Calcium–Permeable Channels and Endothelial Dysfunction in Acute Lung Injury
Abstract
1. Introduction
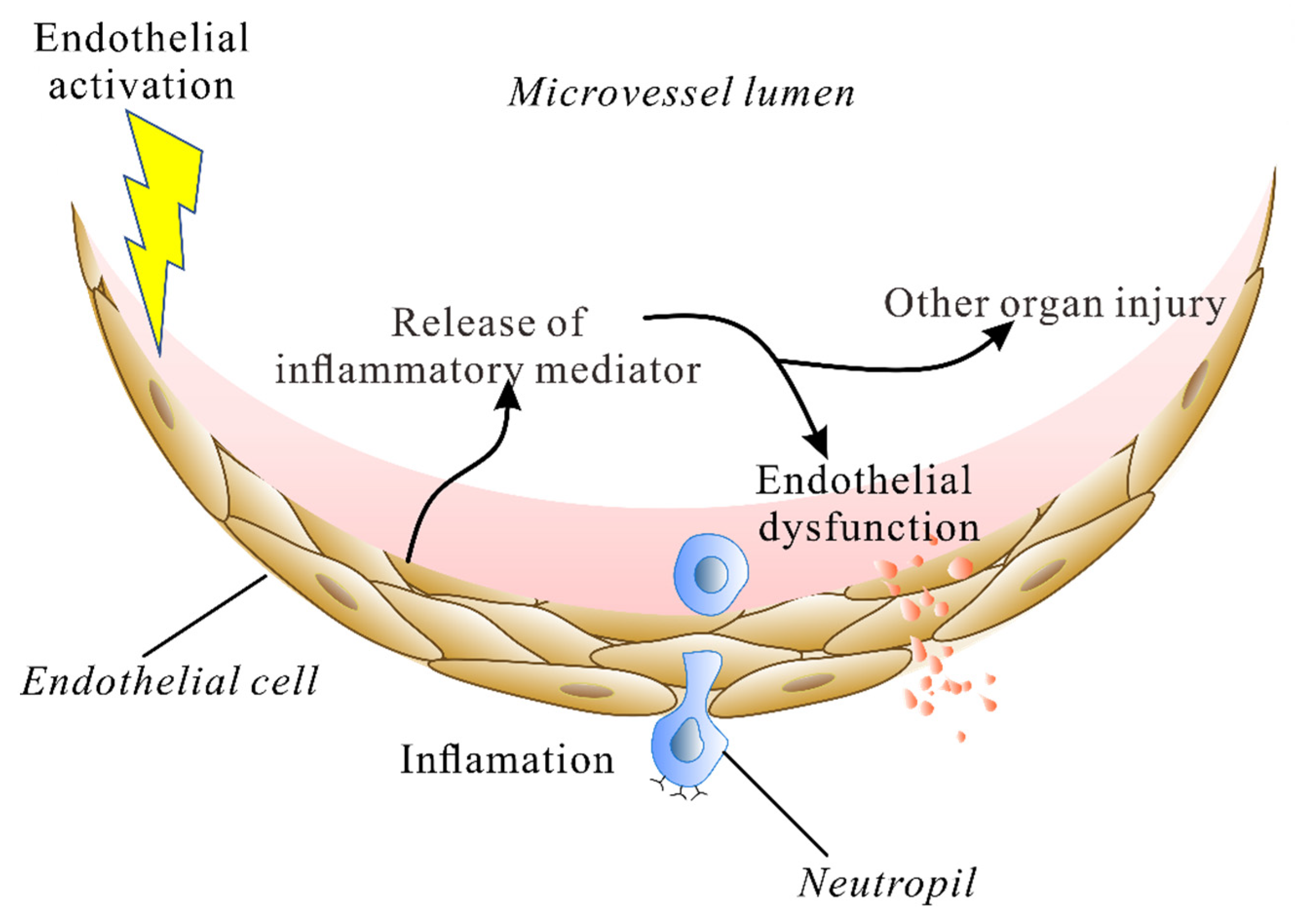
2. The Endothelium during Lung Injury
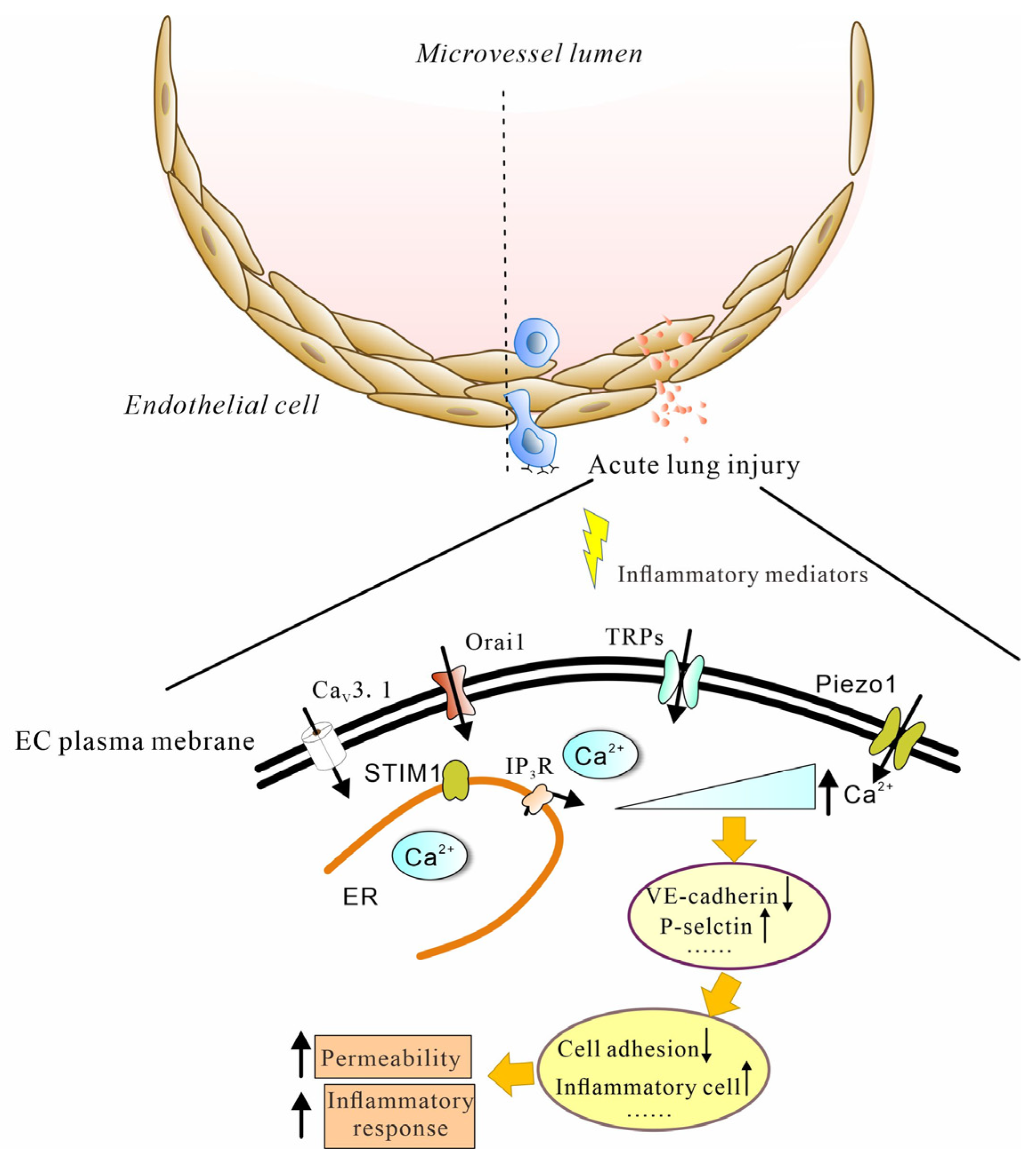
3. Calcium Influx into ECs Mediates Lung Injury
4. Calcium Machinery
4.1. Voltage-Gated Ca2+ Channels
4.2. Store-Operated Calcium (SOC) Channel
4.3. TRP Channels
4.4. Piezo Channel
4.5. Calcium Flux: A New Target for Therapy
5. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Mowery, N.T.; Terzian, W.T.H.; Nelson, A.C. Acute lung injury. Curr. Probl. Surg. 2020, 57, 100777. [Google Scholar] [CrossRef] [PubMed]
- Chan, S.W. Current and Future Direct-Acting Antivirals against COVID-19. Front. Microbiol. 2020, 11, 587944. [Google Scholar] [CrossRef] [PubMed]
- Ji, L.; Cao, C.; Gao, Y.; Zhang, W.; Xie, Y.; Duan, Y.; Kong, S.; You, M.; Ma, R.; Jiang, L.; et al. Prognostic value of bedside lung ultrasound score in patients with COVID-19. Crit. Care 2020, 24, 700. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Yang, Y.; Zhang, C.; Huang, F.; Wang, F.; Yuan, J.; Wang, Z.; Li, J.; Li, J.; Feng, C.; et al. Clinical and biochemical indexes from 2019-nCoV infected patients linked to viral loads and lung injury. Sci. China Life Sci. 2020, 63, 364–374. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Escobar, A.; Vera-Vera, S.; Jurado-Roman, A.; Jimenez-Valero, S.; Galeote, G.; Moreno, R. Calcium Signaling Pathway Is Involved in the Shedding of ACE2 Catalytic Ectodomain: New Insights for Clinical and Therapeutic Applications of ACE2 for COVID-19. Biomolecules 2022, 12, 76. [Google Scholar] [CrossRef]
- Pirahanchi, Y.; Marsidi, J.L.; Brown, K.N. Physiology, Endothelial Derived Relaxation Factor; StatPearls: Treasure Island, FL, USA, 2022. [Google Scholar]
- Townsley, M.I. Permeability and calcium signaling in lung endothelium: Unpack the box. Pulm. Circ. 2018, 8, 2045893217738218. [Google Scholar] [CrossRef]
- Haywood, N.; Ta, H.Q.; Zhang, A.; Charles, E.J.; Rotar, E.; Noona, S.t.; Salmon, M.; Daneva, Z.; Sonkusare, S.K.; Laubach, V.E. Endothelial Transient Receptor Potential Vanilloid 4 Channels Mediate Lung Ischemia-Reperfusion Injury. Ann. Thorac. Surg. 2022, 113, 1256–1264. [Google Scholar] [CrossRef]
- Li, M.; Fang, X.Z.; Zheng, Y.F.; Xie, Y.B.; Ma, X.D.; Liu, X.T.; Xia, Y.; Shao, D.H. Transient receptor potential vanilloid 4 is a critical mediator in LPS mediated inflammation by mediating calcineurin/NFATc3 signaling. Biochem. Biophys. Res. Commun. 2019, 513, 1005–1012. [Google Scholar] [CrossRef]
- Liu, L.; Guo, M.; Lv, X.; Wang, Z.; Yang, J.; Li, Y.; Yu, F.; Wen, X.; Feng, L.; Zhou, T. Role of Transient Receptor Potential Vanilloid 4 in Vascular Function. Front. Mol. Biosci. 2021, 8, 677661. [Google Scholar] [CrossRef]
- Esquivel-Ruiz, S.; Gonzalez-Rodriguez, P.; Lorente, J.A.; Perez-Vizcaino, F.; Herrero, R.; Moreno, L. Extracellular Vesicles and Alveolar Epithelial-Capillary Barrier Disruption in Acute Respiratory Distress Syndrome: Pathophysiological Role and Therapeutic Potential. Front. Physiol. 2021, 12, 752287. [Google Scholar] [CrossRef]
- Birukova, A.A.; Tian, X.; Cokic, I.; Beckham, Y.; Gardel, M.L.; Birukov, K.G. Endothelial barrier disruption and recovery is controlled by substrate stiffness. Microvasc. Res. 2013, 87, 50–57. [Google Scholar] [CrossRef] [PubMed]
- Komarova, Y.; Malik, A.B. Regulation of endothelial permeability via paracellular and transcellular transport pathways. Annu. Rev. Physiol. 2010, 72, 463–493. [Google Scholar] [CrossRef] [PubMed]
- Grimsley-Myers, C.M.; Isaacson, R.H.; Cadwell, C.M.; Campos, J.; Hernandes, M.S.; Myers, K.R.; Seo, T.; Giang, W.; Griendling, K.K.; Kowalczyk, A.P. VE-cadherin endocytosis controls vascular integrity and patterning during development. J. Cell Biol. 2020, 219, e201909081. [Google Scholar] [CrossRef] [PubMed]
- Rotoli, B.M.; Barilli, A.; Visigalli, R.; Ferrari, F.; Dall’Asta, V. Endothelial Cell Activation by SARS-CoV-2 Spike S1 Protein: A Crosstalk between Endothelium and Innate Immune Cells. Biomedicines 2021, 9, 1220. [Google Scholar] [CrossRef]
- Lee, W.; Choi, H.J.; Sim, H.; Choo, S.; Song, G.Y.; Bae, J.S. Barrier protective functions of hederacolchiside-E against HMGB1-mediated septic responses. Pharmacol. Res. 2021, 163, 105318. [Google Scholar] [CrossRef]
- Andonegui, G.; Zhou, H.; Bullard, D.; Kelly, M.M.; Mullaly, S.C.; McDonald, B.; Long, E.M.; Robbins, S.M.; Kubes, P. Mice that exclusively express TLR4 on endothelial cells can efficiently clear a lethal systemic Gram-negative bacterial infection. J. Clin. Investig. 2009, 119, 1921–1930. [Google Scholar] [CrossRef]
- Pirianov, G.; Waddington, S.N.; Lindstrom, T.M.; Terzidou, V.; Mehmet, H.; Bennett, P.R. The cyclopentenone 15-deoxy-delta 12,14-prostaglandin J(2) delays lipopolysaccharide-induced preterm delivery and reduces mortality in the newborn mouse. Endocrinology 2009, 150, 699–706. [Google Scholar] [CrossRef]
- Kuldo, J.M.; Westra, J.; Asgeirsdottir, S.A.; Kok, R.J.; Oosterhuis, K.; Rots, M.G.; Schouten, J.P.; Limburg, P.C.; Molema, G. Differential effects of NF-κB and p38 MAPK inhibitors and combinations thereof on TNF-α- and IL-1β-induced proinflammatory status of endothelial cells in vitro. Am. J. Physiol. Cell Physiol. 2005, 289, C1229–C1239. [Google Scholar] [CrossRef]
- Rayees, S.; Joshi, J.C.; Tauseef, M.; Anwar, M.; Baweja, S.; Rochford, I.; Joshi, B.; Hollenberg, M.D.; Reddy, S.P.; Mehta, D. PAR2-Mediated cAMP Generation Suppresses TRPV4-Dependent Ca(2+) Signaling in Alveolar Macrophages to Resolve TLR4-Induced Inflammation. Cell Rep. 2019, 27, 793–805.e4. [Google Scholar] [CrossRef]
- Escue, R.; Kandasamy, K.; Parthasarathi, K. Thrombin Induces Inositol Trisphosphate-Mediated Spatially Extensive Responses in Lung Microvessels. Am. J. Pathol. 2017, 187, 921–935. [Google Scholar] [CrossRef]
- Ye, X.; Ding, J.; Zhou, X.; Chen, G.; Liu, S.F. Divergent roles of endothelial NF-kappaB in multiple organ injury and bacterial clearance in mouse models of sepsis. J. Exp. Med. 2008, 205, 1303–1315. [Google Scholar] [CrossRef] [PubMed]
- Verdecchia, P.; Cavallini, C.; Spanevello, A.; Angeli, F. COVID-19: ACE2centric Infective Disease? Hypertension 2020, 76, 294–299. [Google Scholar] [CrossRef] [PubMed]
- Kar, M. Vascular Dysfunction and Its Cardiovascular Consequences During and after COVID-19 Infection: A Narrative Review. Vasc. Health Risk Manag. 2022, 18, 105–112. [Google Scholar] [CrossRef]
- Oxley, T.J.; Mocco, J.; Majidi, S.; Kellner, C.P.; Shoirah, H.; Singh, I.P.; De Leacy, R.A.; Shigematsu, T.; Ladner, T.R.; Yaeger, K.A.; et al. Large-Vessel Stroke as a Presenting Feature of COVID-19 in the Young. N. Engl. J. Med. 2020, 382, e60. [Google Scholar] [CrossRef] [PubMed]
- Colas-Algora, N.; Millan, J. How many cadherins do human endothelial cells express? Cell. Mol. Life Sci. 2019, 76, 1299–1317. [Google Scholar] [CrossRef] [PubMed]
- Duong, C.N.; Vestweber, D. Mechanisms Ensuring Endothelial Junction Integrity beyond VE-Cadherin. Front. Physiol. 2020, 11, 519. [Google Scholar] [CrossRef]
- Ichimura, H.; Parthasarathi, K.; Quadri, S.; Issekutz, A.C.; Bhattacharya, J. Mechano-oxidative coupling by mitochondria induces proinflammatory responses in lung venular capillaries. J. Clin. Investig. 2003, 111, 691–699. [Google Scholar] [CrossRef]
- Ye, X.; Beckett, T.; Bagher, P.; Garland, C.J.; Dora, K.A. VEGF-A inhibits agonist-mediated Ca(2+) responses and activation of IKCa channels in mouse resistance artery endothelial cells. J. Physiol. 2018, 596, 3553–3566. [Google Scholar] [CrossRef]
- White, L.E.; Cui, Y.; Shelak, C.M.; Lie, M.L.; Hassoun, H.T. Lung endothelial cell apoptosis during ischemic acute kidney injury. Shock 2012, 38, 320–327. [Google Scholar] [CrossRef]
- Richter, F.; Williams, S.K.; John, K.; Huber, C.; Vaslin, C.; Zanker, H.; Fairless, R.; Pichi, K.; Marhenke, S.; Vogel, A.; et al. The TNFR1 Antagonist Atrosimab Is Therapeutic in Mouse Models of Acute and Chronic Inflammation. Front. Immunol. 2021, 12, 705485. [Google Scholar] [CrossRef]
- Rohrmann, S.; Garmo, H.; Malmstrom, H.; Hammar, N.; Jungner, I.; Walldius, G.; Van Hemelrijck, M. Association between serum calcium concentration and risk of incident and fatal cardiovascular disease in the prospective AMORIS study. Atherosclerosis 2016, 251, 85–93. [Google Scholar] [CrossRef] [PubMed]
- Kang, K. Serum calcium and phosphate concentrations and intracranial atherosclerosis. Atherosclerosis 2014, 232, 249–253. [Google Scholar] [CrossRef] [PubMed]
- Catterall, W.A. Voltage-gated calcium channels. Cold Spring Harb. Perspect. Biol. 2011, 3, a003947. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Haynes, J., Jr.; Taylor, J.T.; Obiako, B.O.; Stubbs, J.R.; Li, M.; Stevens, T. Cav3.1 (alpha1G) T-type Ca2+ channels mediate vaso-occlusion of sickled erythrocytes in lung microcirculation. Circ. Res. 2003, 93, 346–353. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.; Wu, S. T-type calcium channels in pulmonary vascular endothelium. Microcirculation 2006, 13, 645–656. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Z.; Wang, X.; Wang, Y.; King, J.A.C.; Xie, P.; Wu, S. CaMK4 is a downstream effector of the alpha1G T-type calcium channel to determine the angiogenic potential of pulmonary microvascular endothelial cells. Am. J. Physiol. Cell Physiol. 2021, 321, C964–C977. [Google Scholar] [CrossRef]
- Wei, Z.; Manevich, Y.; Al-Mehdi, A.B.; Chatterjee, S.; Fisher, A.B. Ca2+ flux through voltage-gated channels with flow cessation in pulmonary microvascular endothelial cells. Microcirculation 2004, 11, 517–526. [Google Scholar] [CrossRef]
- Gilbert, G.; Courtois, A.; Dubois, M.; Cussac, L.A.; Ducret, T.; Lory, P.; Marthan, R.; Savineau, J.P.; Quignard, J.F. T-type voltage gated calcium channels are involved in endothelium-dependent relaxation of mice pulmonary artery. Biochem. Pharmacol. 2017, 138, 61–72. [Google Scholar] [CrossRef]
- Zheng, Z.; Chen, H.; Xie, P.; Dickerson, C.A.; King, J.A.C.; Alexeyev, M.F.; Wu, S. alpha1G T-type calcium channel determines the angiogenic potential of pulmonary microvascular endothelial cells. Am. J. Physiol. Cell. Physiol. 2019, 316, C353–C364. [Google Scholar] [CrossRef]
- Zhou, C.; Chen, H.; King, J.A.; Sellak, H.; Kuebler, W.M.; Yin, J.; Townsley, M.I.; Shin, H.S.; Wu, S. Alpha1G T-type calcium channel selectively regulates P-selectin surface expression in pulmonary capillary endothelium. Am. J. Physiol. Lung Cell Mol. Physiol. 2010, 299, L86–L97. [Google Scholar] [CrossRef]
- Wan, L.; Wu, W.; Jiang, S.; Wan, S.; Meng, D.; Wang, Z.; Zhang, J.; Wei, L.; Yu, P. Mibefradil and Flunarizine, Two T-Type Calcium Channel Inhibitors, Protect Mice against Lipopolysaccharide-Induced Acute Lung Injury. Mediat. Inflamm. 2020, 2020, 3691701. [Google Scholar] [CrossRef] [PubMed]
- Hogan, P.G.; Lewis, R.S.; Rao, A. Molecular basis of calcium signaling in lymphocytes: STIM and ORAI. Annu. Rev. Immunol. 2010, 28, 491–533. [Google Scholar] [CrossRef]
- Nwokonko, R.M.; Cai, X.; Loktionova, N.A.; Wang, Y.; Zhou, Y.; Gill, D.L. The STIM-Orai Pathway: Conformational Coupling between STIM and Orai in the Activation of Store-Operated Ca(2+) Entry. Adv. Exp. Med. Biol. 2017, 993, 83–98. [Google Scholar] [CrossRef] [PubMed]
- Gandhirajan, R.K.; Meng, S.; Chandramoorthy, H.C.; Mallilankaraman, K.; Mancarella, S.; Gao, H.; Razmpour, R.; Yang, X.F.; Houser, S.R.; Chen, J.; et al. Blockade of NOX2 and STIM1 signaling limits lipopolysaccharide-induced vascular inflammation. J. Clin. Investig. 2013, 123, 887–902. [Google Scholar] [CrossRef]
- Seeley, E.J.; Rosenberg, P.; Matthay, M.A. Calcium flux and endothelial dysfunction during acute lung injury: A STIMulating target for therapy. J. Clin. Investig. 2013, 123, 1015–1018. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Qiu, X.; Dong, K.; Sun, R. STIM1 Regulates Endothelial Calcium Overload and Cytokine Upregulation during Sepsis. J. Surg. Res. 2021, 263, 236–244. [Google Scholar] [CrossRef] [PubMed]
- Qiu, X.; Liang, X.; Li, H.; Sun, R. LPS-induced vein endothelial cell injury and acute lung injury have Btk and Orai 1 to regulate SOC-mediated calcium influx. Int. Immunopharmacol. 2021, 90, 107039. [Google Scholar] [CrossRef]
- Shinde, A.V.; Motiani, R.K.; Zhang, X.; Abdullaev, I.F.; Adam, A.P.; Gonzalez-Cobos, J.C.; Zhang, W.; Matrougui, K.; Vincent, P.A.; Trebak, M. STIM1 controls endothelial barrier function independently of Orai1 and Ca2+ entry. Sci. Signal. 2013, 6, ra18. [Google Scholar] [CrossRef]
- Tian, C.; Zhang, P.; Yang, J.; Zhang, Z.; Wang, H.; Guo, Y.; Liu, M. The protective effect of the flavonoid fraction of Abutilon theophrasti Medic. leaves on LPS-induced acute lung injury in mice via the NF-kappaB and MAPK signalling pathways. Biomed. Pharmacother. 2019, 109, 1024–1031. [Google Scholar] [CrossRef]
- Lin, T.; Luo, W.; Li, Z.; Zhang, L.; Zheng, X.; Mai, L.; Yang, W.; Guan, G.; Su, Z.; Liu, P.; et al. Rhamnocitrin extracted from Nervilia fordii inhibited vascular endothelial activation via miR-185/STIM-1/SOCE/NFATc3. Phytomedicine 2020, 79, 153350. [Google Scholar] [CrossRef]
- Li, J.; Zhang, X.; Song, X.; Liu, R.; Zhang, J.; Li, Z. The structure of TRPC ion channels. Cell Calcium 2019, 80, 25–28. [Google Scholar] [CrossRef]
- Hicks, G.A. TRP channels as therapeutic targets: Hot property, or time to cool down? Neurogastroenterol. Motil. 2006, 18, 590–594. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Sooch, G.; Demaree, I.S.; White, F.A.; Obukhov, A.G. Transient Receptor Potential Canonical (TRPC) Channels: Then and Now. Cells 2020, 9, 1983. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Cao, R.; Zhong, W. Host Calcium Channels and Pumps in Viral Infections. Cells 2019, 9, 94. [Google Scholar] [CrossRef] [PubMed]
- Achanta, S.; Jordt, S.E. Transient receptor potential channels in pulmonary chemical injuries and as countermeasure targets. Ann. N. Y. Acad. Sci. 2020, 1480, 73–103. [Google Scholar] [CrossRef] [PubMed]
- Dietrich, A.; Steinritz, D.; Gudermann, T. Transient receptor potential (TRP) channels as molecular targets in lung toxicology and associated diseases. Cell Calcium 2017, 67, 123–137. [Google Scholar] [CrossRef]
- Mehta, D.; Malik, A.B. Signaling mechanisms regulating endothelial permeability. Physiol. Rev. 2006, 86, 279–367. [Google Scholar] [CrossRef]
- Tauseef, M.; Farazuddin, M.; Sukriti, S.; Rajput, C.; Meyer, J.O.; Ramasamy, S.K.; Mehta, D. Transient receptor potential channel 1 maintains adherens junction plasticity by suppressing sphingosine kinase 1 expression to induce endothelial hyperpermeability. FASEB J. 2016, 30, 102–110. [Google Scholar] [CrossRef]
- Weissmann, N.; Sydykov, A.; Kalwa, H.; Storch, U.; Fuchs, B.; Mederos y Schnitzler, M.; Brandes, R.P.; Grimminger, F.; Meissner, M.; Freichel, M.; et al. Activation of TRPC6 channels is essential for lung ischaemia-reperfusion induced oedema in mice. Nat. Commun. 2012, 3, 649. [Google Scholar] [CrossRef]
- Tauseef, M.; Knezevic, N.; Chava, K.R.; Smith, M.; Sukriti, S.; Gianaris, N.; Obukhov, A.G.; Vogel, S.M.; Schraufnagel, D.E.; Dietrich, A.; et al. TLR4 activation of TRPC6-dependent calcium signaling mediates endotoxin-induced lung vascular permeability and inflammation. J. Exp. Med. 2012, 209, 1953–1968. [Google Scholar] [CrossRef]
- Rajan, S.; Schremmer, C.; Weber, J.; Alt, P.; Geiger, F.; Dietrich, A. Ca(2+) Signaling by TRPV4 Channels in Respiratory Function and Disease. Cells 2021, 10, 822. [Google Scholar] [CrossRef] [PubMed]
- Jian, M.Y.; King, J.A.; Al-Mehdi, A.B.; Liedtke, W.; Townsley, M.I. High vascular pressure-induced lung injury requires P450 epoxygenase-dependent activation of TRPV4. Am. J. Respir. Cell Mol. Biol. 2008, 38, 386–392. [Google Scholar] [CrossRef] [PubMed]
- Yin, J.; Michalick, L.; Tang, C.; Tabuchi, A.; Goldenberg, N.; Dan, Q.; Awwad, K.; Wang, L.; Erfinanda, L.; Nouailles, G.; et al. Role of Transient Receptor Potential Vanilloid 4 in Neutrophil Activation and Acute Lung Injury. Am. J. Respir. Cell Mol. Biol. 2016, 54, 370–383. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, H.; Vriens, J.; Prenen, J.; Droogmans, G.; Voets, T.; Nilius, B. Anandamide and arachidonic acid use epoxyeicosatrienoic acids to activate TRPV4 channels. Nature 2003, 424, 434–438. [Google Scholar] [CrossRef]
- Watanabe, H.; Vriens, J.; Janssens, A.; Wondergem, R.; Droogmans, G.; Nilius, B. Modulation of TRPV4 gating by intra- and extracellular Ca2+. Cell Calcium 2003, 33, 489–495. [Google Scholar] [CrossRef]
- Willette, R.N.; Bao, W.; Nerurkar, S.; Yue, T.L.; Doe, C.P.; Stankus, G.; Turner, G.H.; Ju, H.; Thomas, H.; Fishman, C.E.; et al. Systemic activation of the transient receptor potential vanilloid subtype 4 channel causes endothelial failure and circulatory collapse: Part 2. J. Pharmacol. Exp. Ther. 2008, 326, 443–452. [Google Scholar] [CrossRef]
- Alvarez, D.F.; King, J.A.; Weber, D.; Addison, E.; Liedtke, W.; Townsley, M.I. Transient receptor potential vanilloid 4-mediated disruption of the alveolar septal barrier: A novel mechanism of acute lung injury. Circ. Res. 2006, 99, 988–995. [Google Scholar] [CrossRef]
- Baratchi, S.; Keov, P.; Darby, W.G.; Lai, A.; Khoshmanesh, K.; Thurgood, P.; Vahidi, P.; Ejendal, K.; McIntyre, P. The TRPV4 Agonist GSK1016790A Regulates the Membrane Expression of TRPV4 Channels. Front. Pharmacol. 2019, 10, 6. [Google Scholar] [CrossRef]
- Bihari, S.; Dixon, D.L.; Lawrence, M.D.; De Bellis, D.; Bonder, C.S.; Dimasi, D.P.; Bersten, A.D. Fluid-induced lung injury-role of TRPV4 channels. Pflugers Arch. 2017, 469, 1121–1134. [Google Scholar] [CrossRef]
- Suresh, K.; Servinsky, L.; Jiang, H.; Bigham, Z.; Yun, X.; Kliment, C.; Huetsch, J.; Damarla, M.; Shimoda, L.A. Reactive oxygen species induced Ca(2+) influx via TRPV4 and microvascular endothelial dysfunction in the SU5416/hypoxia model of pulmonary arterial hypertension. Am. J. Physiol. Lung Cell Mol. Physiol. 2018, 314, L893–L907. [Google Scholar] [CrossRef]
- Balakrishna, S.; Song, W.; Achanta, S.; Doran, S.F.; Liu, B.; Kaelberer, M.M.; Yu, Z.; Sui, A.; Cheung, M.; Leishman, E.; et al. TRPV4 inhibition counteracts edema and inflammation and improves pulmonary function and oxygen saturation in chemically induced acute lung injury. Am. J. Physiol. Lung Cell Mol. Physiol. 2014, 307, L158–L172. [Google Scholar] [CrossRef] [PubMed]
- Swain, S.M.; Liddle, R.A. Piezo1 acts upstream of TRPV4 to induce pathological changes in endothelial cells due to shear stress. J. Biol. Chem. 2021, 296, 100171. [Google Scholar] [CrossRef] [PubMed]
- Lawhorn, B.G.; Brnardic, E.J.; Behm, D.J. TRPV4 antagonists: A patent review (2015–2020). Expert Opin. Ther. Pat. 2021, 31, 773–784. [Google Scholar] [CrossRef] [PubMed]
- Pairet, N.; Mang, S.; Fois, G.; Keck, M.; Kuhnbach, M.; Gindele, J.; Frick, M.; Dietl, P.; Lamb, D.J. TRPV4 inhibition attenuates stretch-induced inflammatory cellular responses and lung barrier dysfunction during mechanical ventilation. PLoS ONE 2018, 13, e0196055. [Google Scholar] [CrossRef] [PubMed]
- Thorneloe, K.S.; Cheung, M.; Bao, W.; Alsaid, H.; Lenhard, S.; Jian, M.Y.; Costell, M.; Maniscalco-Hauk, K.; Krawiec, J.A.; Olzinski, A.; et al. An orally active TRPV4 channel blocker prevents and resolves pulmonary edema induced by heart failure. Sci. Transl. Med. 2012, 4, 159ra148. [Google Scholar] [CrossRef] [PubMed]
- Dagenais, A.; Desjardins, J.; Shabbir, W.; Roy, A.; Filion, D.; Sauve, R.; Berthiaume, Y. Loss of barrier integrity in alveolar epithelial cells downregulates ENaC expression and activity via Ca(2+) and TRPV4 activation. Pflugers Arch. 2018, 470, 1615–1631. [Google Scholar] [CrossRef]
- Weber, J.; Rajan, S.; Schremmer, C.; Chao, Y.K.; Krasteva-Christ, G.; Kannler, M.; Yildirim, A.O.; Brosien, M.; Schredelseker, J.; Weissmann, N.; et al. TRPV4 channels are essential for alveolar epithelial barrier function as protection from lung edema. JCI Insight 2020, 5, e134464. [Google Scholar] [CrossRef]
- Kuebler, W.M.; Jordt, S.E.; Liedtke, W.B. Urgent reconsideration of lung edema as a preventable outcome in COVID-19: Inhibition of TRPV4 represents a promising and feasible approach. Am. J. Physiol. Lung Cell Mol. Physiol. 2020, 318, L1239–L1243. [Google Scholar] [CrossRef]
- Cheung, M.; Bao, W.; Behm, D.J.; Brooks, C.A.; Bury, M.J.; Dowdell, S.E.; Eidam, H.S.; Fox, R.M.; Goodman, K.B.; Holt, D.A.; et al. Discovery of GSK2193874: An Orally Active, Potent, and Selective Blocker of Transient Receptor Potential Vanilloid 4. ACS Med. Chem. Lett. 2017, 8, 549–554. [Google Scholar] [CrossRef]
- Brooks, C.A.; Barton, L.S.; Behm, D.J.; Eidam, H.S.; Fox, R.M.; Hammond, M.; Hoang, T.H.; Holt, D.A.; Hilfiker, M.A.; Lawhorn, B.G.; et al. Discovery of GSK2798745: A Clinical Candidate for Inhibition of Transient Receptor Potential Vanilloid 4 (TRPV4). ACS Med. Chem. Lett. 2019, 10, 1228–1233. [Google Scholar] [CrossRef]
- Goyal, N.; Skrdla, P.; Schroyer, R.; Kumar, S.; Fernando, D.; Oughton, A.; Norton, N.; Sprecher, D.L.; Cheriyan, J. Clinical Pharmacokinetics, Safety, and Tolerability of a Novel, First-in-Class TRPV4 Ion Channel Inhibitor, GSK2798745, in Healthy and Heart Failure Subjects. Am. J. Cardiovasc. Drugs 2019, 19, 335–342. [Google Scholar] [CrossRef] [PubMed]
- Mole, S.; Harry, A.; Fowler, A.; Hotee, S.; Warburton, J.; Waite, S.; Beerahee, M.; Behm, D.J.; Badorrek, P.; Muller, M.; et al. Investigating the effect of TRPV4 inhibition on pulmonary-vascular barrier permeability following segmental endotoxin challenge. Pulm. Pharmacol. Ther. 2020, 64, 101977. [Google Scholar] [CrossRef] [PubMed]
- Hecquet, C.M.; Ahmmed, G.U.; Malik, A.B. TRPM2 channel regulates endothelial barrier function. Adv. Exp. Med. Biol. 2010, 661, 155–167. [Google Scholar] [CrossRef] [PubMed]
- Hecquet, C.M.; Zhang, M.; Mittal, M.; Vogel, S.M.; Di, A.; Gao, X.; Bonini, M.G.; Malik, A.B. Cooperative interaction of trp melastatin channel transient receptor potential (TRPM2) with its splice variant TRPM2 short variant is essential for endothelial cell apoptosis. Circ. Res. 2014, 114, 469–479. [Google Scholar] [CrossRef]
- Wang, T.; Wang, L.; Moreno-Vinasco, L.; Lang, G.D.; Siegler, J.H.; Mathew, B.; Usatyuk, P.V.; Samet, J.M.; Geyh, A.S.; Breysse, P.N.; et al. Particulate matter air pollution disrupts endothelial cell barrier via calpain-mediated tight junction protein degradation. Part Fibre Toxicol. 2012, 9, 35. [Google Scholar] [CrossRef]
- Qin, L.; He, T.; Chen, S.; Yang, D.; Yi, W.; Cao, H.; Xiao, G. Roles of mechanosensitive channel Piezo1/2 proteins in skeleton and other tissues. Bone Res. 2021, 9, 44. [Google Scholar] [CrossRef]
- Coste, B.; Mathur, J.; Schmidt, M.; Earley, T.J.; Ranade, S.; Petrus, M.J.; Dubin, A.E.; Patapoutian, A. Piezo1 and Piezo2 are essential components of distinct mechanically activated cation channels. Science 2010, 330, 55–60. [Google Scholar] [CrossRef]
- Friedrich, E.E.; Hong, Z.; Xiong, S.; Zhong, M.; Di, A.; Rehman, J.; Komarova, Y.A.; Malik, A.B. Endothelial cell Piezo1 mediates pressure-induced lung vascular hyperpermeability via disruption of adherens junctions. Proc. Natl. Acad. Sci. USA 2019, 116, 12980–12985. [Google Scholar] [CrossRef]
- Zhang, Y.; Jiang, L.; Huang, T.; Lu, D.; Song, Y.; Wang, L.; Gao, J. Mechanosensitive cation channel Piezo1 contributes to ventilator-induced lung injury by activating RhoA/ROCK1 in rats. Respir. Res. 2021, 22, 250. [Google Scholar] [CrossRef]
- Jiang, L.; Zhang, Y.; Lu, D.; Huang, T.; Yan, K.; Yang, W.; Gao, J. Mechanosensitive Piezo1 channel activation promotes ventilator-induced lung injury via disruption of endothelial junctions in ARDS rats. Biochem. Biophys. Res. Commun. 2021, 556, 79–86. [Google Scholar] [CrossRef]
- Zhong, M.; Wu, W.; Kang, H.; Hong, Z.; Xiong, S.; Gao, X.; Rehman, J.; Komarova, Y.A.; Malik, A.B. Alveolar Stretch Activation of Endothelial Piezo1 Protects Adherens Junctions and Lung Vascular Barrier. Am. J. Respir. Cell. Mol. Biol. 2020, 62, 168–177. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.W.; Zhang, H.; Huang, J.Q.; Wang, S.N.; Lu, Y.; Cheng, B.; Dong, S.H.; Wang, Y.Y.; Li, F.S.; Li, Y.W. PIEZO1 Ion Channel Mediates Ionizing Radiation-Induced Pulmonary Endothelial Cell Ferroptosis via Ca(2+)/Calpain/VE-Cadherin Signaling. Front. Mol. Biosci. 2021, 8, 725274. [Google Scholar] [CrossRef] [PubMed]
| Target Channel | Compounds | Application | Reference |
|---|---|---|---|
| CaV3.1 | Mibefradil | LPS-induced lung injury | [36,42] |
| Flunarizine | LPS-induced lung injury | [36,42] | |
| SOCs | Rhamnocitrin | A potent inhibitor of endothelial activation | [51] |
| TRPV4 | GSK2798745 | LPS-induced lung inflammation * | [77,84] |
| GSK2193874 | High-pulmonary-venous-pressure (PVP)-induced edema | [77] | |
| HC-067047 | LPS-induced mouse lung injury | [9] | |
| Piezo1 | GSMTx4 | Ionizing-radiation (IR)-induced lung injury | [93] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hao, Y.; Wang, Z.; Frimpong, F.; Chen, X. Calcium–Permeable Channels and Endothelial Dysfunction in Acute Lung Injury. Curr. Issues Mol. Biol. 2022, 44, 2217-2229. https://doi.org/10.3390/cimb44050150
Hao Y, Wang Z, Frimpong F, Chen X. Calcium–Permeable Channels and Endothelial Dysfunction in Acute Lung Injury. Current Issues in Molecular Biology. 2022; 44(5):2217-2229. https://doi.org/10.3390/cimb44050150
Chicago/Turabian StyleHao, Ying, Zhuang Wang, Francis Frimpong, and Xingjuan Chen. 2022. "Calcium–Permeable Channels and Endothelial Dysfunction in Acute Lung Injury" Current Issues in Molecular Biology 44, no. 5: 2217-2229. https://doi.org/10.3390/cimb44050150
APA StyleHao, Y., Wang, Z., Frimpong, F., & Chen, X. (2022). Calcium–Permeable Channels and Endothelial Dysfunction in Acute Lung Injury. Current Issues in Molecular Biology, 44(5), 2217-2229. https://doi.org/10.3390/cimb44050150





