E-Cadherin Is Expressed in Epithelial Cells of the Choroid Plexus in Human and Mouse Brains
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Human Tissues
2.3. Immunohistochemical Procedure in Mouse Brains
2.4. Western Blotting in Choroid Plexus Tissues of Mice
2.5. RT-PCR Analysis of Choroid Plexus Tissues in Mice
2.6. Immunohistochemical Procedure in Human Brains
3. Results
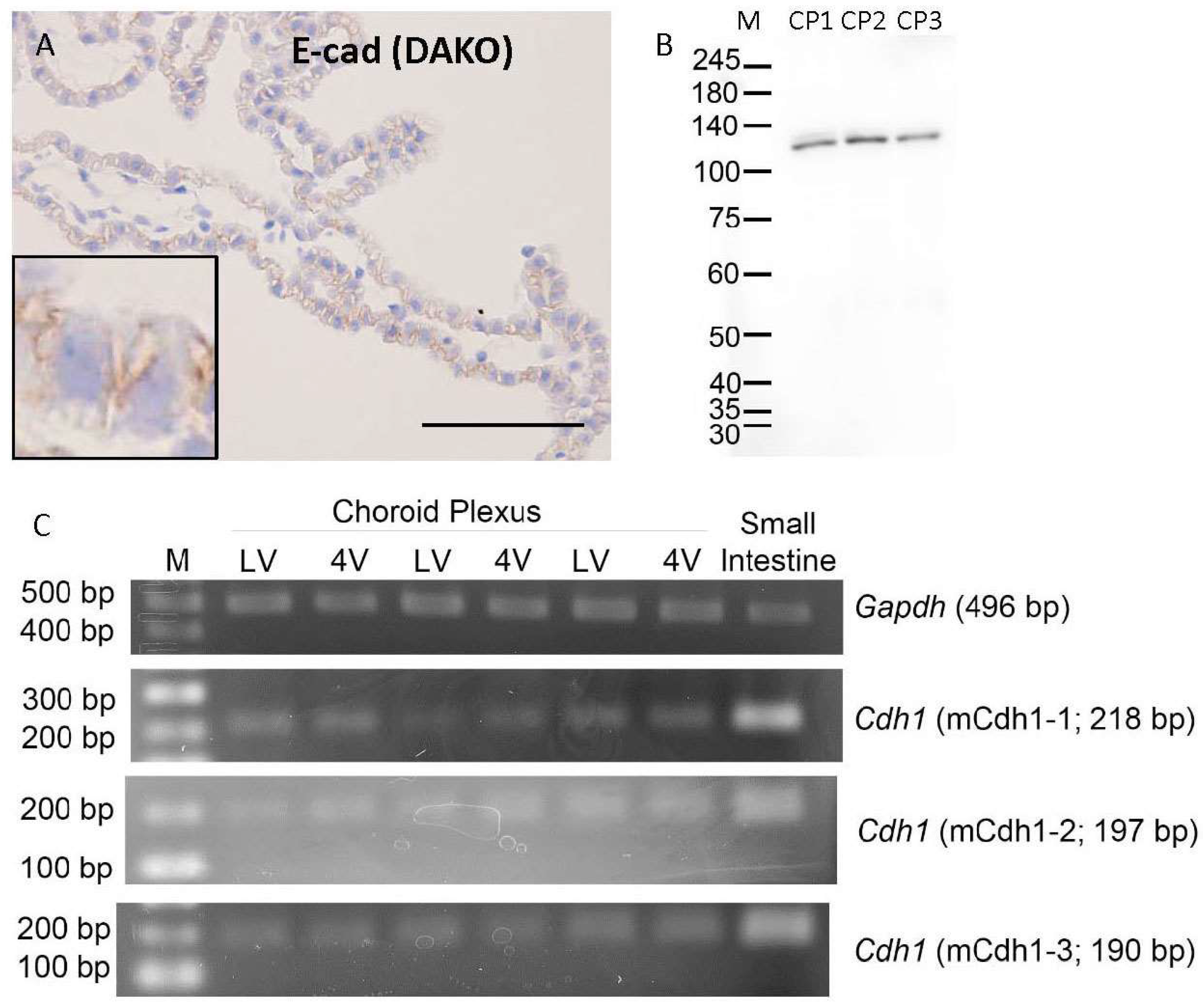
3.1. Cadherin Expression in Mouse Choroid Plexus
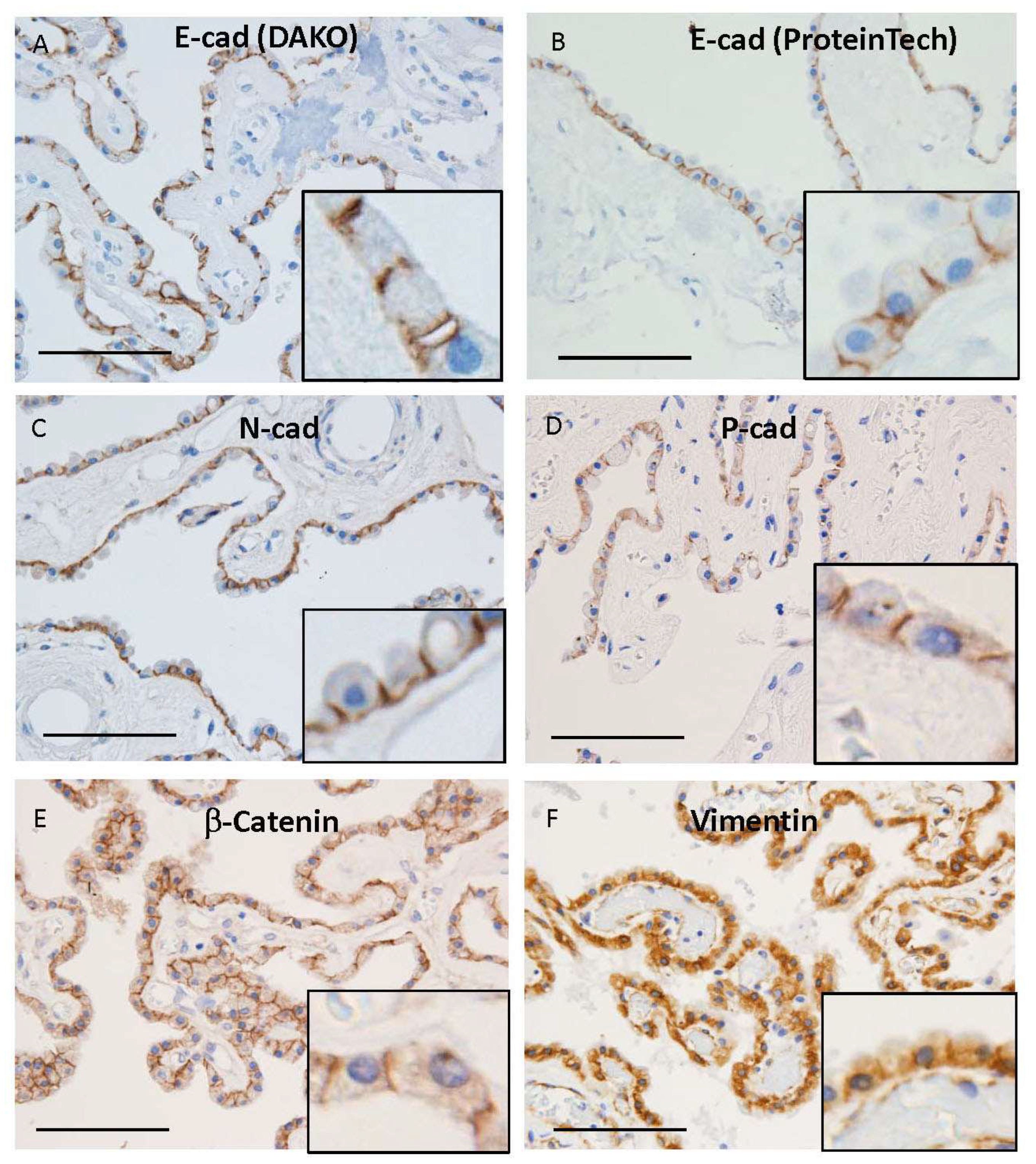
3.2. Cadherin Expression in Human Choroid Plexus
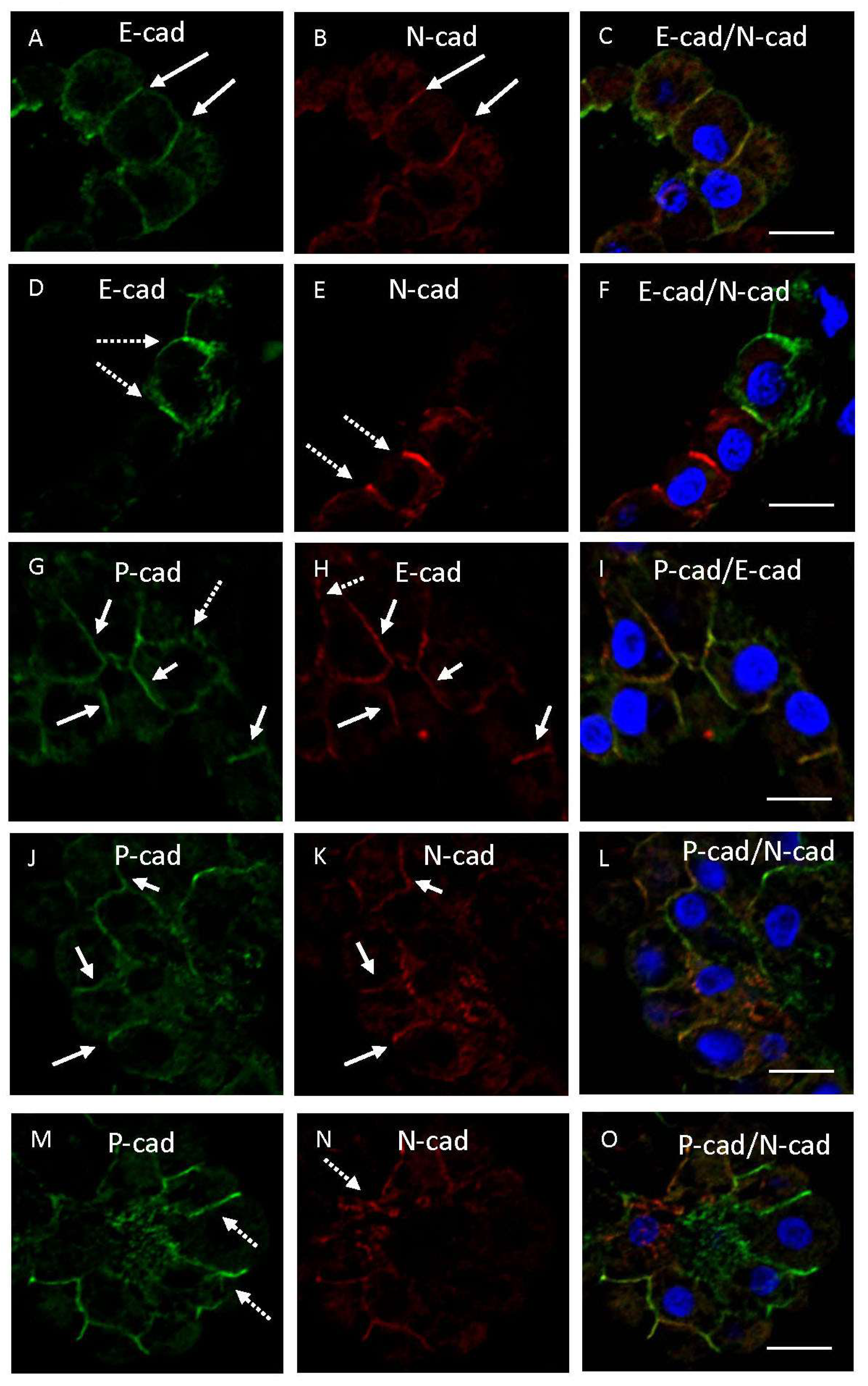
3.3. Double Immunofluorescence Examination of Cadherin Expression
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lun, M.P.; Johnson, M.B.; Broadbelt, K.G.; Watanabe, M.; Kang, Y.-J.; Chau, K.F.; Springel, M.W.; Malesz, A.; Sousa, A.M.M.; Pletikos, M.; et al. Spatially heterogeneous choroid plexus transcriptomes encode positional identity and contribute to regional CSF production. J. Neurosci. 2015, 35, 4903–4916. [Google Scholar] [CrossRef] [PubMed]
- Bryniarski, M.A.; Ren, T.; Rizvi, A.R.; Snyder, A.M.; Morris, M.E. Targeting the choroid plexuses for protein drug delivery. Pharmaceutics 2020, 12, 963. [Google Scholar] [CrossRef] [PubMed]
- González-Marrero, I.; Giménez-Llort, L.; Johanson, C.E.; Carmona-Calero, E.M.; Castañeyra-Ruiz, L.; Brito-Armas, J.M.; Castañeyra-Perdomo, A.; Castro-Fuentes, R. Choroid plexus dysfunction impairs beta-amyloid clearance in a triple transgenic mouse model of Alzheimer’s disease. Front. Cell Neurosci. 2015, 9, 17. [Google Scholar] [CrossRef] [PubMed]
- Myung, J.; Schmal, C.; Hong, S.; Tsukizawa, Y.; Rose, P.; Zhang, Y.; Holtzman, M.J.; Schutter, E.D.; Herzel, H.; Bordyugov, G.; et al. The choroid plexus is an important circadian clock component. Nat. Commun. 2018, 9, 1062. [Google Scholar] [CrossRef] [PubMed]
- Mishima, K.; Tozawa, T.; Satoh, K.; Matsumoto, Y.; Hishikawa, Y.; Okawa, M. Melatonin secretion rhythm disorders in patients with senile dementia of Alzheimer’s type with disturbed sleep-waking. Biol. Psychiatry 1999, 45, 417–421. [Google Scholar] [CrossRef] [PubMed]
- Jovanović, I.; Ugrenović, S.; Antić, S.; Stefanović, N.; Mihailović, D. Morphometric and some immunohistochemical characteris tics of human choroids plexus stroma and psammoma bodies. Microsc. Res. Tech. 2007, 70, 617–627. [Google Scholar] [CrossRef]
- Jovanović, I.; Ugrenović, S.; Vasović, L.; Petrović, D.; Cekić, S. Psammoma bodies—Friends or foes of the aging choroid plexus. Med. Hypotheses 2010, 74, 1017–1020. [Google Scholar] [CrossRef]
- Pearson, A.; Ajoy, R.; Crynen, G.; Reed, J.M.; Algamal, M.; Mullan, M.; Purohit, D.; Crawford, F.; Ojo, J.O. Molecular abnormali ties in autopsied brain tissue from the inferior horn of the lateral ventricles of nonagenarians and Alzheimer disease patients. BMC Neurol. 2020, 20, 317. [Google Scholar] [CrossRef]
- Christensen, I.B.; Mogensen, E.N.; Damkier, H.H.; Praetorius, J. Choroid plexus epithelial cells express the adhesion protein P-cadherin at cell-cell contacts and syntaxin-4 in the luminal membrane domain. Am. J. Physiol. Cell Physiol. 2018, 314, C519–C533. [Google Scholar] [CrossRef]
- Damkier, H.; Praetorius, J. Structure of the mammalian choroid plexus. In Role of the Choroid Plexus in Health and Disease; Prae torius, J., Blazer-Yost, B., Damkier, H., Eds.; Springer: New York, NY, USA, 2020; pp. 19–24. [Google Scholar]
- Vorbrodt, A.W.; Dobrogowska, D.H. Molecular anatomy of intercellular junctions in brain endothelial and epithelial barriers: Electron microscopist’s view. Brain Res. Brain Res. Rev. 2003, 42, 221–242. [Google Scholar] [CrossRef]
- Lippoldt, A.; Liebner, S.; Andbjer, B.; Kalbacher, H.; Wolburg, H.; Haller, H.; Fuxe, K. Organization of choroid plexus epithelial and endothelial cell tight junctions and regulation of claudin-1, -2, and -5 expression by protein kinase C. Neuroreport 2000, 11, 1427–1431. [Google Scholar] [CrossRef] [PubMed]
- Wolburg, H.; Wolburg-Buchholz, K.; Liebner, S.; Engelhardt, B. Claudin-1, claudin-2, and claudin-11 are present in tight junc tions of choroid plexus epithelium of the mouse. Neurosci. Lett. 2001, 307, 77–80. [Google Scholar] [CrossRef] [PubMed]
- Steinemann, A.; Galm, I.; Chip, S.; Nitsch, C.; Maly, I.P. Claudin-1, -2 and -3 are selectively expressed in the epithelia of the choroid plexus of the mouse from early development and into adulthood while claudin-5 is restricted to endothelial cells. Front. Neuroanat. 2016, 10, 16. [Google Scholar] [CrossRef] [PubMed]
- Rosenthal, R.; Milatz, S.; Krug, S.M.; Oelrich, B.; Schulzke, J.D.; Amasheh, S.; Günzel, D.; Fromm, M. Claudin-2, a component of the tight junction, form a paracellular water channel. J. Cell Sci. 2010, 123, 1913–1921. [Google Scholar] [CrossRef] [PubMed]
- Lagunowich, L.A.; Schneider, J.C.; Chasen, S.; Grunwald, G.B. Immunohistochemical and biochemical analysis of N-cadherin expression during CNS development. J. Neurosci. Res. 1992, 32, 202–208. [Google Scholar] [CrossRef]
- Marrs, J.; Napolitano, E.W.; Murphy-Erdosh, C.; Mays, R.W.; Reichardt, L.F.; Nelson, W.J. Distinguishing roles of the mem branes-cytoskeleton and cadherin mediated cell-cell adhesion in generating different Na+,K+-ATPase distributions in polarized epithelia. J. Cell Biol. 1993, 123, 149–164. [Google Scholar] [CrossRef]
- Kaji, C.; Tomooka, M.; Kato, Y.; Kojima, H.; Sawa, Y. The expression of podoplanin and classic cadherins in the mouse brain. J. Anat. 2012, 220, 435–446. [Google Scholar] [CrossRef]
- Christensen, I.B.; Gyldenholm, T.; Damkier, H.H.; Praetorius, J. Polarization of membrane associated proteins in the choroid plexus epithelium from normal and slc4a10 knockout mice. Front. Physiol. 2013, 4, 344. [Google Scholar] [CrossRef]
- Figarella-Branger, D.; Lepidi, H.; Poncet, C.; Gambarelli, D.; Bianco, N.; Rougon, G.; Pellissier, J.-F. Differential expression of cell adhesion molecules (CAM), neural CAM and epithelial cadherin in ependymomas and choroid plexus tumors. Acta Neuropathol. 1995, 89, 248–257. [Google Scholar] [CrossRef]
- Szmydynger-Chodobska, J.; Pascale, C.L.; Pfeffer, A.N.; Coulter, C.; Chodobski, A. Expression of junctional proteins in choroid plexus epithelial cell lines: A comparative study. Cerebrospinal Fluid. Res. 2007, 4, 11. [Google Scholar] [CrossRef]
- Chiba, Y.; Sugiyama, Y.; Nishi, N.; Nonaka, W.; Murakami, R.; Ueno, M. Sodium/glucose cotransporter 2 is expressed in choroid plexus epithelial cells and ependymal cells in human and mouse brain. Neuropathology 2020, 40, 482–491. [Google Scholar] [CrossRef] [PubMed]
- Wakamatsu, K.; Chiba, Y.; Murakami, R.; Matsumoto, K.; Miyai, Y.; Kawauchi, M.; Yanase, K.; Uemura, N.; Ueno, M. Immuno histochemical expression of osteopontin and collagens in choroid plexus of human brains. Neuropathology 2022, 42, 117–125. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Ma, X.; Li, Y.; He, Y.; Huang, D.; Cai, S.; Peng, J. The characteristics and prognostic effect of E-cadherin expression in colorectal signet ring cell carcinoma. PLoS ONE 2016, 11, e0160527. [Google Scholar] [CrossRef]
- Khadri, F.-Z.; Issac, M.S.M.; Gaboury, L.A. Impact of epithelial-mesenchymal transition on the immune landscape in breast cancer. Cancers 2021, 13, 5099. [Google Scholar] [CrossRef]
- He, S.; Zhao, Z.; Yang, Y.; O’Connell, D.; Zhang, X.; Oh, S.; Ma, B.; Lee, J.-H.; Zhang, T.; Varghese, B.; et al. Truncating mutation in the autophagy gene UVRAG confers oncogenic properties and chemosensitivity in colorectal cancers. Nat. Commun. 2015, 6, 7839. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Z.; Zhai, X.; Shi, B.; Luo, D.; Jin, B. KIAA1199 overexpression is associated with abnormal expression on EMT markers and is a novel independent prognostic biomarker for hepatocellular carcinoma. Onco. Targets Ther. 2018, 11, 8341–8348. [Google Scholar] [CrossRef]
- Wakamatsu, K.; Chiba, Y.; Murakami, R.; Miyai, Y.; Matsumoto, K.; Kamada, M.; Nonaka, W.; Uemura, N.; Yanase, K.; Ueno, M. Metabolites and biomarker compounds of neurodegenerative diseases in cerebrospinal fluid. Metabolites 2022, 12, 343. [Google Scholar] [CrossRef]
- Mescher, M.; Jeong, P.; Knapp, S.K.; Rübsam, M.; Saynisch, M.; Kranen, M.; Landsberg, J.; Schlaak, M.; Mauch, C.; Tüting, T.; et al. The epidermal polarity protein Par3 is a non-cell autonomous suppressor of malignant melanoma. J. Exp. Med. 2017, 214, 339–358. [Google Scholar] [CrossRef]
- Khan, S.; Jena, G.; Tikoo, K.; Kumar, V. Valproate attenuate the proteinuria, podocyte and renal injury by facilitating autophagy and inactivation of NF-κB/iNOS signaling in diabetic rat. Biochimie 2015, 110, 1–16. [Google Scholar] [CrossRef]
- Einecke, G.; Fairhead, T.; Hidalgo, L.G.; Sis, B.; Turner, P.; Zhu, L.-F.; Bleackley, R.C.; Hadley, G.A.; Famulski, K.S.; Halloran, P.F. Tubulitis and epithelial cell alterations in mouse kidney transplant rejection are independent of CD103, perforin or granzymes A/B. Am. J. Transplant. 2006, 6, 2109–2120. [Google Scholar] [CrossRef]
- Nose, A.; Takeichi, M. A novel cadherin cell adhesion molecule: Its expression patterns associated with implantation and organ ogenesis of mouse embryos. J. Cell Biol. 1986, 103, 2649–2658. [Google Scholar] [CrossRef]
- Takeichi, M.; Hatta, K.; Nose, A.; Nagafuchi, A. Identification of a gene family of cadherin cell adhesion molecules. Cell Difffer. Dev. 1988, 25, 91–94. [Google Scholar] [CrossRef] [PubMed]
- Takeichi, M. The cadherins: Cell-cell adhesion molecules controlling animal morphogenesis. Development 1988, 102, 639–655. [Google Scholar] [CrossRef] [PubMed]
- Noronha, C.; Ribeiro, A.S.; Taipa, R.; Castro, D.S.; Reis, J.; Faria, C.; Paredes, J. Cadherin expression and EMT: A focus on glioma. Biomedicines 2021, 9, 1328. [Google Scholar] [CrossRef] [PubMed]
- Hirai, Y.; Nose, A.; Kobayashi, S.; Takeichi, M. Expression and role of E- and P-cadherin adhesion molecules in embryonic histo genesis. I. Lung epithelial morphogenesis. Development 1989, 105, 263–270. [Google Scholar] [CrossRef] [PubMed]
- Furukawa, F.; Fujii, K.; Horiguchi, Y.; Matsuyoshi, N.; Fujita, M.; Toda, K.; Imamura, S.; Wakita, H.; Shirahama, S.; Takigawa, M. Roles of E- and P-cadherin in the human skin. Microsc. Res. Tech. 1997, 38, 343–352. [Google Scholar] [CrossRef]
- Paredes, J.; Figueiredo, J.; Albergaria, A.; Oliveira, P.; Carvalho, J.; Ribeiro, A.S.; Caldeira, J.; Costa, A.M.; Simões-Correia, J.; Oliveira, M.J.; et al. Epithelial E- and P-cadherins: Role and clinical significance in cancer. Biochim. Biophys. Acta 2012, 1826, 297–311. [Google Scholar] [CrossRef]
- Vieira, A.F.; Paredes, J. P-cadherin and the journey to cancer metastasis. Mol. Cancer 2015, 14, 178. [Google Scholar] [CrossRef]
- Naydenov, N.G.; Lechuga, S.; Zalavadia, A.; Mukherjee, P.K.; Gordon, H.O.; Skvasik, D.; Vidovic, P.; Huang, E.; Rieder, F.; Ivanov, A.I. P-cadherin regulates intestinal epithelial cell migration and mucosal repair, but is dispensable for colitis associated colon cancer. Cells 2022, 11, 1467. [Google Scholar] [CrossRef]
- Sanders, D.S.A.; Perry, I.; Hardy, R.; Jankowski, J. aberrant P-cadherin expression is a feature of clonal expansion in the gastrointestinal tract associated with repair and neoplasia. J. Pathol. 2000, 190, 526–530. [Google Scholar] [CrossRef]
- Zbar, A.P.; Simopoulos, C.; Karayiannakis, A.J. Cadherins: An integral role in inflammatory bowel disease and mucosal restitution. J. Gastroenterol. 2004, 39, 413–421. [Google Scholar] [CrossRef] [PubMed]
- Matsuo, M.; Seo, K.; Taruno, A.; Mizoro, Y.; Yamaguchi, Y.; Doi, M.; Nakao, R.; Kori, H.; Abe, T.; Ohmori, H.; et al. A light-induced small G-protein gem limits the circadian clock phase-shift magnitude by inhibiting voltage-dependent calcium channels. Cell Rep. 2022, 39, 110844. [Google Scholar] [CrossRef] [PubMed]
- The Human Protein Atlas (Human Protein Atlas proteinatlas.org). Available online: https://www.proteinatlas.org/ENSG00000039068-CDH1 (accessed on 17 August 2023).

| (No.) | Age/Sex | Main Diagnosis |
|---|---|---|
| 1 | 42/M | Pulmonary hypertension, Heart failure |
| 2 | 57/F | Psychiatric disorder, Liver abscess, Sepsis |
| 3 | 64/M | Multiple system atrophy, Pneumonia |
| 4 | 68/F | Thalamic hemorrhage |
| 5 | 70/M | Myocardial infarction |
| 6 | 72/F | Pneumonia |
| 7 | 74/M | Lung cancer |
| 8 | 75/M | Gastric cancer |
| 9 | 75/M | Multiple system atrophy, Pneumonia |
| 10 | 84/M | Myocardial infarction, Cerebral infarction |
| Antibody | Cat. No. (Clone Name) | Host Species and Usage | References |
|---|---|---|---|
| E-cadherin | Dako, M3612 (NCH-38) | mouse, 1:250 (¶1) | [24,25] |
| E-cadherin | ProteinTech, 20874-1-AP | rabbit, 1:2000 (¶2) | [26,27] |
| N-cadherin | ProteinTech, 22018-1-AP | rabbit, 1:1000 (¶2) | [27,28] |
| P-cadherin | Invitrogen, 13-2000Z (PCD-1) | rat, 1:200 (¶2) | [29] |
| P-cadherin | Santa Cruz, sc-74545 (A-10) | mouse, 1:100 (¶1) | [28] |
| β-catenin | Santa Cruz, sc-7199 | rabbit, 1:50 (¶2) | [30] |
| vimentin | Dako, M0725 (V9) | mouse, 1:50 (¶1) | [6,23] |
| Genes | Primers (5’-3’) | Location | Ampicon Size (bp) | Reference |
|---|---|---|---|---|
| Cdh1 (mCdf1-1) | F: GGAGACCAGTTTCCTCGTCC R: CATTTTCGGGGCAGCTGATG | 433–452 650–631 | 218 | |
| Cdh1 (mCdf1-2) | F: GCTCTCATCATCGCCACAGA R: GCAGTAAAGGGGGACGTGTT | 1838–1857 2034–2015 | 197 | |
| Cdh1 (mCdh1-3) | F: CTGCCATCCTCGGAATCCTT R: TGGCTCAAATCAAAGTCCTGGT | 2274–2293 2463–2442 | 190 | [31] |
| Gapdh | F: CAAGGTCATCCATGACAACTTTG R: GTCCACCACCCTGTTGCTGTAG | 527–549 1022–1001 | 496 | [22] |
| (No.) | Age/Sex | A | B | C | D | E | F |
|---|---|---|---|---|---|---|---|
| 1 | 42/M | 79 | 91 | 81 | 31 | 98 | 94 |
| 2 | 57/F | 47 | 74 | 84 | 25 | 73 | 54 |
| 3 | 64/M | 68 | 81 | 57 | 29 | 77 | 98 |
| 4 | 68/F | 12 | 68 | 87 | 24 | 28 | 42 |
| 5 | 70/M | 73 | 94 | 84 | 38 | 96 | 98 |
| 6 | 72/F | 51 | 86 | 73 | 15 | 82 | 88 |
| 7 | 74/M | 64 | 95 | 46 | 19 | 86 | 70 |
| 8 | 75/M | 56 | 92 | 61 | 22 | 74 | 98 |
| 9 | 75/M | 43 | 68 | 67 | 40 | 72 | 99 |
| 10 | 84/M | 67 | 98 | 81 | 37 | 76 | 97 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Takebayashi, G.; Chiba, Y.; Wakamatsu, K.; Murakami, R.; Miyai, Y.; Matsumoto, K.; Uemura, N.; Yanase, K.; Shirakami, G.; Ogino, Y.; et al. E-Cadherin Is Expressed in Epithelial Cells of the Choroid Plexus in Human and Mouse Brains. Curr. Issues Mol. Biol. 2023, 45, 7813-7826. https://doi.org/10.3390/cimb45100492
Takebayashi G, Chiba Y, Wakamatsu K, Murakami R, Miyai Y, Matsumoto K, Uemura N, Yanase K, Shirakami G, Ogino Y, et al. E-Cadherin Is Expressed in Epithelial Cells of the Choroid Plexus in Human and Mouse Brains. Current Issues in Molecular Biology. 2023; 45(10):7813-7826. https://doi.org/10.3390/cimb45100492
Chicago/Turabian StyleTakebayashi, Genta, Yoichi Chiba, Keiji Wakamatsu, Ryuta Murakami, Yumi Miyai, Koichi Matsumoto, Naoya Uemura, Ken Yanase, Gotaro Shirakami, Yuichi Ogino, and et al. 2023. "E-Cadherin Is Expressed in Epithelial Cells of the Choroid Plexus in Human and Mouse Brains" Current Issues in Molecular Biology 45, no. 10: 7813-7826. https://doi.org/10.3390/cimb45100492
APA StyleTakebayashi, G., Chiba, Y., Wakamatsu, K., Murakami, R., Miyai, Y., Matsumoto, K., Uemura, N., Yanase, K., Shirakami, G., Ogino, Y., & Ueno, M. (2023). E-Cadherin Is Expressed in Epithelial Cells of the Choroid Plexus in Human and Mouse Brains. Current Issues in Molecular Biology, 45(10), 7813-7826. https://doi.org/10.3390/cimb45100492






