Anxiety and Metabolic Disorders: The Role of Botanicals
Abstract
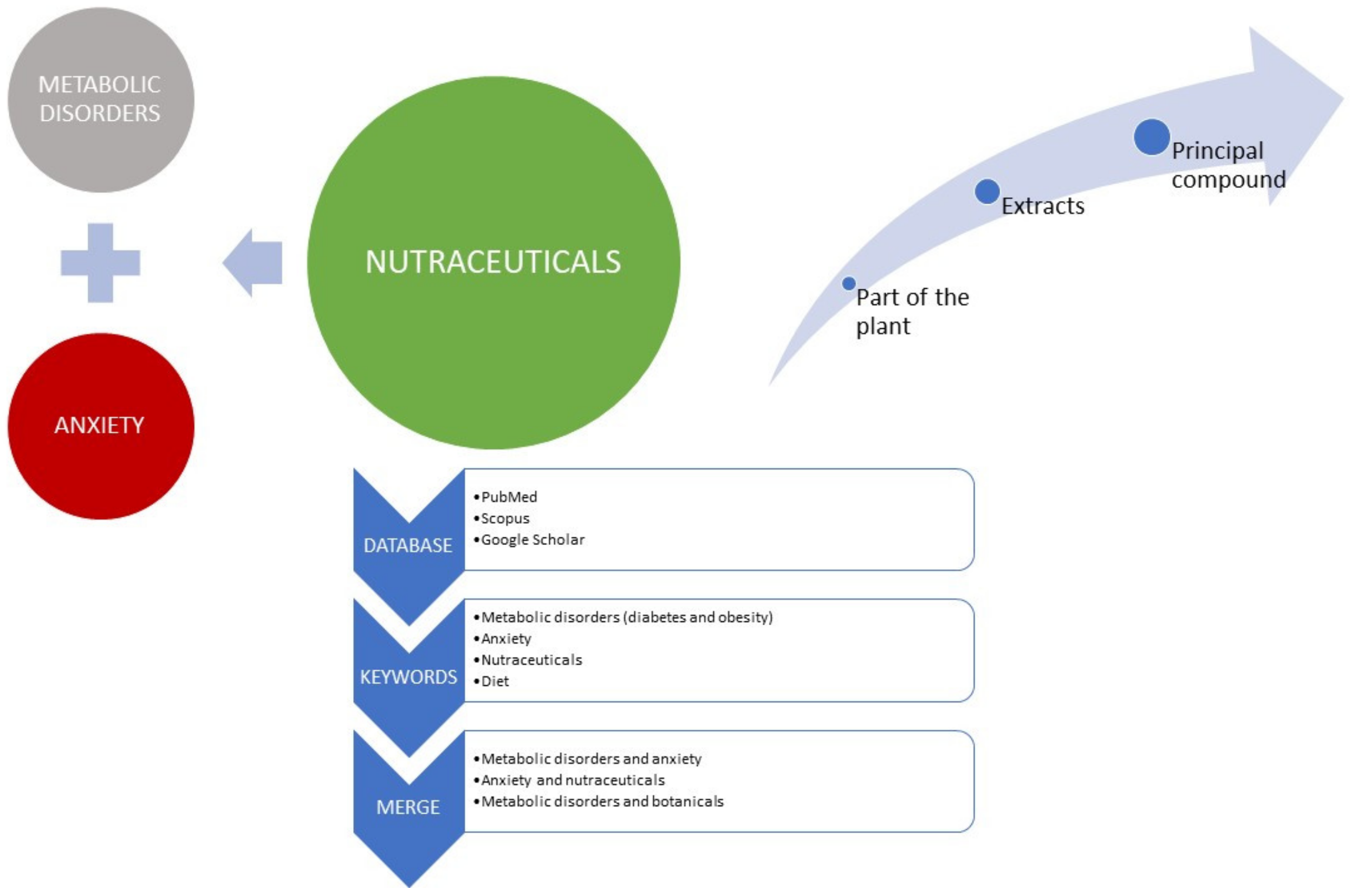
1. Introduction
2. Supporting Data
3. Metabolic Disorders and Anxiety
4. Botanicals
4.1. Curcuma Longa
4.2. Pimpinella Anisum
4.3. Trigonella Foenum-Graecum
4.4. Withania Somnifera
4.5. Genus Tilia
4.6. Zingiber Officinale
| Botanicals | Principal Secondary Metabolites Involved in the Anxiolytic-like Effect | Principal Secondary Metabolites Involved in the MetS Effect and in Anxiolytic Effect | Note | Recommendation for MetS Effect | Cautions |
|---|---|---|---|---|---|
Curcuma longa (radix) | Curcumin | Nano-formulation of curcumin seems crucial to improve its bioavailability. Different effects are mediated by the metabolism induced by different microbiota from person to person | 80 mg/day of curcumin in the form of nano-micelles [40] | In case of impaired liver function, biliary or biliary tract stones, the use of the product is not recommended. Do not use during pregnancy and breastfeeding. It is recommended to consult a doctor before its use to avoid possible drug interactions. | |
| Curcumin as C3 Complex® formula (obtained from Sami Labs Ltd., Bangalore, India) was used as the source of curcuminoids (comprising curcumin, demethoxycurcumin and bisdemethoxycurcumin) | 1 g/day for a period of 30 days [66] | In case of impaired liver function, biliary or biliary tract stones, the use of the product is not recommended. Do not use during pregnancy and breastfeeding. It is recommended to consult a doctor before its use to avoid potential interactions with drugs | |||
Pimpinella anisum (seeds) | 70% hydro-ethanolic extract of previously defatted P. anisum powder (mainly containing: p-coumaric acid and catechin, followed by oleuropein, gallic acid, caffeic acid, chlorogenic acid, trans-4-hydroxy-3-methoxycinnamic acid, quercetin, and myricetin) | Study on mice model 100 and 200 mg/kg (21 days) [45] | |||
| Aqueous crude extract | Study on rat model 500 mg/kg orally once daily [46] | ||||
Trigonella foenum-graecum (seeds) | (2S, 3R, 4S)-4-hydroxyisoleucine (4-HI), a major amino acid from fenugreek seeds in saline (0.9% NaCl) solution | Study on rat model 10, 30, 100 mg/kg once a day for 14 days [47] | |||
| Study on high-fat-fed mice fed a low- or high-fat diet for 16 weeks with or without 2% (w/w) fenugreek supplementation [48] | |||||
Withania somnifera (radix) | Radix dry extract | 300 mg, twice daily (600 mg/day), orally for 8 weeks [49] | |||
| Radix dry extract (standardized in a complex group of steroidal lactones known as withanolides, which also occur as glycosides withanosides) | commercially available preparations of WS (KSM-66®, Sensoril®, Essentra®, or Shoden®) | 125–1000 mg daily using capsules for 6–12 weeks | If used as ingredients in dietary supplements in some European Countries (i.e., Poland) the following recommendation has been highlighted “Withania somnifera (L.) Dunal root powder can be used in an amount less than 3 g per day; The maximum content of withanolides must not exceed 10 mg in the recommended daily portion of the product; An entity placing a given food on the market should include a quantitative specification confirming the total content of withanolides per recommended daily portion of the product “ | ||
Tilia cordata (flowers) | Tilia cordata (1%) [67] | ||||
Tilia tomentosa Moench (buds) | Glyceric Macerate: benzoic acids (47% of the total phytocomplex), catechins (36% of the total phytocomplex) flavonols (14% of the total phytocomplex) and cinnamic acids (3% of the total phytocomplex) | Study on mice Glyceric Macerate dissolved in the drinkingwater (1 µL/mL) for 21 days [56] | |||
Zingiber officinale (radix) | gingerenone A, 6-shogaol, and 6-gingerol [58] | ||||
| ginger root extracts rich in gingerols (GEG) and shogaols (SEG) | Study on rats +0.75%GEG +0.75%SEG in diet for 30 days [61] |
5. Plant Bioactive Compounds
5.1. Quercetin
5.2. Berberine
5.3. Caffeic Acid
5.4. Ellagic Acid
6. Discussion and Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Charlson, F.; van Ommeren, M.; Flaxman, A.; Cornett, J.; Whiteford, H.; Saxena, S. New WHO Prevalence Estimates of Mental Disorders in Conflict Settings: A Systematic Review and Meta-Analysis. Lancet 2019, 394, 240–248. [Google Scholar] [CrossRef]
- Rozenman, M.; Piacentini, J.; O’Neill, J.; Bergman, R.L.; Chang, S.; Peris, T.S. Improvement in Anxiety and Depression Symptoms Following Cognitive Behavior Therapy for Pediatric Obsessive Compulsive Disorder. Psychiatry Res. 2019, 276, 115–123. [Google Scholar] [CrossRef] [PubMed]
- Boggia, R.; Zunin, P.; Turrini, F. Functional Foods and Food Supplements. Appl. Sci. 2020, 10, 8538. [Google Scholar] [CrossRef]
- Colombo, F.; Restani, P.; Biella, S.; Di Lorenzo, C. Botanicals in Functional Foods and Food Supplements: Tradition, Efficacy and Regulatory Aspects. Appl. Sci. 2020, 10, 2387. [Google Scholar] [CrossRef]
- Schieber, A. Botanicals—Challenges Abound, Solutions in Sight? Curr. Opin. Food Sci. 2020, 32, 144–148. [Google Scholar] [CrossRef]
- Noubiap, J.J.; Nansseu, J.R.; Lontchi-Yimagou, E.; Nkeck, J.R.; Nyaga, U.F.; Ngouo, A.T.; Tounouga, D.N.; Tianyi, F.L.; Foka, A.J.; Ndoadoumgue, A.L.; et al. Geographic Distribution of Metabolic Syndrome and Its Components in the General Adult Population: A Meta-Analysis of Global Data from 28 Million Individuals. Diabetes Res. Clin. Pract. 2022, 188, 109924. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Vera, D.; Vergara-Castañeda, A.; Lazcano-Orozco, D.K.; Ramírez-Vélez, G.; Vivar-Sierra, A.; Araiza-Macías, M.J.; Hernández-Contreras, J.P.; Naranjo-Navarro, C.R.; Salazar, J.R.; Loza-Mejía, M.A.; et al. Inflammation Parameters Associated with Metabolic Disorders: Relationship Between Diet and Microbiota. Metab. Syndr. Relat. Disord. 2021, 19, 469–482. [Google Scholar] [CrossRef]
- Moszak, M.; Szulińska, M.; Bogdański, P. You Are What You Eat—The Relationship between Diet, Microbiota, and Metabolic Disorders—A Review. Nutrients 2020, 12, 1096. [Google Scholar] [CrossRef]
- Liang, Y.; Zou, L.; Tian, Y.; Zhou, S.; Chen, X.; Lin, C. Dietary and Metabolic Risk of Neuropsychiatric Disorders: Insights from Animal Models. Br. J. Nutr. 2021, 126, 1771–1787. [Google Scholar] [CrossRef]
- Xia, G.; Han, Y.; Meng, F.; He, Y.; Srisai, D.; Farias, M.; Dang, M.; Palmiter, R.D.; Xu, Y.; Wu, Q. Reciprocal Control of Obesity and Anxiety–Depressive Disorder via a GABA and Serotonin Neural Circuit. Mol. Psychiatry 2021, 26, 2837–2853. [Google Scholar] [CrossRef]
- Artasensi, A.; Pedretti, A.; Vistoli, G.; Fumagalli, L. Type 2 Diabetes Mellitus: A Review of Multi-Target Drugs. Molecules 2020, 25, 1987. [Google Scholar] [CrossRef] [PubMed]
- Lewgood, J.; Oliveira, B.; Korzepa, M.; Forbes, S.C.; Little, J.P.; Breen, L.; Bailie, R.; Candow, D.G. Efficacy of Dietary and Supplementation Interventions for Individuals with Type 2 Diabetes. Nutrients 2021, 13, 2378. [Google Scholar] [CrossRef]
- Sharma, S.; Zhuang, Y.; Gomez-Pinilla, F. Diet Transition to a High-Fat Diet for 3 Weeks Reduces Brain Omega-3-Fatty Acid Levels, Alters BDNF Signaling and Induces Anxiety & Depression-like Behavior in Adult Rats. Nat. Preced. 2012, 1. [Google Scholar] [CrossRef]
- Sun, G.Y.; Simonyi, A.; Fritsche, K.L.; Chuang, D.Y.; Hannink, M.; Gu, Z.; Greenlief, C.M.; Yao, J.K.; Lee, J.C.; Beversdorf, D.Q. Docosahexaenoic Acid (DHA): An Essential Nutrient and a Nutraceutical for Brain Health and Diseases. Prostaglandins Leukot. Essent. Fat. Acids 2018, 136, 3–13. [Google Scholar] [CrossRef] [PubMed]
- Oviedo-Ojeda, M.F.; Roque-Jiménez, J.A.; Whalin, M.; Lee-Rangel, H.A.; Relling, A.E. Effect of Supplementation with Different Fatty Acid Profile to the Dam in Early Gestation and to the Offspring on the Finishing Diet on Offspring Growth and Hypothalamus MRNA Expression in Sheep. J. Anim. Sci. 2021, 99, skab064. [Google Scholar] [CrossRef] [PubMed]
- Aron-Wisnewsky, J.; Warmbrunn, M.V.; Nieuwdorp, M.; Clément, K. Metabolism and Metabolic Disorders and the Microbiome: The Intestinal Microbiota Associated With Obesity, Lipid Metabolism, and Metabolic Health—Pathophysiology and Therapeutic Strategies. Gastroenterology 2021, 160, 573–599. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.T.; Walsh, R.F.L.; Sheehan, A.E. Prebiotics and Probiotics for Depression and Anxiety: A Systematic Review and Meta-Analysis of Controlled Clinical Trials. Neurosci. Biobehav. Rev. 2019, 102, 13–23. [Google Scholar] [CrossRef]
- Scheithauer, T.P.M.; Rampanelli, E.; Nieuwdorp, M.; Vallance, B.A.; Verchere, C.B.; van Raalte, D.H.; Herrema, H. Gut Microbiota as a Trigger for Metabolic Inflammation in Obesity and Type 2 Diabetes. Front. Immunol. 2020, 11, 2546. [Google Scholar] [CrossRef]
- Mukherjee, K.; Biswas, R.; Chaudhary, S.K.; Mukherjee, P.K. Botanicals as Medicinal Food and Their Effects against Obesity. In Evidence-Based Validation of Herbal Medicine; Elsevier: Amsterdam, The Netherlands, 2015; pp. 373–403. [Google Scholar] [CrossRef]
- Zu, G.; Sun, K.; Li, L.; Zu, X.; Han, T.; Huang, H. Mechanism of Quercetin Therapeutic Targets for Alzheimer Disease and Type 2 Diabetes Mellitus. Sci. Rep. 2021, 11, 22959. [Google Scholar] [CrossRef] [PubMed]
- Johansson, M.; Stomrud, E.; Lindberg, O.; Westman, E.; Johansson, P.M.; van Westen, D.; Mattsson, N.; Hansson, O. Apathy and Anxiety Are Early Markers of Alzheimer’s Disease. Neurobiol. Aging 2020, 85, 74–82. [Google Scholar] [CrossRef]
- Hannon, B.A.; Fairfield, W.D.; Adams, B.; Kyle, T.; Crow, M.; Thomas, D.M. Use and Abuse of Dietary Supplements in Persons with Diabetes. Nutr. Diabetes 2020, 10, 14. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Y.; Hu, M.; Wang, Q.; Yang, Z.; Zhu, N.; Wang, F.; Zhang, X.; Zhang, C.; Min, J.; Wang, H.; et al. The Prevalence and Related Factors of Metabolic Syndrome in Outpatients with First-Episode Drug-Naive Major Depression Comorbid with Anxiety. Sci. Rep. 2021, 11, 3324. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Ren, W.; Sun, Q.; Yu, K.M.; Lang, X.; Li, Z.; Zhang, X.Y. The Association of Clinical Correlates, Metabolic Parameters, and Thyroid Hormones with Suicide Attempts in First-Episode and Drug-Naïve Patients with Major Depressive Disorder Comorbid with Anxiety: A Large-Scale Cross-Sectional Study. Transl. Psychiatry 2021, 11, 97. [Google Scholar] [CrossRef]
- EFSA Scientific Committee. Scientific Opinion on a Qualified Presumption of Safety (QPS) Approach for the Safety Assessment of Botanicals and Botanical Preparations. EFSA J. 2014, 12, 3593. [Google Scholar] [CrossRef]
- Tesoriere, L. Update on Nutraceuticals. Nutraceuticals 2021, 1, 1. [Google Scholar] [CrossRef]
- Kušar, A.; Pravst, I. Exploitation of the Traditional Evidence for Botanical Health Claims on Foodstuffs in Europe. J. Funct. Foods 2022, 89, 104936. [Google Scholar] [CrossRef]
- Konstantinidi, M.; Koutelidakis, A.E. Functional Foods and Bioactive Compounds: A Review of Its Possible Role on Weight Management and Obesity’s Metabolic Consequences. Medicines 2019, 6, 94. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Wang, Q.; Chen, K.; Zou, S.; Shu, S.; Lu, C.; Wang, S.; Jiang, Y.; Fan, C.; Luo, Y. Red Yeast Rice for Hyperlipidemia: A Meta-Analysis of 15 High-Quality Randomized Controlled Trials. Front. Pharmacol. 2022, 12, 4016. [Google Scholar] [CrossRef] [PubMed]
- Banach, M.; Patti, A.M.; Giglio, R.V.; Cicero, A.F.G.; Atanasov, A.G.; Bajraktari, G.; Bruckert, E.; Descamps, O.; Djuric, D.M.; Ezhov, M.; et al. The Role of Nutraceuticals in Statin Intolerant Patients. J. Am. Coll. Cardiol. 2018, 72, 96–118. [Google Scholar] [CrossRef] [PubMed]
- European Commission (Text with EEA Relevance) 21.6.2017. Off. J. Eur. Un. 2017, 2016, 48–119. Available online: http://data.europa.eu/eli/reg/2017/1017/oj (accessed on 21 December 2022).
- Shabbir, U.; Rubab, M.; Banan-Mwine Daliri, E.; Chelliah, R.; Javed, A.; Oh, D.-H. Nutrients Curcumin, Quercetin, Catechins and Metabolic Diseases: The Role of Gut Microbiota. Nutrients 2021, 13, 206. [Google Scholar] [CrossRef]
- Djedjibegovic, J.; Marjanovic, A.; Panieri, E.; Saso, L. Ellagic Acid-Derived Urolithins as Modulators of Oxidative Stress. Oxid. Med. Cell. Longev. 2020, 2020, 5194508. [Google Scholar] [CrossRef]
- Allio, A.; Calorio, C.; Franchino, C.; Gavello, D.; Carbone, E.; Marcantoni, A. Bud Extracts from Tilia Tomentosa Moench Inhibit Hippocampal Neuronal Firing through GABAA and Benzodiazepine Receptors Activation. J. Ethnopharmacol. 2015, 172, 288–296. [Google Scholar] [CrossRef] [PubMed]
- Calorio, C.; Donno, D.; Franchino, C.; Carabelli, V.; Marcantoni, A. Bud Extracts from Salix Caprea, L. Inhibit Voltage Gated Calcium Channels and Catecholamines Secretion in Mouse Chromaffin Cells. Phytomedicine 2017, 36, 168–175. [Google Scholar] [CrossRef]
- Stanciauskaite, M.; Marksa, M.; Babickaite, L.; Majiene, D.; Ramanauskiene, K. Comparison of Ethanolic and Aqueous Populus Balsamifera l. Bud Extracts by Different Extraction Methods: Chemical Composition, Antioxidant and Antibacterial Activities. Pharmaceuticals 2021, 14, 1018. [Google Scholar] [CrossRef] [PubMed]
- Donno, D.; Turrini, F.; Boggia, R.; Guido, M.; Gamba, G.; Mellano, M.G.; Riondato, I.; Beccaro, G.L. Vitis Vinifera l. Pruning Waste for Bud-Preparations as Source of Phenolic Compounds–Traditional and Innovative Extraction Techniques to Produce New Natural Products. Plants 2021, 10, 2233. [Google Scholar] [CrossRef] [PubMed]
- Turrini, F.; Donno, D.; Beccaro, G.L.; Pittaluga, A.; Grilli, M.; Zunin, P.; Boggia, R. Bud-Derivatives, a Novel Source of Polyphenols and How Different Extraction Processes Affect Their Composition. Foods 2020, 9, 1343. [Google Scholar] [CrossRef]
- Singh, L.; Sharma, S.; Xu, S.; Tewari, D.; Fang, J. Curcumin as a Natural Remedy for Atherosclerosis: A Pharmacological Review. Molecules 2021, 26, 4036. [Google Scholar] [CrossRef]
- Jabczyk, M.; Nowak, J.; Hudzik, B.; Zubelewicz-Szkodzińska, B. Curcumin in Metabolic Health and Disease. Nutrients 2021, 13, 4440. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.; Lee, H. Systemic Administration of Curcumin Affect Anxiety-Related Behaviors in a Rat Model of Posttraumatic Stress Disorder via Activation of Serotonergic Systems. Evid. Based Complement. Altern. Med. 2018, 2018, 9041309. [Google Scholar] [CrossRef] [PubMed]
- EFSA Panel on Nutrition, Novel Foods and Food Allergens (NDA); Turck, D.; Bohn, T.; Castenmiller, J.; De Henauw, S.; Hirsch-Ernst, K.I.; Maciuk, A.; Mangelsdorf, I.; McArdle, H.J.; Naska, A.; et al. Safety of Tetrahydrocurcuminoids from Turmeric (Curcuma Longa L.) as a Novel Food Pursuant to Regulation (EU) 2015/2283. EFSA J. 2021, 19, e06936. [Google Scholar] [CrossRef] [PubMed]
- Zeng, L.; Yu, G.; Hao, W.; Yang, K.; Chen, H. The Efficacy and Safety of Curcuma Longa Extract and Curcumin Supplements on Osteoarthritis: A Systematic Review and Meta-Analysis. Biosci. Rep. 2021, 41, BSR20210817. [Google Scholar] [CrossRef]
- Rapporteur, M.; Assessor, D.; Calapai, G.; Delbò, M.; Silano, V. Assessment Report on Pimpinella Anisum L., Fructus and Pimpinella Anisum L., Aetheroleum. Eur. Med. Agency 2013, 44, 1–25. [Google Scholar]
- Es-Safi, I.; Mechchate, H.; Amaghnouje, A.; Elbouzidi, A.; Bouhrim, M.; Bencheikh, N.; Hano, C.; Bousta, D.; Hussein, A.; Cheikhyouseef, A.; et al. Assessment of Antidepressant-like, Anxiolytic Effects and Impact on Memory of Pimpinella Anisum L. Total Extract on Swiss Albino Mice Academic Editors: Ahmed. Plants 2021, 10, 1573. [Google Scholar] [CrossRef] [PubMed]
- Faried, M.A.; El-Mehi, A.E.S. Aqueous Anise Extract Alleviated the Pancreatic Changes in Streptozotocin-Induced Diabetic Rat Model via Modulation of Hyperglycaemia, Oxidative Stress, Apoptosis and Autophagy: A Biochemical, Histological and Immunohistochemical Study. Folia Morphol. 2020, 78, 489–502. [Google Scholar] [CrossRef]
- Knott, E.J.; Richard, A.J.; Mynatt, R.L.; Ribnicky, D.; Stephens, J.M.; Bruce-Keller, A. Fenugreek Supplementation during High-Fat Feeding Improves Specific Markers of Metabolic Health. Sci. Rep. 2017, 7, 12770. [Google Scholar] [CrossRef]
- Kalshetti, P.; Alluri, R.; Mohan, V.; Thakurdesai, P. Effects of 4-Hydroxyisoleucine from Fenugreek Seeds on Depression-like Behavior in Socially Isolated Olfactory Bulbectomized Rats. Pharmacogn. Mag. 2015, 11, 388–396. [Google Scholar] [CrossRef]
- Verma, N.; Gupta, S.K.; Tiwari, S.; Mishra, A.K. Safety of Ashwagandha Root Extract: A Randomized, Placebo-Controlled, Study in Healthy Volunteers. Complement. Ther. Med. 2021, 57, 102642. [Google Scholar] [CrossRef] [PubMed]
- Shah, P.C.; Trivedi, N.A.; Bhatt, J.D.; Hemavathi, K.G. Effect of Withania Somnifera on Forced Swimming Test Induced Immobility in Mice and Its Interaction with Various Drugs. Indian J. Physiol. Pharmacol. 2006, 50, 409–415. [Google Scholar]
- Alzoubi, K.H.; Al Hilo, A.S.; Al-Balas, Q.A.; El-Salem, K.; El-Elimat, T.; Alali, F.Q. Withania Somnifera Root Powder Protects Againist Post-Traumatic Stress Disorder-Induced Memory Impairment. Mol. Biol. Rep. 2019, 46, 4709–4715. [Google Scholar] [CrossRef]
- Speers, A.B.; Cabey, K.A.; Soumyanath, A.; Wright, K.M. Effects of Withania Somnifera (Ashwagandha) on Stress and the Stress- Related Neuropsychiatric Disorders Anxiety, Depression, and Insomnia. Curr. Neuropharmacol. 2021, 19, 1468–1495. [Google Scholar] [CrossRef]
- Choudhary, D.; Bhattacharyya, S.; Joshi, K. Body Weight Management in Adults Under Chronic Stress Through Treatment With Ashwagandha Root Extract. J. Evid. Based Complement. Altern. Med. 2017, 22, 96–106. [Google Scholar] [CrossRef]
- Bonilla, D.A.; Moreno, Y.; Gho, C.; Petro, J.L.; Odriozola-Martínez, A.; Kreider, R.B. Effects of Ashwagandha (Withania Somnifera) on Physical Performance: Systematic Review and Bayesian Meta-Analysis. J. Funct. Morphol. Kinesiol. 2021, 6, 20. [Google Scholar] [CrossRef]
- Wróbel, K.; Milewska, A.J.; Marczak, M.; Kozłowski, R. Assessment of the Impact of Scientific Reports Published by EFSA and GIS on Functional Foods Newly Placed on the Market in Poland. Int. J. Environ. Res. Public Health 2022, 19, 4057. [Google Scholar] [CrossRef]
- Turrini, F.; Vallarino, G.; Cisani, F.; Donno, D.; Beccaro, G.L.; Zunin, P.; Boggia, R.; Pittaluga, A.; Grilli, M. Use of an Animal Model to Evaluate Anxiolytic Effects of Dietary Supplementation with Tilia Tomentosa Moench Bud Extracts. Nutrients 2020, 12, 3328. [Google Scholar] [CrossRef]
- Cerantola, S.; Faggin, S.; Annaloro, G.; Mainente, F.; Filippini, R.; Savarino, E.V.; Piovan, A.; Zoccatelli, G.; Giron, M.C. Influence of Tilia Tomentosa Moench Extract on Mouse Small Intestine Neuromuscular Contractility. Nutrients 2021, 13, 3505. [Google Scholar] [CrossRef]
- Mao, Q.-Q.; Xu, X.-Y.; Cao, S.-Y.; Gan, R.-Y.; Corke, H.; Beta, T.; Li, H.-B. Bioactive Compounds and Bioactivities of Ginger (Zingiber Officinale Roscoe). Foods 2019, 8, 185. [Google Scholar] [CrossRef]
- Simon, A.; Darcsi, A.; Kéry, Á.; Riethmüller, E. Blood-Brain Barrier Permeability Study of Ginger Constituents. J. Pharm. Biomed. Anal. 2020, 177, 112820. [Google Scholar] [CrossRef]
- Vishwakarma, S.L.; Pal, S.C.; Kasture, V.S.; Kasture, S.B. Anxiolytic and Antiemetic Activity of Zingiber Officinale. Phytother. Res. 2002, 16, 621–626. [Google Scholar] [CrossRef]
- Shen, C.-L.; Wang, R.; Ji, G.; Elmassry, M.M.; Zabet-Moghaddam, M.; Vellers, H.; Hamood, A.N.; Gong, X.; Mirzaei, P.; Sang, S.; et al. Dietary Supplementation of Gingerols- and Shogaols-Enriched Ginger Root Extract Attenuate Pain-Associated Behaviors While Modulating Gut Microbiota and Metabolites in Rats with Spinal Nerve Ligation. J. Nutr. Biochem. 2022, 100, 108904. [Google Scholar] [CrossRef]
- Yang, C.; Fang, X.; Zhan, G.; Huang, N.; Li, S.; Bi, J.; Jiang, R.; Yang, L.; Miao, L.; Zhu, B.; et al. Key Role of Gut Microbiota in Anhedonia-like Phenotype in Rodents with Neuropathic Pain. Transl. Psychiatry 2019, 9, 57. [Google Scholar] [CrossRef]
- Carvalho, G.C.N.; Lira-Neto, J.C.G.; De Araújo, M.F.M.; De Freitas, R.W.J.F.; Zanetti, M.L.; Damasceno, M.M.C. Effectiveness of Ginger in Reducing Metabolic Levels in People with Diabetes: A Randomized Clinical Trial. Rev. Lat. Am. Enferm. 2020, 28, e3369. [Google Scholar] [CrossRef]
- Ebrahimzadeh, A.; Ebrahimzadeh, A.; Mirghazanfari, S.M.; Hazrati, E.; Hadi, S.; Milajerdi, A. The Effect of Ginger Supplementation on Metabolic Profiles in Patients with Type 2 Diabetes Mellitus: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Complement. Ther. Med. 2022, 65, 102802. [Google Scholar] [CrossRef] [PubMed]
- Huang, F.-Y.; Deng, T.; Meng, L.-X.; Ma, X.-L. Dietary Ginger as a Traditional Therapy for Blood Sugar Control in Patients with Type 2 Diabetes Mellitus: A Systematic Review and Meta-Analysis. Medicine 2019, 98, e15054. [Google Scholar] [CrossRef] [PubMed]
- Esmaily, H.; Sahebkar, A.; Iranshahi, M.; Ganjali, S.; Mohammadi, A.; Ferns, G.; Ghayour-Mobarhan, M. An Investigation of the Effects of Curcumin on Anxiety and Depression in Obese Individuals: A Randomized Controlled Trial. Chin. J. Integr. Med. 2015, 21, 332–338. [Google Scholar] [CrossRef]
- Otoom, S.A.; Al-Safi, S.A.; Kerem, Z.K.; Alkofahi, A. The Use of Medicinal Herbs by Diabetic Jordanian Patients. J. Herb. Pharmacother. 2006, 6, 31–41. [Google Scholar] [CrossRef] [PubMed]
- Andres, S.; Pevny, S.; Ziegenhagen, R.; Bakhiya, N.; Schäfer, B.; Hirsch-Ernst, K.I.; Lampen, A. Safety Aspects of the Use of Quercetin as a Dietary Supplement. Mol. Nutr. Food Res. 2018, 62, 1700447. [Google Scholar] [CrossRef] [PubMed]
- Hosseini, A.; Razavi, B.M.; Banach, M.; Hosseinzadeh, H. Quercetin and Metabolic Syndrome: A Review. Phytother. Res. 2021, 35, 5352–5364. [Google Scholar] [CrossRef] [PubMed]
- Carullo, G.; Cappello, A.R.; Frattaruolo, L.; Badolato, M.; Armentano, B.; Aiello, F. Quercetin and Derivatives: Useful Tools in Inflammation and Pain Management. Future Med. Chem. 2016, 9, 79–93. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Jiang, H.; Wu, X.; Fang, J. Therapeutic Effects of Quercetin on Inflammation, Obesity, and Type 2 Diabetes. Mediat. Inflamm. 2016, 2016, 9343460. [Google Scholar] [CrossRef]
- Samad, N.; Saleem, A.; Yasmin, F.; Shehzad, M.A. Quercetin Protects against Stress-Induced Anxiety- and Depression- like Behavior and Improves Memory in Male Mice. Physiol. Res. 2018, 67, 795–808. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Sun, J.; Chen, C.; Zhang, Y.; Zou, L.; Zhang, Z.; Chen, M.; Wu, H.; Tian, W.; Liu, Y.; et al. Quercetin Mitigates Methamphetamine-Induced Anxiety-Like Behavior Through Ameliorating Mitochondrial Dysfunction and Neuroinflammation. Front. Mol. Neurosci. 2022, 15, 829886. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Feng, X.; Chai, L.; Cao, S.; Qiu, F. The Metabolism of Berberine and Its Contribution to the Pharmacological Effects. Drug Metab. Rev. 2017, 49, 139–157. [Google Scholar] [CrossRef]
- Cicero, A.F.G.; Baggioni, A. Berberine and Its Role in Chronic Disease. In Anti-Inflammatory Nutraceuticals and Chronic Diseases; Gupta, S.C., Prasad, S., Aggarwal, B.B., Eds.; Springer International Publishing: Cham, Switzerland, 2016; pp. 27–45. ISBN 978-3-319-41334-1. [Google Scholar]
- Song, D.; Hao, J.; Fan, D. Biological Properties and Clinical Applications of Berberine. Front. Med. 2020, 14, 564–582. [Google Scholar] [CrossRef] [PubMed]
- Marcheluzzo, S.; Faggian, M.; Zancato, M.; Peron, G. Analysis of Monacolins and Berberine in Food Supplements for Lipid Control: An Overview of Products Sold on the Italian Market. Molecules 2021, 26, 2222. [Google Scholar] [CrossRef]
- Hsu, Y.Y.; Tseng, Y.T.; Lo, Y.C. Berberine, a Natural Antidiabetes Drug, Attenuates Glucose Neurotoxicity and Promotes Nrf2-Related Neurite Outgrowth. Toxicol. Appl. Pharmacol. 2013, 272, 787–796. [Google Scholar] [CrossRef] [PubMed]
- WenXiao, J.; ShiHua, L.; XiaoJiang, L. SCIENCE CHINA Therapeutic Potential of Berberine against Neurodegenerative Diseases. Sci. China Life Sci. 2015, 58, 564–569. [Google Scholar] [CrossRef]
- Fan, J.; Zhang, K.; Jin, Y.; Li, B.; Gao, S.; Zhu, J.; Cui, R.; Ranji Cui, C.; ming Zhu, J.; Provincial, J. Pharmacological Effects of Berberine on Mood Disorders. J. Cell. Mol. Med. 2018, 23, 21–28. [Google Scholar] [CrossRef]
- Lee, B.; Shim, I.; Lee, H.; Hahm, D.H. Berberine Alleviates Symptoms of Anxiety by Enhancing Dopamine Expression in Rats with Post-Traumatic Stress Disorder. Korean J. Physiol. Pharmacol. 2018, 22, 183–192. [Google Scholar] [CrossRef]
- Namela, N.; Kadar, M.A.; Ahmad, F.; Teoh, S.L.; Fairuz Yahaya, M. Molecules Caffeic Acid on Metabolic Syndrome: A Review. Molecules 2021, 26, 5490. [Google Scholar] [CrossRef]
- Olgierd, B.; Kamila, Z.; Emilia, M. Molecules The Pluripotent Activities of Caffeic Acid Phenethyl Ester. Molecules 2021, 26, 1335. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, N.P.; Vaidya, B.; Narula, A.S.; Sharma, S.S. Neuroprotective Potential of Caffeic Acid Phenethyl Ester (CAPE) in CNS Disorders: Mechanistic and Therapeutic Insights. Curr. Neuropharmacol. 2021, 19, 1401–1415. [Google Scholar] [CrossRef] [PubMed]
- Behl, T.; Kaur, G.; Sehgal, A.; Singh, S.; Bhatia, S.; Al-Harrasi, A.; Zengin, G.; Bungau, S.G.; Munteanu, M.A.; Brisc, M.C.; et al. Elucidating the Multi-Targeted Role of Nutraceuticals: A Complementary Therapy to Starve Neurodegenerative Diseases. Int. J. Mol. Sci. 2021, 22, 4045. [Google Scholar] [CrossRef] [PubMed]
- Girish, C.; Raj, V.; Arya, J.; Balakrishnan, S. Involvement of the GABAergic System in the Anxiolytic-like Effect of the Flavonoid Ellagic Acid in Mice. Eur. J. Pharmacol. 2013, 710, 49–58. [Google Scholar] [CrossRef] [PubMed]
- Farbood, Y.; Rashno, M.; Ghaderi, S.; Khoshnam, S.E.; Sarkaki, A.; Rashidi, K.; Rashno, M.; Badavi, M. Ellagic Acid Protects against Diabetes-Associated Behavioral Deficits in Rats: Possible Involved Mechanisms. Life Sci. 2019, 225, 8–19. [Google Scholar] [CrossRef] [PubMed]
- Kábelová, A.; Malínská, H.; Marková, I.; Oliyarnyk, O.; Chylíková, B.; Šeda, O. Ellagic Acid Affects Metabolic and Transcriptomic Profiles and Attenuates Features of Metabolic Syndrome in Adult Male Rats. Nutrients 2021, 13, 804. [Google Scholar] [CrossRef]
- Tomás-Barberán, F.A.; García-Villalba, R.; González-Sarrías, A.; Selma, M.V.; Espín, J.C. Ellagic Acid Metabolism by Human Gut Microbiota: Consistent Observation of Three Urolithin Phenotypes in Intervention Trials, Independent of Food Source, Age, and Health Status. J. Agric. Food Chem. 2014, 62, 6535–6538. [Google Scholar] [CrossRef] [PubMed]
- Santini, A.; Cammarata, S.M.; Capone, G.; Ianaro, A.; Tenore, G.C.; Pani, L.; Novellino, E. Nutraceuticals: Opening the Debate for a Regulatory Framework. Br. J. Clin. Pharmacol. 2018, 84, 659–672. [Google Scholar] [CrossRef]
- Tong, J.; Satyanarayanan, S.K.; Su, H. Nutraceuticals and Probiotics in the Management of Psychiatric and Neurological Disorders: A Focus on Microbiota-Gut-Brain-Immune Axis. Brain Behav. Immun. 2020, 90, 403–419. [Google Scholar] [CrossRef]
- Andrew, R.; Izzo, A.A. Highlights into the Pharmacology of Nutraceuticals. Br. J. Pharmacol. 2020, 177, 1209–1211. [Google Scholar] [CrossRef]
- Kunnumakkara, A.B.; Bordoloi, D.; Padmavathi, G.; Monisha, J.; Roy, N.K.; Prasad, S.; Aggarwal, B.B. Curcumin, the Golden Nutraceutical: Multitargeting for Multiple Chronic Diseases. Br. J. Pharmacol. 2017, 174, 1325–1348. [Google Scholar] [CrossRef]
- Micheli, L.; Pacini, A.; Di Cesare Mannelli, L.; Trallori, E.; D’Ambrosio, R.; Bianchini, C.; Lampertico, P.; Ghelardini, C. Treatment of Non-Alcoholic Steatosis: Preclinical Study of a New Nutraceutical Multitarget Formulation. Nutrients 2020, 12, 1819. [Google Scholar] [CrossRef] [PubMed]
- Milani, C.; Duranti, S.; Bottacini, F.; Casey, E.; Turroni, F.; Mahony, J.; Belzer, C.; Delgado Palacio, S.; Arboleya Montes, S.; Mancabelli, L.; et al. The First Microbial Colonizers of the Human Gut: Composition, Activities, and Health Implications of the Infant Gut Microbiota. Microbiol. Mol. Biol. Rev. 2017, 81, e00036-17. [Google Scholar] [CrossRef] [PubMed]
- Roy Sarkar, S.; Banerjee, S. Gut Microbiota in Neurodegenerative Disorders. J. Neuroimmunol. 2019, 328, 98–104. [Google Scholar] [CrossRef]
- Quigley, E.M.M. Microbiota-Brain-Gut Axis and Neurodegenerative Diseases. Curr. Neurol. Neurosci. Rep. 2017, 17, 94. [Google Scholar] [CrossRef]
- Illiano, P.; Brambilla, R.; Parolini, C. The Mutual Interplay of Gut Microbiota, Diet and Human Disease. FEBS J. 2020, 287, 833–855. [Google Scholar] [CrossRef]
- Sergeev, I.N.; Aljutaily, T.; Walton, G.; Huarte, E. Effects of Synbiotic Supplement on Human Gut Microbiota, Body Composition and Weight Loss in Obesity. Nutrients 2020, 12, 222. [Google Scholar] [CrossRef]
- Duranti, S.; Ruiz, L.; Lugli, G.A.; Tames, H.; Milani, C.; Mancabelli, L.; Mancino, W.; Longhi, G.; Carnevali, L.; Sgoifo, A.; et al. Bifidobacterium Adolescentis as a Key Member of the Human Gut Microbiota in the Production of GABA. Sci. Rep. 2020, 10, 14112. [Google Scholar] [CrossRef]
- Liu, W.; Lau, H.K.; Son, D.O.; Jin, T.; Yang, Y.; Zhang, Z.; Li, Y.; Prud’homme, G.J.; Wang, Q. Combined Use of GABA and Sitagliptin Promotes Human β-Cell Proliferation and Reduces Apoptosis. J. Endocrinol. 2021, 248, 133–143. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Trebesova, H.; Orlandi, V.; Boggia, R.; Grilli, M. Anxiety and Metabolic Disorders: The Role of Botanicals. Curr. Issues Mol. Biol. 2023, 45, 1037-1053. https://doi.org/10.3390/cimb45020068
Trebesova H, Orlandi V, Boggia R, Grilli M. Anxiety and Metabolic Disorders: The Role of Botanicals. Current Issues in Molecular Biology. 2023; 45(2):1037-1053. https://doi.org/10.3390/cimb45020068
Chicago/Turabian StyleTrebesova, Hanna, Valentina Orlandi, Raffaella Boggia, and Massimo Grilli. 2023. "Anxiety and Metabolic Disorders: The Role of Botanicals" Current Issues in Molecular Biology 45, no. 2: 1037-1053. https://doi.org/10.3390/cimb45020068
APA StyleTrebesova, H., Orlandi, V., Boggia, R., & Grilli, M. (2023). Anxiety and Metabolic Disorders: The Role of Botanicals. Current Issues in Molecular Biology, 45(2), 1037-1053. https://doi.org/10.3390/cimb45020068








