TRPA1 Polymorphisms Modify the Hypotensive Responses to Propofol with No Change in Nitrite or Nitrate Levels
Abstract
1. Introduction
2. Materials and Methods
2.1. Subjects and Study Design
2.2. Measurement of Plasma Nitrite and Plasma Nitrate Concentrations
2.3. Genotyping
2.4. Haplotype Inference
2.5. Statistical Analysis
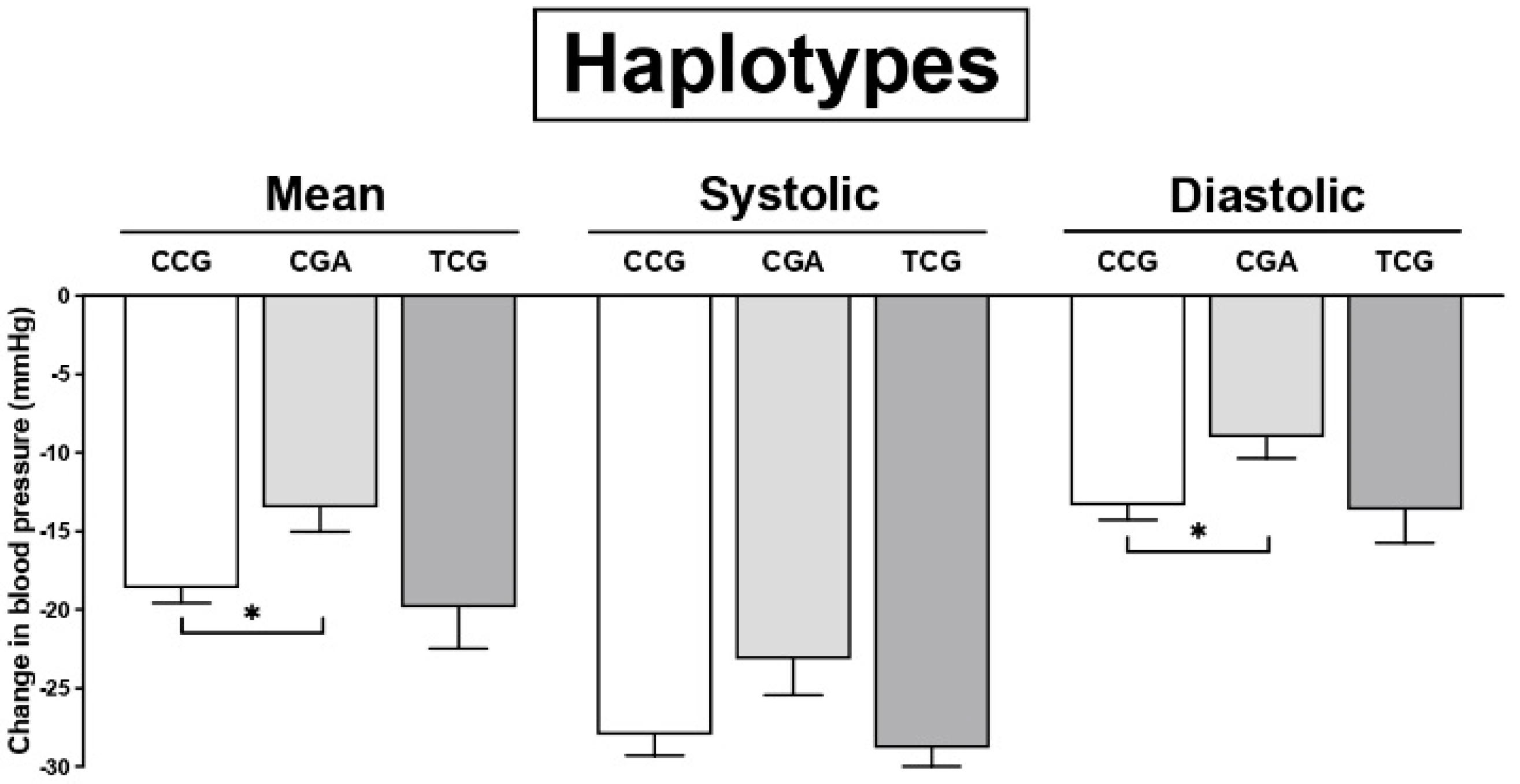
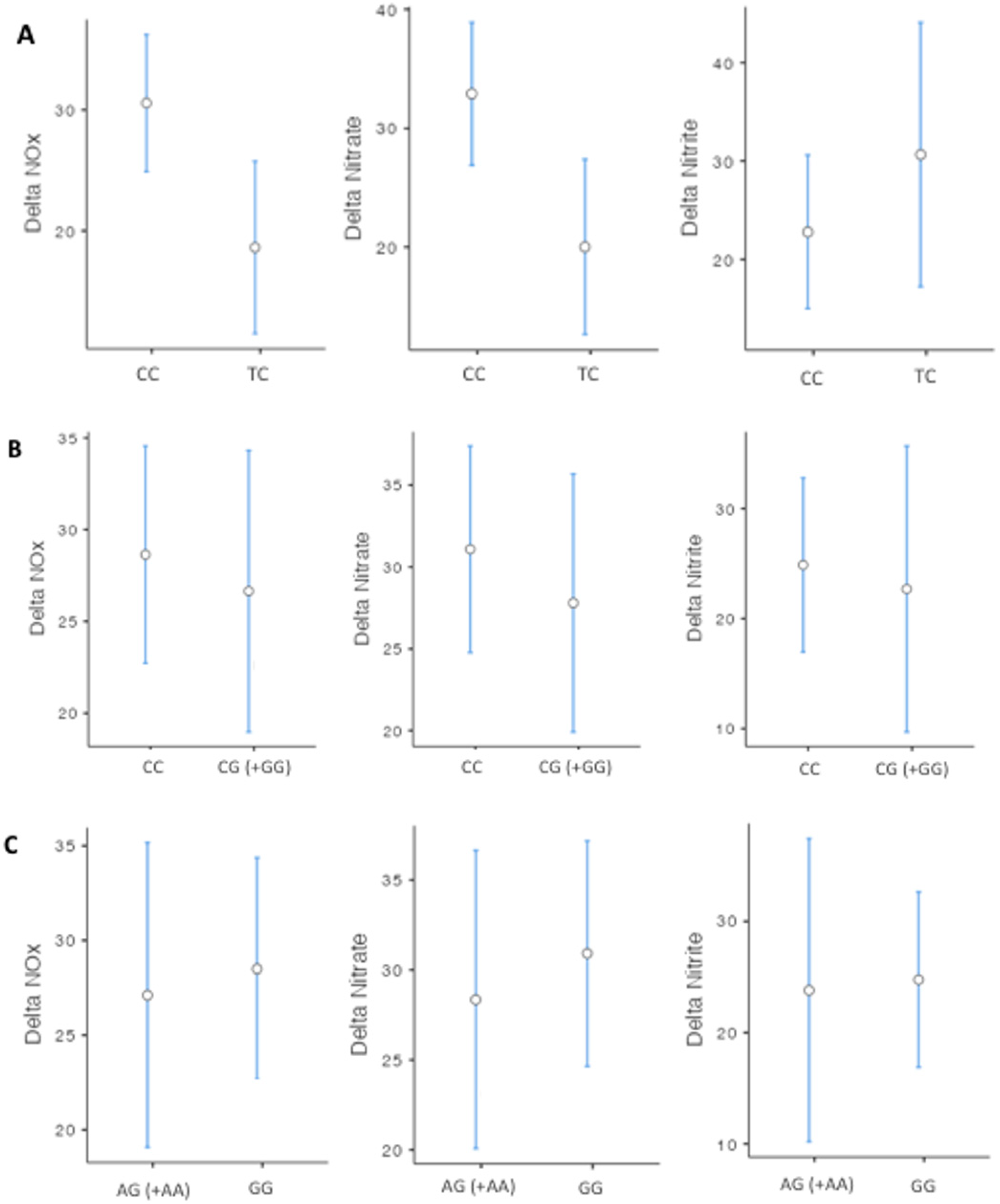
3. Results
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Marik, P. Propofol: Therapeutic Indications and Side-Effects. Curr. Pharm. Des. 2004, 10, 3639–3649. [Google Scholar] [CrossRef] [PubMed]
- Smith, I.; White, P.F.; Nathanson, M.; Gouldson, R. Propofol. An update on its clinical use. Anesthesiology 1994, 81, 1005–1043. [Google Scholar] [PubMed]
- Ferrier, D.C.; Kiely, J.; Luxton, R. Propofol detection for monitoring of intravenous anaesthesia: A review. Int. J. Clin. Monit. Comput. 2021, 36, 315–323. [Google Scholar] [CrossRef] [PubMed]
- Doursout, M.F.; Joseph, P.M.; Liang, Y.Y.; Hartley, C.J.; Chelly, J.E. Role of propofol and its solvent, intralipid, in nitric oxide-induced peripheral vasodilatation in dogs. Br. J. Anaesth. 2002, 89, 492–498. [Google Scholar] [CrossRef]
- Klockgether-Radke, A.P.; Schulze, H.; Neumann, P.; Hellige, G. Activation of the K+ channel BKCa is involved in the relaxing effect of propofol on coronary arteries. J. Anaesthesiol. 2004, 21, 226–230. [Google Scholar] [CrossRef]
- Park, K.W.; Dai, H.B.; Lowenstein, E.; Sellke, F.W. Propofol-associated dilation of rat distal coronary arteries is mediated by multiple substances, including endothelium-derived nitric oxide. Anesth. Analg. 1995, 81, 1191–1196. [Google Scholar]
- Hug, C.C., Jr.; McLeskey, C.H.; Nahrwold, M.L.; Roizen, M.F.; Stanley, T.H.; Thisted, R.A.; Walawander, C.A.; White, P.F.; Apfelbaum, J.L.; Grasela, T.H. Hemodynamic effects of propofol: Data from over 25,000 patients. Anesth. Analg. 1993, 77, S21–S29. [Google Scholar]
- Monk, T.G.; Bronsert, M.R.; Henderson, W.G.; Mangione, M.P.; Sum-Ping, S.J.; Bentt, D.R.; Nguyen, J.D.; Richman, J.S.; Meguid, R.A.; Hammermeister, K.E. Association between Intraoperative Hypotension and Hypertension and 30-day Postoperative Mortality in Noncardiac Surgery. Anesthesiology 2015, 123, 307–319. [Google Scholar] [CrossRef]
- Wesselink, E.; Kappen, T.; Torn, H.; Slooter, A.; van Klei, W. Intraoperative hypotension and the risk of postoperative adverse outcomes: A systematic review. Br. J. Anaesth. 2018, 121, 706–721. [Google Scholar] [CrossRef]
- Story, G.M. The Emerging Role of TRP Channels in Mechanisms of Temperature and Pain Sensation. Curr. Neuropharmacol. 2006, 4, 183–196. [Google Scholar] [CrossRef]
- Ton, H.T.; Phan, T.X.; Abramyan, A.M.; Shi, L.; Ahern, G.P. Identification of a putative binding site critical for general anesthetic activation of TRPA1. Proc. Natl. Acad. Sci. USA 2017, 114, 3762–3767. [Google Scholar] [CrossRef] [PubMed]
- Woll, K.A.; Skinner, K.; Gianti, E.; Bhanu, N.V.; Garcia, B.A.; Carnevale, V.; Eckenhoff, R.G.; Gaudet, R. Sites Contributing to TRPA1 Activation by the Anesthetic Propofol Identified by Photoaffinity Labeling. Biophys. J. 2017, 113, 2168–2172. [Google Scholar] [CrossRef]
- Fischer, M.J.; Leffler, A.; Niedermirtl, F.; Kistner, K.; Eberhardt, M.; Reeh, P.W.; Nau, C. The general anesthetic propofol excites nociceptors by activating TRPV1 and TRPA1 rather than GABAA receptors. J. Biol. Chem. 2010, 285, 34781–34792. [Google Scholar] [CrossRef] [PubMed]
- Matta, J.A.; Cornett, P.M.; Miyares, R.L.; Abe, K.; Sahibzada, N.; Ahern, G.P. General anesthetics activate a nociceptive ion channel to enhance pain and inflammation. Proc. Natl. Acad. Sci. USA 2008, 105, 8784–8789. [Google Scholar] [CrossRef] [PubMed]
- Ton, H.T.; Phan, T.X.; Ahern, G.P. Inhibition of Ligand-Gated TRPA1 by General Anesthetics. Mol. Pharmacol. 2020, 98, 185–191. [Google Scholar] [CrossRef]
- Tsutsumi, S.; Tomioka, A.; Sudo, M.; Nakamura, A.; Shirakura, K.; Takagishi, K.; Kohama, K. Propofol activates vanilloid receptor channels expressed in human embryonic kidney 293 cells. Neurosci. Lett. 2001, 312, 45–49. [Google Scholar] [CrossRef]
- Fischer, M.; Carli, G.; Raboisson, P.; Reeh, P. The interphase of the formalin test. Pain 2014, 155, 511–521. [Google Scholar] [CrossRef]
- Talavera, K.; Startek, J.B.; Alvarez-Collazo, J.; Boonen, B.; Alpizar, Y.A.; Sanchez, A.; Naert, R.; Nilius, B. Mammalian Transient Receptor Potential TRPA1 Channels: From Structure to Disease. Physiol. Rev. 2020, 100, 725–803. [Google Scholar] [CrossRef]
- Sinha, S.; Sinharoy, P.; Bratz, I.N.; Damron, D.S. Propofol Causes Vasodilation In Vivo via TRPA1 Ion Channels: Role of Nitric Oxide and BKCa Channels. PLoS ONE 2015, 10, e0122189. [Google Scholar] [CrossRef]
- Earley, S.; Gonzales, A.L.; Crnich, R. Endothelium-dependent cerebral artery dilation mediated by TRPA1 and Ca2+-Activated K+ channels. Circ. Res. 2009, 104, 987–994. [Google Scholar] [CrossRef]
- Earley, S.; Brayden, J.E. Transient receptor potential channels and vascular function. Clin. Sci. 2010, 119, 19–36. [Google Scholar] [CrossRef] [PubMed]
- Pozsgai, G.; Bodkin, J.V.; Graepel, R.; Bevan, S.; Andersson, D.A.; Brain, S.D. Evidence for the pathophysiological relevance of TRPA1 receptors in the cardiovascular system in vivo. Cardiovasc. Res. 2010, 87, 760–768. [Google Scholar] [CrossRef] [PubMed]
- Jhun, E.H.; Hu, X.; Sadhu, N.; Yao, Y.; He, Y.; Wilkie, D.J.; Molokie, R.E.; Wang, Z.J. Transient receptor potential polymorphism and haplotype associate with crisis pain in sickle cell disease. Pharmacogenomics 2018, 19, 401–411. [Google Scholar] [CrossRef] [PubMed]
- Deering-Rice, C.E.; Shapiro, D.; Romero, E.G.; Stockmann, C.; Bevans, T.S.; Phan, Q.M.; Stone, B.L.; Fassl, B.; Nkoy, F.; Uchida, D.A.; et al. Activation of Transient Receptor Potential Ankyrin-1 by Insoluble Particulate Material and Association with Asthma. Am. J. Respir. Cell Mol. Biol. 2015, 53, 893–901. [Google Scholar] [CrossRef] [PubMed]
- May, D.; Baastrup, J.; Nientit, M.R.; Binder, A.; Schünke, M.; Baron, R.; Cascorbi, I. Differential Expression and Functionality of TRPA1 Protein Genetic Variants in Conditions of Thermal Stimulation. J. Biol. Chem. 2012, 287, 27087–27094. [Google Scholar] [CrossRef] [PubMed]
- Metzger, I.F.; Sertorio, J.T.C.; Tanus-Santos, J.E. Relationship between systemic nitric oxide metabolites and cyclic GMP in healthy male volunteers. Acta Physiol. 2006, 188, 123–127. [Google Scholar] [CrossRef]
- Silva, P.S.; Fontana, V.; Palei, A.C.T.; Sertório, J.T.C.; Biagi, C.; Tanus-Santos, J.E. Antihypertensive effects exerted by enalapril in mild to moderate hypertension are not associated with changes in the circulating levels of nitric oxide-related markers. Eur. J. Clin. Pharmacol. 2011, 67, 365–370. [Google Scholar] [CrossRef] [PubMed]
- Pereira, L.M.; Lima-Junior, R.C.; Bem, A.X.; Teixeira, C.G.; Grassi, L.S.; Medeiros, R.P.; Marques-Neto, R.D.; Callado, R.B.; Aragão, K.S.; Wong, D.V.T.; et al. Blockade of TRPA1 with HC-030031 at-tenuates visceral nociception by a mechanism independent of inflammatory resident cells, nitric oxide and the opioid system. Eur. J Pain 2013, 17, 223–233. [Google Scholar] [CrossRef]
- Kassam, S.I.; Lu, C.; Buckley, N.; Lee, R.M.K.W. The Mechanisms of Propofol-Induced Vascular Relaxation and Modulation by Perivascular Adipose Tissue and Endothelium. Anesth. Analg. 2011, 112, 1339–1345. [Google Scholar] [CrossRef]
- Klockgether-Radke, A.P.; Frerichs, A.; Kettler, D.; Hellige, G. Propofol and thiopental attenuate the contractile response to vasoconstrictors in human and porcine coronary artery segments. Eur. J. Anaesthesiol. 2000, 17, 485–490. [Google Scholar] [CrossRef]
- Zhang, M.H.; Wickley, P.J.; Sinha, M.S.; Bratz, I.N.; Damron, D.S. Propofol Restores Transient Receptor Potential Vanilloid Receptor Subtype-1 Sensitivity via Activation of Transient Receptor Potential Ankyrin Receptor Subtype-1 in Sensory Neurons. Anesthesiology 2011, 114, 1169–1179. [Google Scholar] [CrossRef] [PubMed]
- Park, W.K.; Lynch, C., 3rd; Johns, R.A. Effects of propofol and thiopental in isolated rat aorta and pulmonary artery. Anesthesiology 1992, 77, 956–963. [Google Scholar] [CrossRef] [PubMed]
- Earley, S. Endothelium-dependent Cerebral Artery Dilation Mediated by Transient Receptor Potential and Ca2+-activated K+ Channels. J. Cardiovasc. Pharmacol. 2011, 57, 148–153. [Google Scholar] [CrossRef] [PubMed]
- Oliveira-Paula, G.H.; Pinheiro, L.C.; Ferreira, G.C.; Garcia, W.N.; Lacchini, R.; Garcia, L.V.; Tanus-Santos, J.E. Angiotensin converting enzyme inhibitors enhance the hypotensive effects of propofol by increasing nitric oxide production. Free Radic. Biol. Med. 2018, 115, 10–17. [Google Scholar] [CrossRef]
- Xu, H.; Aibiki, M.; Yokono, S.; Ogli, K. Dose-dependent effects of propofol on renal sympathetic nerve activity, blood pressure and heart rate in urethane-anesthetized rabbits. Eur. J. Pharmacol. 2000, 387, 79–85. [Google Scholar] [CrossRef]
- Krassioukov, A.V.; Gelb, A.W.; Weaver, L.C. Action of propofol on central sympathetic mechanisms controlling blood pressure. Can. J. Anaesth. 1993, 40, 761–769. [Google Scholar] [CrossRef][Green Version]
- Wang, B.; Luo, T.; Chen, D.; Ansley, D.M. Propofol Reduces Apoptosis and Up-Regulates Endothelial Nitric Oxide Synthase Protein Expression in Hydrogen Peroxide-Stimulated Human Umbilical Vein Endothelial Cells. Anesth. Analg. 2007, 105, 1027–1033. [Google Scholar] [CrossRef]
- Wang, L.; Wu, B.; Sun, Y.; Xu, T.; Zhang, X.; Zhou, M.; Jiang, W. Translocation of protein kinase C isoforms is involved in propofol-induced endothelial nitric oxide synthase activation. Br. J. Anaesth. 2010, 104, 606–612. [Google Scholar] [CrossRef]
- Wang, Y.; Zhou, H.; Wu, B.; Zhou, Q.; Cui, D.; Wang, L. Protein Kinase C Isoforms Distinctly Regulate Propofol-induced Endothelium-dependent and Endothelium-independent Vasodilation. J. Cardiovasc. Pharmacol. 2015, 66, 276–284. [Google Scholar] [CrossRef]
- Thomas, D.D.; Liu, X.; Kantrow, S.P.; Lancaster, J.R., Jr. The biological lifetime of nitric oxide: Implications for the perivascular dynamics of NO and O2. Proc. Natl. Acad. Sci. USA 2001, 98, 355–360. [Google Scholar] [CrossRef]
- Ellis, G.; Adatia, I.; Yazdanpanah, M.; Makela, S.K. Nitrite and Nitrate Analyses: A Clinical Biochemistry Perspective. Clin. Biochem. 1998, 31, 195–220. [Google Scholar] [CrossRef]
- Kleinbongard, P.; Dejam, A.; Lauer, T.; Jax, T.; Kerber, S.; Gharini, P.; Balzer, J.; Zotz, R.B.; Scharf, R.E.; Willers, R.; et al. Plasma nitrite concentrations reflect the degree of endothelial dysfunction in humans. Free Radic. Biol. Med. 2006, 40, 295–302. [Google Scholar] [CrossRef] [PubMed]
- Kleinbongard, P.; Dejam, A.; Lauer, T.; Rassaf, T.; Schindler, A.; Picker, O.; Scheeren, T.; Gödecke, A.; Schrader, J.; Schulz, R.; et al. Plasma nitrite reflects constitutive nitric oxide synthase activity in mammals. Free Radic. Biol. Med. 2003, 35, 790–796. [Google Scholar] [CrossRef] [PubMed]
- Metzger, I.F.; Luizon, M.R.; Lacchini, R.; Ishizawa, M.H.; Tanus-Santos, J.E. Effects of endothelial nitric oxide synthase tagSNPs haplotypes on nitrite levels in black subjects. Nitric Oxide 2013, 28, 33–38. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.P.; Buber, M.T.; Yang, Q.; Cerne, R.; Cortés, R.Y.; Sprous, D.G.; Bryant, R.W. Thymol and related alkyl phenols activate the hTRPA1 channel. J. Cereb. Blood Flow Metab. 2008, 153, 1739–1749. [Google Scholar] [CrossRef] [PubMed]
- Sinharoy, P.; Bratz, I.N.; Sinha, S.; Showalter, L.E.; Andrei, S.R.; Damron, D.S. TRPA1 and TRPV1 contribute to propofol-mediated antagonism of U46619-induced constriction in murine coronary arteries. PLoS ONE 2017, 12, e0180106. [Google Scholar] [CrossRef]
- Sinharoy, P.; Zhang, H.; Sinha, S.; Prudner, B.C.; Bratz, I.N.; Damron, D.S. Propofol restores TRPV1 sensitivity via a TRPA1-, nitric oxide synthase-dependent activation of PKCepsilon. Pharmacol. Res. Perspect. 2015, 3, e00153. [Google Scholar] [CrossRef]
- Qian, X.; Francis, M.; Solodushko, V.; Earley, S.; Taylor, M.S. Recruitment of Dynamic Endothelial Ca2+ Signals by the TRPA1 Channel Activator AITC in Rat Cerebral Arteries. Microcirculation 2012, 20, 138–148. [Google Scholar] [CrossRef]
- Sullivan, M.N.; Gonzales, A.L.; Pires, P.W.; Bruhl, A.; Leo, M.D.; Li, W.; Oulidi, A.; Boop, F.A.; Feng, Y.; Jaggar, J.H.; et al. Localized TRPA1 channel Ca 2+ signals stimulated by reactive oxygen species promote cerebral artery dilation. Sci. Signal. 2015, 8, ra2. [Google Scholar] [CrossRef]
- Lam, C.-F.; Chang, P.-J.; Chen, Y.-A.; Yeh, C.-Y.; Tsai, Y.-C. Inhibition of ATP-sensitive potassium channels attenuates propofol-induced vasorelaxation. Crit. Care Resusc. 2010, 12, 186–190. [Google Scholar]
- Liu, Y.; Chang, H.; Niu, L.; Xue, W.; Zhang, X.; Liang, Y.; Zhang, M. Effects of propofol on responses of rat isolated renal arteriole to vasoactive agents. Vasc. Pharmacol. 2009, 51, 182–189. [Google Scholar] [CrossRef] [PubMed]
- Iwasaki, Y.; Tanabe, M.; Kobata, K.; Watanabe, T. TRPA1 agonists—Allyl isothiocyanate and cinnamaldehyde—Induce adrenaline secretion. Biosci. Biotechnol. Biochem. 2008, 72, 2608–2614. [Google Scholar] [CrossRef] [PubMed]
- Delgermurun, D.; Yamaguchi, S.; Ichii, O.; Kon, Y.; Ito, S.; Otsuguro, K.-I. Hydrogen sulfide activates TRPA1 and releases 5-HT from epithelioid cells of the chicken thoracic aorta. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2016, 187, 43–49. [Google Scholar] [CrossRef] [PubMed]
- Aubdool, A.A.; Kodji, X.; Abdul-Kader, N.; Heads, R.; Fernandes, E.S.; Bevan, S.; Brain, S.D. TRPA1 activation leads to neurogenic vasodilatation: Involvement of reactive oxygen nitrogen species in addition to CGRP and NO. Br. J. Pharmacol. 2016, 173, 2419–2433. [Google Scholar] [CrossRef]
- Bodkin, J.V.; Thakore, P.; Aubdool, A.A.; Liang, L.; Fernandes, E.S.; Nandi, M.; Spina, D.; Clark, J.E.; Aaronson, P.I.; Shattock, M.J.; et al. Investigating the potential role of TRPA1 in locomotion and cardiovascular control during hypertension. Pharmacol. Res. Perspect. 2014, 2, e00052. [Google Scholar] [CrossRef]
- Oliveira-Paula, G.H.; Luizon, M.R.; Lacchini, R.; Fontana, V.; Silva, P.S.; Biagi, C.; Tanus-Santos, J.E. Gene-Gene Interactions Among PRKCA, NOS3 and BDKRB2 Polymorphisms Affect the Antihypertensive Effects of Enalapril. Basic Clin. Pharmacol. Toxicol. 2017, 120, 284–291. [Google Scholar] [CrossRef]
- Ackland, G.L.; Harrington, J.; Downie, P.; Holding, J.W.; Singh-Ranger, D.; Griva, K.; Mythen, M.G.; Newman, S.P. Dehydration induced by bowel preparation in older adults does not result in cognitive dysfunction. Anesth. Analg. 2008, 106, 924–929. [Google Scholar] [CrossRef]
- Colson, P.; Saussine, M.; Séguin, J.R.; Cuchet, D.; Chaptal, P.A.; Roquefeuil, B. Hemodynamic effects of anesthesia in patients chronically treated with angiotensin-converting en-zyme inhibitors. Anesth. Analg. 1992, 74, 805–808. [Google Scholar] [CrossRef]
- Bertrand, M.; Godet, G.; Meersschaert, K. Should the angiotensin II antagonists be discontinued before surgery? Anesth. Analg. 2001, 92, 26–30. [Google Scholar] [CrossRef]
- Rau, R.-H.; Li, Y.-C.; Cheng, J.-K.; Chen, C.-C.; Ko, Y.-P.; Huang, C.-J. Predicting blood pressure change caused by rapid injection of propofol during anesthesia induction with a logistic regression model. Acta Anaesthesiol. Taiwanica 2004, 42, 81–86. [Google Scholar]
- Kawasaki, S.; Kiyohara, C.; Tokunaga, S.; Hoka, S. Prediction of hemodynamic fluctuations after induction of general anesthesia using propofol in non-cardiac surgery: A retrospective cohort study. BMC Anesthesiol. 2018, 18, 167. [Google Scholar] [CrossRef] [PubMed]
- Visigalli, R.; Barilli, A.; Parolari, A.; Sala, R.; Rotoli, B.M.; Bussolati, O.; Gazzola, G.C.; Dall’Asta, V. Regulation of arginine transport and metabolism by protein kinase Cα in endothelial cells: Stimulation of CAT2 transporters and arginase activity. J. Mol. Cell. Cardiol. 2010, 49, 260–270. [Google Scholar] [CrossRef] [PubMed]
- Oliveira-Paula, G.H.; Lacchini, R.; Fontana, V.; Silva, P.S.; Biagi, C.; Tanus-Santos, J.E. Polymorphisms in VEGFA gene affect the antihypertensive responses to enalapril. Eur. J. Clin. Pharmacol. 2015, 71, 949–957. [Google Scholar] [CrossRef] [PubMed]

| Characteristics | Total (n = 164) |
|---|---|
| Male/female | 70/94 |
| Age (years) (mean ± SD) | 55 ± 14 |
| Ethnicity (Caucasian Yes/No) | 134/30 |
| Body mass index (kg/m2) (mean ± SD) | 25.9 ± 4.7 |
| Total cholesterol (mg/dL (mean ± SD) | 192 ± 48 |
| Glucose (mg/dL) (mean ± SD) | 96 ± 19 |
| Urea (mg/dL) (mean ± SD) | 32 ± 12 |
| Creatinine (mg/dl) (mean ± SD) | 0.90 ± 0.3 |
| Potassium (mM) (mean ± SD) | 4.3 ± 0.5 |
| Hemoglobin (g/dL) (mean ± SD) | 12.7± 1.9 |
| Total propofol use (mg) (mean ± SD) | 192 ± 68 |
| Mean blood pressure (mmHg) (mean ± SD) | |
| Baseline | 93.7 ± 16.5 |
| After propofol | 75.6 ± 15.8 * |
| Systolic blood pressure (mmHg) (mean ± SD) | |
| Baseline | 133 ± 19.7 |
| After propofol | 105 ± 18.2 * |
| Diastolic blood pressure (mmHg) (mean ± SD) | |
| Baseline | 76.8 ± 15.6 |
| After propofol | 64.1 ± 15.6 * |
| Heart rate (beats/min) (mean ± SD) | |
| Baseline | 81.4 ± 13.8 |
| After propofol | 79.4 ± 14.1 |
| Change in SBP (mmHg) | Change in MBP (mmHg) | Change in DBP (mmHg) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Rs920829 | R2 = 0.40 RMSE = 15.00 | R2 = 0.28 RMSE = 12.20 | R2 = 0.23 RMSE = 11.90 | ||||||
| Source | ß | 95% CI | p | ß | 95% CI | p | ß | 95% CI | p |
| Use of ACEi | +1.64 | −6.65 to 9.94 | 0.695 | −2.18 | −8.84 to 4.47 | 0.518 | −1.36 | −7.87 to 5.15 | 0.680 |
| Age (years) | −0.02 | −0.24 to 0.19 | 0.804 | −0.04 | −0.21 to 0.13 | 0.636 | +0.00 | −0.15 to 0.17 | 0.943 |
| BMI (kg/m2) | +0.58 | −0.05 to 1.21 | 0.071 | +0.72 | 0.21 to 1.24 | <0.006 * | +0.67 | 0.17 to 1.18 | 0.009 * |
| BBP | −0.61 | −0.76 to −0.47 | <0.001 * | −0.43 | −0.56 to −0.31 | <0.001 * | −0.40 | −0.53 to −0.27 | <0.001 * |
| TC | −3.61 | −10.09 to 2.65 | 0.251 | −2.65 | −7.83 to 2.51 | 0.311 | −2.77 | −7.83 to 2.29 | 0.281 |
| Change in SBP (mmHg) | Change in MBP (mmHg) | Change in DBP (mmHg) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Rs16937976 | R2 = 0.42 RMSE = 15.40 | R2 = 0.32 RMSE = 11.80 | R2 = 0.26 RMSE = 11.70 | ||||||
| Source | ß | 95% CI | p | ß | 95% CI | p | ß | 95% CI | p |
| Use of ACEi | +0.97 | −7.49 to 9.44 | 0.820 | −1.98 | −8.43 to 4.45 | 0.543 | −1.20 | −7.60 to 5.18 | 0.709 |
| Age (years) | −0.11 | −0.33 to 0.11 | 0.318 | −0.04 | −0.20 to 0.12 | 0.612 | +0.00 | −0.15 to 0.16 | 0.954 |
| BMI (kg/m2) | +1.20 | 0.55 to 1.85 | <0.001 * | +0.82 | 0.32 to 1.13 | 0.001 * | +0.75 | 0.25 to 1.25 | 0.003 * |
| BBP | −0.62 | −0.77 to −0.47 | <0.001 * | −0.44 | −0.56 to −0.32 | <0.001 * | −0.40 | −0.53 to −0.28 | <0.001 * |
| CG (+GG) | +7.53 | 1.62 to 13.43 | 0.013 * | +7.15 | 2.61 to 11.70 | 0.002 * | +5.18 | 0.67 to 9.70 | 0.025 * |
| Change in SBP (mmHg) | Change in MBP (mmHg) | Change in DBP (mmHg) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Rs13218757 | R2 = 0.42 RMSE = 15.30 | R2 = 0.33 RMSE = 11.70 | R2 = 0.26 RMSE = 11.70 | ||||||
| Source | ß | 95% CI | p | ß | 95% CI | p | ß | 95% CI | p |
| Use of ACEi | +0.82 | −7.62 to 9.26 | 0.848 | −2.13 | −8.55 to 4.27 | 0.511 | −1.31 | −7.70 to 5.07 | 0.685 |
| Age (years) | −0.10 | −0.32 to 0.11 | 0.339 | −0.03 | −0.20 to 0.12 | 0.637 | +0.00 | −0.15 to 0.16 | 0.932 |
| BMI (kg/m2) | +1.24 | 0.59 to 1.89 | <0.001 * | +0.86 | 0.36 to 1.36 | <0.001 * | +0.77 | 0.28 to 1.27 | 0.002 * |
| BBP | −0.62 | −0.77 to −0.47 | <0.001 * | −0.44 | −0.56 to −0.31 | <0.001 * | −0.40 | −0.53 to −0.27 | <0.001 * |
| AG (+AA) | +8.11 | 2.12 to 14.10 | 0.008 * | +7.66 | 3.06 to 12.27 | 0.001 * | +5.62 | 1.04 to 10.21 | 0.016 * |
| Change in SBP (mmHg) | Change in MBP (mmHg) | Change in DBP (mmHg) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| R2 = 0.39 RMSE = 15.40 | R2 = 0.28 RMSE = 11.70 | R2 = 0.24 RMSE = 11.70 | |||||||
| Source | ß | 95% CI | p | ß | 95% CI | p | ß | 95% CI | p |
| Use of ACEi | −0.74 | −4.78 to 6.27 | 0.790 | −1.46 | −5.64 to 2.72 | 0.493 | +0.10 | −4.06 to 4.26 | 0.962 |
| Age (years) | −0.09 | −0.23 to 0.05 | 0.207 | −0.07 | −0.17 to 0.03 | 0.199 | −0.00 | −0.11 to 0.09 | 0.877 |
| BMI (kg/m2) | +0.98 | +0.56 to 1.41 | <0.001 * | +0.72 | 0.39 to 1.04 | <0.001 * | +0.63 | 0.31 to 0.95 | <0.001 * |
| BBP | −0.60 | −0.70 to −0.50 | <0.001 * | −0.40 | −0.49 to −0.32 | <0.001 * | −0.38 | −0.47 to 0.30 | <0.001 * |
| Haplotypes | |||||||||
| CGA | +6.04 | 0.90 to 11.08 | 0.021 * | +5.74 | 1.81 to 9.67 | 0.004 * | +4.55 | 0.65 to 8.45 | 0.022 * |
| CGG | +3.73 | −27.16 to 34.63 | 0.812 | +2.02 | −21.58 to 25.62 | 0.866 | −0.04 | −23.52 to 23.42 | 0.997 |
| TCG | +0.41 | −5.58 to 6.41 | 0.892 | −1.27 | −5.854 to 3.31 | 0.586 | −1.90 | −5.64 to 3.45 | 0.636 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
de Melo, I.B.; Oliveira-Paula, G.H.; Ferezin, L.P.; Ferreira, G.C.; Pinheiro, L.C.; Tanus-Santos, J.E.; Garcia, L.V.; Lacchini, R.; Paula-Garcia, W.N. TRPA1 Polymorphisms Modify the Hypotensive Responses to Propofol with No Change in Nitrite or Nitrate Levels. Curr. Issues Mol. Biol. 2022, 44, 6333-6345. https://doi.org/10.3390/cimb44120432
de Melo IB, Oliveira-Paula GH, Ferezin LP, Ferreira GC, Pinheiro LC, Tanus-Santos JE, Garcia LV, Lacchini R, Paula-Garcia WN. TRPA1 Polymorphisms Modify the Hypotensive Responses to Propofol with No Change in Nitrite or Nitrate Levels. Current Issues in Molecular Biology. 2022; 44(12):6333-6345. https://doi.org/10.3390/cimb44120432
Chicago/Turabian Stylede Melo, Isabela Borges, Gustavo H. Oliveira-Paula, Letícia Perticarrara Ferezin, Graziele C. Ferreira, Lucas C. Pinheiro, Jose E. Tanus-Santos, Luis V. Garcia, Riccardo Lacchini, and Waynice N. Paula-Garcia. 2022. "TRPA1 Polymorphisms Modify the Hypotensive Responses to Propofol with No Change in Nitrite or Nitrate Levels" Current Issues in Molecular Biology 44, no. 12: 6333-6345. https://doi.org/10.3390/cimb44120432
APA Stylede Melo, I. B., Oliveira-Paula, G. H., Ferezin, L. P., Ferreira, G. C., Pinheiro, L. C., Tanus-Santos, J. E., Garcia, L. V., Lacchini, R., & Paula-Garcia, W. N. (2022). TRPA1 Polymorphisms Modify the Hypotensive Responses to Propofol with No Change in Nitrite or Nitrate Levels. Current Issues in Molecular Biology, 44(12), 6333-6345. https://doi.org/10.3390/cimb44120432








