Heat Shock Proteins (HSPs) and Cardiovascular Complications of Obesity: Searching for Potential Biomarkers
Abstract
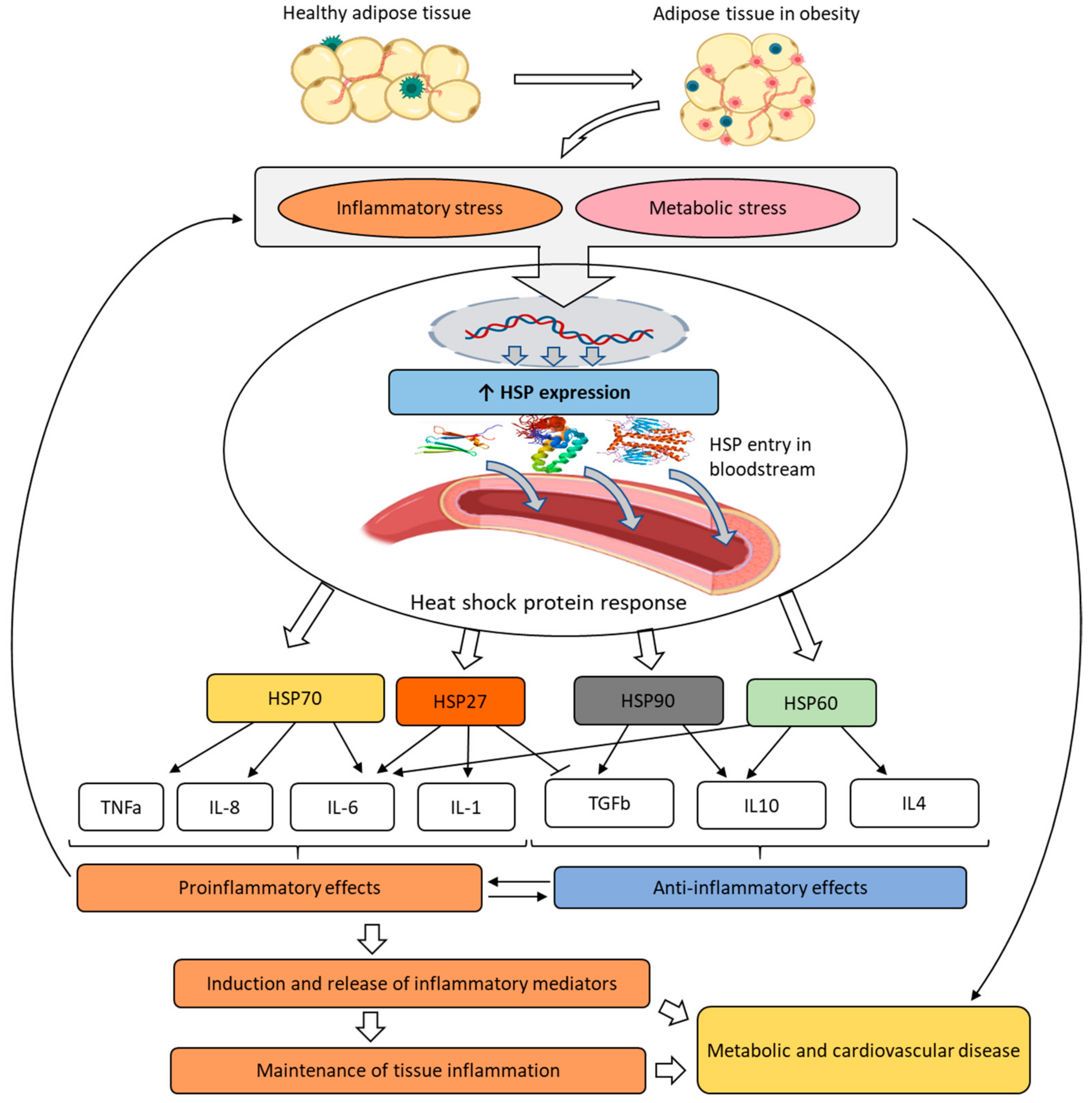
:1. Introduction
2. HSP Family: Biochemistry and Clinical Significance
2.1. HSP27
2.2. HSP40
2.3. HSP60
2.4. HSP70 and HSP72
2.5. HSP90
3. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Valenzuela, P.L.; Carrera-Bastos, P.; Castillo-Garcia, A.; Lieberman, D.E.; Santos-Lozano, A.; Lucia, A. Obesity and the risk of cardiometabolic diseases. Nat. Rev. Cardiol. 2023, 20, 475–494. [Google Scholar] [CrossRef] [PubMed]
- Piche, M.E.; Tchernof, A.; Despres, J.P. Obesity phenotypes, diabetes, and cardiovascular diseases. Circ. Res. 2020, 126, 1477–1500. [Google Scholar] [CrossRef] [PubMed]
- Kinlen, D.; Cody, D.; O’Shea, D. Complications of obesity. QJM 2018, 111, 437–443. [Google Scholar] [CrossRef] [PubMed]
- Powell-Wiley, T.M.; Poirier, P.; Burke, L.E.; Despres, J.P.; Gordon-Larsen, P.; Lavie, C.J.; Lear, S.A.; Ndumele, C.E.; Neeland, I.J.; Sanders, P.; et al. Obesity and cardiovascular disease: A scientific statement from the American Heart Association. Circulation 2021, 143, e984–e1010. [Google Scholar] [CrossRef]
- Koliaki, C.; Liatis, S.; Kokkinos, A. Obesity and cardiovascular disease: Revisiting an old relationship. Metabolism 2019, 92, 98–107. [Google Scholar] [CrossRef]
- Tutor, A.W.; Lavie, C.J.; Kachur, S.; Milani, R.V.; Ventura, H.O. Updates on obesity and the obesity paradox in cardiovascular diseases. Prog. Cardiovasc. Dis. 2023, 78, 2–10. [Google Scholar] [CrossRef]
- Barakat, B.; Almeida, M.E.F. Biochemical and immunological changes in obesity. Arch. Biochem. Biophys. 2021, 708, 108951. [Google Scholar] [CrossRef]
- Mayoral, L.P.; Andrade, G.M.; Mayoral, E.P.; Huerta, T.H.; Canseco, S.P.; Rodal Canales, F.J.; Cabrera-Fuentes, H.A.; Cruz, M.M.; Perez Santiago, A.D.; Alpuche, J.J.; et al. Obesity subtypes, related biomarkers & heterogeneity. Indian J. Med. Res. 2020, 151, 11–21. [Google Scholar] [CrossRef]
- Hu, C.; Yang, J.; Qi, Z.; Wu, H.; Wang, B.; Zou, F.; Mei, H.; Liu, J.; Wang, W.; Liu, Q. Heat shock proteins: Biological functions, pathological roles, and therapeutic opportunities. MedComm 2022, 3, e161. [Google Scholar] [CrossRef]
- Bascos, N.A.D.; Landry, S.J. A History of molecular chaperone structures in the Protein Data Bank. Int. J. Mol. Sci. 2019, 20, 6195. [Google Scholar] [CrossRef]
- Almalki, A.F.Y.; Arabdin, M.; Khan, A. The role of heat shock proteins in cellular homeostasis and cell survival. Cureus 2021, 13, e18316. [Google Scholar] [CrossRef]
- De Maio, A.; Hightower, L.E. Heat shock proteins and the biogenesis of cellular membranes. Cell Stress Chaperones 2021, 26, 15–18. [Google Scholar] [CrossRef] [PubMed]
- Haslbeck, M.; Weinkauf, S.; Buchner, J. Small heat shock proteins: Simplicity meets complexity. J. Biol. Chem. 2019, 294, 2121–2132. [Google Scholar] [CrossRef]
- Shan, Q.; Ma, F.; Wei, J.; Li, H.; Ma, H.; Sun, P. Physiological functions of heat shock proteins. Curr. Protein Pept. Sci. 2020, 21, 751–760. [Google Scholar] [CrossRef] [PubMed]
- Dubrez, L.; Causse, S.; Bonan, N.B.; Dumetier, B.; Garrido, C. Heat-shock proteins: Chaperoning DNA repair. Oncogene 2020, 39, 516–529. [Google Scholar] [CrossRef]
- Henstridge, D.C.; Whitham, M.; Febbraio, M.A. Chaperoning to the metabolic party: The emerging therapeutic role of heat-shock proteins in obesity and type 2 diabetes. Mol. Metab. 2014, 3, 781–793. [Google Scholar] [CrossRef] [PubMed]
- van Marion, D.M.S.; Lanters, E.A.H.; Ramos, K.S.; Li, J.; Wiersma, M.; Bulte, L.B.-T.; Muskens, A.; Boersma, E.; de Groot, N.M.S.; Brundel, B.J.J.M. Evaluating serum heat shock protein levels as novel biomarkers for atrial fibrillation. Cells 2020, 9, 2105. [Google Scholar] [CrossRef]
- Moura, C.S.; Lollo, P.C.B.; Morato, P.N.; Amaya-Farfan, J. Dietary nutrients and bioactive substances modulate heat shock protein (HSP) expression: A review. Nutrients 2018, 10, 683. [Google Scholar] [CrossRef]
- Molina, M.N.; Ferder, L.; Manucha, W. Emerging role of nitric oxide and heat shock proteins in insulin resistance. Curr. Hypertens. Rep. 2016, 18, 1. [Google Scholar] [CrossRef]
- Habich, C.; Sell, H. Heat shock proteins in obesity: Links to cardiovascular disease. Horm. Mol. Biol. Clin. Investig. 2015, 21, 117–124. [Google Scholar] [CrossRef]
- Kochetkova, O.Y.; Yurinskaya, M.M.; Evgen’ev, M.B.; Zatsepina, O.G.; Shabarchina, L.I.; Suslikov, A.V.; Tikhonenko, S.A.; Vinokurov, M.G. Influence of encapsulated heat shock protein HSP70 on the basic functional properties of blood phagocytes. Dokl. Biol. Sci. 2015, 465, 299–302. [Google Scholar] [CrossRef] [PubMed]
- Tiss, A.; Khadir, A.; Abubaker, J.; Abu-Farha, M.; Al-Khairi, I.; Cherian, P.; John, J.; Kavalakatt, S.; Warsame, S.; Al-Ghimlas, F.; et al. Immunohistochemical profiling of the heat shock response in obese non-diabetic subjects revealed impaired expression of heat shock proteins in the adipose tissue. Lipids Health Dis. 2014, 13, 106. [Google Scholar] [CrossRef] [PubMed]
- Ludwig, M.S.; Minguetti-Camara, V.C.; Heck, T.G.; Scomazzon, S.P.; Nunes, P.R.; Bazotte, R.B.; de Bittencourt, P.I.H., Jr. Short-term but not long-term hypoglycaemia enhances plasma levels and hepatic expression of HSP72 in insulin-treated rats: An effect associated with increased IL-6 levels but not with IL-10 or TNF-α. Mol. Cell. Biochem. 2014, 397, 97–107. [Google Scholar] [CrossRef] [PubMed]
- Jee, H. Size dependent classification of heat shock proteins: A mini-review. J. Exerc. Rehabil. 2016, 12, 255–259. [Google Scholar] [CrossRef]
- Rodriguez-Iturbe, B.; Johnson, R.J. Heat shock proteins and cardiovascular disease. Physiol. Int. 2018, 105, 19–37. [Google Scholar] [CrossRef] [PubMed]
- Schopf, F.H.; Biebl, M.M.; Buchner, J. The HSP90 chaperone machinery. Nat. Rev. Mol. Cell Biol. 2017, 18, 345–360. [Google Scholar] [CrossRef]
- Kurop, M.K.; Huyen, C.M.; Kelly, J.H.; Blagg, B.S.J. The heat shock response and small molecule regulators. Eur. J. Med. Chem. 2021, 226, 113846. [Google Scholar] [CrossRef]
- Collier, M.P.; Benesch, J.L.P. Small heat-shock proteins and their role in mechanical stress. Cell Stress Chaperones 2020, 25, 601–613. [Google Scholar] [CrossRef]
- Pavlova, T.; Novak, J.; Zlamal, F.; Bienertova-Vasku, J. HSPB7 gene polymorphism associated with anthropometric parameters of obesity and fat intake in a Central European population. Cent. Eur. J. Public Health 2018, 26, 272–277. [Google Scholar] [CrossRef]
- Singh, M.K.; Sharma, B.; Tiwari, P.K. The small heat shock protein Hsp27: Present understanding and future prospects. J. Therm. Biol. 2017, 69, 149–154. [Google Scholar] [CrossRef]
- Muranova, L.K.; Shatov, V.M.; Bukach, O.V.; Gusev, N.B. Cardio-vascular heat shock protein (cvHsp, HspB7), an unusual representative of small heat shock protein family. Biochemistry 2021, 86, S1–S11. [Google Scholar] [CrossRef] [PubMed]
- Oliva, K.; Barker, G.; Rice, G.E.; Bailey, M.J.; Lappas, M. 2D-DIGE to identify proteins associated with gestational diabetes in omental adipose tissue. J. Endocrinol. 2013, 218, 165–178. [Google Scholar] [CrossRef] [PubMed]
- Tavallaie, S.; Rahsepar, A.A.; Abdi, H.; Moohebati, M.; Moodi, F.; Pourghadamyari, H.; Esmaily, H.; Khorashadizadeh, F.; Ghayour-Mobarhan, M.; Ferns, G.A. Association between indices of body mass and antibody titers to heat-shock protein-27 in healthy subjects. Clin. Biochem. 2012, 45, 144–147. [Google Scholar] [CrossRef] [PubMed]
- Islam, A.; Hait, S.H.; Andrews-Shigaki, B.; Carus, S.; Deuster, P.A. Plasma HSP70 levels correlate with health risk factors and insulin resistance in African American subjects. Exp. Clin. Endocrinol. Diabetes 2014, 122, 496–501. [Google Scholar] [CrossRef] [PubMed]
- Kargari, M.; Tavassoli, S.; Avan, A.; Ebrahimi, M.; Azarpazhooh, M.R.; Asoodeh, R.; Nematy, M.; Hassanian, S.M.; Rahmani, F.; Mohammadzade, E.; et al. Relationship between serum anti-heat shock protein 27 antibody levels and obesity. Clin. Biochem. 2017, 50, 690–695. [Google Scholar] [CrossRef]
- Abaspour, A.R.; Taghikhani, M.; Parizadeh, S.M.R.; Seyedi, S.M.R.; Ghazizadeh, H.; Kazemi, E.; Moohebati, M.; Ghafoori, F.; Mardannik, M.; Avan, A.; et al. HSP27 expression in the human peripheral blood mononuclear cells as an early prognostic biomarker in coronary artery disease patients. Diabetes Metab. Syndr. 2019, 13, 1791–1795. [Google Scholar] [CrossRef]
- Muranova, L.K.; Shatov, V.M.; Gusev, N.B. Role of small heat shock proteins in the remodeling of actin microfilaments. Biochemistry 2022, 87, 800–811. [Google Scholar] [CrossRef]
- Sabbah, N.A.; Rezk, N.A.; Saad, M.S.S. Relationship between heat shock protein expression and obesity with and without metabolic syndrome. Genet. Test. Mol. Biomark. 2019, 23, 737–743. [Google Scholar] [CrossRef]
- Cherian, P.T.; Al-Khairi, I.; Sriraman, D.; Al-Enezi, A.; Al-Sultan, D.; AlOtaibi, M.; Al-Enezi, S.; Tuomilehto, J.; Al-Mulla, F.; Abubaker, J.A.; et al. Increased circulation and adipose tissue Levels of DNAJC27/RBJ in obesity and type 2-diabetes. Front. Endocrinol. 2018, 9, 423. [Google Scholar] [CrossRef]
- He, Y.; Wang, Z. The roles of HSP40/DNAJ protein family in neurodegenerative diseases. Zhejiang Da Xue Xue Bao Yi Xue Ban 2022, 51, 640–646. [Google Scholar] [CrossRef]
- Jais, A.; Einwallner, E.; Sharif, O.; Gossens, K.; Lu, T.T.; Soyal, S.M.; Medgyesi, D.; Neureiter, D.; Paier-Pourani, J.; Dalgaard, K.; et al. Heme oxygenase-1 drives metaflammation and insulin resistance in mouse and man. Cell 2014, 158, 25–40. [Google Scholar] [CrossRef] [PubMed]
- Diane, A.; Abunada, H.; Khattab, N.; Moin, A.S.M.; Butler, A.E.; Dehbi, M. Role of the DNAJ/HSP40 family in the pathogenesis of insulin resistance and type 2 diabetes. Ageing Res. Rev. 2021, 67, 101313. [Google Scholar] [CrossRef] [PubMed]
- Abubaker, J.; Tiss, A.; Abu-Farha, M.; Al-Ghimlas, F.; Al-Khairi, I.; Baturcam, E.; Cherian, P.; Elkum, N.; Hammad, M.; John, J.; et al. DNAJB3/HSP-40 cochaperone is downregulated in obese humans and is restored by physical exercise. PLoS ONE 2013, 8, e69217. [Google Scholar] [CrossRef] [PubMed]
- Bavisotto, C.C.; Alberti, G.; Vitale, A.M.; Paladino, L.; Campanella, C.; Rappa, F.; Gorska, M.; de Macario, E.C.; Cappello, F.; Macario, A.J.L.; et al. HSP60 post-translational modifications: Functional and pathological consequences. Front. Mol. Biosci. 2020, 7, 95. [Google Scholar] [CrossRef] [PubMed]
- Krishnan-Sivadoss, I.; Mijares-Rojas, I.A.; Villarreal-Leal, R.A.; Torre-Amione, G.; Knowlton, A.A.; Guerrero-Beltran, C.E. Heat shock protein 60 and cardiovascular diseases: An intricate love-hate story. Med. Res. Rev. 2021, 41, 29–71. [Google Scholar] [CrossRef] [PubMed]
- Märker, T.; Sell, H.; Zilleßen, P.; Glöde, A.; Kriebel, J.; Ouwens, D.M.; Pattyn, P.; Ruige, J.; Famulla, S.; Roden, M.; et al. Heat shock protein 60 as a mediator of adipose tissue inflammation and insulin resistance. Diabetes 2012, 61, 615–625. [Google Scholar] [CrossRef]
- Juwono, J.; Martinus, R.D. Does HSP60 provide a link between mitochondrial stress and inflammation in diabetes mellitus? J. Diabetes Res. 2016, 2016, 8017571. [Google Scholar] [CrossRef]
- Duan, Y.; Tang, H.; Mitchell-Silbaugh, K.; Fang, X.; Han, Z.; Ouyang, K. Heat shock protein 60 in cardiovascular physiology and diseases. Front. Mol. Biosci. 2020, 7, 73. [Google Scholar] [CrossRef]
- Martinus, R.D.; Goldsbury, J. Endothelial TNF-α induction by HSP60 secreted from THP-1 monocytes exposed to hyperglycaemic conditions. Cell Stress Chaperones 2018, 23, 519–525. [Google Scholar] [CrossRef]
- Märker, T.; Kriebel, J.; Wohlrab, U.; Burkart, V.; Habich, C. Adipocytes from New Zealand obese mice exhibit aberrant proinflammatory reactivity to the stress signal heat shock protein 60. J. Diabetes Res. 2014, 2014, 187153. [Google Scholar] [CrossRef]
- Sell, H.; Poitou, C.; Habich, C.; Bouillot, J.L.; Eckel, J.; Clement, K. Heat shock protein 60 in obesity: Effect of bariatric surgery and its relation to inflammation and cardiovascular risk. Obesity 2017, 25, 2108–2114. [Google Scholar] [CrossRef]
- Kuka, P.; Bucova, M.; Penz, P.; Paulovicova, E.; Blazicek, P.; Atalay, M.; Lietava, J. HSP60, oxidative stress parameters and cardiometabolic risk markers in hypertensive and normotensive Slovak females. Bratisl. Lek. Listy 2010, 111, 527–534. [Google Scholar] [PubMed]
- Damluji, A.A.; Ramireddy, A.; Al-Damluji, M.S.; Marzouka, G.R.; Otalvaro, L.; Viles-Gonzalez, J.F.; Dong, C.; Alfonso, C.E.; Hendel, R.C.; Cohen, M.G.; et al. Association between anti-human heat shock protein-60 and interleukin-2 with coronary artery calcium score. Heart 2015, 101, 436–441. [Google Scholar] [CrossRef]
- Khadir, A.; Kavalakatt, S.; Cherian, P.; Warsame, S.; Abubaker, J.A.; Dehbi, M.; Tiss, A. Physical Exercise Enhanced Heat Shock Protein 60 Expression and Attenuated Inflammation in the Adipose Tissue of Human Diabetic Obese. Front. Endocrinol. 2018, 9, 16. [Google Scholar] [CrossRef] [PubMed]
- Nahas, E.A.; Nahas-Neto, J.; Orsatti, C.L.; Tardivo, A.P.; Uemura, G.; Peracoli, M.T.; Witkin, S.S. The 60- and 70-kDa heat-shock proteins and their correlation with cardiovascular risk factors in postmenopausal women with metabolic syndrome. Cell Stress Chaperones 2014, 19, 559–568. [Google Scholar] [CrossRef] [PubMed]
- Yıldırım, O.; Tatar, E. The Roles of heat shock protein-60 and 70 and inflammation in obesity-related kidney disease. Cureus 2022, 14, e28675. [Google Scholar] [CrossRef]
- De Maio, A. Extracellular heat shock proteins, cellular export vesicles, and the stress observation system: A form of communication during injury, infection, and cell damage. It is never known how far a controversial finding will go! Dedicated to Ferruccio Ritossa. Cell Stress Chaperones 2011, 16, 235–249. [Google Scholar] [CrossRef]
- Ranek, M.J.; Stachowski, M.J.; Kirk, J.A.; Willis, M.S. The role of heat shock proteins and co-chaperones in heart failure. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2018, 373, 20160530. [Google Scholar] [CrossRef]
- De Lemos Muller, C.H.; Schroeder, H.T.; Rodrigues-Krause, J.; Krause, M. Extra and intra cellular HSP70 levels in adults with and without metabolic disorders: A systematic review and meta-analysis. Cell Stress Chaperones 2023, 1–11. [Google Scholar] [CrossRef]
- Gonzalez-Ramos, M.; Calleros, L.; Lopez-Ongil, S.; Raoch, V.; Griera, M.; Rodriguez-Puyol, M.; de Frutos, S.; Rodriguez-Puyol, D. HSP70 increases extracellular matrix production by human vascular smooth muscle through TGF-β1 up-regulation. Int. J. Biochem. Cell Biol. 2013, 45, 232–242. [Google Scholar] [CrossRef]
- Mulyani, W.R.W.; Sanjiwani, M.I.D.; Sandra; Prabawa, I.P.Y.; Lestari, A.A.W.; Wihandani, D.M.; Suastika, K.; Saraswati, M.R.; Bhargah, A.; Manuaba, I.B.A.P. Chaperone-based therapeutic target innovation: Heat shock protein 70 (HSP70) for type 2 diabetes mellitus. Diabetes Metab. Syndr. Obes. 2020, 13, 559–568. [Google Scholar] [CrossRef] [PubMed]
- Rogers, R.S.; Morris, E.M.; Wheatley, J.L.; Archer, A.E.; McCoin, C.S.; White, K.S.; Wilson, D.R.; Meers, G.M.; Koch, L.G.; Britton, S.L.; et al. Deficiency in the heat stress response could underlie susceptibility to metabolic disease. Diabetes 2016, 65, 3341–3351. [Google Scholar] [CrossRef]
- Alemi, H.; Khaloo, P.; Rabizadeh, S.; Mansournia, M.A.; Mirmiranpour, H.; Salehi, S.S.; Esteghamati, A.; Nakhjavani, M. Association of extracellular heat shock protein 70 and insulin resistance in type 2 diabetes; Independent of obesity and C-reactive protein. Cell Stress Chaperones 2019, 24, 69–75. [Google Scholar] [CrossRef]
- Lubkowska, A.; Dudzińska, W.; Pluta, W. Antioxidant enzyme activity and serum HSP70 concentrations in relation to insulin resistance and lipid profile in lean and overweight young men. Antioxidants 2023, 12, 655. [Google Scholar] [CrossRef] [PubMed]
- Costa-Beber, L.C.; Heck, T.G.; Fiorin, P.B.G.; Ludwig, M.S. HSP70 as a biomarker of the thin threshold between benefit and injury due to physical exercise when exposed to air pollution. Cell Stress Chaperones 2021, 26, 889–915. [Google Scholar] [CrossRef]
- Archer, A.E.; Von Schulze, A.T.; Geiger, P.C. Exercise, heat shock proteins and insulin resistance. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2018, 373, 20160529. [Google Scholar] [CrossRef]
- Vulf, M.A.; Kirienkova, E.V.; Skuratovskaia, D.A.; Levada, E.V.; Volkova, L.V.; Zatolokin, P.A.; Gazatova, N.D.; Litvinova, L.S. Factors governing development of nonalcoholic fatty liver disease and insulin resistance in obesity. Biomed. Khimiia 2018, 64, 444–450. (In Russian) [Google Scholar] [CrossRef]
- Di Naso, F.C.; Porto, R.R.; Fillmann, H.S.; Maggioni, L.; Padoin, A.V.; Ramos, R.J.; Mottin, C.C.; Bittencourt, A.; Marroni, N.A.; de Bittencourt, P.I.H., Jr. Obesity depresses the anti-inflammatory HSP70 pathway, contributing to NAFLD progression. Obesity 2015, 23, 120–129. [Google Scholar] [CrossRef]
- Soliman, N.A.; Ghafar, M.T.A.; El Kolaley, R.M.; Hafez, Y.M.; Elgheit, R.E.A.; Atef, M.M. Cross talk between Hsp72, HMGB1 and RAGE/ERK1/2 signaling in the pathogenesis of bronchial asthma in obese patients. Mol. Biol. Rep. 2020, 47, 4109–4116. [Google Scholar] [CrossRef]
- Mardan-Nik, M.; Pasdar, A.; Jamialahmadi, K.; Avan, A.; Mohebati, M.; Esmaily, H.; Biabangard-Zak, A.; Javan, F.A.; Rivandi, M.; Ferns, G.A.; et al. Association of heat shock protein70-2 (HSP70-2) gene polymorphism with obesity. Ann. Hum. Biol. 2016, 43, 542–546. [Google Scholar] [CrossRef]
- Hagymasi, A.T.; Dempsey, J.P.; Srivastava, P.K. Heat-shock proteins. Curr. Protoc. 2022, 2, e592. [Google Scholar] [CrossRef] [PubMed]
- Hoter, A.; El-Sabban, M.E.; Naim, H.Y. The HSP90 family: Structure, regulation, function, and implications in health and disease. Int. J. Mol. Sci. 2018, 19, 2560. [Google Scholar] [CrossRef] [PubMed]
- Balanescu, A.; Stan, I.; Codreanu, I.; Comanici, V.; Balanescu, E.; Balanescu, P. Circulating HSP90 isoform levels in overweight and obese children and the relation to nonalcoholic fatty liver disease: Results from a cross-sectional study. Dis. Markers 2019, 2019, 9560247. [Google Scholar] [CrossRef] [PubMed]
- Skorzynska-Dziduszko, K.E.; Olszewska, A.; Prendecka, M.; Malecka-Massalska, T. Serum heat shock protein 90 alpha: A new marker of hypertension-induced endothelial injury? Adv. Clin. Exp. Med. 2016, 25, 255–261. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Timofeev, Y.S.; Kiselev, A.R.; Dzhioeva, O.N.; Drapkina, O.M. Heat Shock Proteins (HSPs) and Cardiovascular Complications of Obesity: Searching for Potential Biomarkers. Curr. Issues Mol. Biol. 2023, 45, 9378-9389. https://doi.org/10.3390/cimb45120588
Timofeev YS, Kiselev AR, Dzhioeva ON, Drapkina OM. Heat Shock Proteins (HSPs) and Cardiovascular Complications of Obesity: Searching for Potential Biomarkers. Current Issues in Molecular Biology. 2023; 45(12):9378-9389. https://doi.org/10.3390/cimb45120588
Chicago/Turabian StyleTimofeev, Yuriy S., Anton R. Kiselev, Olga N. Dzhioeva, and Oxana M. Drapkina. 2023. "Heat Shock Proteins (HSPs) and Cardiovascular Complications of Obesity: Searching for Potential Biomarkers" Current Issues in Molecular Biology 45, no. 12: 9378-9389. https://doi.org/10.3390/cimb45120588
APA StyleTimofeev, Y. S., Kiselev, A. R., Dzhioeva, O. N., & Drapkina, O. M. (2023). Heat Shock Proteins (HSPs) and Cardiovascular Complications of Obesity: Searching for Potential Biomarkers. Current Issues in Molecular Biology, 45(12), 9378-9389. https://doi.org/10.3390/cimb45120588





