discovery.ucl.ac.uk/id/eprint/1560225/ (accessed on 30 December 2022).
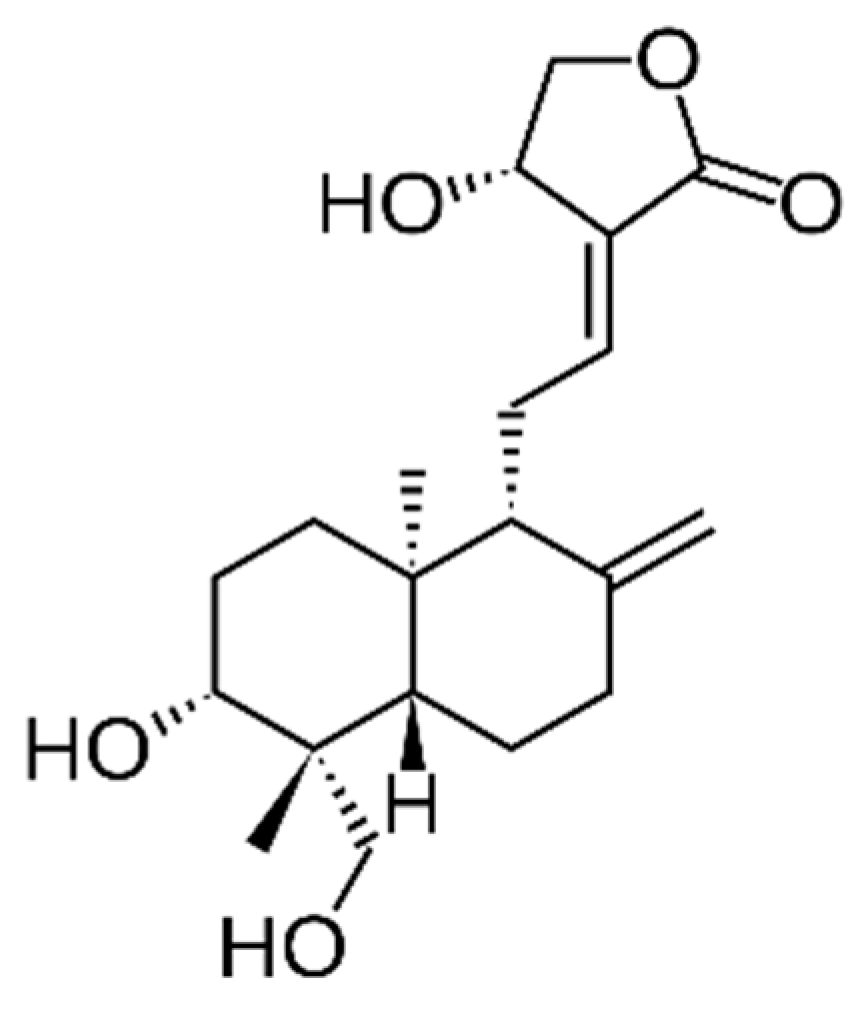
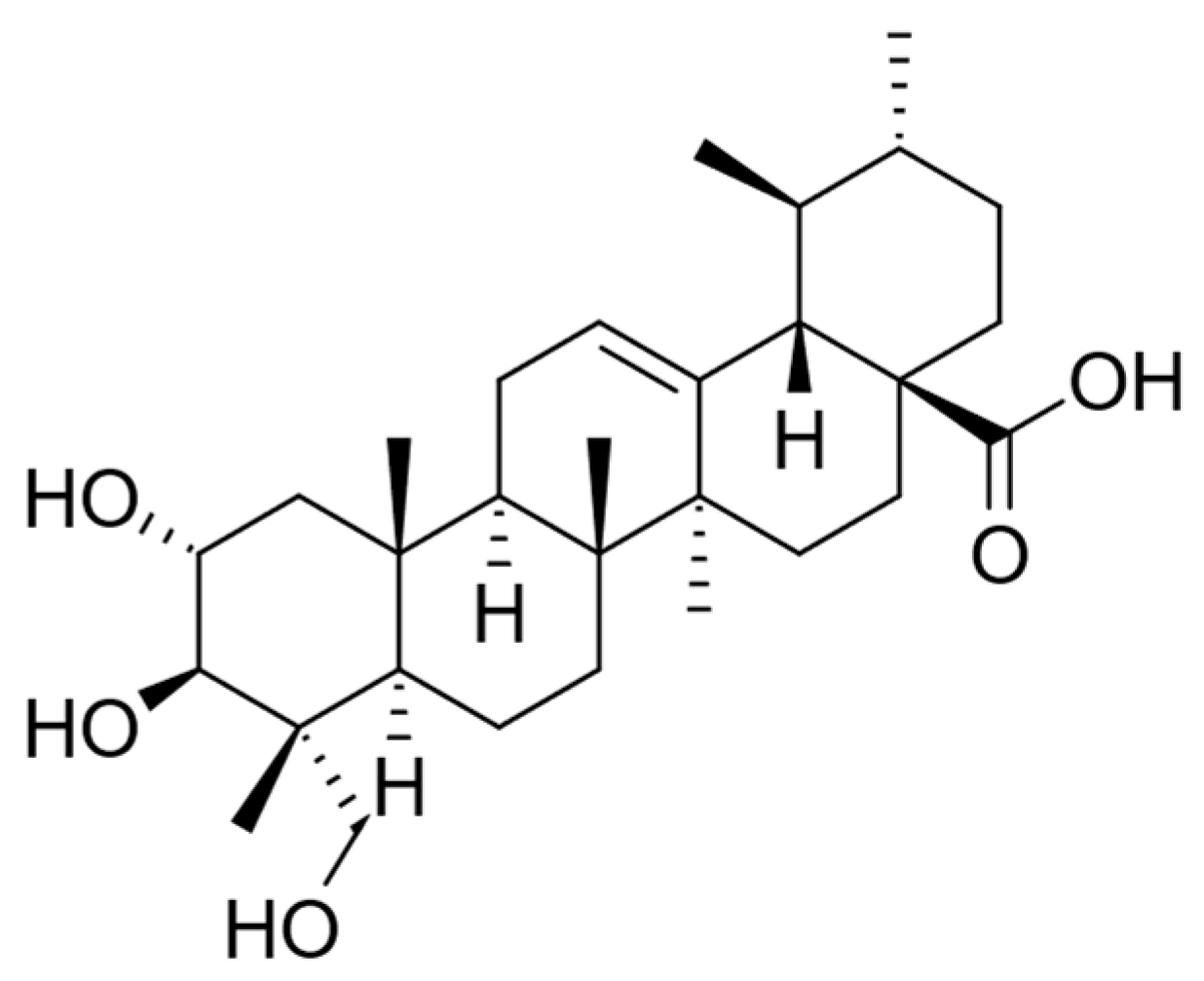
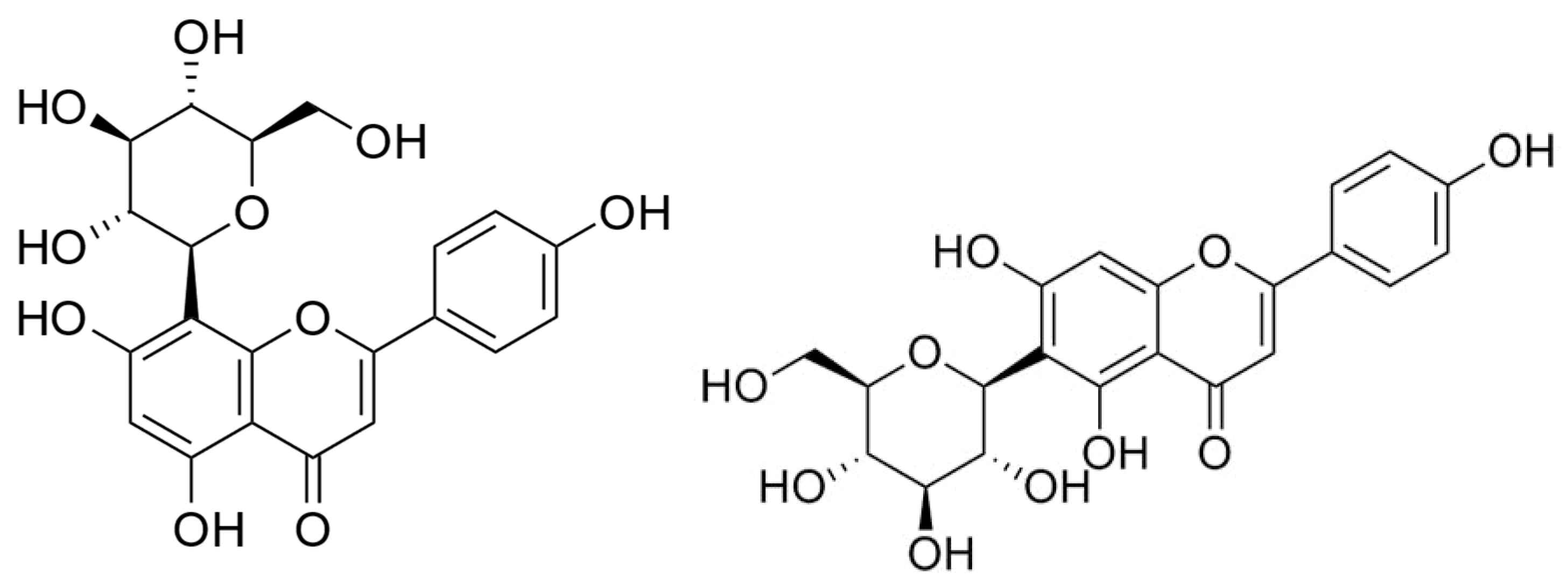
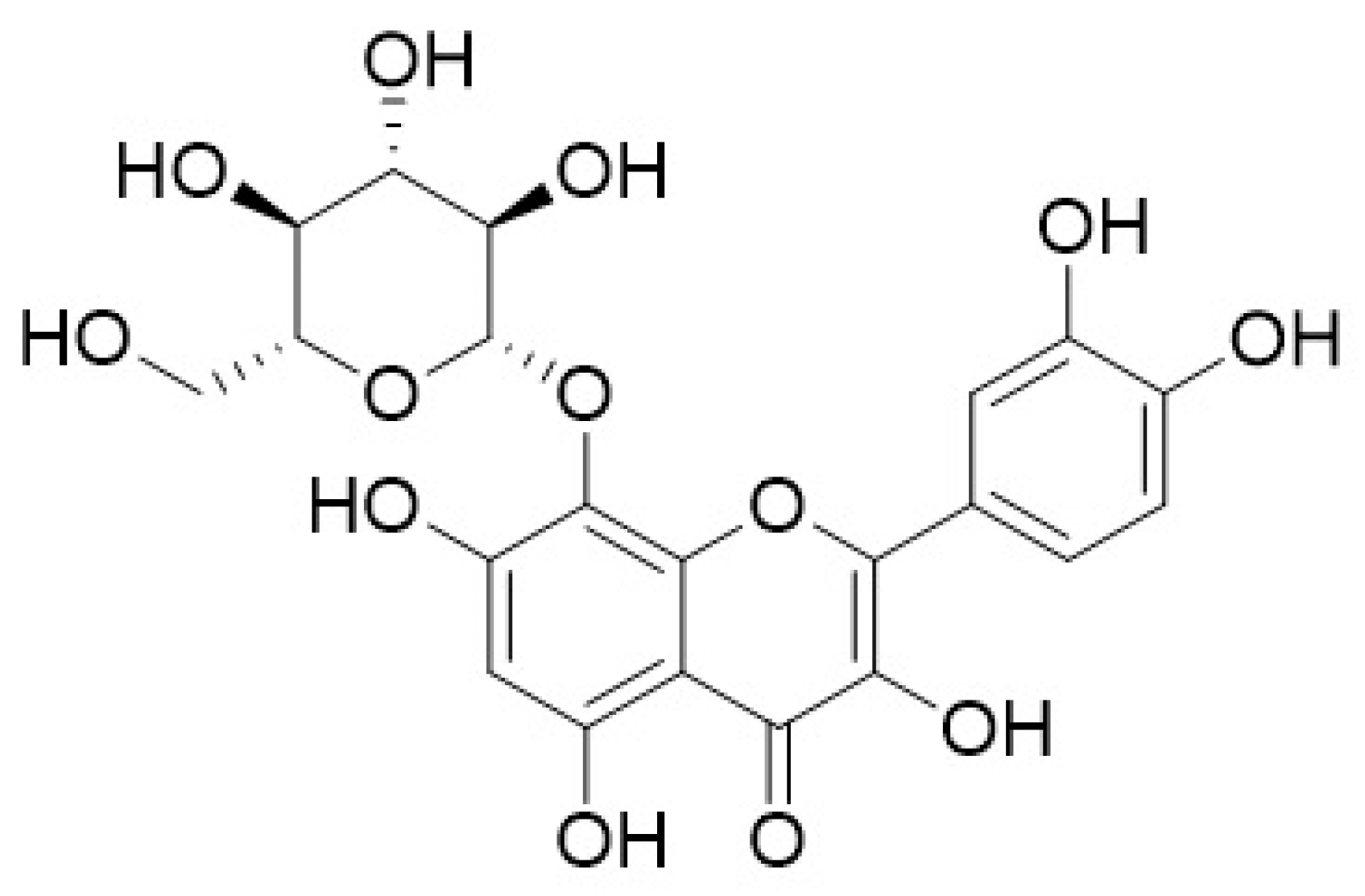

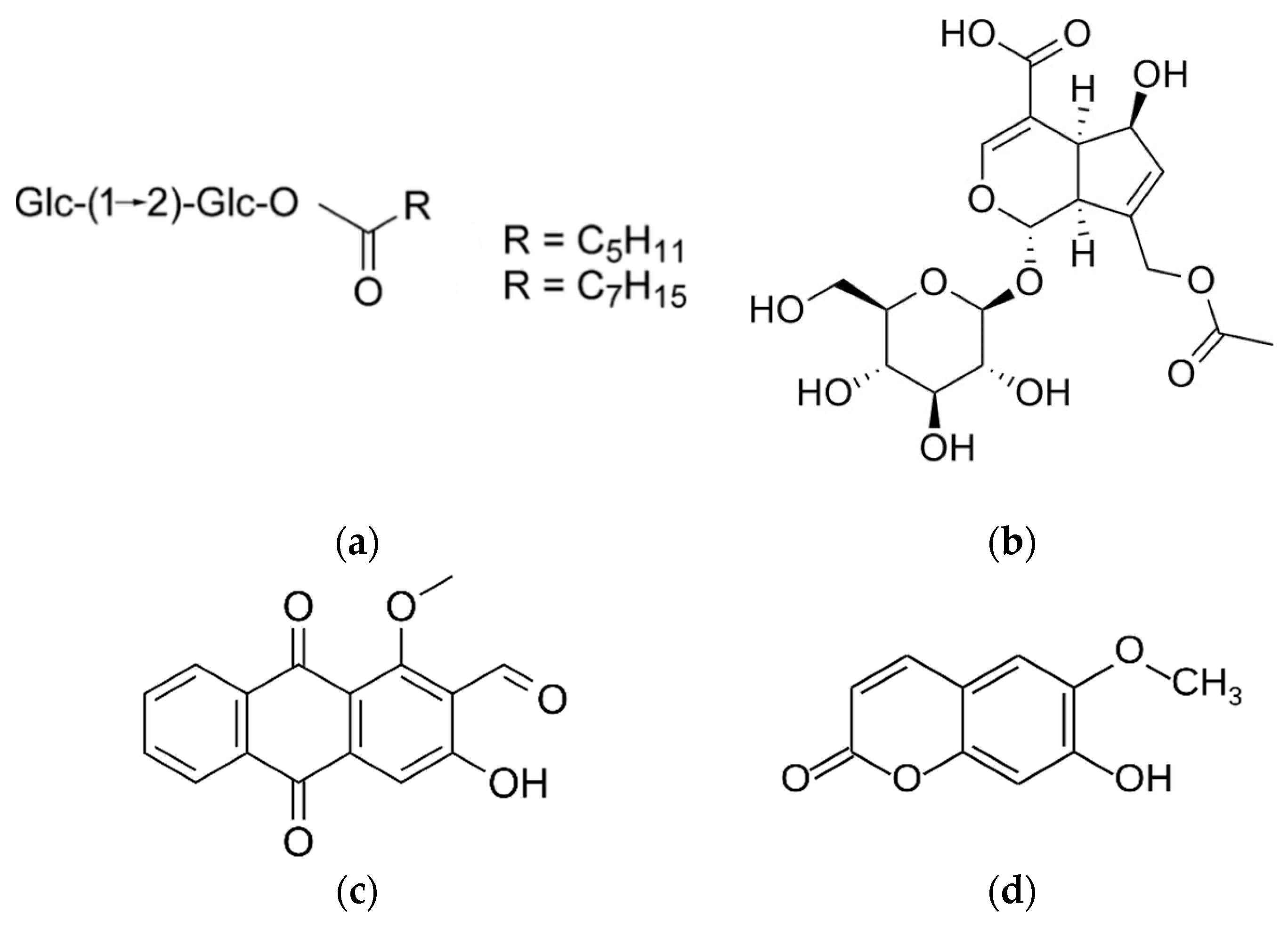
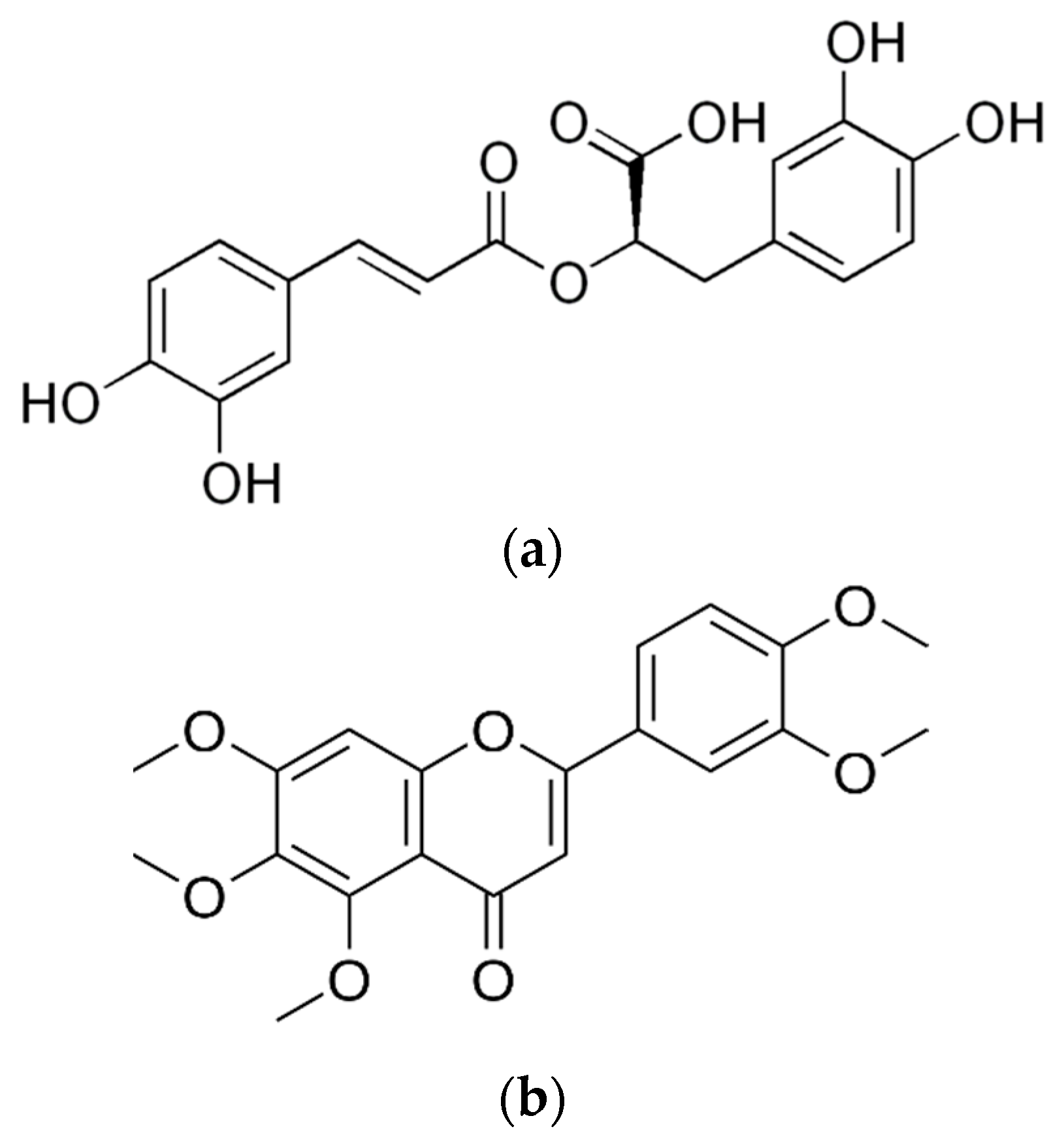
| Latin Name | Family | Malay Name | Part Used | Targeted Indications |
|---|---|---|---|---|
| Andrographis paniculata (Burm.f.) Nees | Acanthaceae | Hempedu Bumi | Leaves | Antidiabetic tonic and supplement |
| Centella asiatica (L.) Urb. | Apiaceae | Pegaga | Leaves, stem | Skincare ingredients, herbal drink |
| Clinacanthus nutans (Burm.f.) Lindau | Acanthaceae | Belalai Gajah | Leaves | Herbal drink, anticancer supplement |
| Eurycoma longifolia Jack | Simaroubaceae | Tongkat Ali | Roots | Male tonic, coffee, energy drink |
| Ficus deltoidea Jack | Moraceae | Mas Cotek | Fruit, roots, and leaves | Afterbirth tonic, whitening serum |
| Hibiscus sabdariffa L. | Malvaceae | Roselle | Fruit, leaves | Functional drinks, skincare |
| Marantodes pumilum (Blume) Kuntze (syn. Labisia pumila (Blume) Mez) | Primulaceae | Kacip Fatimah | Leaves and roots | Gynecological, antiaging serum, women’s tonic, and supplement |
| Morinda citrifolia L. | Rubiaceae | Mengkudu | Fruits, leaves, and roots | Herbal drinks, coffee |
| Orthosiphon aristatus (Blume) Miq. | Lamiaceae | Misai Kucing | Stem and leaves | Diuretics, tea, and herbal supplement for kidney disease |
| Phyllanthus niruri L. | Phyllanthaceae. | Dukung Anak | Whole plant | Liver tonic |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Prieto, J.M.; Hanafi, M.M.M. Advances in Molecular Regulation of Prostate Cancer Cells by Top Natural Products of Malaysia. Curr. Issues Mol. Biol. 2023, 45, 1536-1567. https://doi.org/10.3390/cimb45020099
Prieto JM, Hanafi MMM. Advances in Molecular Regulation of Prostate Cancer Cells by Top Natural Products of Malaysia. Current Issues in Molecular Biology. 2023; 45(2):1536-1567. https://doi.org/10.3390/cimb45020099
Chicago/Turabian StylePrieto, Jose M., and Mohd Mukrish Mohd Hanafi. 2023. "Advances in Molecular Regulation of Prostate Cancer Cells by Top Natural Products of Malaysia" Current Issues in Molecular Biology 45, no. 2: 1536-1567. https://doi.org/10.3390/cimb45020099
APA StylePrieto, J. M., & Hanafi, M. M. M. (2023). Advances in Molecular Regulation of Prostate Cancer Cells by Top Natural Products of Malaysia. Current Issues in Molecular Biology, 45(2), 1536-1567. https://doi.org/10.3390/cimb45020099






