Tremella fuciformis Crude Polysaccharides Attenuates Steatosis and Suppresses Inflammation in Diet-Induced NAFLD Mice
Abstract
1. Introduction
2. Materials and Methods
2.1. TFCP Preparation and Characterization
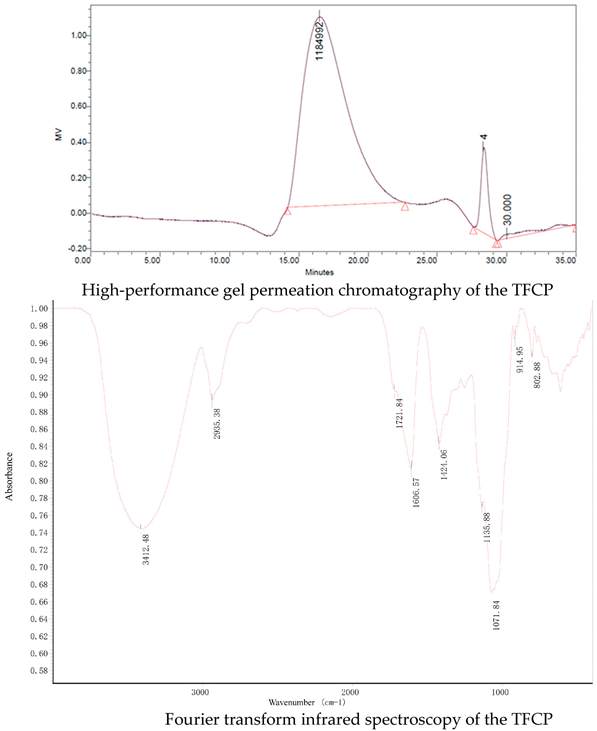
2.2. Animal Experiments
2.3. Collection of Liver Tissue and Their Histopathological Analysis
2.4. Serum and Blood Measurements
2.5. Real-Time Quantitative Polymerase Chain Reaction
2.6. Statistical Analysis
3. Results
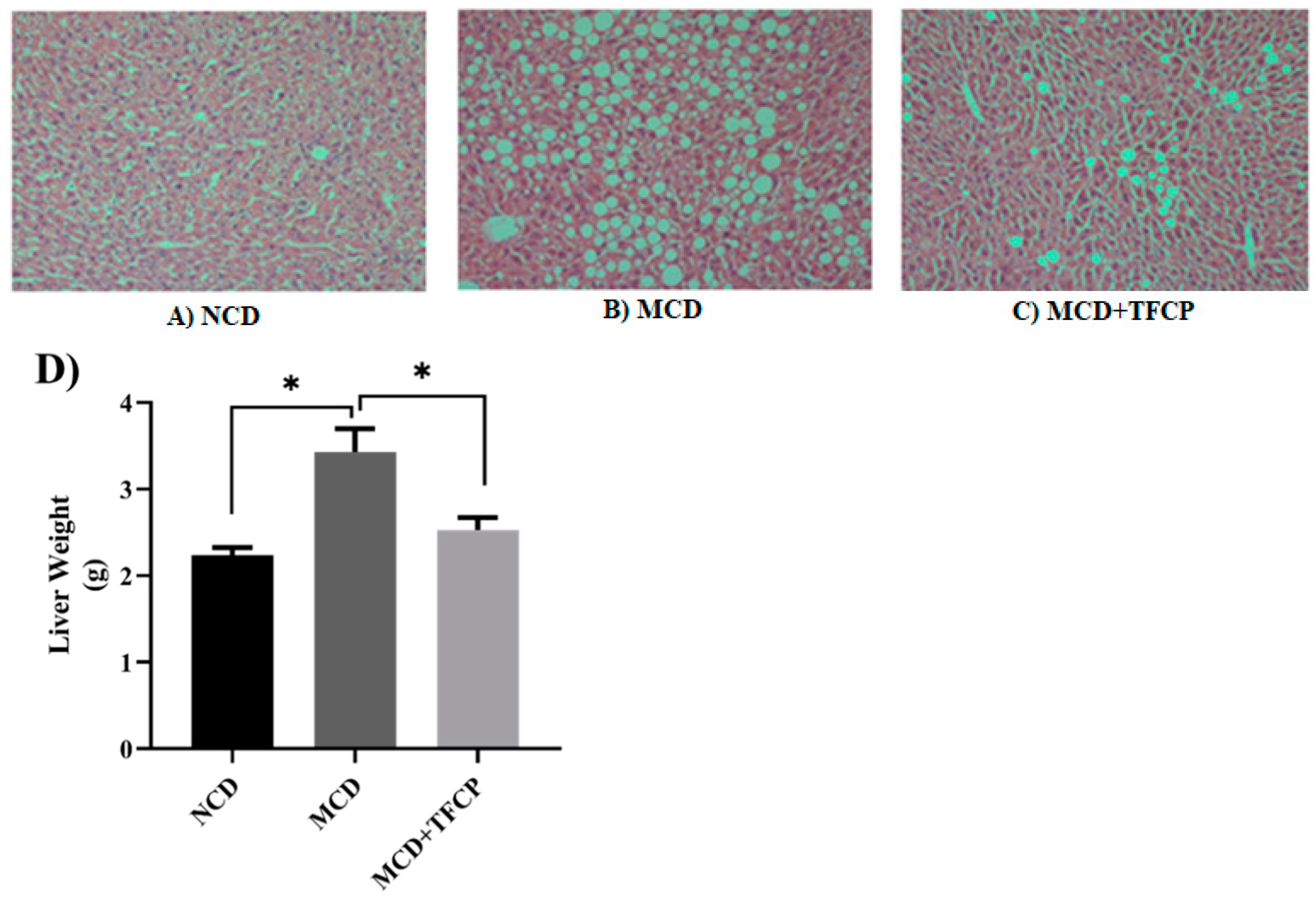
3.1. Inhibition of Hepatic Steatosis and Reduction in Liver Weight through TFCP Administration
3.2. Effects of TFCP on Triglyceride (TG), Total Cholesterol (TC), and High-Density Lipoprotein Cholesterol (HDL-C) Levels in the Serum
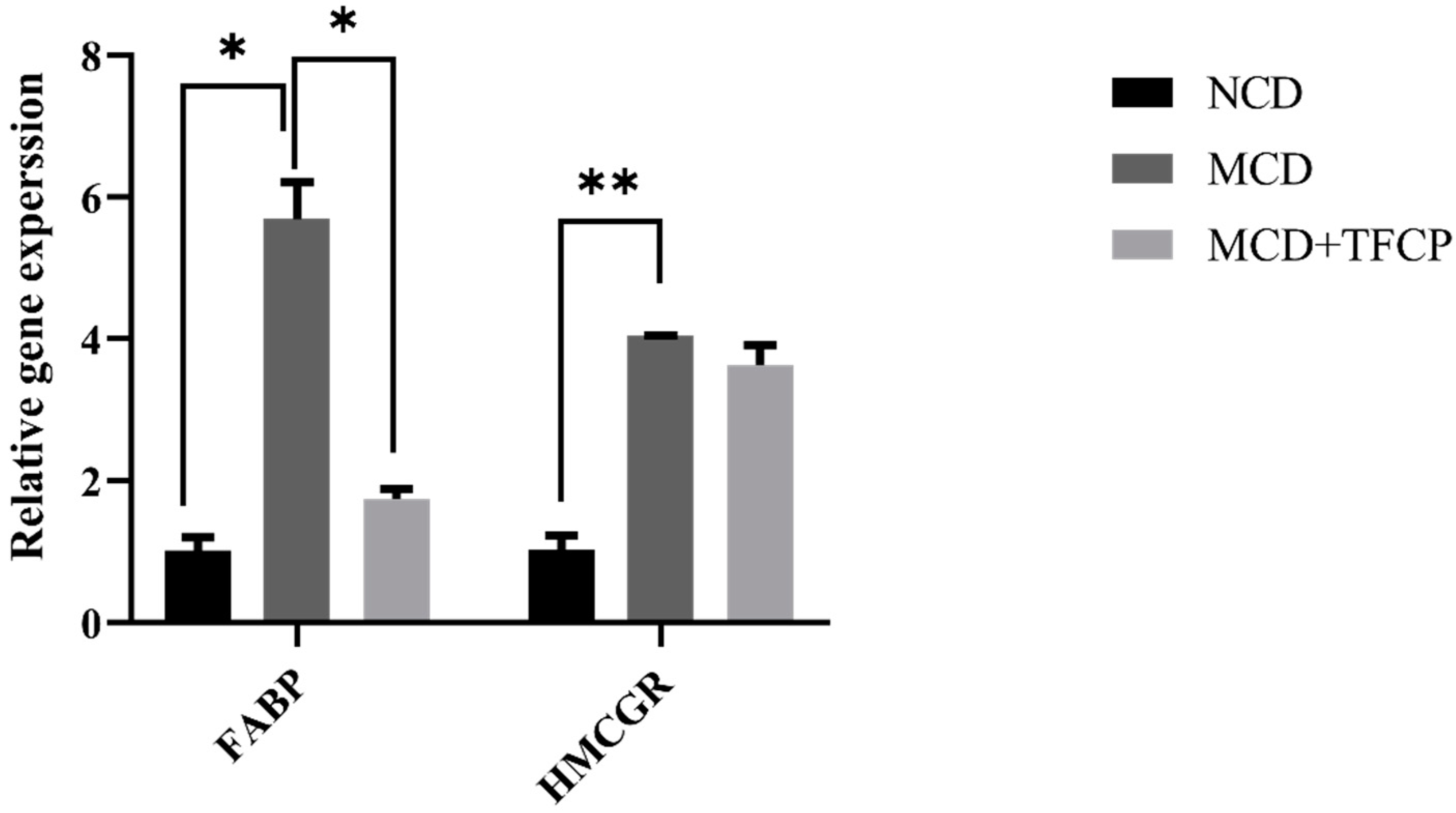
3.3. TFCP Administration Reduced the Expression of the Genes Involved in Cholesterol Metabolism and Transport
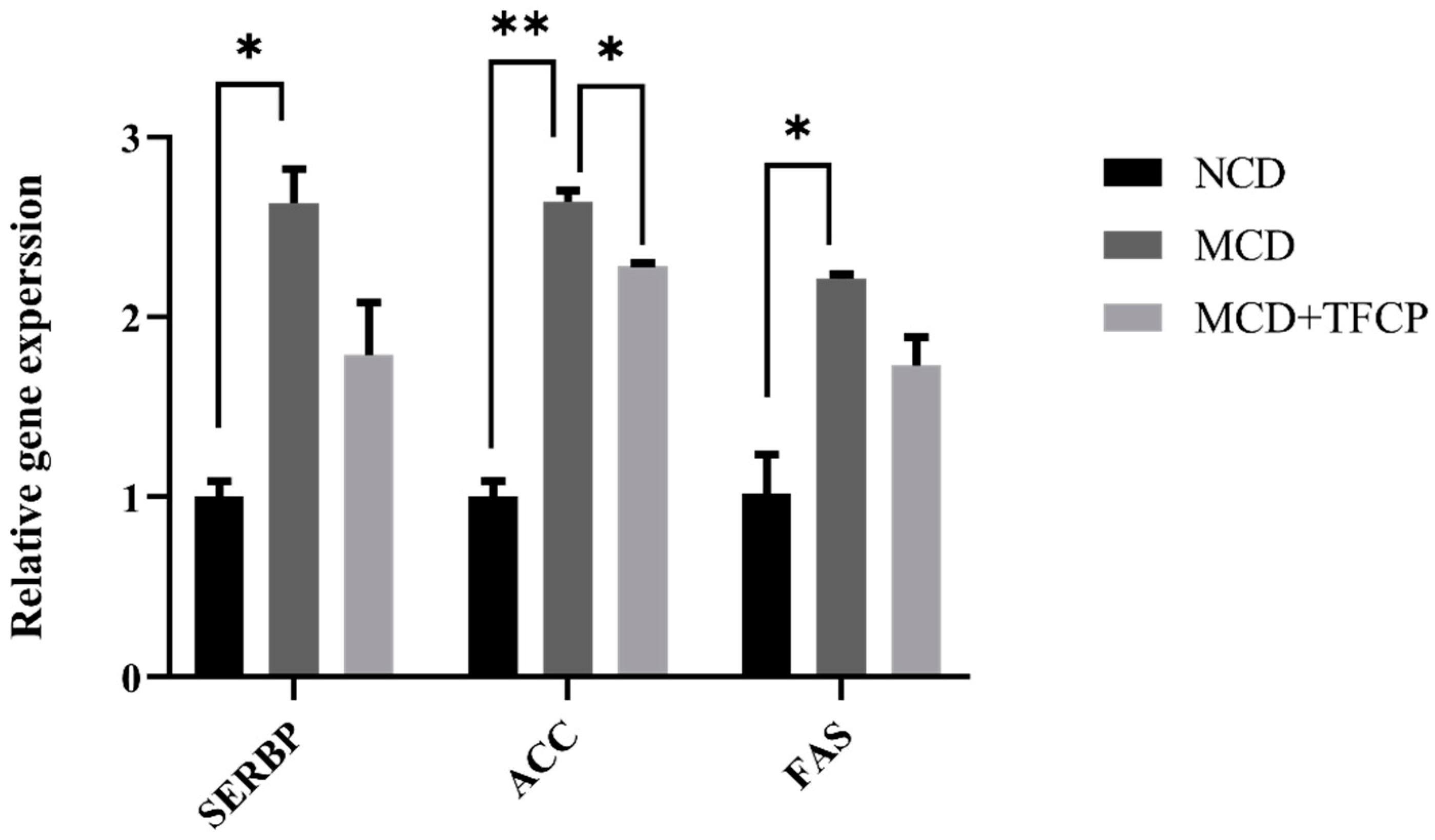
3.4. TFCP Administration Reduces the Expression of Lipid-Metabolism-Associated Genes
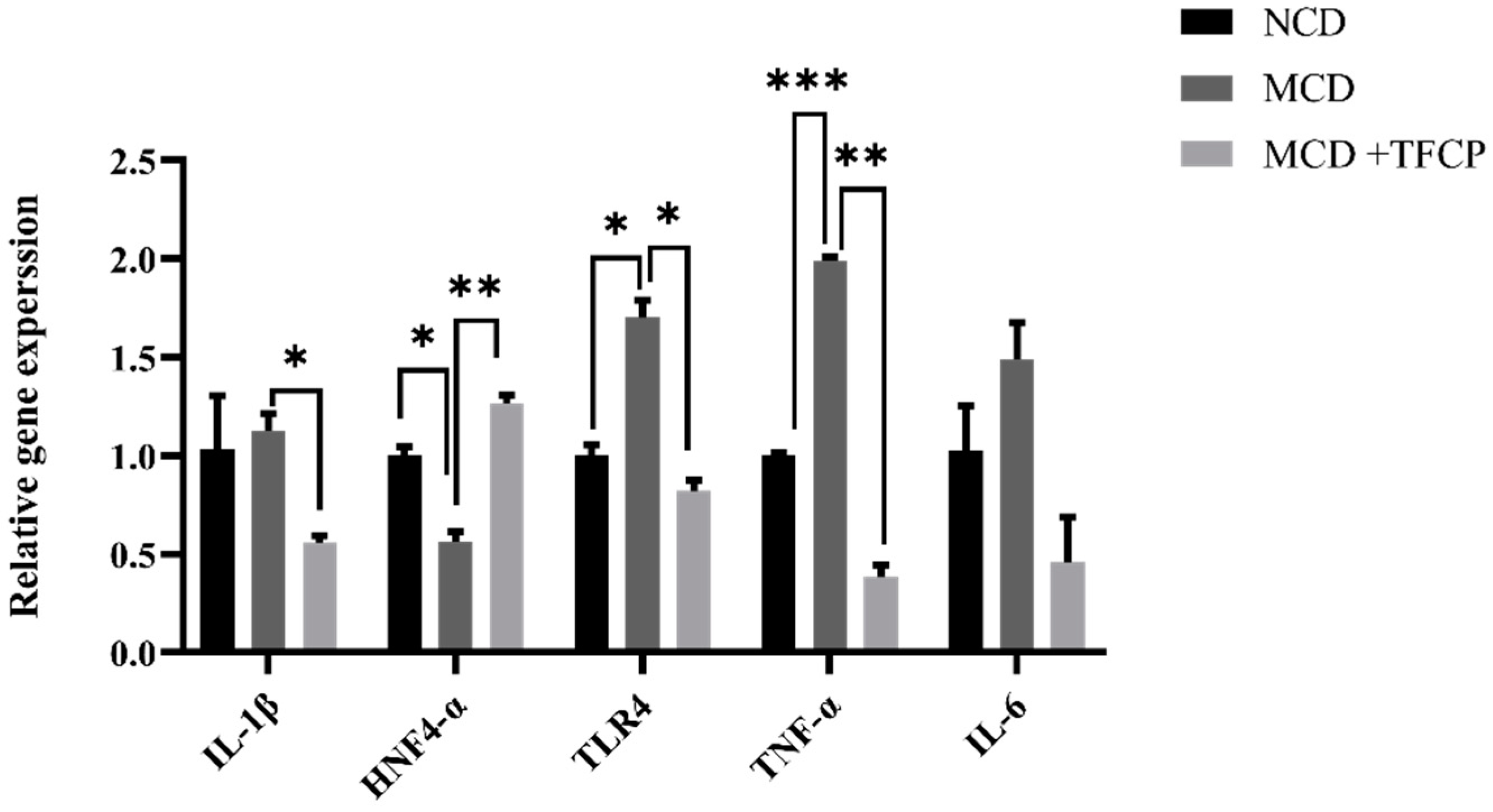
3.5. TFCP Downregulates the Expression of Inflammation-Associated Genes in the Liver
3.6. Effect of TFCP on the ALT, AST, MDA, and SOD Levels in the Mice Serum
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sanyal, A.J.; Brunt, E.M.; Kleiner, D.E.; Kowdley, K.V.; Chalasani, N.; Lavine, J.E.; Ratziu, V.; McCullough, A. Endpoints and clinical trial design for nonalcoholic steatohepatitis. Hepatol. (Baltim. Md.) 2011, 54, 344–353. [Google Scholar] [CrossRef]
- Rosmond, R.; Björntorp, P. The interactions between hypothalamic-pituitary-adrenal axis activity, testosterone, insulin-like growth factor I and abdominal obesity with metabolism and blood pressure in men. Int. J. Obes. Relat. Metab. Disord. J. Int. Assoc. Study Obes. 1998, 22, 1184–1196. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Browning, J.D.; Szczepaniak, L.S.; Dobbins, R.; Nuremberg, P.; Horton, J.D.; Cohen, J.C.; Grundy, S.M.; Hobbs, H.H. Prevalence of hepatic steatosis in an urban population in the United States: Impact of ethnicity. Hepatol. (Baltim. Md.) 2004, 40, 1387–1395. [Google Scholar] [CrossRef]
- Milosevic, I.; Vujovic, A.; Barac, A.; Djelic, M.; Korac, M.; Radovanovic Spurnic, A.; Gmizic, I.; Stevanovic, O.; Djordjevic, V.; Lekic, N.; et al. Gut-Liver Axis, Gut Microbiota, and Its Modulation in the Management of Liver Diseases: A Review of the Literature. Int. J. Mol. Sci. 2019, 20, 395. [Google Scholar] [CrossRef] [PubMed]
- Ceccarelli, S.; Panera, N.; Mina, M.; Gnani, D.; De Stefanis, C.; Crudele, A.; Rychlicki, C.; Petrini, S.; Bruscalupi, G.; Agostinelli, L.; et al. LPS-induced TNF-α factor mediates pro-inflammatory and pro-fibrogenic pattern in non-alcoholic fatty liver disease. Oncotarget 2015, 6, 41434–41452. [Google Scholar] [CrossRef] [PubMed]
- Compare, D.; Coccoli, P.; Rocco, A.; Nardone, O.M.; De Maria, S.; Cartenì, M.; Nardone, G. Gut--liver axis: The impact of gut microbiota on non alcoholic fatty liver disease. Nutr. Metab. Cardiovasc. Dis. NMCD 2012, 22, 471–476. [Google Scholar] [CrossRef] [PubMed]
- Ma, T.Y.; Iwamoto, G.K.; Hoa, N.T.; Akotia, V.; Pedram, A.; Boivin, M.A.; Said, H.M. TNF-alpha-induced increase in intestinal epithelial tight junction permeability requires NF-kappa B activation. Am. J. Physiol. Gastrointest. Liver Physiol. 2004, 286, G367–G376. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Chen, X.; Yi, R.; Li, G.; Sun, P.; Qian, Y.; Zhao, X. Immunomodulatory Effect of Tremella Polysaccharides against Cyclophosphamide-Induced Immunosuppression in Mice. Molecules 2018, 23, 239. [Google Scholar] [CrossRef]
- Han, C.K.; Chiang, H.C.; Lin, C.Y.; Tang, C.H.; Lee, H.; Huang, D.D.; Zeng, Y.R.; Chuang, T.N.; Huang, Y.L. Comparison of Immunomodulatory and Anticancer Activities in Different Strains of Tremella fuciformis Berk. Am. J. Chin. Med. 2015, 43, 1637–1655. [Google Scholar] [CrossRef]
- Xu, Y.; Xie, L.; Zhang, Z.; Zhang, W.; Tang, J.; He, X.; Zhou, J.; Peng, W. Tremella fuciformis Polysaccharides Inhibited Colonic Inflammation in Dextran Sulfate Sodium-Treated Mice via Foxp3+ T Cells, Gut Microbiota, and Bacterial Metabolites. Front. Immunol. 2021, 12, 648162. [Google Scholar] [CrossRef]
- Akiyama, T.E.; Ward, J.M.; Gonzalez, F.J. Regulation of the liver fatty acid-binding protein gene by hepatocyte nuclear factor 1alpha (HNF1alpha). Alterations in fatty acid homeostasis in HNF1alpha-deficient mice. J. Biol. Chem. 2000, 275, 27117–27122. [Google Scholar] [CrossRef]
- Harnish, D.C.; Malik, S.; Kilbourne, E.; Costa, R.; Karathanasis, S.K. Control of apolipoprotein AI gene expression through synergistic interactions between hepatocyte nuclear factors 3 and 4. J. Biol. Chem. 1996, 271, 13621–13628. [Google Scholar] [CrossRef] [PubMed]
- Ye, Z.; Lu, W.; Liang, L.; Tang, M.; Wang, Y.; Li, Z.; Zeng, H.; Wang, A.; Lin, M.; Huang, L.; et al. Mesenchymal stem cells overexpressing hepatocyte nuclear factor-4 alpha alleviate liver injury by modulating anti-inflammatory functions in mice. Stem Cell Res. Ther. 2019, 10, 149. [Google Scholar] [CrossRef] [PubMed]
- Muriel, P.; Ramos-Tovar, E.; Montes-Páez, G.; Buendía-Montaño, L.D. Chapter 40—Experimental Models of Liver Damage Mediated by Oxidative Stress. In Liver Pathophysiology; Muriel, P., Ed.; Academic Press: Boston, UK, 2017; pp. 529–546. [Google Scholar]
- Li, R.; Li, J.; Huang, Y.; Li, H.; Yan, S.; Lin, J.; Chen, Y.; Wu, L.; Liu, B.; Wang, G.; et al. Polydatin attenuates diet-induced nonalcoholic steatohepatitis and fibrosis in mice. Int. J. Biol. Sci. 2018, 14, 1411–1425. [Google Scholar] [CrossRef]
- Liu, M.X.; Gao, M.; Li, C.Z.; Yu, C.Z.; Yan, H.; Peng, C.; Li, Y.; Li, C.G.; Ma, Z.L.; Zhao, Y.; et al. Dicer1/miR-29/HMGCR axis contributes to hepatic free cholesterol accumulation in mouse non-alcoholic steatohepatitis. Acta Pharmacol. Sin. 2017, 38, 660–671. [Google Scholar] [CrossRef]
- Gyamfi, M.A.; Damjanov, I.; French, S.; Wan, Y.J. The pathogenesis of ethanol versus methionine and choline deficient diet-induced liver injury. Biochem. Pharmacol. 2008, 75, 981–995. [Google Scholar] [CrossRef]
- Chyau, C.-C.; Wang, H.-F.; Zhang, W.-J.; Chen, C.-C.; Huang, S.-H.; Chang, C.-C.; Peng, R.Y. Antrodan Alleviates High-Fat and High-Fructose Diet-Induced Fatty Liver Disease in C57BL/6 Mice Model via AMPK/Sirt1/SREBP-1c/PPARγ Pathway. Int. J. Mol. Sci. 2020, 21, 360. [Google Scholar] [CrossRef]
- Eberlé, D.; Hegarty, B.; Bossard, P.; Ferré, P.; Foufelle, F. SREBP transcription factors: Master regulators of lipid homeostasis. Biochimie 2004, 86, 839–848. [Google Scholar] [CrossRef]
- Guo, Y.; Yu, J.; Wang, C.; Li, K.; Liu, B.; Du, Y.; Xiao, F.; Chen, S.; Guo, F. miR-212-5p suppresses lipid accumulation by targeting FAS and SCD1. J. Mol. Endocrinol. 2017, 59, 205–217. [Google Scholar] [CrossRef]
- Bates, J.; Vijayakumar, A.; Ghoshal, S.; Marchand, B.; Yi, S.; Kornyeyev, D.; Zagorska, A.; Hollenback, D.; Walker, K.; Liu, K.; et al. Acetyl-CoA carboxylase inhibition disrupts metabolic reprogramming during hepatic stellate cell activation. J. Hepatol. 2020, 73, 896–905. [Google Scholar] [CrossRef]
- Gaballah, H.H.; El-Horany, H.E.; Helal, D.S. Mitigative effects of the bioactive flavonol fisetin on high-fat/high-sucrose induced nonalcoholic fatty liver disease in rats. J. Cell. Biochem. 2019, 120, 12762–12774. [Google Scholar] [CrossRef] [PubMed]
- Ohashi, T.; Nakade, Y.; Ibusuki, M.; Kitano, R.; Yamauchi, T.; Kimoto, S.; Inoue, T.; Kobayashi, Y.; Sumida, Y.; Ito, K.; et al. Conophylline inhibits high fat diet-induced non-alcoholic fatty liver disease in mice. PLoS ONE 2019, 14, e0210068. [Google Scholar]
- Barbier-Torres, L.; Fortner, K.A.; Iruzubieta, P.; Delgado, T.C.; Giddings, E.; Chen, Y.; Champagne, D.; Fernández-Ramos, D.; Mestre, D.; Gomez-Santos, B.; et al. Silencing hepatic MCJ attenuates non-alcoholic fatty liver disease (NAFLD) by increasing mitochondrial fatty acid oxidation. Nat. Commun. 2020, 11, 3360. [Google Scholar] [CrossRef]
- Zhang, X.; Han, J.; Man, K.; Li, X.; Du, J.; Chu, E.S.; Go, M.Y.; Sung, J.J.; Yu, J. CXC chemokine receptor 3 promotes steatohepatitis in mice through mediating inflammatory cytokines, macrophages and autophagy. J. Hepatol. 2016, 64, 160–170. [Google Scholar] [CrossRef]
- Assmann, N.; O’Brien, K.L.; Donnelly, R.P.; Dyck, L.; Zaiatz-Bittencourt, V.; Loftus, R.M.; Heinrich, P.; Oefner, P.J.; Lynch, L.; Gardiner, C.M.; et al. Srebp-controlled glucose metabolism is essential for NK cell functional responses. Nat. Immunol. 2017, 18, 1197–1206. [Google Scholar] [CrossRef] [PubMed]
- Park, S.Y.; Jeon, E.; Lee, S.; Lee, J.; Lee, T.; Won, J.I. An analysis of the concentration change of intermediate metabolites by gene manipulation in fatty acid biosynthesis. Enzym. Microb. Technol. 2012, 51, 95–99. [Google Scholar] [CrossRef] [PubMed]
- Alkhouri, N.; Lawitz, E.; Noureddin, M.; DeFronzo, R.; Shulman, G.I. GS-0976 (Firsocostat): An investigational liver-directed acetyl-CoA carboxylase (ACC) inhibitor for the treatment of non-alcoholic steatohepatitis (NASH). Expert Opin. Investig. Drugs 2020, 29, 135–141. [Google Scholar] [CrossRef] [PubMed]
- Pacifico, L.; Bonci, E.; Andreoli, G.; Romaggioli, S.; Di Miscio, R.; Lombardo, C.V.; Chiesa, C. Association of serum triglyceride-to-HDL cholesterol ratio with carotid artery intima-media thickness, insulin resistance and nonalcoholic fatty liver disease in children and adolescents. Nutr. Metab. Cardiovasc. Dis. NMCD 2014, 24, 737–743. [Google Scholar] [CrossRef]
- Kannel, W.B.; Vasan, R.S.; Keyes, M.J.; Sullivan, L.M.; Robins, S.J. Usefulness of the triglyceride-high-density lipoprotein versus the cholesterol-high-density lipoprotein ratio for predicting insulin resistance and cardiometabolic risk (from the Framingham Offspring Cohort). Am. J. Cardiol. 2008, 101, 497–501. [Google Scholar] [CrossRef]
- Lin, L.; Hao, Z.; Zhang, S.; Shi, L.; Lu, B.; Xu, H.; Ji, L. Study on the protection of water extracts of Polygoni Multiflori Radix and Polygoni Multiflori Radix Praeparata against NAFLD and its mechanism. J. Ethnopharmacol. 2020, 252, 112577. [Google Scholar] [CrossRef]
- Petrasek, J.; Csak, T.; Szabo, G. Toll-like receptors in liver disease. Adv. Clin. Chem. 2013, 59, 155–201. [Google Scholar] [PubMed]
- Luo, M.-C.; Zhou, S.-Y.; Feng, D.-Y.; Xiao, J.; Li, W.-Y.; Xu, C.-D.; Wang, H.-Y.; Zhou, T. Runt-related Transcription Factor 1 (RUNX1) Binds to p50 in Macrophages and Enhances TLR4-triggered Inflammation and Septic Shock. J. Biol. Chem. 2016, 291, 22011–22020. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Miura, K.; Zhang, B.; Matsushita, H.; Yang, Y.M.; Liang, S.; Song, J.; Roh, Y.S.; Seki, E. TRIF Differentially Regulates Hepatic Steatosis and Inflammation/Fibrosis in Mice. Cell. Mol. Gastroenterol. Hepatol. 2017, 3, 469–483. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Baker, R.D.; Baker, S.S. Gut microbiome and nonalcoholic fatty liver diseases. Pediatric Res. 2015, 77, 245–251. [Google Scholar] [CrossRef]
- Lu, Q.; Jiang, M.H.; Jiang, J.G.; Zhang, R.F.; Zhang, M.W. Isolation and identification of compounds from Penthorum chinense Pursh with antioxidant and antihepatocarcinoma properties. J. Agric. Chem. 2012, 60, 11097–11103. [Google Scholar] [CrossRef]
- Guo, C.J.; Xiao, X.; Sheng, L.; Chen, L.; Zhong, W.; Li, H.; Hua, J.; Ma, X. RNA Sequencing and Bioinformatics Analysis Implicate the Regulatory Role of a Long Noncoding RNA-mRNA Network in Hepatic Stellate Cell Activation. Cell. Physiol. Biochem. Int. J. Exp. Cell. Physiol. Biochem. Pharmacol. 2017, 42, 2030–2042. [Google Scholar] [CrossRef]
- Thymiakou, E.; Othman, A.; Hornemann, T.; Kardassis, D. Defects in High Density Lipoprotein metabolism and hepatic steatosis in mice with liver-specific ablation of Hepatocyte Nuclear Factor 4A. Metab. Clin. Exp. 2020, 110, 154307. [Google Scholar] [CrossRef]
- Huang, K.-W.; Reebye, V.; Czysz, K.; Ciriello, S.; Dorman, S.; Reccia, I.; Lai, H.-S.; Peng, L.; Kostomitsopoulos, N.; Nicholls, J.; et al. Liver Activation of Hepatocellular Nuclear Factor-4α by Small Activating RNA Rescues Dyslipidemia and Improves Metabolic Profile. Mol. Ther. Nucleic Acids 2020, 19, 361–370. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khan, T.J.; Xu, X.; Xie, X.; Dai, X.; Sun, P.; Xie, Q.; Zhou, X. Tremella fuciformis Crude Polysaccharides Attenuates Steatosis and Suppresses Inflammation in Diet-Induced NAFLD Mice. Curr. Issues Mol. Biol. 2022, 44, 1224-1234. https://doi.org/10.3390/cimb44030081
Khan TJ, Xu X, Xie X, Dai X, Sun P, Xie Q, Zhou X. Tremella fuciformis Crude Polysaccharides Attenuates Steatosis and Suppresses Inflammation in Diet-Induced NAFLD Mice. Current Issues in Molecular Biology. 2022; 44(3):1224-1234. https://doi.org/10.3390/cimb44030081
Chicago/Turabian StyleKhan, Tariq Jamal, Xiaofei Xu, Xiaoling Xie, Ximing Dai, Pingnan Sun, Qingdong Xie, and Xiaoling Zhou. 2022. "Tremella fuciformis Crude Polysaccharides Attenuates Steatosis and Suppresses Inflammation in Diet-Induced NAFLD Mice" Current Issues in Molecular Biology 44, no. 3: 1224-1234. https://doi.org/10.3390/cimb44030081
APA StyleKhan, T. J., Xu, X., Xie, X., Dai, X., Sun, P., Xie, Q., & Zhou, X. (2022). Tremella fuciformis Crude Polysaccharides Attenuates Steatosis and Suppresses Inflammation in Diet-Induced NAFLD Mice. Current Issues in Molecular Biology, 44(3), 1224-1234. https://doi.org/10.3390/cimb44030081





