Lycopus lucidus Turcz Water Extract Ameliorates the Metabolic Disorder by Up-Regulated Major Urinary Protein Expression in High-Fat Diet-Induced Obesity
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Substance
2.2. Experimental Animals and Diets
2.3. Energy Expenditure
2.4. Histology and Immunohistochemistry
2.5. Plasma Biomarkers
2.6. Fasting Blood Glucose, Intraperitoneal Glucose Tolerance Test, and Homeostatic Index of Insulin Resistance
2.7. Hepatic Lipid Content
2.8. Hepatic Lipid-Regulating and Glucose-Regulating Enzyme Activities
2.9. Real-Time qPCR
2.10. Hepatic mRNA Sequencing
2.11. Statistical Analysis
3. Results
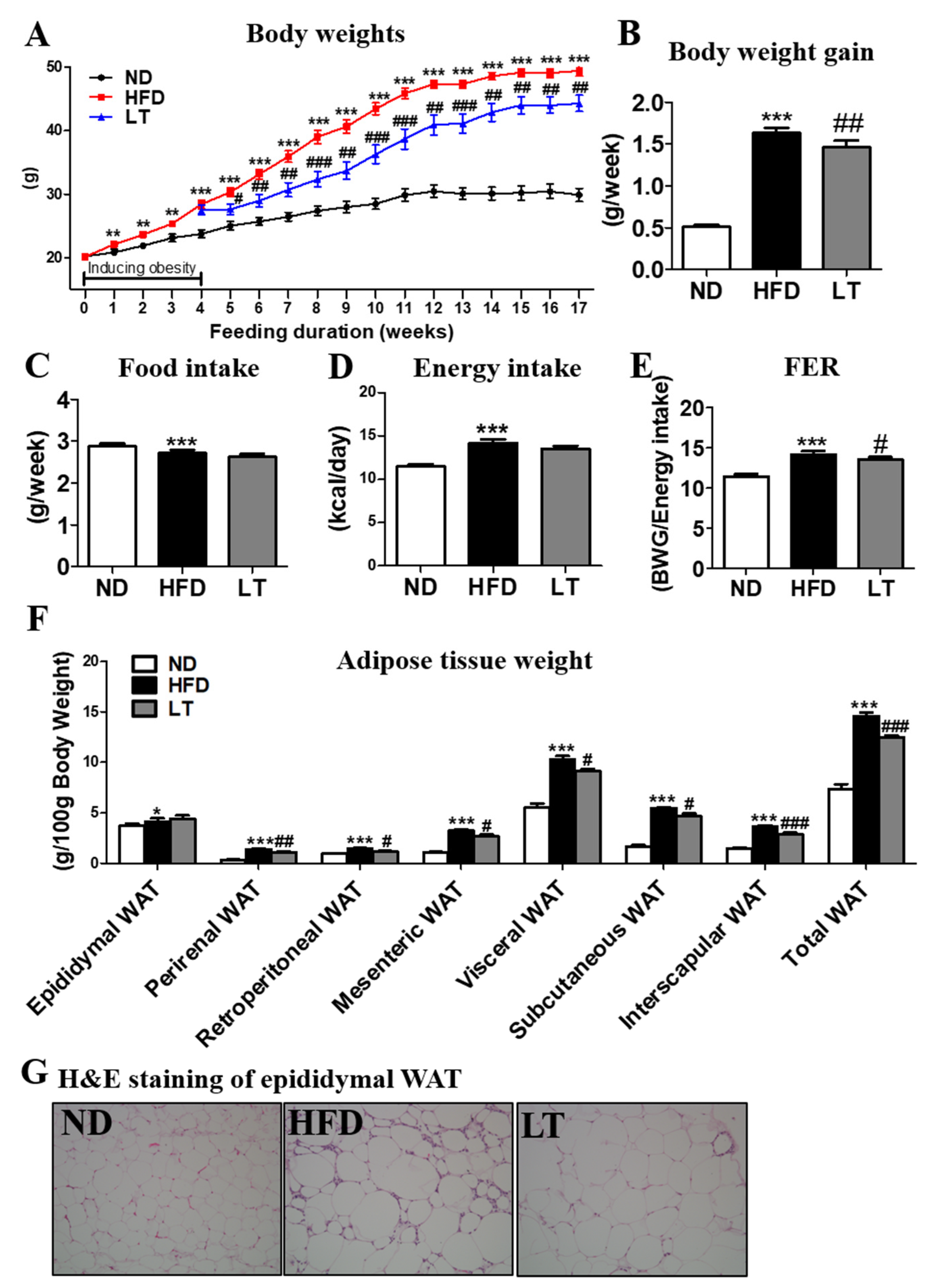
3.1. LT Supplementation Reduced Body Weight and Body Fat Mass
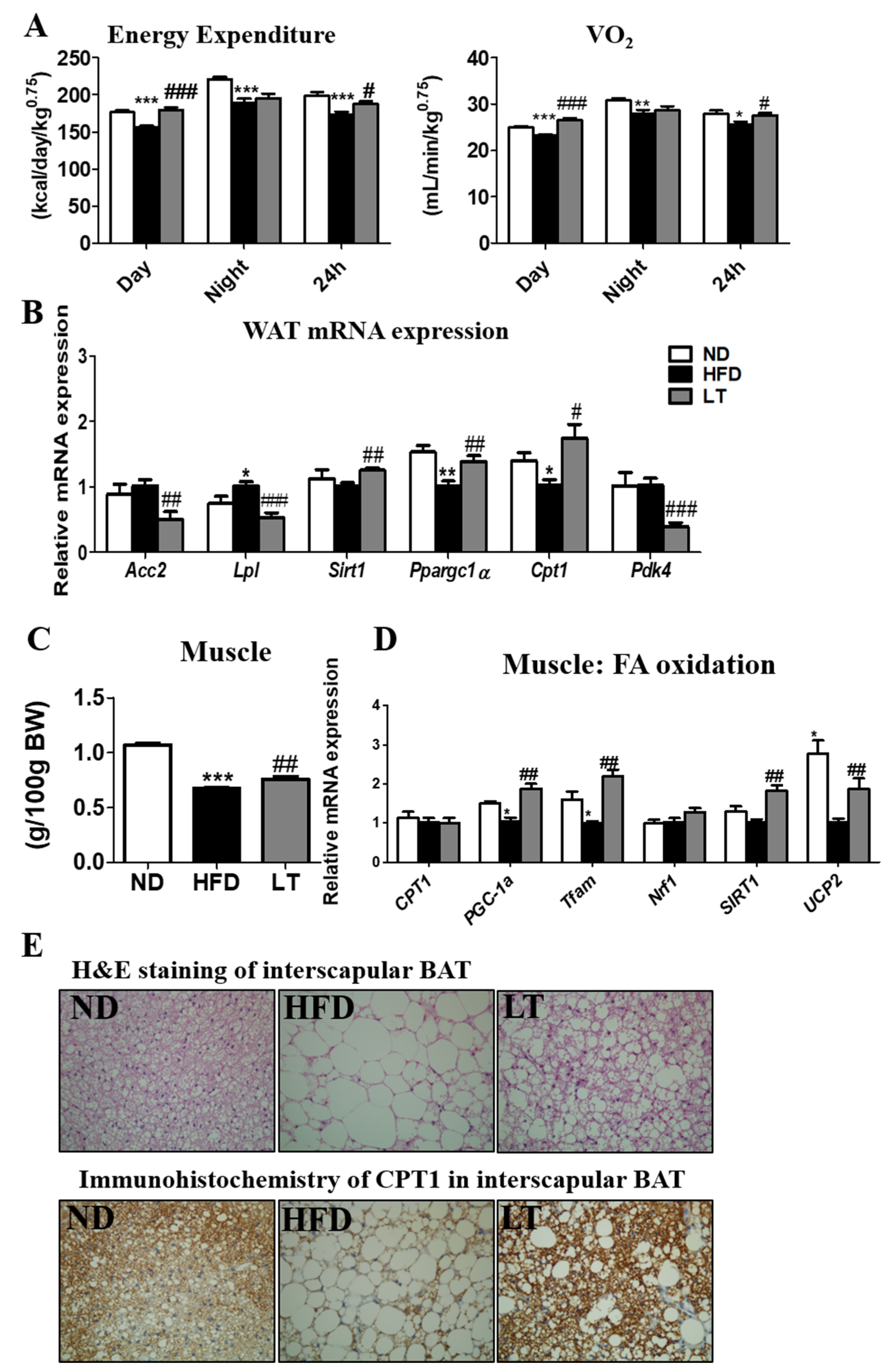
3.2. LT Supplementation Increased EE and Regulated mRNA Expression of WAT and Muscle
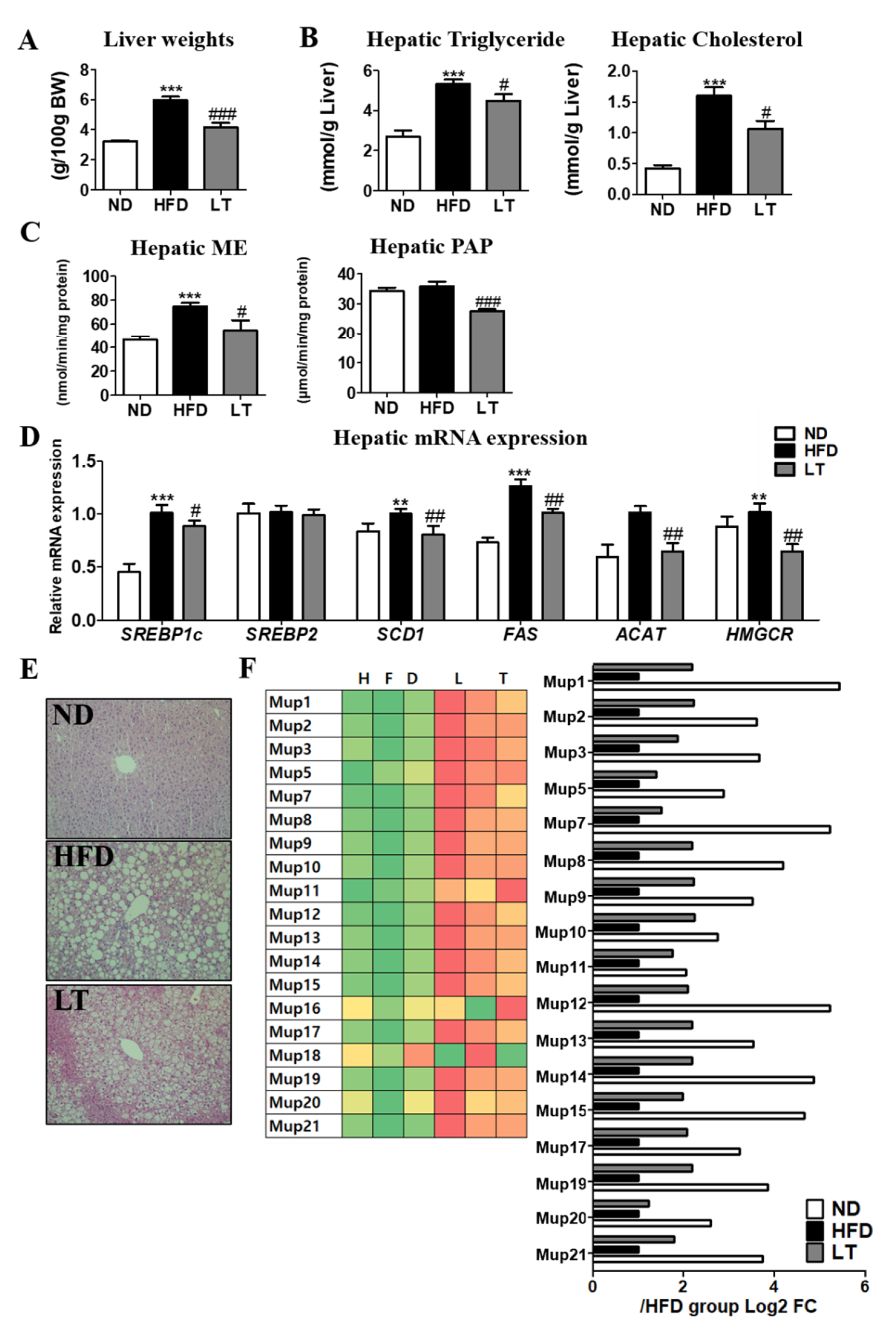
3.3. LT Supplementation Improved Hepatic Steatosis
3.4. LT Supplement Improved the Impaired Glucose Metabolism-Related Obesity
3.5. LT Supplementation Ameliorated Inflammation
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Polero, P.; Rebollo-Seco, C.; Adsuar, J.C.; Pérez-Gómez, J.; Rojo-Ramos, J.; Manzano-Redondo, F.; Garcia-Gordillo, M.; Carlos-Vivas, J. Physical activity recommendations during COVID-19: Narrative review. Int. J. Environ. Res. Public Health 2021, 18, 65. [Google Scholar] [CrossRef] [PubMed]
- Gesta, S.; Tseng, Y.-H.; Kahn, C.R. Developmental origin of fat: Tracking obesity to its source. Cell 2007, 131, 242–256. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gao, F.; Zheng, K.I.; Wang, X.-B.; Sun, Q.-F.; Pan, K.-H.; Wang, T.-Y.; Chen, Y.-P.; Targher, G.; Byrne, C.D.; George, J.; et al. Obesity is a risk factor for greater COVID-19 severity. Diabetes Care 2020, 43, e72–e74. [Google Scholar] [CrossRef] [PubMed]
- Simonnet, A.; Chetboun, M.; Poissy, J.; Raverdy, V.; Noulette, J.; Duhamel, A.; Labreuche, J.; Mathieu, D.; Pattou, F.; Jourdain, M.; et al. High prevalence of obesity in severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) requiring invasive mechanical ventilation. Obesity 2020, 28, 1195–1199. [Google Scholar] [CrossRef] [PubMed]
- Cuschieri, S.; Grech, S. Obesity population at risk of COVID-19 complications. Glob. Health Epidemiol. Genom. 2020, 5, e6. [Google Scholar] [CrossRef]
- Kompaniyets, L.; Goodman, A.B.; Belay, B.; Freedman, D.S.; Sucosky, M.S.; Lange, S.J.; Gundlapalli, A.V.; Boehmer, T.K.; Blanck, H.M. Body mass index and risk for COVID-19—Related hospitalization, intensive care unit admission, invasive mechanical ventilation, and death—United States, March–December 2020. Morb. Mortal. Wkly. Rep. 2021, 70, 355. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Zhao, Y.; He, N.; Croft, K.D. Isolation, characterization, and immunological effects of α-galacto-oligosaccharides from a new source, the herb Lycopus lucidus Turcz. J. Agric. Food Chem. 2010, 58, 8253–8258. [Google Scholar] [CrossRef]
- Lu, Y.-h.; Huang, J.-h.; Li, Y.-c.; Ma, T.T.; Sang, P.; Wang, W.J.; Gao, C.Y. Variation in nutritional compositions, antioxidant activity and microstructure of Lycopus lucidus Turcz. root at different harvest times. Food Chem. 2015, 183, 91–100. [Google Scholar] [CrossRef]
- Ślusarczyk, S.; Hajnos, M.; Skalicka-Woźniak, K.; Matkowski, A. Antioxidant activity of polyphenols from Lycopus lucidus Turcz. Food Chem. 2009, 113, 134–138. [Google Scholar] [CrossRef]
- Lee, Y.J.; Kang, D.G.; Kim, J.S.; Lee, H.S. Lycopus lucidus inhibits high glucose-induced vascular inflammation in human umbilical vein endothelial cells. Vasc. Pharmacol. 2008, 48, 38–46. [Google Scholar] [CrossRef]
- Folch, J.; Lees, M.; Sloane-Stanley, G. A simple method for the isolation and purification of total lipids from animal tissues. J. Biol. Chem. 1957, 226, 497–509. [Google Scholar] [CrossRef]
- Hulcher, F.H.; Oleson, W.H. Simplified spectrophotometric assay for microsomal 3-hydroxy-3-methylglutaryl CoA reductase by measurement of coenzyme A. J. Lipid Res. 1973, 14, 625–631. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Walton, P.A.; Possmayer, F. The role of Mg2+-dependent phosphatidate phosphohydrolase in pulmonary glycerolipid biosynthesis. Biochim. Biophys. Acta (BBA) Lipids Lipid Metab. 1984, 796, 364–372. [Google Scholar] [CrossRef]
- Ochoa, S.; Mehler, A.H.; Kornberg, A. Biosynthesis of dicarboxylic acids by carbon dioxide fixation i. isolation and properties of an enzyme from pigeon liver catalyzing the reversible oxidative decarboxylation of l-malic acid. J. Biol. Chem. 1948, 174, 979–1000. [Google Scholar] [CrossRef]
- Bentle, L.; Lardy, H.A. Interaction of anions and divalent metal ions with phosphoenolpyruvate carboxykinase. J. Biol. Chem. 1976, 251, 2916–2921. [Google Scholar] [CrossRef]
- Schmittgen, T.D.; Livak, K.J. Analyzing real-time PCR data by the comparative CT method. Nat. Protoc. 2008, 3, 1101–1108. [Google Scholar] [CrossRef]
- Hill, J.O.; Wyatt, H.R.; Peters, J.C. Energy balance and obesity. Circulation 2012, 126, 126–132. [Google Scholar] [CrossRef]
- Baker, J.S.; McCormick, M.C.; Robergs, R.A. Interaction among skeletal muscle metabolic energy systems during intense exercise. J. Nutr. Metab. 2010, 2010, 905612. [Google Scholar] [CrossRef] [Green Version]
- Westerblad, H.; Bruton, J.D.; Katz, A. Skeletal muscle: Energy metabolism, fiber types, fatigue and adaptability. Exp. Cell Res. 2010, 316, 3093–3099. [Google Scholar] [CrossRef]
- Horowitz, J.F.; Klein, S. Lipid metabolism during endurance exercise. Am. J. Clin. Nutr. 2000, 72, 558S–563S. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, J.-Y.; Hickner, R.C.; Cortright, R.L.; Dohm, G.L.; Houmard, J.A. Lipid oxidation is reduced in obese human skeletal muscle. Am. J. Physiol.-Endocrinol. Metab. 2000, 279, E1039–E1044. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yki-Järvinen, H. Non-alcoholic fatty liver disease as a cause and a consequence of metabolic syndrome. Lancet Diabetes Endocrinol. 2014, 2, 901–910. [Google Scholar] [CrossRef]
- Van Herpen, N.; Schrauwen-Hinderling, V. Lipid accumulation in non-adipose tissue and lipotoxicity. Physiol. Behav. 2008, 94, 231–241. [Google Scholar] [CrossRef]
- Klaus, S. Adipose tissue as a regulator of energy balance. Curr. Drug Targets 2004, 5, 241–250. [Google Scholar] [CrossRef]
- Lee, M.R.; Yang, H.J.; Park, K.I.; Ma, J.Y. Lycopus lucidus Turcz. ex Benth. Attenuates free fatty acid-induced steatosis in HepG2 cells and non-alcoholic fatty liver disease in high-fat diet-induced obese mice. Phytomedicine 2019, 55, 14–22. [Google Scholar] [CrossRef]
- Fatani, S.; Itua, I.; Clark, P.; Wong, C.; Naderali, E.K. The effects of diet-induced obesity on hepatocyte insulin signaling pathways and induction of non-alcoholic liver damage. Int. J. Gen. Med. 2011, 4, 211. [Google Scholar]
- Garg, A.; Misra, A. Hepatic steatosis, insulin resistance, and adipose tissue disorders. J. Clin. Endocrinol. Metab. 2002, 87, 3019–3022. [Google Scholar] [CrossRef]
- Zhou, Y.; Rui, L. Major urinary protein regulation of chemical communication and nutrient metabolism. Vitam. Horm. 2010, 83, 151–163. [Google Scholar]
- Hui, X.; Zhu, W.; Wang, Y.; Lam, K.S.L.; Zhang, J.; Wu, D.; Kraegen, E.W.; Li, Y.; Xu, A. Major urinary protein-1 increases energy expenditure and improves glucose intolerance through enhancing mitochondrial function in skeletal muscle of diabetic mice. J. Biol. Chem. 2009, 284, 14050–14057. [Google Scholar] [CrossRef] [Green Version]
- Wu, Y.; Kim, J.Y.; Zhou, S.; Smas, C.M. Differential screening identifies transcripts with depot-dependent expression in white adipose tissues. BMC Genom. 2008, 9, 397. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shin, T.-Y.; Kim, S.-H.; Suk, K.; Ha, J.-H.; Kim, I.; Lee, M.-G.; Jun, C.-D.; Lim, J.-P.; Eun, J.-S. Anti-allergic effects of Lycopus lucidus on mast cell-mediated allergy model. Toxicol. Appl. Pharmacol. 2005, 209, 255–262. [Google Scholar] [CrossRef] [PubMed]


| Amount | |
|---|---|
| Total polyphenolic content | 480.86 ± 1.70 mg (GAE)/g |
| Total flavonoid content | 76.61 ± 0.16 mg (QE)/g |
| Luteolin | 2.426 mg/100 g |
| ND | HFD | LT | |
|---|---|---|---|
| FFA (mmol/L) | 0.44 ± 0.05 | 0.40 ± 0.02 | 0.36 ± 0.02 |
| TG (mmol/L) | 1.15 ± 0.10 | 1.61 ± 0.11 ** | 1.50 ± 0.11 |
| PL (mg/dL) | 228.39 ± 10.82 | 352.49 ± 11.46 *** | 253.27 ± 10.11 ### |
| Total-C (mmol/L) | 3.59 ± 0.09 | 7.85 ± 0.26 *** | 4.85 ± 0.10 ### |
| HDL-C (mmol/L) | 0.59 ± 0.05 | 1.39 ± 0.07 *** | 0.99 ± 0.09 ## |
| nonHDL-C (mmol/L) | 3.13 ± 0.20 | 6.49 ± 0.26 *** | 4.01 ± 0.23 ### |
| Apo B (mg/dL) | 6.39 ± 0.37 | 8.51 ± 0.51 ** | 6.42 ± 0.31 ## |
| Apo A-1(mg/dL) | 63.72 ± 1.85 | 68.64 ± 0.99 * | 62.10 ± 1.46 ## |
| Apo B/Apo A-1 | 0.102 ± 0.007 | 0.127 ± 0.006 * | 0.107 ± 0.004 # |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Han, Y.; Choi, J.-Y.; Kwon, E.-Y. Lycopus lucidus Turcz Water Extract Ameliorates the Metabolic Disorder by Up-Regulated Major Urinary Protein Expression in High-Fat Diet-Induced Obesity. Curr. Issues Mol. Biol. 2022, 44, 2417-2430. https://doi.org/10.3390/cimb44050165
Han Y, Choi J-Y, Kwon E-Y. Lycopus lucidus Turcz Water Extract Ameliorates the Metabolic Disorder by Up-Regulated Major Urinary Protein Expression in High-Fat Diet-Induced Obesity. Current Issues in Molecular Biology. 2022; 44(5):2417-2430. https://doi.org/10.3390/cimb44050165
Chicago/Turabian StyleHan, Youngji, Ji-Young Choi, and Eun-Young Kwon. 2022. "Lycopus lucidus Turcz Water Extract Ameliorates the Metabolic Disorder by Up-Regulated Major Urinary Protein Expression in High-Fat Diet-Induced Obesity" Current Issues in Molecular Biology 44, no. 5: 2417-2430. https://doi.org/10.3390/cimb44050165
APA StyleHan, Y., Choi, J.-Y., & Kwon, E.-Y. (2022). Lycopus lucidus Turcz Water Extract Ameliorates the Metabolic Disorder by Up-Regulated Major Urinary Protein Expression in High-Fat Diet-Induced Obesity. Current Issues in Molecular Biology, 44(5), 2417-2430. https://doi.org/10.3390/cimb44050165






