3,3′,4,5′-Tetramethoxy-trans-stilbene Improves Insulin Resistance by Activating the IRS/PI3K/Akt Pathway and Inhibiting Oxidative Stress
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Cell Culture
2.3. MTT Assay
2.4. Glucose Consumption Assay
2.5. Glycogen Synthesis Assay
2.6. Western Blot Assay
2.7. ROS Assay
2.8. Statistical Analysis
3. Results
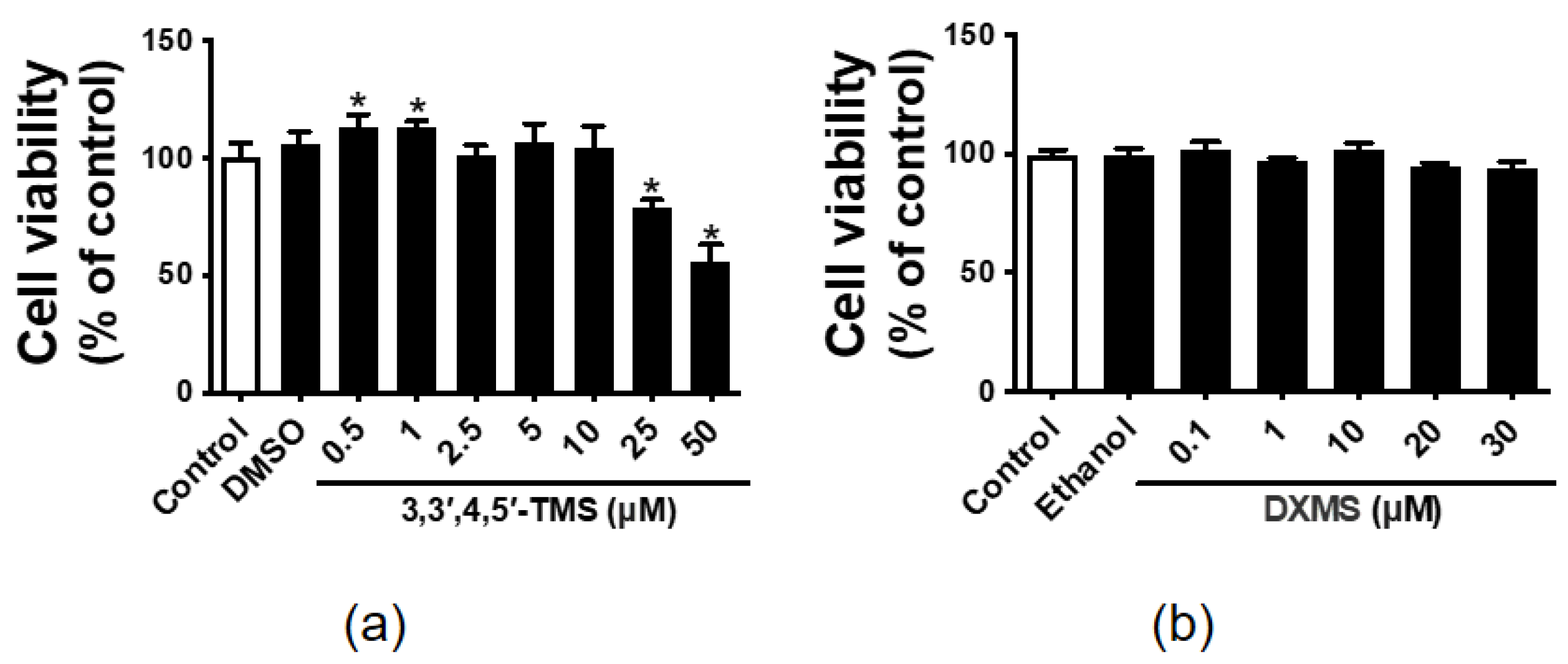
3.1. The Effect of 3,3′,4,5′-TMS and DXMS on the HepG2 Cell Viability
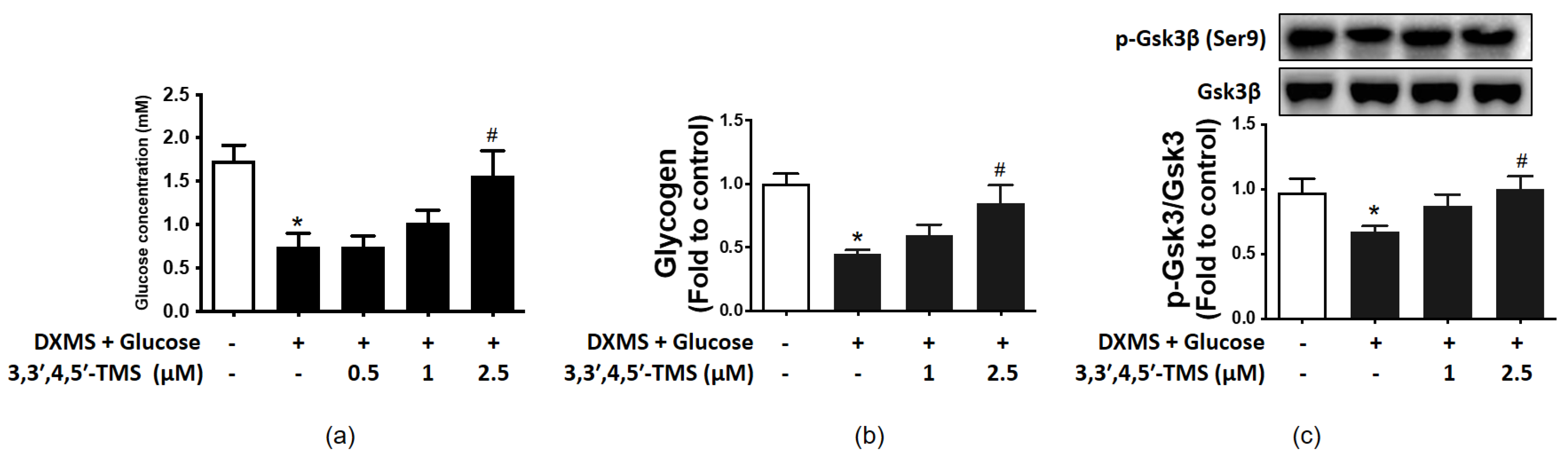
3.2. Effect of 3,3′,4,5′-TMS on Glucose Consumption
3.3. 3,3′,4,5′-TMS Enhanced Glycogen Synthesis Ability by Up-Regulating p-GSK3β
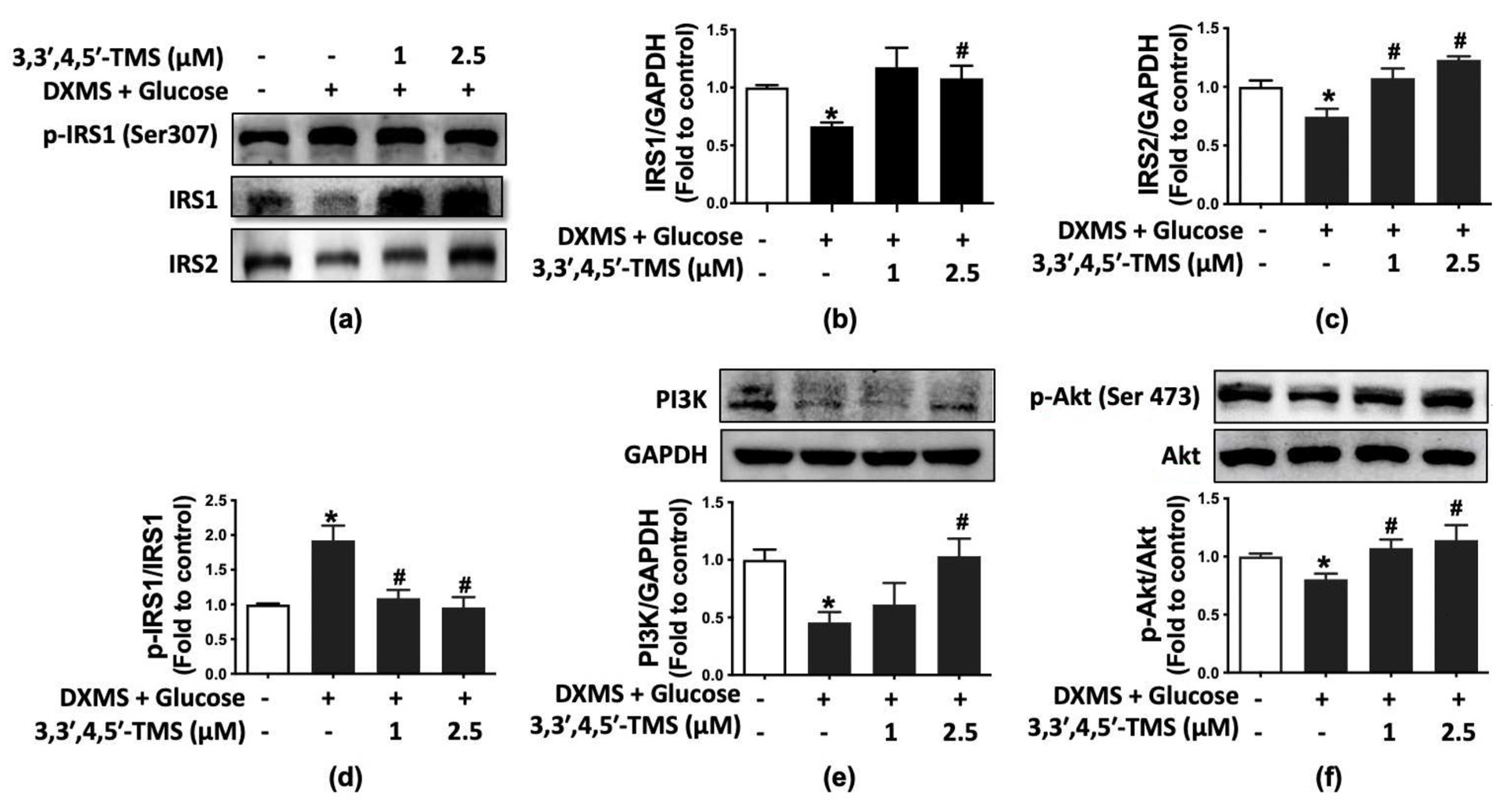
3.4. 3,3′,4,5′-TMS Activated IRS/PI3K/Akt Pathway
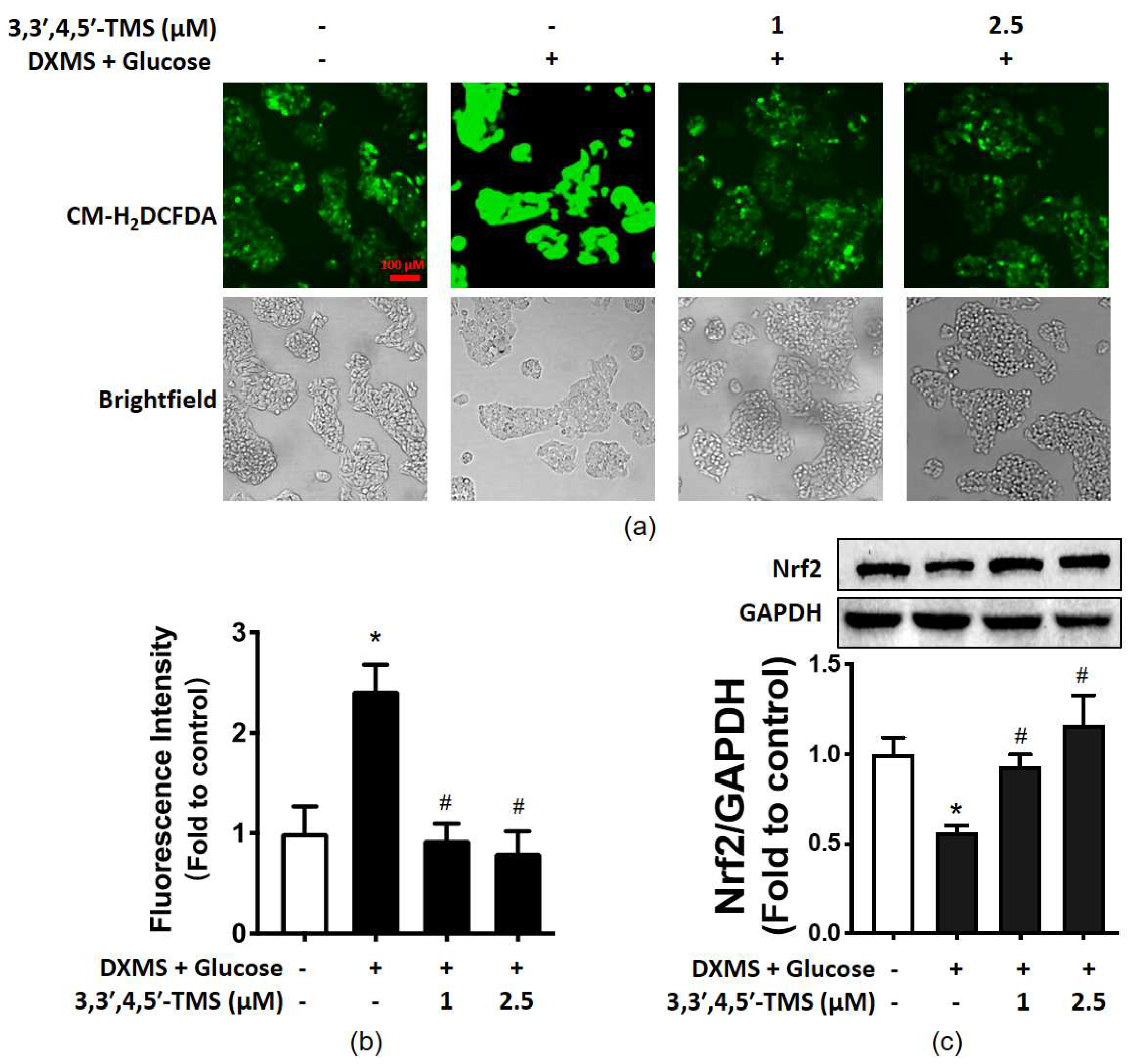
3.5. 3,3′,4,5′-TMS Alleviated Oxidative Stress by Upregulating Nrf2
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gregg, E.W.; Sattar, N.; Ali, M.K. The changing face of diabetes complications. Lancet Diabetes Endocrinol. 2016, 4, 537–547. [Google Scholar] [CrossRef]
- Taylor, R. Insulin Resistance and Type 2 Diabetes. Diabetes 2012, 61, 778–779. [Google Scholar] [CrossRef] [PubMed]
- Saadeldeen, F.S.A.; Niu, Y.; Wang, H.; Zhou, L.; Meng, L.; Chen, S.; Sun-Waterhouse, D.; Waterhouse, G.I.N.; Liu, Z.; Kang, W. Natural products: Regulating glucose metabolism and improving insulin resistance. Food Sci. Hum. Wellness 2020, 9, 214–228. [Google Scholar] [CrossRef]
- Wojcik, M.; Krawczyk, M.; Zieleniak, A.; Marcjanek, K.M.; Wozniak, L.A. Associations of high blood sugar with oxidative stress and inflammation in patients with type 2 diabetes. In Dietary Sugar, Salt and Fat in Human Health; Preuss, H.G., Bagchi, D., Eds.; Academic Press: Cambridge, MA, USA, 2020; pp. 305–323. [Google Scholar]
- Tiganis, T. Reactive oxygen species and insulin resistance: The good, the bad and the ugly. Trends Pharmacol. Sci. 2011, 32, 82–89. [Google Scholar] [CrossRef]
- Klanjscek, T.; Muller, E.B.; Nisbet, R.M. Feedbacks and tipping points in organismal response to oxidative stress. J. Theor. Biol. 2016, 404, 361–374. [Google Scholar] [CrossRef]
- Volpe, C.M.O.; Villar-Delfino, P.H.; dos Anjos, P.M.F.; Nogueira-Machado, J.A. Cellular death, reactive oxygen species (ROS) and diabetic complications. Cell Death Dis. 2018, 9, 119. [Google Scholar] [CrossRef]
- Satake, S.; Moore, M.C.; Igawa, K.; Converse, M.; Farmer, B.; Neal, D.W.; Cherrington, A.D. Direct and Indirect Effects of Insulin on Glucose Uptake and Storage by the Liver. Diabetes 2002, 51, 1663. [Google Scholar] [CrossRef]
- Girard, J. The Inhibitory Effects of Insulin on Hepatic Glucose Production Are Both Direct and Indirect. Diabetes 2006, 55, 65–69. [Google Scholar] [CrossRef]
- Iyer, V.V.; Hong, Y.; Ierapetritou, M.G.; Roth, C.M. Effects of glucose and insulin on HepG2-C3A cell metabolism. Biotechnol. Bioeng. 2010, 107, 347–356. [Google Scholar] [CrossRef]
- Suh, S.; Park, M.K. Glucocorticoid-Induced Diabetes Mellitus: An Important but Overlooked Problem. Endocrinol. Metab. 2017, 32, 180–189. [Google Scholar] [CrossRef]
- Ma, J.-Z.; Yang, L.-X.; Xiao-Ling, S.; Ji-Huan, Q.; Li-Lan, D.; Ahmed, S.; Xu, H.-X.; Xue, D.-Y.; Ye, J.-X.; Xu, G. Effects of Traditional Chinese Medicinal Plants on Anti-insulin Resistance Bioactivity of DXMS-Induced Insulin Resistant HepG2 Cells. Nat. Prod. Bioprospect. 2014, 4, 197–206. [Google Scholar] [CrossRef]
- Xia, T.; Duan, W.; Zhang, Z.; Fang, B.; Xiao, J. Polyphenol-rich extract of Zhenjiang aromatic vinegar ameliorates high glucose-induced insulin resistance by regulating JNK-IRS-1 and PI3K/Akt signaling pathways. Food Chem. 2020, 335, 127513. [Google Scholar] [CrossRef]
- Ren, L.; Zhou, X.; Huang, X.; Wang, C.; Li, Y. The IRS/PI3K/Akt signaling pathway mediates olanzapine-induced hepatic insulin resistance in male rats. Life Sci. 2019, 217, 229–236. [Google Scholar] [CrossRef]
- Liu, H.X.; He, B. Glucocorticoid Aggravates High Glucose-Induced Insulin Resistance. J. Chin. Physician 2001, 12, wpr-519140. [Google Scholar]
- Franco-Colín, M.; Villanueva, I.; Piñón, M.; Racotta, R. The effects of sympathectomy and dexamethasone in rats ingesting sucrose. Int. J. Biol. Sci. 2006, 2, 17–22. [Google Scholar] [CrossRef][Green Version]
- Costa, M.M.; Violato, N.M.; Taboga, S.R.; Góes, R.M.; Bosqueiro, J.R. Reduction of insulin signalling pathway IRS-1/IRS-2/AKT/mTOR and decrease of epithelial cell proliferation in the prostate of glucocorticoid-treated rats. Int. J. Exp. Pathol. 2012, 93, 188–195. [Google Scholar] [CrossRef]
- Eckstein, S.S.; Weigert, C.; Lehmann, R. Divergent Roles of IRS (Insulin Receptor Substrate) 1 and 2 in Liver and Skeletal Muscle. Curr. Med. Chem. 2017, 24, 1827–1852. [Google Scholar] [CrossRef]
- Taniguchi, C.M.; Ueki, K.; Kahn, C.R. Complementary roles of IRS-1 and IRS-2 in the hepatic regulation of metabolism. J. Clin. Investig. 2005, 115, 718. [Google Scholar] [CrossRef]
- Lee, J.; Kim, M.-S. The role of GSK3 in glucose homeostasis and the development of insulin resistance. Diabetes Res. Clin. Pract. 2007, 77, S49–S57. [Google Scholar] [CrossRef]
- Rains, J.L.; Jain, S.K. Oxidative stress, insulin signaling, and diabetes. Free Radic. Biol. Med. 2011, 50, 567–575. [Google Scholar] [CrossRef]
- Wang, Y.; Miao, X.; Sun, J.; Cai, L. Chapter 6—Oxidative stress in diabetes: Molecular basis for diet supplementation. In Molecular Nutrition and Diabetes; Mauricio, D., Ed.; Academic Press: San Diego, CA, USA, 2016; pp. 65–72. [Google Scholar]
- Szkudelski, T.; Szkudelska, K. Resveratrol and diabetes: From animal to human studies. Biochim. Biophys. Acta 2015, 1852, 1145–1154. [Google Scholar] [CrossRef]
- Zhu, X.; Wu, C.; Qiu, S.; Yuan, X.; Li, L. Effects of resveratrol on glucose control and insulin sensitivity in subjects with type 2 diabetes: Systematic review and meta-analysis. Nutr. Metab. 2017, 14, 60. [Google Scholar] [CrossRef]
- Lin, H.-S.; Tringali, C.; Spatafora, C.; Wu, C.; Ho, P.C. A simple and sensitive HPLC-UV method for the quantification of piceatannol analog trans-3,5,3′,4′-tetramethoxystilbene in rat plasma and its application for a pre-clinical pharmacokinetic study. J. Pharm. Biomed. Anal. 2010, 51, 679–684. [Google Scholar] [CrossRef]
- Sinha, D.; Sarkar, N.; Biswas, J.; Bishayee, A. Resveratrol for breast cancer prevention and therapy: Preclinical evidence and molecular mechanisms. Semin. Cancer Biol. 2016, 40–41, 209–232. [Google Scholar] [CrossRef]
- Zhou, C.; Zhang, X.; Ruan, C.-C.; Cheang, W.S. Two methoxy derivatives of resveratrol, 3,3′,4,5′-tetramethoxy-trans-stilbene and 3,4′,5-trimethoxy-trans-stilbene, suppress lipopolysaccharide-induced inflammation through inactivation of MAPK and NF-κB pathways in RAW 264.7 cells. Chin. Med. 2021, 16, 69. [Google Scholar] [CrossRef]
- Oparka, M.; Walczak, J.; Malinska, D.; van Oppen, L.; Szczepanowska, J.; Koopman, W.J.H.; Wieckowski, M.R. Quantifying ROS levels using CM-H2DCFDA and HyPer. Methods 2016, 109, 3–11. [Google Scholar] [CrossRef]
- Bugianesi, E.; McCullough, A.J.; Marchesini, G. Insulin resistance: A metabolic pathway to chronic liver disease. Hepatology 2005, 42, 987–1000. [Google Scholar] [CrossRef] [PubMed]
- Luan, G.; Li, G.; Ma, X.; Jin, Y.; Hu, N.; Li, J.; Wang, Z.; Wang, H. Dexamethasone-Induced Mitochondrial Dysfunction and Insulin Resistance-Study in 3T3-L1 Adipocytes and Mitochondria Isolated from Mouse Liver. Molecules 2019, 24, 1982. [Google Scholar] [CrossRef]
- Di Meo, S.; Iossa, S.; Venditti, P. Skeletal muscle insulin resistance: Role of mitochondria and other ROS sources. J. Endocrinol. 2017, 233, R15–R42. [Google Scholar] [CrossRef]
- Hurrle, S.; Hsu, W.H. The etiology of oxidative stress in insulin resistance. Biomed. J. 2017, 40, 257–262. [Google Scholar] [CrossRef]
- Morris, V.L.; Toseef, T.; Nazumudeen, F.B.; Rivoira, C.; Spatafora, C.; Tringali, C.; Rotenberg, S.A. Anti-tumor properties of cis-resveratrol methylated analogs in metastatic mouse melanoma cells. Mol. Cell. Biochem. 2015, 402, 83–91. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Kuang, W.; Xu, X.; Li, D.; Zhu, W.; Lan, Z.; Zhang, X. Putative identification of components in Zengye Decoction and their effects on glucose consumption and lipogenesis in insulin-induced insulin-resistant HepG2 cells. J. Chromatogr. B 2018, 1073, 145–153. [Google Scholar] [CrossRef] [PubMed]
- Fadaka, A.; Ajiboye, B.; Ojo, O.; Adewale, O.; Olayide, I.; Emuowhochere, R. Biology of glucose metabolization in cancer cells. J. Oncol. Sci. 2017, 3, 45–51. [Google Scholar] [CrossRef]
- Bouché, C.; Serdy, S.; Kahn, C.R.; Goldfine, A.B. The Cellular Fate of Glucose and Its Relevance in Type 2 Diabetes. Endocr. Rev. 2004, 25, 807–830. [Google Scholar] [CrossRef] [PubMed]
- Mao, Z.-J.; Lin, M.; Zhang, X.; Qin, L.-P. Combined Use of Astragalus Polysaccharide and Berberine Attenuates Insulin Resistance in IR-HepG2 Cells via Regulation of the Gluconeogenesis Signaling Pathway. Front. Pharmacol. 2019, 10, 1508. [Google Scholar] [CrossRef] [PubMed]
- Xuguang, H.; Aofei, T.; Tao, L.; Longyan, Z.; Weijian, B.; Jiao, G. Hesperidin ameliorates insulin resistance by regulating the IRS1-GLUT2 pathway via TLR4 in HepG2 cells. Phytother. Res. 2019, 33, 1697–1705. [Google Scholar] [CrossRef] [PubMed]
- Thorens, B. GLUT2, glucose sensing and glucose homeostasis. Diabetologia 2015, 58, 221–232. [Google Scholar] [CrossRef]
- Rui, L. Energy metabolism in the liver. Compr. Physiol. 2014, 4, 177–197. [Google Scholar] [CrossRef]
- Jiang, S.; Young, J.L.; Wang, K.; Qian, Y.; Cai, L. Diabeticinduced alterations in hepatic glucose and lipid metabolism: The role of type 1 and type 2 diabetes mellitus (Review). Mol. Med. Rep. 2020, 22, 603–611. [Google Scholar] [CrossRef]
- McManus, E.J.; Sakamoto, K.; Armit, L.J.; Ronaldson, L.; Shpiro, N.; Marquez, R.; Alessi, D.R. Role that phosphorylation of GSK3 plays in insulin and Wnt signalling defined by knockin analysis. EMBO J. 2005, 24, 1571–1583. [Google Scholar] [CrossRef]
- Groot, L.D.; Chrousos, G.; Dungan, K.; Grossman, A.; Hershman, J.M.; Koch, C.; Korbonits, M.; Mclachlan, R.; New, M.; Purnell, J. The Insulin Receptor and Its Signal Transduction Network—Endotext. 2000. Available online: https://www.ncbi.nlm.nih.gov/sites/books/NBK378978/ (accessed on 2 December 2021).
- Metz, H.E.; McGarry Houghton, A. Insulin Receptor Substrate Regulation of Phosphoinositide 3-Kinase. Clin. Cancer Res. 2011, 17, 206. [Google Scholar] [CrossRef]
- Groeneveld, M.P.; Brierley, G.V.; Rocha, N.M.; Siddle, K.; Semple, R.K. Acute knockdown of the insulin receptor or its substrates Irs1 and 2 in 3T3-L1 adipocytes suppresses adiponectin production. Sci. Rep. 2016, 6, 21105. [Google Scholar] [CrossRef]
- Krause, C.; Geißler, C.; Tackenberg, H.; El Gammal, A.T.; Wolter, S.; Spranger, J.; Mann, O.; Lehnert, H.; Kirchner, H. Multi-layered epigenetic regulation of IRS2 expression in the liver of obese individuals with type 2 diabetes. Diabetologia 2020, 63, 2182–2193. [Google Scholar] [CrossRef]
- Park, K.; Gross, M.; Lee, D.H.; Holvoet, P.; Himes, J.H.; Shikany, J.M.; Jacobs, D.R. Oxidative Stress and Insulin Resistance. Diabetes Care 2009, 32, 1302. [Google Scholar] [CrossRef]
- Zhong, R.; Chen, L.; Liu, Y.; Xie, S.; Li, S.; Liu, B.; Zhao, C. Anti-diabetic effect of aloin via JNK-IRS1/PI3K pathways and regulation of gut microbiota. Food Sci. Hum. Wellness 2022, 11, 189–198. [Google Scholar] [CrossRef]
- Li, S.; Eguchi, N.; Lau, H.; Ichii, H. The Role of the Nrf2 Signaling in Obesity and Insulin Resistance. Int. J. Mol. Sci. 2020, 21, 6973. [Google Scholar] [CrossRef]
- Chen, J.; Zhang, Z.; Cai, L. Diabetic cardiomyopathy and its prevention by nrf2: Current status. Diabetes Metab. J. 2014, 38, 337–345. [Google Scholar] [CrossRef]
- Ding, X.; Jian, T.; Wu, Y.; Zuo, Y.; Li, J.; Lv, H.; Ma, L.; Ren, B.; Zhao, L.; Li, W.; et al. Ellagic acid ameliorates oxidative stress and insulin resistance in high glucose-treated HepG2 cells via miR-223/keap1-Nrf2 pathway. Biomed. Pharmacother. 2019, 110, 85–94. [Google Scholar] [CrossRef]
- Hyun-Ae, S.; In-Kyu, L. The Role of Nrf2: Adipocyte Differentiation, Obesity, and Insulin Resistance. Oxidative Med. Cell. Longev. 2013, 2013, 184598. [Google Scholar]
- Jiménez-Osorio, A.S.; González-Reyes, S.; Pedraza-Chaverri, J. Natural Nrf2 activators in diabetes. Clin. Chim. Acta 2015, 448, 182–192. [Google Scholar] [CrossRef]
- Li, Y.; Guo, Y.; Feng, Z.; Bergan, R.; Li, B.; Qin, Y.; Zhao, L.; Zhang, Z.; Shi, M. Involvement of the PI3K/Akt/Nrf2 Signaling Pathway in Resveratrol-Mediated Reversal of Drug Resistance in HL-60/ADR Cells. Nutr. Cancer 2019, 71, 1007–1018. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Li, W.; Li, Y.; Wang, Q.; Gao, L.; Zhao, J. Dietary Lycium barbarum polysaccharide induces Nrf2/ARE pathway and ameliorates insulin resistance induced by high-fat via activation of PI3K/AKT signaling. Oxid. Med. Cell. Longev. 2014, 2014, 145641. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.N.; Lim, J.H.; Kim, M.Y.; Ban, T.H.; Choi, B.S. Resveratrol, an Nrf2 activator, ameliorates aging-related progressive renal injury. Aging 2018, 10, 83–99. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tan, Y.; Miao, L.; Xiao, J.; Cheang, W.S. 3,3′,4,5′-Tetramethoxy-trans-stilbene Improves Insulin Resistance by Activating the IRS/PI3K/Akt Pathway and Inhibiting Oxidative Stress. Curr. Issues Mol. Biol. 2022, 44, 2175-2185. https://doi.org/10.3390/cimb44050147
Tan Y, Miao L, Xiao J, Cheang WS. 3,3′,4,5′-Tetramethoxy-trans-stilbene Improves Insulin Resistance by Activating the IRS/PI3K/Akt Pathway and Inhibiting Oxidative Stress. Current Issues in Molecular Biology. 2022; 44(5):2175-2185. https://doi.org/10.3390/cimb44050147
Chicago/Turabian StyleTan, Yi, Lingchao Miao, Jianbo Xiao, and Wai San Cheang. 2022. "3,3′,4,5′-Tetramethoxy-trans-stilbene Improves Insulin Resistance by Activating the IRS/PI3K/Akt Pathway and Inhibiting Oxidative Stress" Current Issues in Molecular Biology 44, no. 5: 2175-2185. https://doi.org/10.3390/cimb44050147
APA StyleTan, Y., Miao, L., Xiao, J., & Cheang, W. S. (2022). 3,3′,4,5′-Tetramethoxy-trans-stilbene Improves Insulin Resistance by Activating the IRS/PI3K/Akt Pathway and Inhibiting Oxidative Stress. Current Issues in Molecular Biology, 44(5), 2175-2185. https://doi.org/10.3390/cimb44050147







