cAMP Signalling Pathway in Biocontrol Fungi
Abstract
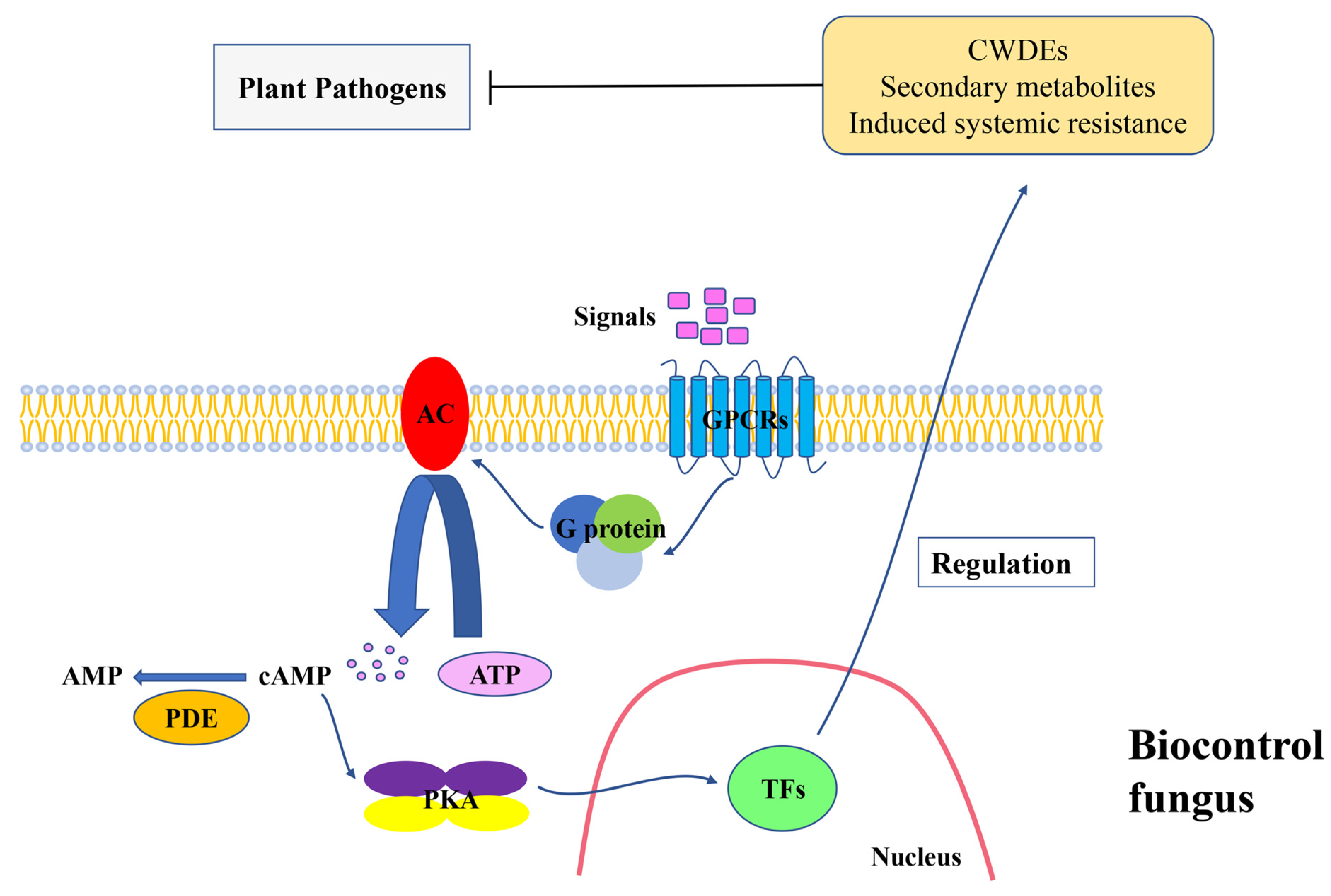
1. Introduction
2. G Protein System
| Protein | Gene | Biocontrol Fungi | Pathogens | |
|---|---|---|---|---|
| G protein system | G-protein alpha subunit | tgaA | Trichoderma virens | Sclerotium rolfsii [63] |
| G-protein alpha subunit | thga1 | T. harzianum | Rhizoctonia solani [65] | |
| G-protein alpha subunit | thga3 | T. harzianum | R. solani [64] | |
| G-protein alpha subunit | tga3 | T. atroviride | Botrytis cinerea, R. solani [62] | |
| G-protein alpha subunit | tga1 | T. atroviride | R. solani, B. cinerea, S. sclerotiorum [59] | |
| Seven-transmembrane receptor Gpr1 | gpr1 | T. atroviride | R. solani, B. cinerea, S. sclerotiorum [58] | |
| G-protein alpha subunit | gna3 | T. reesei | Pythium ultimum [60] | |
| G-protein alpha subunit | MrGpa1 | Metarhizium robertsii | Galleria mellonella [67] | |
| G-protein coupled receptor | MrGpr8 | M. robertsii | G. mellonella, Tenebrio molitor, Bombyx mori [66] | |
| G-protein coupled receptor | BbGPCR3 | Beauveria bassiana | G. mellonella [68] | |
| G-protein coupled receptor | BBA_00828 BBA_05185 | B. bassiana | Anophele stephensi [71] | |
| G-protein coupled receptor | CmEST-463 | Coniothyrium minitans | S. sclerotiorum [70] | |
| G-protein coupled receptor | BN869_T00001016_1 | Clonostachys rosea | Helminthosporium solani [69] | |
| Adenylate cyclase | Adenylate cyclase | tac1 | T. virens | S. rolfsii, R. solani, Pythium sp. [72] |
| Adenylate cyclase | MaAC | M. acridum | Locusta migratoria [73] | |
| Adenylate cyclase | MrAC | M. robertsii | T. molitor [74] | |
| Adenylate cyclase | BcAC | B. bassiana | G. mellonella [74] | |
| Phosphodiesterase | Phosphodiesterase | AopdeH | Arthrobotrys oligospora | Caenorhabditis elegans [75] |
| Protein kinase A | Protein kinase A | MaPKA1 | M. anisopliae | G. mellonella [76] |
| Protein kinase A | CMZSB_03553 | C. minitans | S. sclerotiorum [77] | |
| Transcription factor | Transcription factor | MrStuA | M. rileyi | Spodoptera litura [78] |
| Transcription factor | MaSom1 | M. acridum | L. migratoria manilensis [79] | |
3. Adenylate Cyclases
4. cAMP-Dependent Protein Kinase A
5. Transcription Factors
6. Conclusions and Perspectives
- (1)
- The expression levels of cAMP signalling pathway upstream genes, such as the G protein system components (GPCRs and G protein), should be investigated in biocontrol-fungi-infecting pathogens. The expression levels could be detected through transcriptome sequencing or proteomic analysis or directly monitored by RT-qPCR. Among all the G protein system-encoding genes, those genes with significant differential expression would be selected for gene knockout or silencing to clarify their functions in biocontrol.
- (2)
- The expression levels of all AC-encoding genes should be investigated in the wild-type strain and G protein system null-mutant-infecting pathogens. AC genes that are dramatically differentially expressed after G protein system-encoding gene deletion would be chosen for the gene functional analysis. Gene knockout or silencing was used to investigate the functions of AC-encoding genes in biocontrol. Thus, genes encoding the G protein system and AC in the same cAMP signalling pathway would be connected.
- (3)
- Accordingly, PKA-encoding genes involved in the consistent cAMP signalling pathway would be selected using the above strategy through the process of comparison of the wild strain with the AC null mutant against pathogens. Hence, genes encoding the G protein system, AC, and PKA in the same cAMP signalling pathway would be connected.
- (4)
- The most important components in the cAMP signalling pathway are the downstream TFs. Because a variety of TF families exist, identifying TF-encoding genes consistent with the same cAMP signalling pathway using the above strategy is very important. Thus far, a complete cAMP signalling pathway involved in biocontrol behaviour has been constructed.
- (5)
- Finally, the expression levels of some downstream effector protein genes, such as genes encoding cell-wall-degrading enzymes or secondary metabolite production, should be investigated through comparison of the wild-type strain with the TF null mutant against pathogens. Future work is vital to deeply clear the mechanism of the biocontrol signals transduced through the cAMP signalling pathway in biocontrol fungi.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Karlsson, M.; Atanasova, L.; Jensen, D.F.; Zeilinger, S. Necrotrophic Mycoparasites and Their Genomes. Microbiol. Spectr. 2017, 5. FUNK-0016-2016. [Google Scholar] [CrossRef] [PubMed]
- Qualhato, T.F.; Lopes, F.A.; Steindorff, A.S.; Brand~ao, R.S.; Jesuino, R.S.; Ulhoa, C.J. Mycoparasitism studies of Trichoderma species against three phytopathogenic fungi: Evaluation of antagonism and hydrolytic enzyme production. Biotechnol. Lett. 2013, 35, 1461–1468. [Google Scholar] [CrossRef] [PubMed]
- Reino, J.L.; Guerriero, R.F.; Hernandez-Gala, R.; Collado, I.G. Secondary metabolites from species of the biocontrol agent Trichoderma. Phytochem. Rev. 2008, 7, 89–123. [Google Scholar] [CrossRef]
- Vinale, F.; Sivasithamparam, K.; Ghisalberti, E.L.; Marra, R.; Barbetti, M.J.; Li, H.; Woo, S.L.; Lorito, M. A novel role for Trichoderma secondary metabolites in the interactions with plants. Physiol. Mol. Plant Pathol. 2008, 72, 80–86. [Google Scholar] [CrossRef]
- Zeilinger, S.; Omann, M. Trichoderma biocontrol: Signal transduction pathways involved in host sensing and mycoparasitism. Gene Regul. Syst. Biol. 2007, 1, 227–234. [Google Scholar] [CrossRef]
- Schmoll, M. Assessing the relevance of light for fungi: Implications and insights into the network of signal transmission. Adv. Appl. Microbiol. 2011, 76, 27–78. [Google Scholar]
- Blanco, E.; Fortunato, S.; Viggiano, L.; de Pinto, M.C. Cyclic AMP: A polyhedral signalling molecule in plants. Int. J. Mol. Sci. 2020, 21, 4862. [Google Scholar] [CrossRef]
- Lin, C.J.; Chen, Y.L. Conserved and divergent functions of the cAMP/PKA signaling pathway in Candida albicans and Candida tropicalis. J. Fungi 2018, 4, 68. [Google Scholar] [CrossRef]
- Huang, G.H.; Huang, Q.; Wei, Y.J.; Wang, Y.; Du, H. Multiple roles and diverse regulation of the Ras/cAMP/protein kinase A pathway in Candida albicans. Mol. Microbiol. 2019, 111, 6–16. [Google Scholar] [CrossRef]
- Arumugham, V.B.; Baldari, C.T. cAMP: A multifaceted modulator of immune synapse assembly and T cell activation. J. Leukoc. Biol. 2017, 101, 1301–1316. [Google Scholar] [CrossRef]
- Horvat, A.; Vardjan, N. Astroglial cAMP signalling in space and time. Neurosci. Lett. 2019, 689, 5–10. [Google Scholar] [CrossRef] [PubMed]
- Abel, T.; Nguyen, P.V. Regulation of hippocampus-dependent memory by cyclic AMP-dependent protein kinase. Prog. Brain Res. 2008, 169, 97–115. [Google Scholar] [PubMed]
- Yao, H.; Gu, L.J.; Guo, J.Y. Study on effect of Astragali Radix polysaccharides in improving learning and memory functions in aged rats and its mechanism. China J. Chin. Mater. Med. 2014, 39, 2071–2075. [Google Scholar]
- Zhou, Z.; Ikegaya, Y.; Koyama, R. The Astrocytic cAMP Pathway in Health and Disease. Int. J. Mol. Sci. 2019, 20, 779. [Google Scholar] [CrossRef]
- Dwivedi, Y.; Pandey, G.N. Adenylyl cyclase-cyclicAMP signaling in mood disorders: Role of the crucial phosphorylating enzyme protein kinase A. Neuropsychiatr. Dis. Treat. 2008, 4, 161–176. [Google Scholar] [CrossRef]
- Nestler, E.J. Cellular responses to chronic treatment with drugs of abuse. Crit. Rev. Neurobiol. 1993, 7, 23–39. [Google Scholar]
- Lee, E.H.; Seo, S.R. Neuroprotective roles of pituitary adenylate cyclase-activating polypeptide in neurodegenerative diseases. BMB Rep. 2014, 47, 369–375. [Google Scholar] [CrossRef]
- Delaunay, M.; Osman, H.; Kaiser, S.; Diviani, D. The role of cyclic AMP signaling in cardiac fibrosis. Cells 2019, 9, 69. [Google Scholar] [CrossRef]
- Lv, T.T.; Du, Y.H.; Cao, N.; Zhang, S.L.; Gong, Y.L.; Bai, Y.; Wang, W.; Liu, H.R. Proliferation in cardiac fibroblasts induced by beta(1)-adrenoceptor autoantibody and the underlying mechanisms. Sci. Rep. 2016, 6, 32430. [Google Scholar] [CrossRef]
- Yokoyama, U.; Patel, H.H.; Lai, N.C.; Aroonsakool, N.; Roth, D.M.; Insel, P.A. The cyclic AMP effector Epac integrates pro- and anti-fibrotic signals. Proc. Natl. Acad. Sci. USA 2008, 105, 6386–6391. [Google Scholar] [CrossRef]
- Ghigo, A.; Mika, D. cAMP/PKA signaling compartmentalization in cardiomyocytes: Lessons from FRET-based biosensors. J. Mol. Cell Cardiol. 2019, 131, 112–121. [Google Scholar] [CrossRef] [PubMed]
- Buxton, I.L.; Brunton, L.L. Compartments of cyclic AMP and protein kinase in mammalian cardiomyocytes. J. Biol. Chem. 1983, 258, 10233–10239. [Google Scholar] [CrossRef]
- Ravnskjaer, K.; Madiraju, A.; Montminy, M. Role of the cAMP pathway in glucose and lipid metabolism. Handb. Exp. Pharmacol. 2016, 233, 29–49. [Google Scholar] [PubMed]
- Peverelli, E.; Busnelli, M.; Vitali, E.; Giardino, E.; Gales, C.; Lania, A.G.; Beck-Peccoz, P.; Chini, B.; Mantovani, G.; Spada, A. Specific roles of G(i) protein family members revealed by dissecting SST5 coupling in human pituitary cells. J. Cell Sci. 2013, 126, 638–644. [Google Scholar] [CrossRef][Green Version]
- Pertuit, M.; Barlier, A.; Enjalbert, A.; Gerard, C. Signalling pathway alterations in pituitary adenomas: Involvement of Gsalpha, cAMP and mitogen-activate protein kinases. J. Neuroendocrinol. 2009, 21, 869–877. [Google Scholar] [CrossRef]
- Vortmeyer, A.O.; Glasker, S.; Mehta, G.U.; Abu-Asab, M.S.; Smith, J.H.; Zhuang, Z.; Collins, M.T.; Oldfield, E.H. Somatic GNAS mutation causes widespread and diffuse pituitary disease in acromegalic patients with McCune-Albright syndrome. J. Clin. Endocrinol. Metab. 2012, 97, 2404–2413. [Google Scholar] [CrossRef]
- Hernández-Ramírez, L.C.; Trivellin, G.; Stratakis, C.A. Cyclic 3′,5′-adenosine monophosphate (cAMP) signaling in the anterior pituitary gland in health and disease. Mol. Cell Endocrinol. 2018, 463, 72–86. [Google Scholar] [CrossRef]
- Musheshe, N.; Schmidt, M.; Zaccolo, M. cAMP: From long-range second messenger to nanodomain signalling. Trends Pharmacol. Sci. 2018, 39, 209–222. [Google Scholar] [CrossRef]
- Okumura, S.; Takagi, G.; Kawabe, J.; Yang, G.P.; Lee, M.C.; Hong, C.; Liu, J.; Vatner, D.E.; Sadoshima, J.; Vatner, S.F.; et al. Disruption of type 5 adenylyl cyclase gene preserves cardiac function against pressure overload. Proc. Natl. Acad. Sci. USA 2003, 100, 9986–9990. [Google Scholar] [CrossRef]
- Sprenger, J.U.; Perera, R.K.; Steinbrecher, J.H.; Lehnart, S.E.; Maier, L.S.; Hasenfuss, G.; Nikolaev, V.O. In vivo model with targeted cAMP biosensor reveals changes in receptor-microdomain communication in cardiac disease. Nat. Commun. 2015, 6, 6965. [Google Scholar] [CrossRef]
- Surdo, N.C.; Berrera, M.; Koschinski, A.; Brescia, M.; Machado, M.R.; Carr, C.; Wright, P.; Gorelik, J.; Morotti, S.; Grandi, E.; et al. FRET biosensor uncovers cAMP nano-domains at beta-adrenergic targets that dictate precise tuning of cardiac contractility. Nat. Commun. 2017, 8, 15031. [Google Scholar] [CrossRef] [PubMed]
- Elnagdy, M.; Barve, S.; McClain, C.; Gobejishvili, L. cAMP signaling in pathobiology of alcohol associated liver disease. Biomolecules 2020, 10, 1433. [Google Scholar] [CrossRef] [PubMed]
- Raker, V.K.; Becker, C.; Steinbrink, K. The cAMP pathway as therapeutic target in autoimmune and inflammatory diseases. Front. Immunol. 2016, 7, 123. [Google Scholar] [CrossRef] [PubMed]
- Ding, X.; Cao, C.; Zheng, Q.; Huang, G. The regulatory subunit of protein kinase A (Bcy1) in Candida albicans plays critical roles in filamentation and white-opaque switching but is not essential for cell growth. Front. Microbiol. 2016, 7, 2127. [Google Scholar] [CrossRef]
- Lee, N.; D’Souza, C.A.; Kronstad, J.W. Of smuts, blasts, mildews, and blights: cAMP signaling in phytopathogenic fungi. Annu. Rev. Phytopathol. 2003, 41, 399–427. [Google Scholar] [CrossRef]
- Kayikci, O.; Magwene, P.M. Divergent roles for cAMP-PKA signaling in the regulation of filamentous growth in Saccharomyces cerevisiae and Saccharomyces bayanus. G3: Genes Genomes Genet. 2018, 8, 3529–3538. [Google Scholar] [CrossRef]
- Reinton, N.; Haugen, T.B.; Orstavik, S.; Skalhegg, B.S.; Hansson, V.; Jahnsen, T.; Tasken, K. The gene encoding the C gamma catalytic subunit of cAMP-dependent protein kinase is a transcribed retroposon. Genomics 1998, 297, 290–297. [Google Scholar] [CrossRef]
- Portela, P.; Rossi, S. cAMP-PKA signal transduction specificity in Saccharomyces cerevisiae. Curr. Genet. 2020, 66, 1093–1099. [Google Scholar] [CrossRef]
- Perrin, A.J.; Patel, A.; Flueck, C.; Blackman, M.J.; Baker, D.A. cAMP signalling and its role in host cell invasion by malaria parasites. Curr. Opin. Microbiol. 2020, 58, 69–74. [Google Scholar] [CrossRef]
- Schumacher, J.; Kokkelink, L.; Huesmann, C.; Jimenez-Teja, D.; Collado, I.G.; Barakat, R.; Tudzynski, P.; Tudzynski, B. The cAMP-dependent signaling pathway and its role in conidial germination, growth, and virulence of the gray mold Botrytis cinerea. Mol. Plant Microbe Interact. 2008, 21, 1443–1459. [Google Scholar] [CrossRef]
- Choi, Y.E.; Xu, J.R. The cAMP signaling pathway in Fusarium verticillioides is important for conidiation, plant infection, and stress responses but notfumonisin production. Mol. Plant Microbe Interact. 2010, 23, 522–533. [Google Scholar] [CrossRef] [PubMed]
- Caza, M.; Kronstad, J.W. The cAMP/Protein kinase A pathway regulates virulence and adaptation to host conditions in Cryptococcus neoformans. Front. Cell Infect. Microbiol. 2019, 9, 212. [Google Scholar] [CrossRef] [PubMed]
- Tzima, A.; Paplomatas, E.J.; Rauyaree, P.; Kang, S. Roles of the catalytic subunit of cAMP-dependent protein kinase A in virulence and development of the soilborne plant pathogen Verticillium dahliae. Fungal Genet. Biol. 2010, 47, 406–415. [Google Scholar] [CrossRef] [PubMed]
- Carreras-Villaseñor, N.; Sánchez-Arreguín, J.A.; Herrera-Estrella, A.H. Trichoderma: Sensing the environment for survival and dispersal. Microbiology 2012, 158 Pt 1, 3–16. [Google Scholar] [CrossRef]
- Mukhopadhyay, R.; Kumar, D. Trichoderma: A beneficial antifungal agent and insights into its mechanism of biocontrol potential. Egypt. J. Biol. Pest Control 2020, 30, 133. [Google Scholar] [CrossRef]
- Ortiz-Urquiza, A.; Keyhani, N.O. Stress response signaling and virulence: Insights from entomopathogenic fungi. Curr. Genet. 2015, 61, 239–249. [Google Scholar] [CrossRef]
- Rangel, D.E.N.; Alston, D.G.; Roberts, D.W. Effects of physical and nutritional stress conditions during mycelial growth on conidial germination speed, adhesion to host cuticle, and virulence of Metarhizium anisopliae, an entomopathogenic fungus. Mycol. Res. 2008, 112, 1355–1361. [Google Scholar] [CrossRef]
- Omann, M.; Zeilinger, S. How a mycoparasite employs g-protein signaling: Using the example of Trichoderma. J. Signal Transduct. 2010, 2010, 123126. [Google Scholar] [CrossRef][Green Version]
- Li, L.; Wright, S.J.; Krystofova, S.; Park, G.; Borkovich, K.A. Heterotrimeric G protein signaling in filamentous fungi. Annu. Rev. Microbiol. 2007, 61, 423–452. [Google Scholar] [CrossRef]
- Dohlman, H.G.; Song, J.P.; Ma, D.R.; Courchesne, W.E.; Thorner, J. Sst2, a negative regulator of pheromone signaling in the yeast Saccharomyces cerevisiae: Expression, localization, and genetic interaction and physical association with Gpa1 (the G-protein α subunit). Mol. Cell Biol. 1996, 16, 5194–5209. [Google Scholar] [CrossRef]
- Bölker, M. Sex and crime: Heterotrimeric G proteins in fungal mating and pathogenesis. Fungal Genet. Biol. 1998, 25, 143–156. [Google Scholar] [CrossRef] [PubMed]
- Turner, G.E.; Borkovich, K.A. Identification of a G protein alpha subunit from Neurospora crassa that is a member of the Gi family. J. Biol. Chem. 1993, 268, 14805–14811. [Google Scholar] [CrossRef]
- Gilman, A.G. G Proteins: Transducers of receptor-generated signals. Ann. Rev. Biochem. 1987, 56, 615–649. [Google Scholar] [CrossRef] [PubMed]
- Kays, A.M.; Rowley, P.S.; Baasiri, R.A.; Borkovich, K.A. Regulation of conidiation and adenylyl cyclase levels by the Ga protein GNA-3 in Neurospora crassa. Mol. Cell. Biol. 2000, 20, 7693–7705. [Google Scholar] [CrossRef]
- Versele, M.; Lemaire, K.; Thevelein, J.M. Sex and sugar in yeast: Two distinct GPCR systems. EMBO Rep. 2001, 2, 574–579. [Google Scholar] [CrossRef]
- Gruber, S.; Omann, M.; Zeilinger, S. Comparative analysis of the repertoire of G protein-coupled receptors of three species of the fungal genus Trichoderma. BMC Microbiol. 2013, 13, 108. [Google Scholar] [CrossRef]
- Brunner, K.; Omann, M.; Pucher, M.E.; Delic, M.; Lehner, S.M.; Domnanich, P.; Kratochwill, K.; Druzhinina, I.; Denk, D.; Zeilinger, S. Trichoderma G protein-coupled receptors: Functional characterisation of a cAMP receptor-like protein from Trichoderma atroviride. Curr. Genet. 2008, 54, 283–299. [Google Scholar] [CrossRef]
- Omann, M.R.; Lehner, S.; Rodríguez, C.E.; Brunner, K.; Zeilinger, S. The seven-transmembrane receptor Gpr1 governs processes relevant for the antagonistic interaction of Trichoderma atroviride with its host. Microbiology 2012, 158 Pt 1, 107–118. [Google Scholar] [CrossRef]
- Reithner, B.; Brunner, K.; Schuhmacher, R.; Peissl, I.; Seidl, V.; Krska, R.; Zeilinger, S. The G protein alpha subunit Tga1 of Trichoderma atroviride is involved in chitinase formation and differential production of antifungal metabolites. Fungal Genet. Biol. 2005, 42, 749–760. [Google Scholar] [CrossRef]
- do Nascimento Silva, R.; Steindorff, A.S.; Ulhoa, C.J.; Félix, C.R. Involvement of G-alpha protein GNA3 in production of cell wall-degrading enzymes by Trichoderma reesei (Hypocrea jecorina) during mycoparasitism against Pythium ultimum. Biotechnol. Lett. 2009, 31, 531–536. [Google Scholar] [CrossRef]
- Rocha-Ramirez, V.; Omero, C.; Chet, I.; Horwitz, B.A.; Herrera-Estrella, A. Trichoderma atroviride G-protein alpha-subunit gene tga1 is involved in mycoparasitic coiling and conidiation. Eukaryot. Cell 2002, 1, 594–605. [Google Scholar] [CrossRef] [PubMed]
- Zeilinger, S.; Reithner, B.; Scala, V.; Peissl, I.; Lorito, M.; Mach, R.L. Signal transduction by Tga3, a novel g protein subunit alpha of Trichoderma atroviride. Appl. Environ. Microbiol. 2005, 71, 1591–1597. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, P.K.; Latha, J.; Hadar, R.; Horwitz, B.A. Role of two G-protein alpha subunits, TgaA and TgaB, in the antagonism of plant pathogens by Trichoderma virens. Appl. Environ. Microbiol. 2004, 70, 542–549. [Google Scholar] [CrossRef] [PubMed]
- Ding, J.; Mei, J.; Huang, P.; Tian, Y.; Liang, Y.; Jiang, X.L.; Li, M. Gα3 subunit Thga3 positively regulates conidiation, mycoparasitism, chitinase activity, and hydrophobicity of Trichoderma harzianum. AMB Express 2020, 10, 221. [Google Scholar] [CrossRef]
- Sun, Q.; Jiang, X.L.; Pang, L.; Wang, L.R.; Li, M. Functions of thga1 gene in Trichoderma harzianum based on transcriptome analysis. Biomed. Res. Int. 2016, 2016, 8329513. [Google Scholar] [CrossRef]
- Shang, J.M.; Shang, Y.F.; Tang, G.R.; Wang, C.S. Identification of a key G-protein coupled receptor in mediating appressorium formation and fungal virulence against insects. Sci. China Life Sci. 2021, 64, 466–477. [Google Scholar] [CrossRef]
- Tong, Y.; Wu, H.; Liu, Z.; Wang, Z.; Huang, B. G-Protein Subunit Gαi in mitochondria, MrGPA1, affects conidiation, stress resistance, and virulence of entomopathogenic fungus Metarhizium robertsii. Front. Microbiol. 2020, 11, 1251. [Google Scholar] [CrossRef]
- Ying, S.H.; Feng, M.G.; Keyhani, N.O. A carbon responsive G-protein coupled receptor modulates broad developmental and genetic networks in the entomopathogenic fungus, Beauveria bassiana. Environ. Microbiol. 2013, 15, 2902–2921. [Google Scholar] [CrossRef]
- Lysøe, E.; Dees, M.W.; Brurberg, M.B. A three-way transcriptomic interaction study of a biocontrol agent (Clonostachys rosea), a fungal pathogen (Helminthosporium solani), and a potato host (Solanum tuberosum). Mol. Plant. Microbe Interact. 2017, 30, 646–655. [Google Scholar] [CrossRef]
- Muthumeenakshi, S.; Sreenivasaprasad, S.; Rogers, C.W.; Challen, M.P.; Whipps, J.M. Analysis of cDNA transcripts from Coniothyrium minitans reveals a diverse array of genes involved in key processes during sclerotial mycoparasitism. Fungal Genet. Biol. 2007, 44, 1262–1284. [Google Scholar] [CrossRef]
- Lai, Y.L.; Chen, H.; Wei, G.; Wang, G.D.; Li, F.; Wang, S.B. In vivo gene expression profiling of the entomopathogenic fungus Beauveria bassiana elucidates its infection stratagems in Anopheles mosquito. Sci. China Life Sci. 2017, 60, 839–851. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, M.; Mukherjee, P.K.; Kale, S.P. cAMP signalling is involved in growth, germination, mycoparasitism and secondary metabolism in Trichoderma virens. Microbiology 2007, 153 Pt 6, 1734–1742. [Google Scholar] [CrossRef]
- Liu, S.Y.; Peng, G.X.; Xia, Y.X. The adenylate cyclase gene MaAC is required for virulence and multi-stress tolerance of Metarhizium acridum. BMC Microbiol. 2012, 12, 163. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhou, G.; Ying, S.H.; Feng, M.G. Adenylate cyclase orthologues in two filamentous entomopathogens contribute differentially to growth, conidiation, pathogenicity, and multistress responses. Fungal Biol. 2014, 118, 422–431. [Google Scholar] [CrossRef] [PubMed]
- Ma, N.; Jiang, K.X.; Bai, N.; Li, D.N.; Zhang, K.Q.; Yang, J.K. Functional analysis of two affinity cAMP phosphodiesterases in the nematode-trapping fungus Arthrobotrys oligospora. Pathogens 2022, 11, 405. [Google Scholar] [CrossRef]
- Fang, W.G.; Pava-ripoll, M.; Wang, S.B.; Leger, R.S. Protein kinase A regulates production of virulence determinants by the entomopathogenic fungus, Metarhizium anisopliae. Fungal Genet. Biol. 2009, 46, 277–285. [Google Scholar] [CrossRef]
- Zhao, H.Z.; Zhou, T.; Xie, J.T.; Cheng, J.S.; Chen, T.; Jiang, D.H.; Fu, Y.P. Mycoparasitism illuminated by genome and transcriptome sequencing of Coniothyrium minitans, an important biocontrol fungus of the plant pathogen Sclerotinia sclerotiorum. Microb. Genom. 2020, 6, e000345. [Google Scholar] [CrossRef]
- Xin, C.Y.; Zhang, J.P.; Nian, S.J.; Wang, G.X.; Wang, Z.K.; Song, Z.Y.; Ren, G.W. Analogous and diverse functions of APSES-type transcription factors in the morphogenesis of the entomopathogenic fungus Metarhizium rileyi. Appl. Environ. Microbiol. 2020, 86, e02928-19. [Google Scholar] [CrossRef]
- Du, Y.R.; Jin, K.; Xia, Y.X. Involvement of MaSom1, a downstream transcriptional factor of cAMP/PKA pathway, in conidial yield, stress tolerances, and virulence in Metarhizium acridum. Appl. Microbiol. Biotechnol. 2018, 102, 5611–5623. [Google Scholar] [CrossRef]
- Zhang, F.; Zhang, L.P.; Qi, Y.; Xu, H. Mitochondrial cAMP signaling. Cell. Mol. Life Sci. 2016, 73, 4577–4590. [Google Scholar] [CrossRef]
- Shapiro, R.S.; Uppuluri, P.; Zaas, A.K.; Collins, C.; Senn, H.; Perfect, J.R.; Heitman, J.; Cowen, L.E. Hsp90 orchestrates temperature-dependent Candida albicans morphogenesis via Ras1-PKA signaling. Curr. Biol. 2009, 19, 621–629. [Google Scholar] [CrossRef] [PubMed]
- Fang, H.M.; Wang, Y. RA domain-mediated interaction of Cdc35 with Ras1 is essential for increasing cellular cAMP level for Candida albicans hyphal development. Mol. Microbiol. 2006, 61, 484–496. [Google Scholar] [CrossRef] [PubMed]
- Klengel, T.; Liang, W.J.; Chaloupka, J.; Ruoff, C.; Schroppel, K.; Naglik, J.R.; Eckert, S.E.; Mogensen, E.G.; Haynes, K.; Tuite, M.F.; et al. Fungal adenylyl cyclase integrates CO2 sensing with cAMP signaling and virulence. Curr. Biol. 2005, 15, 2021–2026. [Google Scholar] [CrossRef] [PubMed]
- Adachi, K.; Hamer, J.E. Divergent cAMP signaling pathways regulate growth and pathogenesis in the rice blast fungus Magnaporthe grisea. Plant Cell 1998, 10, 1361–1374. [Google Scholar] [CrossRef] [PubMed]
- Ivey, F.D.; Kays, A.M.; Borkovich, K.A. Shared and independent roles for a G alpha(i) protein and adenylyl cyclase in regulating development and stress responses in Neurospora crassa. Eukaryot. Cell 2002, 1, 634–642. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Tao, L.; Guan, G.; Yue, H.; Liang, W.; Cao, C.; Dai, Y.; Huang, G. Regulation of filamentation in the human fungal pathogen Candida tropicalis. Mol. Microbiol. 2016, 99, 528–545. [Google Scholar] [CrossRef]
- García-Martínez, J.; Adám, A.L.; Avalos, J. Adenylyl cyclase plays a regulatory role in development, stress resistance and secondary metabolism in Fusarium fujikuroi. PLoS ONE 2012, 7, e28849. [Google Scholar] [CrossRef]
- Choi, W.; Dean, R.A. The adenylate cyclase gene MAC1 of Magnaporthe grisea controls appressorium formation and other aspects of growth and development. Plant Cell 1997, 9, 1973–1983. [Google Scholar]
- Chen, X.; Zhang, X.; Zhu, P.; Wang, Y.; Na, Y.; Guo, H.; Cai, Y.; Nie, H.; Jiang, Y.; Xu, L. A single nucleotide mutation in adenylate cyclase affects vegetative growth, sclerotial formation and virulence of Botrytis cinerea. Int. J. Mol. Sci. 2020, 21, 2912. [Google Scholar] [CrossRef]
- Yang, K.L.; Qin, Q.P.; Liu, Y.H.; Zhang, L.M.; Liang, L.L.; Lan, H.H.; Chen, C.H.; You, Y.C.; Zhang, F.; Wang, S.H. Adenylate cyclase AcyA regulates development, aflatoxin biosynthesis and fungal virulence in Aspergillus flavus. Front. Cell Infect. Microbiol. 2016, 6, 190. [Google Scholar] [CrossRef]
- Swaney, J.S.; Roth, D.M.; Olson, E.R.; Naugle, J.E.; Meszaros, J.G.; Insel, P.A. Inhibition of cardiac myofibroblast formation and collagen synthesis by activation and overexpression of adenylyl cyclase. Proc. Natl. Acad. Sci. USA 2005, 102, 437–442. [Google Scholar] [CrossRef] [PubMed]
- Swaney, J.S.; Patel, H.H.; Yokoyama, U.; Head, B.P.; Roth, D.M.; Insel, P.A. Focal adhesions in (myo) fibroblasts scaffold adenylyl cyclase with phosphorylated caveolin. J. Biol. Chem. 2006, 281, 17173–17179. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Lee, H.C.; Kwon, Y.W.; Lee, S.E.; Cho, Y.; Kim, J.; Lee, S.; Kim, J.Y.; Lee, J.; Yang, H.M.; et al. Adenylyl cyclase-associated protein 1 is a receptor for human resistin and mediates inflammatory actions of human monocytes. Cell Metab. 2014, 19, 484–497. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.R.; Lo, C.T.; Liu, S.Y.; Peng, K.C. Involvement of pachybasin and emodin in self-regulation of Trichoderma harzianum mycoparasitic coiling. J. Agric. Food Chem. 2012, 60, 2123–2128. [Google Scholar] [CrossRef] [PubMed]
- Dickman, M.; Yarden, O. Serine/Threonine protein kinases and phosphatases in filamentous fungi. Fungal Genet. Boil. 1999, 26, 99–117. [Google Scholar] [CrossRef]
- Taylor, S.S.; Ilouz, R.; Zhang, P.; Kornev, A.P. Assembly of allosteric macromolecular switches: Lessons from PKA. Nat. Rev. Mol. Cell Biol. 2012, 13, 646–658. [Google Scholar] [CrossRef]
- Zhu, W.J.; Zhou, M.; Xiong, Z.Y.; Peng, F.; Wei, W. The cAMP-PKA signaling pathway regulates pathogenicity, hyphal growth, appressorial formation, conidiation, and stress tolerance in Colletotrichum higginsianum. Front. Microbiol. 2017, 8, 1416. [Google Scholar] [CrossRef]
- Zhao, P.B.; Ren, A.Z.; Xu, H.J.; Li, D.C. The gene fpk1, encoding a cAMP-dependent protein kinase catalytic subunit homolog, is required for hyphal growth, spore germination, and plant infection in Fusarium verticillioides. J. Microbiol. Biotechnol. 2010, 20, 208–216. [Google Scholar]
- Schoberle, T.J.; Nguyen-Coleman, C.K.; Herold, J.; Yang, A.; Weirauch, M.; Hughes, T.R.; MuMurray, J.S.; May, G.S. A novel C2H2 transcription factor that regulates gliA expression interdependently with GliZ in Aspergillus fumigatus. PLoS Genet. 2014, 10, e1004336. [Google Scholar] [CrossRef]
- Matheis, S.; Yemelin, A.; Scheps, D.; Andresen, K.; Jacob, S.; Thines, E.; Foster, A.J. Functions of the Magnaporthe oryzae Flb3p and Flb4p transcription factors in the regulation of conidiation. Microbiol. Res. 2017, 196, 106–117. [Google Scholar] [CrossRef]
- Thiel, G.; Al Sarraj, J.; Vinson, C.; Stefano, L.; Bach, K. Role of basic region leucine zipper transcription factors cyclic AMP response element binding protein (CREB), CREB2, activating transcription factor 2 and CAAT/enhancer binding protein alpha in cyclic AMP response element-mediated transcription. J. Neurochem. 2005, 92, 321–336. [Google Scholar] [CrossRef] [PubMed]
- Song, Z.Y.; Yin, Y.P.; Lin, Y.L.; Du, F.; Ren, G.W.; Wang, Z.K. The bZIP transcriptional factor activator protein-1 regulates Metarhizium rileyi morphology and mediates microsclerotia formation. Appl. Microbiol. Biotechnol. 2018, 102, 4577–4588. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.; Shang, Y.F.; Chen, P.L.; Chen, K.; Wang, C.S. Basic leucine zipper (bZIP) domain transcription factor MBZ1 regulates cell wall integrity, spore adherence, and virulence in Metarhizium robertsii. J. Biol. Chem. 2015, 290, 8218–8231. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.J.; Wang, J.J.; Lin, H.Y.; Ding, J.L.; Feng, M.G.; Ying, S.H. HapX, an indispensable bZIP transcription factor for iron acquisition, regulates infection initiation by orchestrating conidial oleic acid homeostasis and cytomembrane in mycopathogen Beauveria bassiana. Msystems 2020, 5, e00695-20. [Google Scholar] [CrossRef]
- Xu, Y.P.; Wang, Y.C.; Zhao, H.Z.; Wu, M.D.; Zhang, J.; Chen, W.D.; Li, G.Q.; Yang, L. Genome-wide identification and expression analysis of the bZIP transcription factors in the mycoparasite Coniothyrium minitans. Microorganisms 2020, 8, 1045. [Google Scholar] [CrossRef]
- Luo, X.; Mao, H.; Wei, Y.; Cai, J.; Xie, C.; Sui, A.; Yang, X.; Dong, J. The fungal-specific transcription factor Vdpf influences conidia production, melanized microsclerotia formation and pathogenicity in Verticillium dahliae. Mol. Plant. Pathol. 2016, 17, 1364–1381. [Google Scholar] [CrossRef]
- Song, Z.Y.; Yang, J.; Xin, C.Y.; Xing, X.R.; Yuan, Q.; Yin, Y.P.; Wang, Z.K. A transcription factor, MrMsn2, in the dimorphic fungus Metarhizium rileyi is essential for dimorphism transition, aggravated pigmentation, conidiation and microsclerotia formation. Microb. Biotechnol. 2018, 11, 1157–1169. [Google Scholar] [CrossRef]
- Xin, C.Y.; Yang, J.; Mao, Y.Y.; Chen, W.B.; Wang, Z.K.; Song, Z.Y. GATA-type transcription factor MrNsdD regulates dimorphic transition, conidiation, virulence and microsclerotium formation in the entomopathogenic fungus Metarhizium rileyi. Microb. Biotechnol. 2020, 13, 1489–1501. [Google Scholar] [CrossRef]
- Huang, W.; Shang, Y.F.; Chen, P.L.; Gao, Q.; Wang, C.S. MrpacC regulates sporulation, insect cuticle penetration and immune evasion in Metarhizium robertsii. Environ. Microbiol. 2015, 17, 994–1008. [Google Scholar] [CrossRef]
- Zhang, M.G.; Wei, Q.L.; Xia, Y.X.; Jin, K. MaPacC, a pH-responsive transcription factor, negatively regulates thermotolerance and contributes to conidiation and virulence in Metarhizium acridum. Curr. Genet. 2020, 66, 397–408. [Google Scholar] [CrossRef]
- Wang, Z.L.; Pan, H.B.; Huang, J.; Yu, X.P. The zinc finger transcription factors Bbctf1alpha and Bbctf1beta regulate the expression of genes involved in lipid degradation and contribute to stress tolerance and virulence in a fungal insect pathogen. Pest. Manag. Sci. 2020, 76, 2589–2600. [Google Scholar] [CrossRef] [PubMed]
- Luo, Z.B.; Ren, H.; Mousa, J.J.; Rangel, D.E.N.; Zhang, Y.J.; Bruner, S.D.; Keyhani, N.O. The PacC transcription factor regulates secondary metabolite production and stress response, but has only minor effects on virulence in the insect pathogenic fungus Beauveria bassiana. Environ. Microbiol. 2017, 19, 788–802. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Liu, D.; Yu, Z.; Hou, B.; Fan, Y.; Li, Z.; Shang, S.; Qiao, Y.; Fu, J.; Niu, J.; et al. Comparative genome and transcriptome analysis of the nematode-trapping fungus Duddingtonia flagrans reveals high pathogenicity during nematode infection. Biol. Control. 2020, 143, 104159. [Google Scholar] [CrossRef]
- Luo, Y.; Han, Y.C.; Yang, L.; Wu, M.D.; Zhang, J.; Cheng, J.S.; Wang, M.Y.; Jiang, D.H.; Chen, W.D.; Li, G.Q. CmpacC regulates mycoparasitism, oxalate degradation and antifungal activity in the mycoparasitic fungus Coniothyrium minitans. Environ. Microbiol. 2015, 17, 4711–4729. [Google Scholar]
- Trushina, N.; Levin, M.; Mukherjee, P.K.; Horwitz, B.A. PacC and pH-dependent transcriptome of the mycotrophic fungus Trichoderma virens. BMC Genom. 2013, 14, 138. [Google Scholar] [CrossRef] [PubMed]
- Zou, C.G.; Tu, H.H.; Liu, X.Y.; Tao, N.; Zhang, K.Q. PacC in the nematophagous fungus Clonostachys rosea controls virulence to nematodes. Environ. Microbiol. 2010, 12, 1868–1877. [Google Scholar] [CrossRef]
- Ferguson, S.B.; Anderson, E.S.; Harshaw, R.B.; Thate, T.; Craig, N.L.; Nelson, H.C. Protein kinase A regulates constitutive expression of small heat-shock genes in an Msn2/4p-independent and Hsf1p-dependent manner in Saccharomyces cerevisiae. Genetics 2005, 169, 1203–1214. [Google Scholar] [CrossRef][Green Version]
- Zhou, G.; Ying, S.H.; Hu, Y.; Fang, X.; Feng, M.G.; Wang, J. Roles of three HSF domain-containing proteins in mediating heat-shock protein genes and sustaining asexual cycle, stress tolerance, and virulence in Beauveria bassiana. Front. Microbiol. 2018, 9, 1677. [Google Scholar] [CrossRef]
- Shang, Y.F.; Chen, P.L.; Chen, Y.X.; Lu, Y.Z.; Wang, C.S. MrSkn7 controls sporulation, cell wall integrity, autolysis, and virulence in Metarhizium robertsii. Eukaryot. Cell 2015, 14, 396–405. [Google Scholar] [CrossRef]
- Hussain, M.; Hamid, M.I.; Wang, N.N.; Bin, L.; Xiang, M.C.; Liu, X.Z. The transcription factor SKN7 regulates conidiation, thermotolerance, apoptotic-like cell death and parasitism in the nematode endoparasitic fungus Hirsutella minnesotensis. Sci. Rep. 2016, 6, 30047. [Google Scholar] [CrossRef]
- Escalante, R.; Sastre, L. cAMP and DIF-1 repress the expression of the Dictyostelium MADS-box gene srfA at early stages of development. Biochem. Biophys. Res. Commun. 2001, 285, 820–824. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Yang, X.; Lu, Z.; Wang, H.; He, Z.; Zhou, G.; Luo, Z.; Zhang, Y. MADS-box transcription factor Mcm1 controls cell cycle, fungal development, cell integrity and virulence in the filamentous insect pathogenic fungus Beauveria bassiana. Environ. Microbiol. 2019, 21, 3392–3416. [Google Scholar] [CrossRef] [PubMed]
- Sui, Y.; Sun, Z.Q.; Zou, Y.P.; Li, W.H.; Jiang, M.G.; Luo, Y.Z.; Liao, W.J.; Wang, Y.H.; Gao, X.W.; Liu, J.; et al. The Rlm1 transcription factor in Candida oleophila contributes to abiotic stress resistance and biocontrol efficacy against postharvest gray mold of kiwifruit. Postharvest Biol. Tec. 2020, 166, 111222. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, Z.-B.; Yu, S.-F.; Wang, C.-L.; Wang, L. cAMP Signalling Pathway in Biocontrol Fungi. Curr. Issues Mol. Biol. 2022, 44, 2622-2634. https://doi.org/10.3390/cimb44060179
Sun Z-B, Yu S-F, Wang C-L, Wang L. cAMP Signalling Pathway in Biocontrol Fungi. Current Issues in Molecular Biology. 2022; 44(6):2622-2634. https://doi.org/10.3390/cimb44060179
Chicago/Turabian StyleSun, Zhan-Bin, Shu-Fan Yu, Chu-Lun Wang, and Ling Wang. 2022. "cAMP Signalling Pathway in Biocontrol Fungi" Current Issues in Molecular Biology 44, no. 6: 2622-2634. https://doi.org/10.3390/cimb44060179
APA StyleSun, Z.-B., Yu, S.-F., Wang, C.-L., & Wang, L. (2022). cAMP Signalling Pathway in Biocontrol Fungi. Current Issues in Molecular Biology, 44(6), 2622-2634. https://doi.org/10.3390/cimb44060179





