The Core Jasmonic Acid-Signalling Module CoCOI1/CoJAZ1/CoMYC2 Are Involved in Jas Mediated Growth of the Pollen Tube in Camellia oleifera
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials, Pollination Treatment, and Sample Collection
2.2. Pollen Culture
2.3. Extraction of RNA and Quantitative RT-PCR
2.4. Gene Cloning and Bioinformatics Analysis
2.5. Subcellular Localization
2.6. Data Analysis
3. Results
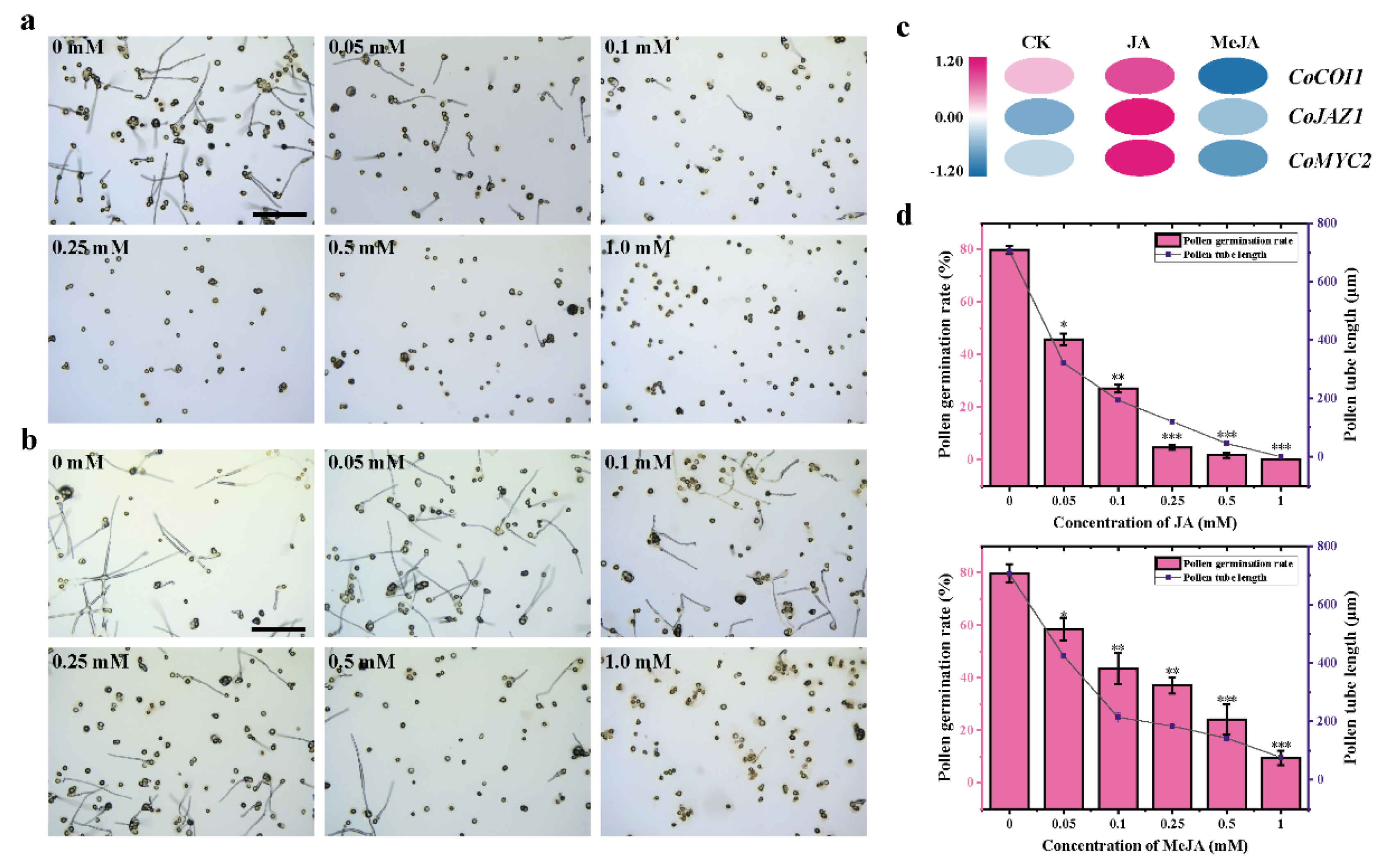
3.1. Exogenous JA and MeJA Inhibit Pollen Germination and Tube Growth
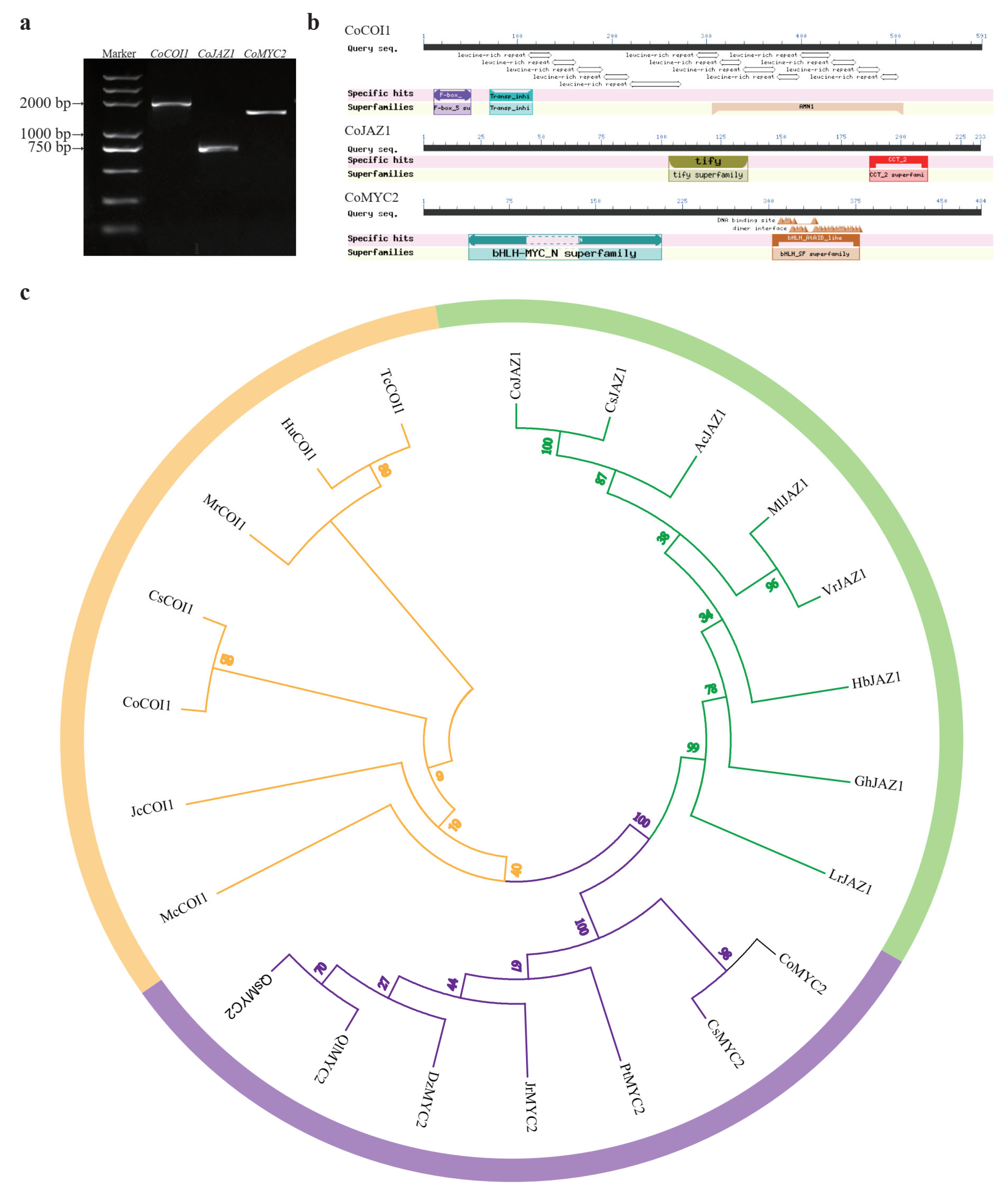
3.2. Cloning and Bioinformatics Analysis of CoCOI1/CoJAZ1/CoMYC2
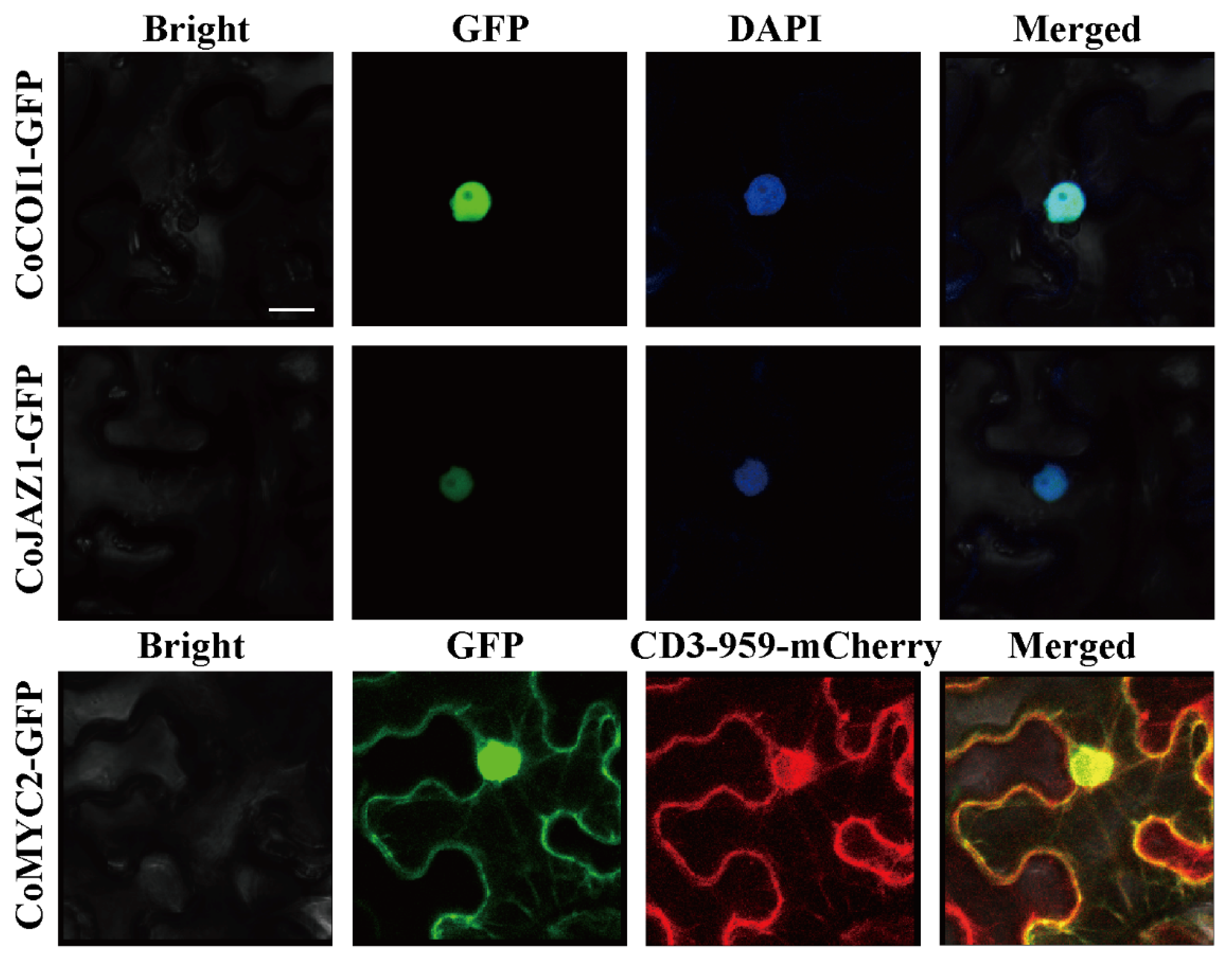
3.3. Subcellular Localization of CoCOI1/CoJAZ1/CoMYC2
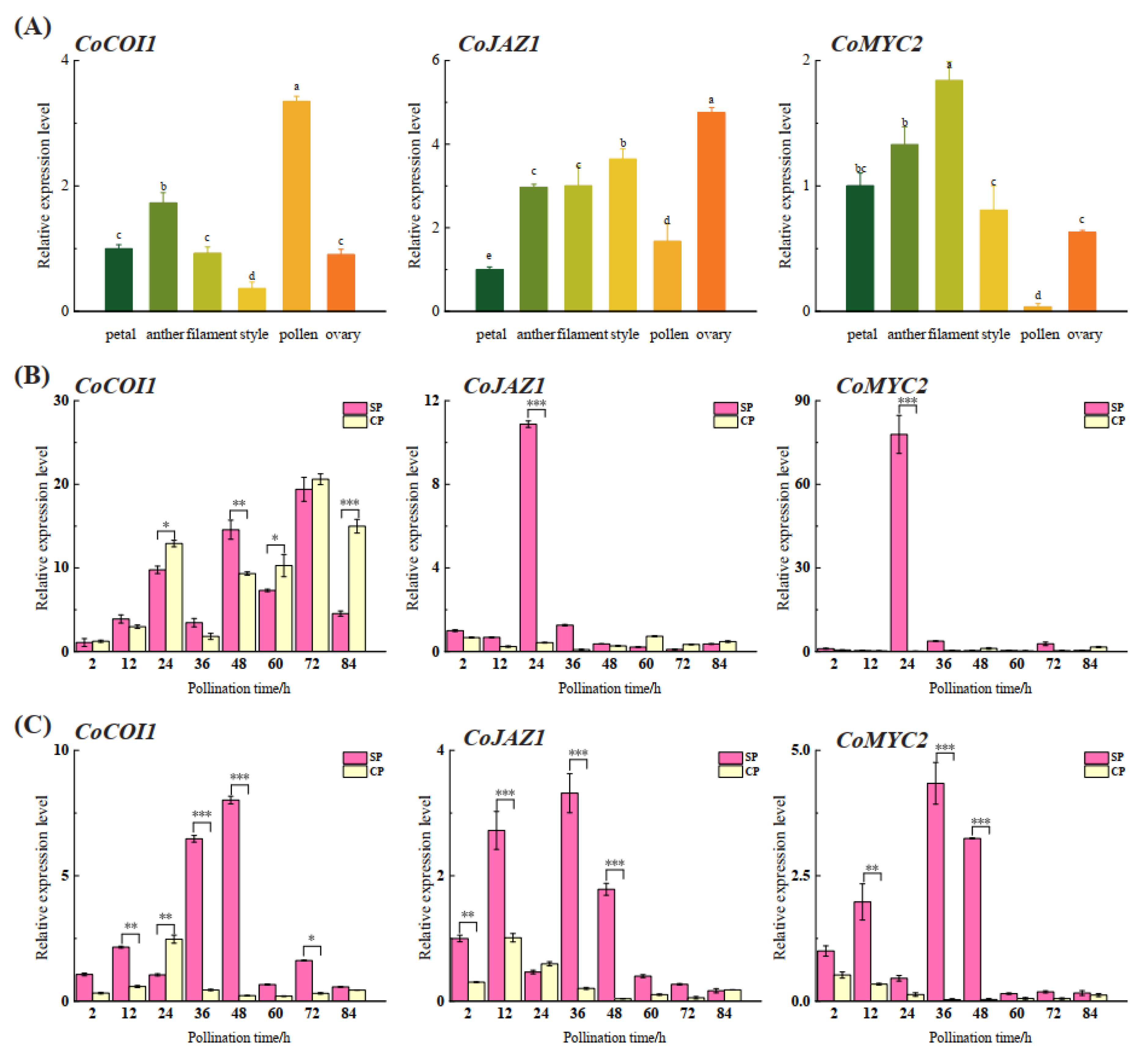
3.4. Expression Patterns of CoCOI1/CoJAZ1/CoMYC2
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhuang, R.L. Oil-tea Camellia in China, 2nd ed.; China Forestry Publishing House: Beijing, China, 2008; pp. 4–30. [Google Scholar]
- Zhang, D.L.; Stack, L.; Zhang, R.Q.; Yu, J.F.; Xie, B.X.; Chen, Y.Z.; Ruter, J.M. Teaoil Camellia-Eastern “Olive” for the World. Acta Hortic. 2006, 769, 43–48. [Google Scholar]
- Xiao, Y.G. Investigation and prospect of bio-active components in vegetable oil. Cereal Food Ind. 2006, 13, 4–8. [Google Scholar]
- Li, Z.J.; Zeng, Y.R.; Dai, W.S. Studies on internal and external factors which related with low yield and poor benefit in Camellia oleifera. J. For. Eng. 2009, 23, 26–31. [Google Scholar]
- Chao, G.; Yuan, D.Y.; Yang, Y.; Wang, B.F.; Liu, D.M.; Zou, F.; Tan, X.F. Anatomical characteristics of self-incompatibility in Camellia oleifera. Sci. Silvae Sin. 2015, 51, 60–68. [Google Scholar]
- Zhou, J.Q.; Lu, M.Q.; Yu, S.S.; Liu, Y.Y.; Yang, J.; Tan, X.F. In-depth understanding of Camellia oleifera self-incompatibility by comparative transcriptome, proteome and metabolome. Int. J. Mol. Sci. 2020, 21, 1600. [Google Scholar] [CrossRef]
- Schopfer, C.R.; Nasrallah, M.E.; Nasrallah, J.B. The male determinant of self-incompatibility in Brassica. Science 1999, 286, 1697–1700. [Google Scholar] [CrossRef]
- Kitashiba, H.; Nasrallah, J.B. Self-incompatibility in Brassicaceae crops: Lessons for interspecific incompatibility. Breed. Sci. 2014, 64, 23–37. [Google Scholar] [CrossRef]
- Campos, M.L.; de Almeida, M.; Rossi, M.L.; Martinelli, A.P.; Litholdo Junior, C.G.; Figueira, A.; Rampelotti-Ferreira, F.T.; Vendramim, J.D.; Benedito, V.A.; Peres, L.E. Brassinosteroids interact negatively with jasmonates in the formation of anti-herbivory traits in tomato. J. Exp. Bot. 2009, 60, 4347–4361. [Google Scholar] [CrossRef]
- Anderson, J.P.; Badruzsaufari, E.; Schenk, P.M.; Manners, J.M.; Desmond, O.J.; Ehlert, C.; Maclean, D.J.; Ebert, P.R.; Kazan, K. Antagonistic interaction between abscisic acid and jasmonate-ethylene signaling pathways modulates defense gene expression and disease resistance in Arabidopsis. Plant Cell. 2004, 16, 3460–3479. [Google Scholar] [CrossRef]
- Melotto, M.; Mecey, C.; Niu, Y.; Chung, H.S.; Katsir, L.; Yao, J.; Zeng, W.; Thines, B.; Staswick, P.; Browse, J.; et al. A critical role of two positively charged amino acids in the Jas motif of Arabidopsis JAZ proteins in mediating coronatine- and jasmonoyl isoleucine-dependent interactions with the COI1 F-box protein. Plant J. 2008, 55, 979–988. [Google Scholar] [CrossRef]
- Lorenzo, O.; Chico, J.M.; Sánchez-Serrano, J.J.; Solano, R. JASMONATE-INSENSITIVE1 encodes a MYC transcription factor essential to discriminate between different jasmonate-regulated defense responses in Arabidopsis. Plant Cell 2004, 16, 1938–1950. [Google Scholar] [CrossRef] [PubMed]
- Orzaez, D.; de Jong, A.J.; Woltering, E.J. A tomato homologue of the human protein PIRIN is induced during programmed cell death. Plant Mol. Biol. 2001, 46, 459–468. [Google Scholar] [CrossRef] [PubMed]
- Ma, W.W.; Xu, W.Z.; Xu, H.; Chen, Y.S.; He, Z.Y.; Ma, M. Nitric oxide modulates cadmium influx during cadmium-induced programmed cell death in tobacco BY-2 cells. Planta 2010, 232, 325–335. [Google Scholar] [CrossRef]
- Wang, C.L.; Xu, G.H.; Jiang, X.T.; Chen, G.; Wu, J.; Wu, H.Q.; Zhang, S.L. S-RNase triggers mitochondrial alteration and DNA degradation in the incompatible pollen tube of Pyrus pyrifolia in vitro. Plant J. 2009, 57, 220–229. [Google Scholar] [CrossRef] [PubMed]
- Thomas, S.G.; Franklin-Tong, V.E. Self-incompatibility triggers programmed cell death in Papaver pollen. Nature 2004, 429, 305–309. [Google Scholar] [CrossRef] [PubMed]
- Ruan, J.J.; Zhou, Y.X.; Zhou, M.L.; Yan, J.; Khurshid, M.; Weng, W.F.; Cheng, J.P.; Zhang, K.X. Jasmonic Acid Signaling Pathway in Plants. Int. J. Mol. Sci. 2019, 20, 2479. [Google Scholar] [CrossRef]
- Yamane, H.; Takagi, H.; Abe, H.; Yokota, T.; Takahashi, N. Identification of Jasmonic acid in three species of higher plants and its biological activities. Plant Cell Physiol. 1981, 22, 689–697. [Google Scholar]
- Pak, H.; Guo, Y.; Chen, M.X.; Chen, K.M.; Li, Y.L.; Hua, S.J.; Shamsi, I.; Meng, H.B.; Shi, C.G.; Jiang, L.X. The effect of exogenous methyl jasmonate on the flowering time, floral organ morphology, and transcript levels of a group of genes implicated in the development of oilseed rape flowers (Brassica napus L.). Planta 2009, 231, 79–91. [Google Scholar] [CrossRef]
- Zeng, X.C.; Jiang, H.Y.; Wu, X.Y.; Fang, J.H.; Xu, F.F. Effects of jasmonates on pollen Germination in rice. Acta. Agric. Univ. Jiangxiensis. 2003, 25, 17–19. [Google Scholar]
- Campos, M.L.; Kang, J.H.; Howe, G.A. Jasmonate-triggered plant immunity. J. Chem. Ecol. 2014, 40, 657–675. [Google Scholar] [CrossRef]
- Feng, M.J.; Xu, H.; Zhang, H.; Zhu, Y. Recent progress in jasmonates regulation of plant growth and development. Plant Physiol. J. 2015, 51, 407–412. [Google Scholar]
- Li, L.; Zhao, Y.F.; McCaig, B.C.; Wingerd, B.A.; Wang, J.H.; Whalon, M.E.; Pichersky, E.; Howe, G.A. The tomato homolog of CORONATINEINSENSITIVE1 is required for the maternal control of seed maturation, jasmonate-signaled defense responses, and glandular trichome development. Plant Cell 2004, 16, 126–143. [Google Scholar] [CrossRef] [PubMed]
- Chini, A.; Fonseca, S.; Fernández, G.; Adie, B.; Chico, J.M.; Lorenzo, O.; García-Casado, G.; López-Vidriero, I.; Lozano, F.M.; Ponce, M.R.; et al. The JAZ family of repressors is the missing link in jasmonate signalling. Nature 2007, 448, 66–671. [Google Scholar] [CrossRef] [PubMed]
- Katsir, L.; Chung, H.S.; Koo, A.J.; Howe, G.A. Jasmonate signaling: A conserved mechanism of hormone sensing. Curr. Opin. Plant Biol. 2008, 11, 428–435. [Google Scholar] [CrossRef]
- Thines, B.; Katsir, L.; Melotto, M.; Niu, Y.; Mandaokar, A.; Liu, G.; Nomura, K.; He, S.Y.; Howe, G.A.; Browse, J. JAZ repressor proteins are targets of the SCFCOI1 complex during jasmonate signalling. Nature 2007, 448, 661–665. [Google Scholar] [CrossRef]
- Xie, D.X.; Feys, B.F.; James, S.; Nieto-Rostro, M.; Turner, J.G. COI1: An Arabidopsis gene required for jasmonate-regulated defense and fertility. Science 1998, 280, 1091–1094. [Google Scholar] [CrossRef]
- Pauwels, L.; Barbero, G.F.; Geerinck, J.; Tilleman, S.; Grunewald, W.; Pérez, A.C.; Chico, J.M.; Bossche, R.V.; Sewell, J.; Gil, E.; et al. NINJA connects the co-repressor TOPLESS to jasmonate signalling. Nature 2010, 464, 788–791. [Google Scholar] [CrossRef]
- Boter, M.; Ruíz-Rivero, O.; Abdeen, A.; Prat, S. Conserved MYC transcription factors play a key role in jasmonate signaling both in tomato and Arabidopsis. Genes Dev. 2004, 18, 1577–1591. [Google Scholar] [CrossRef]
- Cheng, Z.W.; Sun, L.; Qi, T.C.; Zhang, B.S.; Peng, W.; Liu, Y.L.; Xie, D.X. The bHLH transcription factor MYC3 interacts with the jasmonate ZIM-domain proteins to mediate jasmonate response in Arabidopsis. Mol. Plant. 2011, 4, 279–288. [Google Scholar] [CrossRef]
- Goldberg, E.E.; Kohn, J.R.; Lande, R.; Robertson, K.A.; Smith, S.A.; Igić, B. Species selection maintains self-incompatibility. Science 2010, 330, 493–495. [Google Scholar] [CrossRef]
- Liao, T.; Yuan, D.Y.; Zou, F.; Gao, C.; Yang, Y.; Zhang, L.; Tan, X.F. Self-sterility in Camellia oleifera may be due to the prezygotic late-acting self-incompatibility. PLoS ONE 2014, 9, e99639. [Google Scholar]
- Feys, B.; Benedetti, C.E.; Penfold, C.N.; Turner, J.G. Arabidopsis Mutants Selected for Resistance to the Phytotoxin Coronatine Are Male Sterile, Insensitive to Methyl Jasmonate, and Resistant to a Bacterial Pathogen. Plant Cell 1994, 6, 751–759. [Google Scholar] [CrossRef] [PubMed]
- Grunewald, W.; Vanholme, B.; Pauwels, L.; Plovie, E.; Inzé, D.; Gheysen, G.; Goossens, A. Expression of the Arabidopsis jasmonate signalling repressor JAZ1/TIFY10A is stimulated by auxin. EMBO Rep. 2009, 10, 923–928. [Google Scholar] [CrossRef] [PubMed]
- Withers, J.; Yao, J.; Mecey, C.; Howe, G.A.; Melotto, M.; He, S.Y. Transcription factor-dependent nuclear localization of a transcriptional repressor in jasmonate hormone signaling. Proc. Natl. Acad. Sci. USA 2012, 109, 20148–20153. [Google Scholar] [CrossRef]
- Zhang, L.P.; Jia, C.G.; Liu, L.H.; Zhang, Z.M.; Li, C.Y.; Wang, Q.M. The involvement of jasmonates and ethylene in Alternaria alternata f. sp. lycopersici toxin-induced tomato cell death. J. Exp. Bot. 2011, 62, 5405–5418. [Google Scholar] [CrossRef]
- Gu, Z.Y.; Li, W.; Doughty, J.; Meng, D.; Yang, Q.; Yuan, H.; Li, Y.; Chen, Q.J.; Yu, J.; Liu, C.S.; et al. A gamma-thionin protein from apple, MdD1, is required for defense against S-RNase-induced inhibition of pollen tube prior to self/non-self recognition. Plant Biotechnol. J. 2019, 17, 2184–2198. [Google Scholar] [CrossRef]
- Yu, S.S.; Zhou, J.Q.; Lu, M.Q.; Liu, Y.Y.; Yang, J. Cloning and expression analysis of Camellia oleifera transporter gene ABCB26. Plant Physiol. J. 2019, 55, 1664–1672. [Google Scholar]
- Matsumoto, D.; Yamane, H.; Abe, K.; Tao, R. Identification of a Skp1-like protein interacting with SFB, the pollen S determinant of the gametophytic self-incompatibility in Prunus. Plant Physiol. 2012, 159, 1252–1262. [Google Scholar] [CrossRef]
- Xu, C.; Li, M.F.; Wu, J.K.; Guo, H.; Li, Q.; Zhang, Y.; Chai, J.J.; Li, T.Z.; Xue, Y.B. Identification of a canonical SCFSLF complex involved in S-RNase-based self-incompatibility of Pyrus (Rosaceae). Plant Mol. Biol. 2013, 81, 245–257. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Y.; Zhou, J.; Lu, M.; Yang, J.; Tan, X. The Core Jasmonic Acid-Signalling Module CoCOI1/CoJAZ1/CoMYC2 Are Involved in Jas Mediated Growth of the Pollen Tube in Camellia oleifera. Curr. Issues Mol. Biol. 2022, 44, 5405-5415. https://doi.org/10.3390/cimb44110366
Liu Y, Zhou J, Lu M, Yang J, Tan X. The Core Jasmonic Acid-Signalling Module CoCOI1/CoJAZ1/CoMYC2 Are Involved in Jas Mediated Growth of the Pollen Tube in Camellia oleifera. Current Issues in Molecular Biology. 2022; 44(11):5405-5415. https://doi.org/10.3390/cimb44110366
Chicago/Turabian StyleLiu, Yiyao, Junqin Zhou, Mengqi Lu, Jin Yang, and Xiaofeng Tan. 2022. "The Core Jasmonic Acid-Signalling Module CoCOI1/CoJAZ1/CoMYC2 Are Involved in Jas Mediated Growth of the Pollen Tube in Camellia oleifera" Current Issues in Molecular Biology 44, no. 11: 5405-5415. https://doi.org/10.3390/cimb44110366
APA StyleLiu, Y., Zhou, J., Lu, M., Yang, J., & Tan, X. (2022). The Core Jasmonic Acid-Signalling Module CoCOI1/CoJAZ1/CoMYC2 Are Involved in Jas Mediated Growth of the Pollen Tube in Camellia oleifera. Current Issues in Molecular Biology, 44(11), 5405-5415. https://doi.org/10.3390/cimb44110366




