MicroRNA and mRNA Expression Changes in Glioblastoma Cells Cultivated under Conditions of Neurosphere Formation
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Lines
2.2. Patient-Derived Cell Culture
2.3. Cell Culture for Neurosphere Formation
2.4. RNA Isolation
2.5. RNA Sequencing
2.6. Transcriptome Analysis
3. Results
3.1. Neurospheres Formation from Primary Brain Tumor BR3 and Immortalized Cell Line U87MG
3.2. Transcriptome and microRNome Changes of Neurospheres Occurs in Common Way in Patient-Derived and Immortalized Glioblastoma Cell Cultures
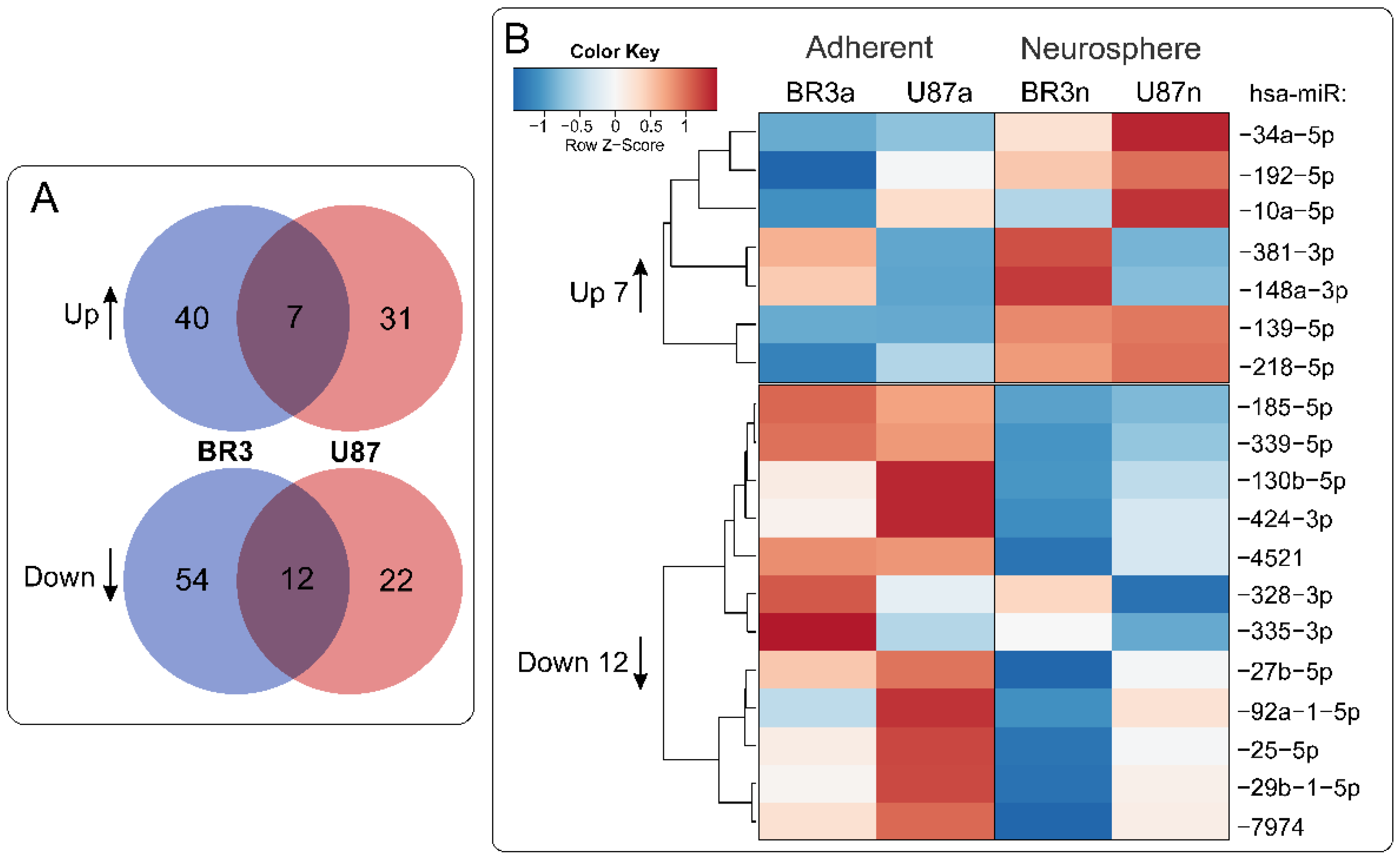
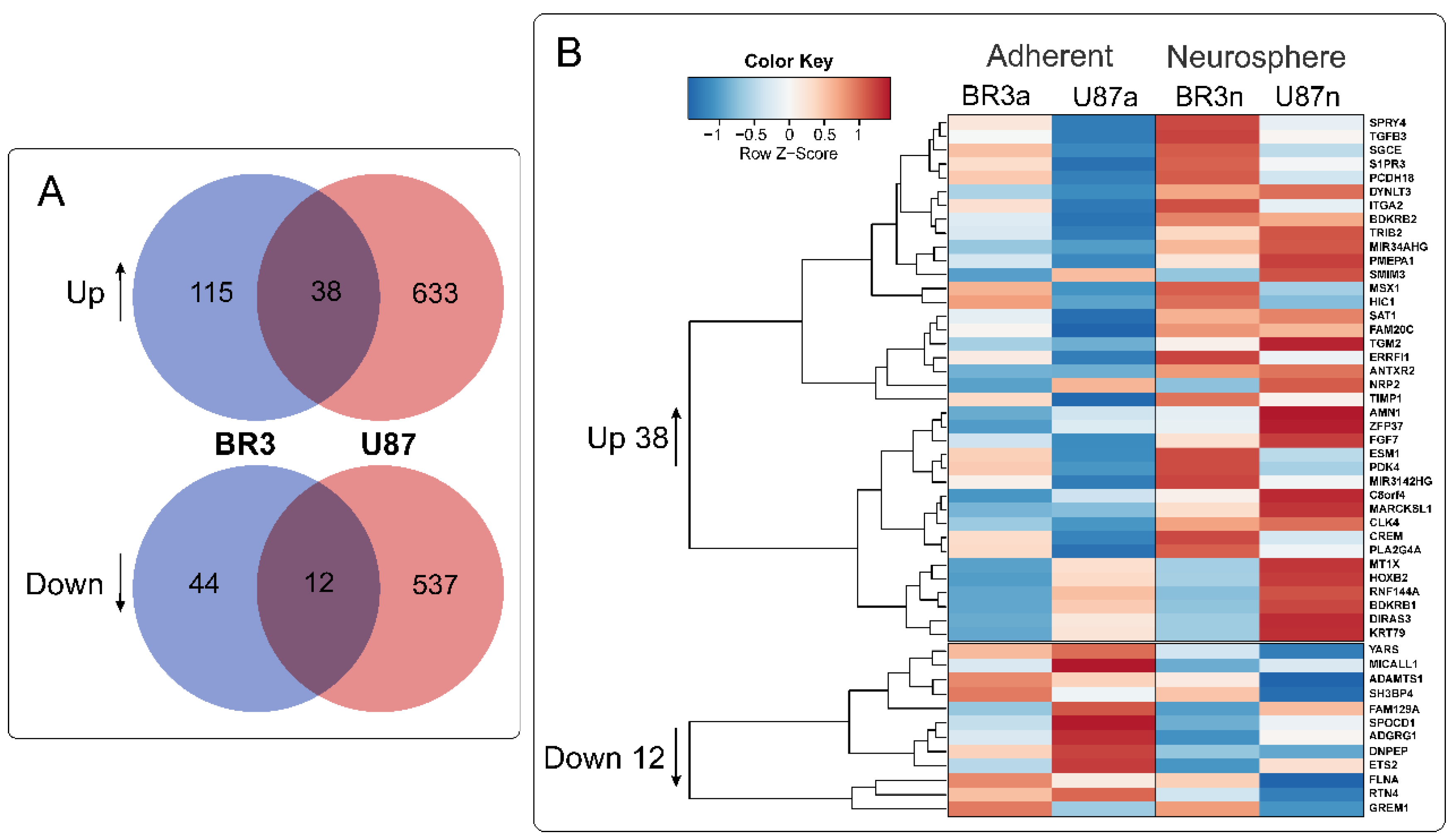
3.3. Common miRNA and mRNA Expression Changes in Both Patient-Derived BR3 and Immortalized U87 MG GBM Cell Cultures
3.4. Relationships between Changes in miRNA and mRNA Levels in GBM Cell Cultures under Conditions of Neurosphere Formation
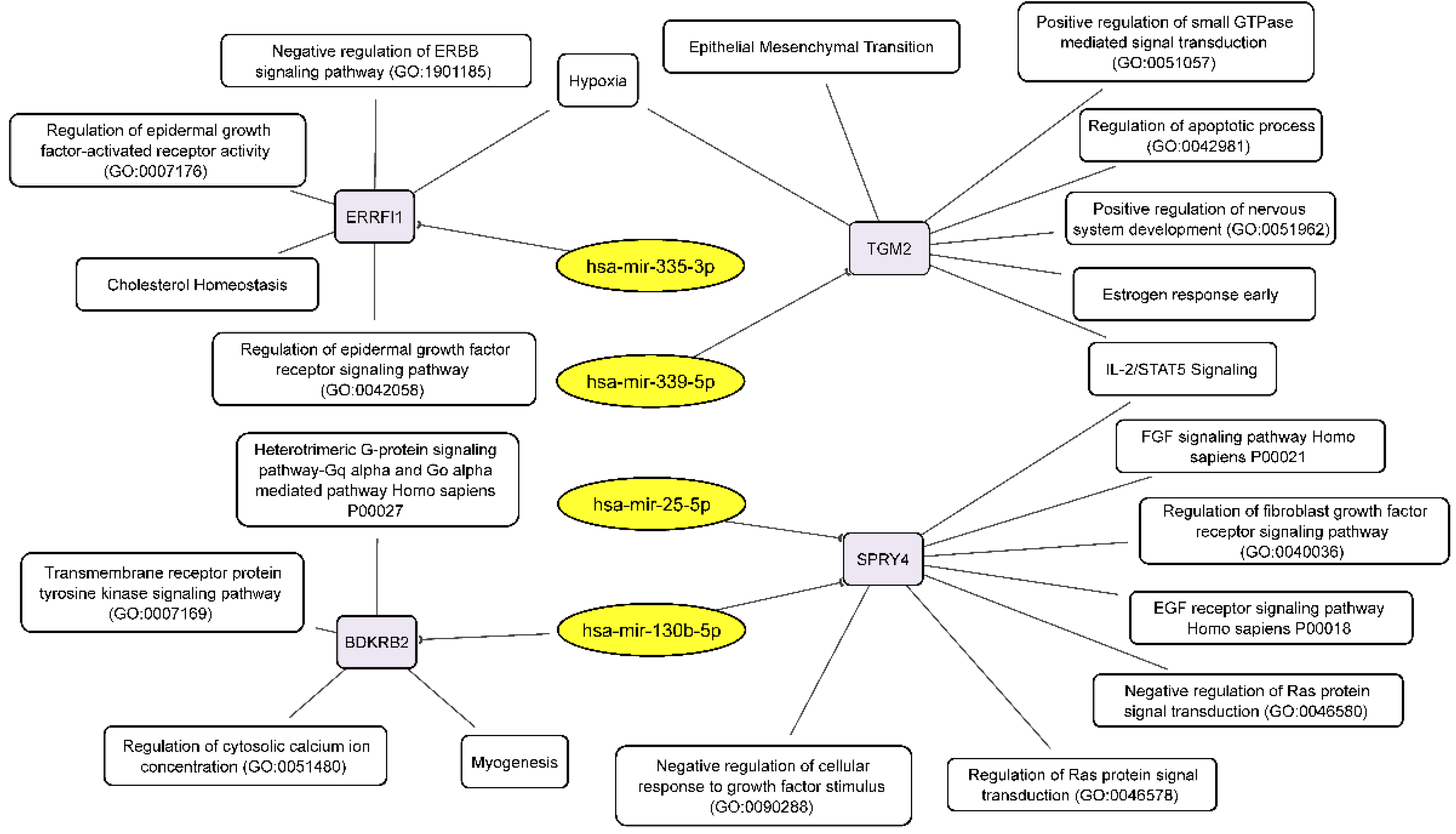
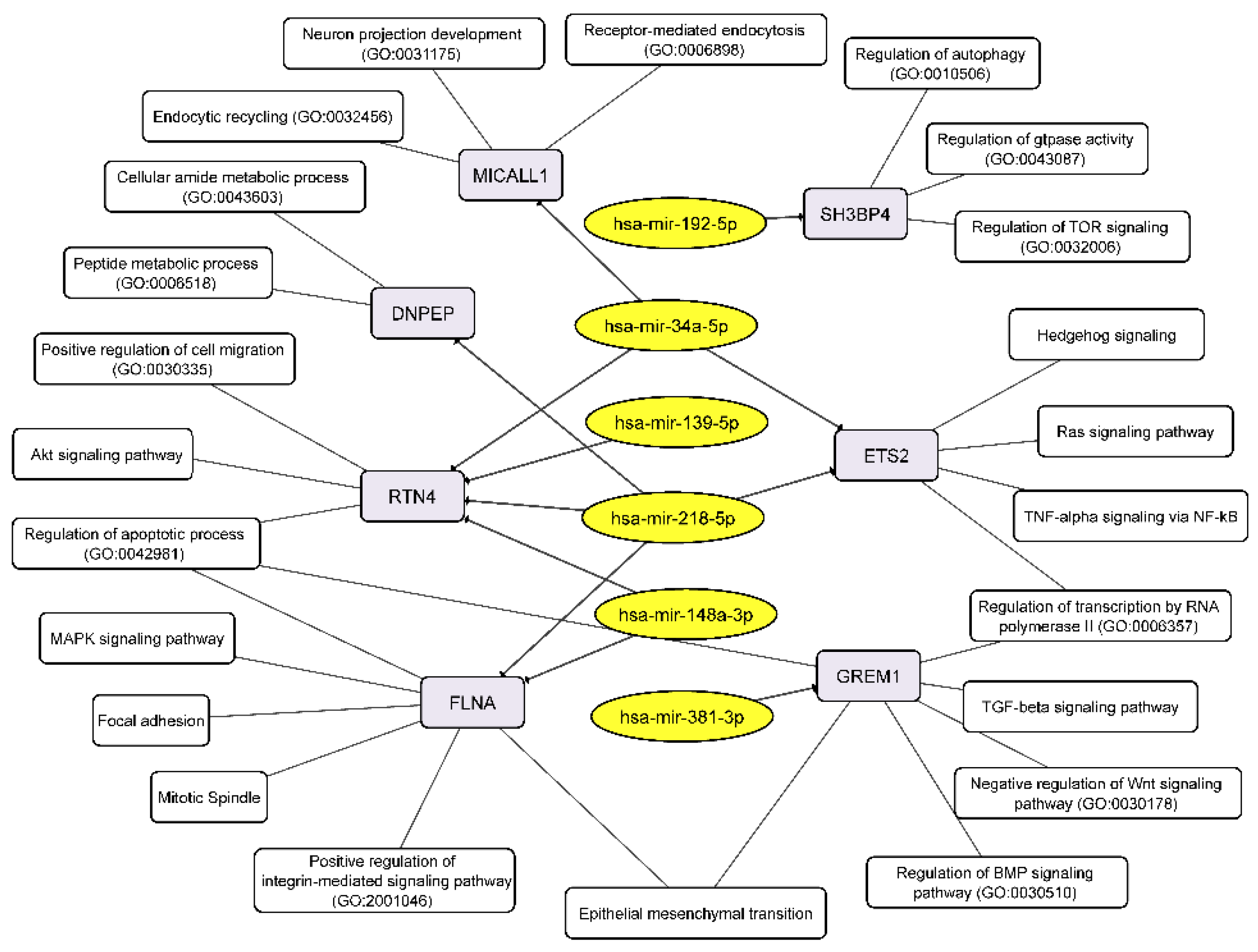
3.5. The miRNA-mRNA Network Allows to Suggest Cell Processes and Signaling Pathways Regulated by miRNAs in Glioblastoma Neurospheres
3.6. In Silico Validation of the Relationship between miRNA and mRNA Expression in Glioblastoma Cells with Data from the TCGA Project
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| CNS | central nervous system |
| CSC | cancer stem cell |
| GBM | glioblastoma |
| HC | hierarchical clustering |
| MN | monolayer |
| NGS | next generation sequencing |
| NS | neurosphere |
| PMID | PubMed identifier |
| RISC | RNA induced silencing complex |
References
- Miller, K.D.; Ostrom, Q.T.; Kruchko, C.; Patil, N.; Tihan, T.; Cioffi, G.; Fuchs, H.E.; Waite, K.A.; Jemal, A.; Siegel, R.L.; et al. Brain and other central nervous system tumor statistics, 2021. CA. Cancer J. Clin. 2021, 71, 381–406. [Google Scholar] [CrossRef]
- Hanif, F.; Muzaffar, K.; Perveen, K.; Malhi, S.M.; Simjee, S.U. Glioblastoma Multiforme: A Review of its Epidemiology and Pathogenesis through Clinical Presentation and Treatment. Asian Pac. J. Cancer Prev. 2017, 18, 3–9. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Graber, J.J. Overview of prognostic factors in adult gliomas. Ann. Palliat. Med. 2021, 10, 863–874. [Google Scholar] [CrossRef] [PubMed]
- Lathia, J.D.; Mack, S.C.; Mulkearns-Hubert, E.E.; Valentim, C.L.L.; Rich, J.N. Cancer stem cells in glioblastoma. Genes Dev. 2015, 29, 1203–1217. [Google Scholar] [CrossRef] [PubMed]
- Bao, S.; Wu, Q.; McLendon, R.E.; Hao, Y.; Shi, Q.; Hjelmeland, A.B.; Dewhirst, M.W.; Bigner, D.D.; Rich, J.N. Glioma stem cells promote radioresistance by preferential activation of the DNA damage response. Nature 2006, 444, 756–760. [Google Scholar] [CrossRef] [PubMed]
- Marzagalli, M.; Fontana, F.; Raimondi, M.; Limonta, P. Cancer Stem Cells—Key Players in Tumor Relapse. Cancers 2021, 13, 376. [Google Scholar] [CrossRef] [PubMed]
- Di Tomaso, T.; Mazzoleni, S.; Wang, E.; Sovena, G.; Clavenna, D.; Franzin, A.; Mortini, P.; Ferrone, S.; Doglioni, C.; Marincola, F.M.; et al. Immunobiological characterization of cancer stem cells isolated from glioblastoma patients. Clin. Cancer Res. 2010, 16, 800–813. [Google Scholar] [CrossRef]
- Pollard, S.M.; Yoshikawa, K.; Clarke, I.D.; Danovi, D.; Stricker, S.; Russell, R.; Bayani, J.; Head, R.; Lee, M.; Bernstein, M.; et al. Glioma Stem Cell Lines Expanded in Adherent Culture Have Tumor-Specific Phenotypes and Are Suitable for Chemical and Genetic Screens. Cell Stem Cell 2009, 4, 568–580. [Google Scholar] [CrossRef]
- Reynolds, B.A.; Rietze, R.L. Neural stem cells and neurospheres—Re-evaluating the relationship. Nat. Methods 2005, 2, 333–336. [Google Scholar] [CrossRef]
- LeBlanc, V.G.; Trinh, D.L.; Aslanpour, S.; Hughes, M.; Livingstone, D.; Jin, D.; Ahn, B.Y.; Blough, M.D.; Cairncross, J.G.; Chan, J.A.; et al. Single-cell landscapes of primary glioblastomas and matched explants and cell lines show variable retention of inter- and intratumor heterogeneity. Cancer Cell 2022, 40, 379–392.e9. [Google Scholar] [CrossRef]
- Peixoto, J.; Janaki-Raman, S.; Schlicker, L.; Schmitz, W.; Walz, S.; Winkelkotte, A.M.; Herold-Mende, C.; Soares, P.; Schulze, A.; Lima, J. Integrated Metabolomics and Transcriptomics Analysis of Monolayer and Neurospheres from Established Glioblastoma Cell Lines. Cancers 2021, 13, 1327. [Google Scholar] [CrossRef] [PubMed]
- Collet, B.; Avril, T.; Aubry, M.; Hamlat, A.; Le Reste, P.-J.; Chiforeanu, D.; Vauleon, E.; Mosser, J.; Quillien, V. Proteomic analysis underlines the usefulness of both primary adherent and stem-like cell lines for studying proteins involved in human glioblastoma. J. Proteom. 2014, 110, 7–19. [Google Scholar] [CrossRef] [PubMed]
- Shea, A.; Harish, V.; Afzal, Z.; Chijioke, J.; Kedir, H.; Dusmatova, S.; Roy, A.; Ramalinga, M.; Harris, B.; Blancato, J.; et al. MicroRNAs in glioblastoma multiforme pathogenesis and therapeutics. Cancer Med. 2016, 5, 1917–1946. [Google Scholar] [CrossRef] [PubMed]
- Nuzziello, N.; Liguori, M. The MicroRNA Centrism in the Orchestration of Neuroinflammation in Neurodegenerative Diseases. Cells 2019, 8, 1193. [Google Scholar] [CrossRef] [PubMed]
- Bartel, D.P. MicroRNAs: Genomics, Biogenesis, Mechanism, and Function. Cell 2004, 116, 281–297. [Google Scholar] [CrossRef]
- Lin, S.L.; Miller, J.D.; Ying, S.Y. Intronic microRNA (miRNA). J. Biomed. Biotechnol. 2006, 2006, 026818. [Google Scholar] [CrossRef]
- Inui, M.; Martello, G.; Piccolo, S. MicroRNA control of signal transduction. Nat. Rev. Mol. Cell Biol. 2010, 11, 252–263. [Google Scholar] [CrossRef]
- Fabian, M.R.; Sonenberg, N. The mechanics of miRNA-mediated gene silencing: A look under the hood of miRISC. Nat. Struct. Mol. Biol. 2012, 19, 586–593. [Google Scholar] [CrossRef]
- Stavast, C.J.; Erkeland, S.J. The Non-Canonical Aspects of MicroRNAs: Many Roads to Gene Regulation. Cells 2019, 8, 1465. [Google Scholar] [CrossRef]
- Henriksen, M.; Johnsen, K.B.; Andersen, H.H.; Pilgaard, L.; Duroux, M. MicroRNA Expression Signatures Determine Prognosis and Survival in Glioblastoma Multiforme—A Systematic Overview. Mol. Neurobiol. 2014, 50, 896–913. [Google Scholar] [CrossRef][Green Version]
- Rezaei, O.; Honarmand, K.; Nateghinia, S.; Taheri, M.; Ghafouri-Fard, S. miRNA signature in glioblastoma: Potential biomarkers and therapeutic targets. Exp. Mol. Pathol. 2020, 117, 104550. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Chen, L.; Li, J.J.; Zhou, Q.; Huang, A.; Liu, W.W.; Wang, K.; Gao, L.; Qi, S.T.; Lu, Y.T. MiR-519a enhances chemosensitivity and promotes autophagy in glioblastoma by targeting STAT3/Bcl2 signaling pathway. J. Hematol. Oncol. 2018, 11, 70. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.-S.; Feng, L.; Hu, B.-G.; Lu, Y.-F.; Wang, W.-M.; Guo, W.; Suen, C.-W.; Jiao, B.-H.; Pang, J.-X.; Fu, W.-M.; et al. miR-133a Promotes TRAIL Resistance in Glioblastoma via Suppressing Death Receptor 5 and Activating NF-κB Signaling. Mol. Ther.-Nucleic Acids 2017, 8, 482–492. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Wang, Y.; Li, Y.-J.; Zeng, C.-C. Chemo-resistance of A172 glioblastoma cells is controlled by miR-1271-regulated Bcl-2. Biomed. Pharmacother. 2018, 108, 734–740. [Google Scholar] [CrossRef]
- Anthiya, S.; Griveau, A.; Loussouarn, C.; Baril, P.; Garnett, M.; Issartel, J.-P.; Garcion, E. MicroRNA-Based Drugs for Brain Tumors. Trends Cancer 2018, 4, 222–238. [Google Scholar] [CrossRef]
- Teplyuk, N.M.; Uhlmann, E.J.; Gabriely, G.; Volfovsky, N.; Wang, Y.; Teng, J.; Karmali, P.; Marcusson, E.; Peter, M.; Mohan, A.; et al. Therapeutic potential of targeting microRNA-10b in established intracranial glioblastoma: First steps toward the clinic. EMBO Mol. Med. 2016, 8, 268–287. [Google Scholar] [CrossRef]
- Bolger, A.M.; Lohse, M.; Usadel, B. Trimmomatic: A flexible trimmer for Illumina sequence data. Bioinformatics 2014, 30, 2114–2120. [Google Scholar] [CrossRef]
- Langmead, B.; Salzberg, S.L. Fast gapped-read alignment with Bowtie 2. Nat. Methods 2012, 9, 357–359. [Google Scholar] [CrossRef]
- Jurka, J.; Kapitonov, V.V.; Pavlicek, A.; Klonowski, P.; Kohany, O.; Walichiewicz, J. Repbase Update, a database of eukaryotic repetitive elements. Cytogenet. Genome Res. 2005, 110, 462–467. [Google Scholar] [CrossRef]
- Dobin, A.; Davis, C.A.; Schlesinger, F.; Drenkow, J.; Zaleski, C.; Jha, S.; Batut, P.; Chaisson, M.; Gingeras, T.R. STAR: Ultrafast universal RNA-seq aligner. Bioinformatics 2013, 29, 15–21. [Google Scholar] [CrossRef]
- Hartley, S.W.; Mullikin, J.C. QoRTs: A comprehensive toolset for quality control and data processing of RNA-Seq experiments. BMC Bioinformatics 2015, 16, 224. [Google Scholar] [CrossRef] [PubMed]
- Kozomara, A.; Birgaoanu, M.; Griffiths-Jones, S. miRBase: From microRNA sequences to function. Nucleic Acids Res. 2019, 47, D155–D162. [Google Scholar] [CrossRef] [PubMed]
- Love, M.I.; Huber, W.; Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef]
- Kuleshov, M.V.; Jones, M.R.; Rouillard, A.D.; Fernandez, N.F.; Duan, Q.; Wang, Z.; Koplev, S.; Jenkins, S.L.; Jagodnik, K.M.; Lachmann, A.; et al. Enrichr: A comprehensive gene set enrichment analysis web server 2016 update. Nucleic Acids Res. 2016, 44, W90–W97. [Google Scholar] [CrossRef] [PubMed]
- Chang, L.; Zhou, G.; Soufan, O.; Xia, J. miRNet 2.0: Network-based visual analytics for miRNA functional analysis and systems biology. Nucleic Acids Res. 2020, 48, W244–W251. [Google Scholar] [CrossRef] [PubMed]
- Iacopino, F.; Angelucci, C.; Piacentini, R.; Biamonte, F.; Mangiola, A.; Maira, G.; Grassi, C.; Sica, G. Isolation of Cancer Stem Cells from Three Human Glioblastoma Cell Lines: Characterization of Two Selected Clones. PLoS ONE 2014, 9, e105166. [Google Scholar] [CrossRef]
- Datta, P.K. Neuronal Cell Culture; Amini, S., White, M.K., Eds.; Methods in Molecular Biology; Humana Press: Totowa, NJ, USA, 2013; Volume 1078, ISBN 978-1-62703-639-9. [Google Scholar]
- Ignatova, T.N.; Kukekov, V.G.; Laywell, E.D.; Suslov, O.N.; Vrionis, F.D.; Steindler, D.A. Human cortical glial tumors contain neural stem-like cells expressing astroglial and neuronal markers in vitro. Glia 2002, 39, 193–206. [Google Scholar] [CrossRef]
- Sasaki, A.; Taketomi, T.; Kato, R.; Saeki, K.; Nonami, A.; Sasaki, M.; Kuriyama, M.; Saito, N.; Shibuya, M.; Yoshimura, A. Mammalian Sprouty4 suppresses Ras-independent ERK activation by binding to Raf1. Nat. Cell Biol. 2003, 5, 427–432. [Google Scholar] [CrossRef]
- Celik-Selvi, B.E.; Stütz, A.; Mayer, C.-E.; Salhi, J.; Siegwart, G.; Sutterlüty, H. Sprouty3 and Sprouty4, Two Members of a Family Known to Inhibit FGF-Mediated Signaling, Exert Opposing Roles on Proliferation and Migration of Glioblastoma-Derived Cells. Cells 2019, 8, 808. [Google Scholar] [CrossRef]
- Duncan, C.G.; Killela, P.J.; Payne, C.A.; Lampson, B.; Chen, W.C.; Liu, J.; Solomon, D.; Waldman, T.; Towers, A.J.; Gregory, S.G.; et al. Integrated genomic analyses identify ERRFI1 and TACC3 as glioblastoma-targeted genes. Oncotarget 2010, 1, 265–277. [Google Scholar] [CrossRef]
- Ying, H.; Zheng, H.; Scott, K.; Wiedemeyer, R.; Yan, H.; Lim, C.; Huang, J.; Dhakal, S.; Ivanova, E.; Xiao, Y.; et al. Mig-6 controls EGFR trafficking and suppresses gliomagenesis. Proc. Natl. Acad. Sci. USA 2010, 107, 6912–6917. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Wang, J.; Shi, F.; Shan, A.; Xu, S.; Lv, W. BDKRB2 is a novel EMT-related biomarker and predicts poor survival in glioma. Aging 2021, 13, 7499–7516. [Google Scholar] [CrossRef] [PubMed]
- Yin, J.; Oh, Y.T.; Kim, J.-Y.; Kim, S.S.; Choi, E.; Kim, T.H.; Hong, J.H.; Chang, N.; Cho, H.J.; Sa, J.K.; et al. Transglutaminase 2 Inhibition Reverses Mesenchymal Transdifferentiation of Glioma Stem Cells by Regulating C/EBPβ Signaling. Cancer Res. 2017, 77, 4973–4984. [Google Scholar] [CrossRef] [PubMed]
- Pathak, G.P.; Shah, R.; Kennedy, B.E.; Murphy, J.P.; Clements, D.; Konda, P.; Giacomantonio, M.; Xu, Z.; Schlaepfer, I.R.; Gujar, S. RTN4 Knockdown Dysregulates the AKT Pathway, Destabilizes the Cytoskeleton, and Enhances Paclitaxel-Induced Cytotoxicity in Cancers. Mol. Ther. 2018, 26, 2019–2033. [Google Scholar] [CrossRef]
- Yuan, D.; Tao, Y.; Chen, G.; Shi, T. Systematic expression analysis of ligand-receptor pairs reveals important cell-to-cell interactions inside glioma. Cell Commun. Signal. 2019, 17, 48. [Google Scholar] [CrossRef]
- Savoy, R.M.; Ghosh, P.M. The dual role of filamin A in cancer: Can’t live with (too much of) it, can’t live without it. Endocr. Relat. Cancer 2013, 20, R341–R356. [Google Scholar] [CrossRef]
- Chantaravisoot, N.; Wongkongkathep, P.; Loo, J.A.; Mischel, P.S.; Tamanoi, F. Significance of filamin A in mTORC2 function in glioblastoma. Mol. Cancer 2015, 14, 127. [Google Scholar] [CrossRef]
- Kim, Y.-M.; Kim, D.-H. dRAGging amino acid-mTORC1 signaling by SH3BP4. Mol. Cells 2013, 35, 1–6. [Google Scholar] [CrossRef]
- Geng, N.; Li, Y.; Zhang, W.; Wang, F.; Wang, X.; Jin, Z.; Xing, Y.; Li, D.; Zhang, H.; Li, Y.; et al. A PAK5–DNPEP–USP4 axis dictates breast cancer growth and metastasis. Int. J. Cancer 2020, 146, 1139–1151. [Google Scholar] [CrossRef]
- Cam, M.; Charan, M.; Welker, A.M.; Dravid, P.; Studebaker, A.W.; Leonard, J.R.; Pierson, C.R.; Nakano, I.; Beattie, C.E.; Hwang, E.I.; et al. ΔNp73/ETS2 complex drives glioblastoma pathogenesis- targeting downstream mediators by rebastinib prolongs survival in preclinical models of glioblastoma. Neuro. Oncol. 2020, 22, 345–356. [Google Scholar] [CrossRef]
- Abou-Zeid, N.; Pandjaitan, R.; Sengmanivong, L.; David, V.; Le Pavec, G.; Salamero, J.; Zahraoui, A. MICAL-like1 mediates epidermal growth factor receptor endocytosis. Mol. Biol. Cell 2011, 22, 3431–3441. [Google Scholar] [CrossRef] [PubMed]
- Yan, K.; Wu, Q.; Yan, D.H.; Lee, C.H.; Rahim, N.; Tritschler, I.; DeVecchio, J.; Kalady, M.F.; Hjelmeland, A.B.; Rich, J.N. Glioma cancer stem cells secrete Gremlin1 to promote their maintenance within the tumor hierarchy. Genes Dev. 2014, 28, 1085–1100. [Google Scholar] [CrossRef] [PubMed]
- Celiku, O.; Johnson, S.; Zhao, S.; Camphausen, K.; Shankavaram, U. Visualizing molecular profiles of glioblastoma with GBM-BioDP. PLoS ONE 2014, 9, e101239. [Google Scholar] [CrossRef] [PubMed]
- Verhaak, R.G.W.; Hoadley, K.A.; Purdom, E.; Wang, V.; Qi, Y.; Wilkerson, M.D.; Miller, C.R.; Ding, L.; Golub, T.; Mesirov, J.P.; et al. Integrated Genomic Analysis Identifies Clinically Relevant Subtypes of Glioblastoma Characterized by Abnormalities in PDGFRA, IDH1, EGFR, and NF1. Cancer Cell 2010, 17, 98–110. [Google Scholar] [CrossRef] [PubMed]
- Dong, H.; Luo, L.; Hong, S.; Siu, H.; Xiao, Y.; Jin, L.; Chen, R.; Xiong, M. Integrated analysis of mutations, miRNA and mRNA expression in glioblastoma. BMC Syst. Biol. 2010, 4, 163. [Google Scholar] [CrossRef]
- Agrawal, R.; Pandey, P.; Jha, P.; Dwivedi, V.; Sarkar, C.; Kulshreshtha, R. Hypoxic signature of microRNAs in glioblastoma: Insights from small RNA deep sequencing. BMC Genom. 2014, 15, 686. [Google Scholar] [CrossRef]
- Yeh, M.; Wang, Y.-Y.; Yoo, J.Y.; Oh, C.; Otani, Y.; Kang, J.M.; Park, E.S.; Kim, E.; Chung, S.; Jeon, Y.-J.; et al. MicroRNA-138 suppresses glioblastoma proliferation through downregulation of CD44. Sci. Rep. 2021, 11, 9219. [Google Scholar] [CrossRef]
- Tomei, S.; Volontè, A.; Ravindran, S.; Mazzoleni, S.; Wang, E.; Galli, R.; Maccalli, C. MicroRNA Expression Profile Distinguishes Glioblastoma Stem Cells from Differentiated Tumor Cells. J. Pers. Med. 2021, 11, 264. [Google Scholar] [CrossRef]
- Bourguignon, L.Y.W.; Earle, C.; Wong, G.; Spevak, C.C.; Krueger, K. Stem cell marker (Nanog) and Stat-3 signaling promote MicroRNA-21 expression and chemoresistance in hyaluronan/CD44-activated head and neck squamous cell carcinoma cells. Oncogene 2012, 31, 149–160. [Google Scholar] [CrossRef]
- Gharbavi, M.; Johari, B.; Rismani, E.; Mousazadeh, N.; Taromchi, A.H.; Sharafi, A. NANOG Decoy Oligodeoxynucleotide–Encapsulated Niosomes Nanocarriers: A Promising Approach to Suppress the Metastatic Properties of U87 Human Glioblastoma Multiforme Cells. ACS Chem. Neurosci. 2020, 11, 4499–4515. [Google Scholar] [CrossRef]
- Fan, B.; Jiao, B.-H.; Fan, F.-S.; Lu, S.-K.; Song, J.; Guo, C.-Y.; Yang, J.-K.; Yang, L. Downregulation of miR-95-3p inhibits proliferation, and invasion promoting apoptosis of glioma cells by targeting CELF2. Int. J. Oncol. 2015, 47, 1025–1033. [Google Scholar] [CrossRef] [PubMed]
- Gu, J.-J.; Fan, K.-C.; Zhang, J.-H.; Chen, H.-J.; Wang, S.-S. Suppression of microRNA-130b inhibits glioma cell proliferation and invasion, and induces apoptosis by PTEN/AKT signaling. Int. J. Mol. Med. 2018, 41, 284–292. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Z.-Q.; Yin, T.-K.; Li, Y.-X.; Zhang, J.-H.; Gu, J.-J. miR-130b regulates the proliferation, invasion and apoptosis of glioma cells via targeting of CYLD. Oncol. Rep. 2017, 38, 167–174. [Google Scholar] [CrossRef] [PubMed]
- Coban, N.; Ozuynuk, A.S.; Erkan, A.F.; Guclu-Geyik, F.; Ekici, B. Levels of miR-130b-5p in peripheral blood are associated with severity of coronary artery disease. Mol. Biol. Rep. 2021, 48, 7719–7732. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Liu, C.; Li, Q.; Li, J.; Wu, Y.; Liu, J. MicroRNA-25-5p counteracts oxidized LDL-induced pathological changes by targeting neuronal growth regulator 1 (NEGR1) in human brain micro-vessel endothelial cells. Biochimie 2019, 165, 141–149. [Google Scholar] [CrossRef]
- Li, P.; Li, Y.; Chen, L.; Ma, X.; Yan, X.; Yan, M.; Qian, B.; Wang, F.; Xu, J.; Yin, J.; et al. Long noncoding RNA uc003pxg.1 regulates endothelial cell proliferation and migration via miR-25-5p in coronary artery disease. Int. J. Mol. Med. 2021, 48, 160. [Google Scholar] [CrossRef]
- Zhao, W.; Chen, T.; Zhao, Y. Upregulated lncRNA CASC9 Contributes to Progression of Non-Small Cell Lung Cancer Through Inhibition of miR-335-3p and Activation S100A14 Expression. OncoTargets Ther. 2020, 13, 6027–6036. [Google Scholar] [CrossRef]
- Zheng, B.; Wang, S.; Shen, H.; Lin, J. Inhibitory Effect of miR-339-5p on Glioma through PTP4A1/HMGB1 Pathway. Dis. Markers 2022, 2022, 2231195. [Google Scholar] [CrossRef]
- Lin, J.; Wang, S.; Shen, H.; Zheng, B. The Expression Profile of miRNA in Glioma and the Role of miR-339-5p in Glioma. Biomed. Res. Int. 2022, 2022, 1–8. [Google Scholar] [CrossRef]
- Wang, L.; Liu, Y.; Yu, Z.; Gong, J.; Deng, Z.; Ren, N.; Zhong, Z.; Cai, H.; Tang, Z.; Cheng, H.; et al. Mir-139-5p inhibits glioma cell proliferation and progression by targeting GABRA1. J. Transl. Med. 2021, 19, 213. [Google Scholar] [CrossRef]
- Wang, W.; Dong, J.; Wang, M.; Yao, S.; Tian, X.; Cui, X.; Fu, S.; Zhang, S. miR-148a-3p suppresses epithelial ovarian cancer progression primarily by targeting c-Met. Oncol. Lett. 2018, 15, 6131–6136. [Google Scholar] [CrossRef] [PubMed]
- Zhu, A.; Xia, J.; Zuo, J.; Jin, S.; Zhou, H.; Yao, L.; Huang, H.; Han, Z. MicroRNA-148a is silenced by hypermethylation and interacts with DNA methyltransferase 1 in gastric cancer. Med. Oncol. 2012, 29, 2701–2709. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Ko, Y.; Park, H.; Zhang, H.; Jeong, Y.; Kim, Y.; Noh, M.; Park, S.; Kim, Y.-M.; Kwon, Y.-G. MicroRNA-148a/b-3p regulates angiogenesis by targeting neuropilin-1 in endothelial cells. Exp. Mol. Med. 2019, 51, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Su, X.; Lin, M.; Fu, B.; Zhou, C.; Ling, C.; Qian, Z.; Yao, Y. Expression of miR-192-5p in colon cancer serum and its relationship with clinicopathologic features. Am. J. Transl. Res. 2021, 13, 9371–9376. [Google Scholar]
- Li, Z.; Qian, R.; Zhang, J.; Shi, X. MiR-218-5p targets LHFPL3 to regulate proliferation, migration, and epithelial-mesenchymal transitions of human glioma cells. Biosci. Rep. 2019, 39, 1–14. [Google Scholar] [CrossRef]
- Vaitkiene, P.; Pranckeviciene, A.; Stakaitis, R.; Steponaitis, G.; Tamasauskas, A.; Bunevicius, A. Association of miR-34a Expression with Quality of Life of Glioblastoma Patients: A Prospective Study. Cancers 2019, 11, 300. [Google Scholar] [CrossRef]
- Dong, Z.; Zhang, J.; Niu, L.; Hou, G.; Gao, Z.; Yang, Q. miR-381-3p Involves in Glioma Progression by Suppressing Tumor-Promoter Factor ANTXR1. Comput. Math. Methods Med. 2021, 2021, 1–7. [Google Scholar] [CrossRef]
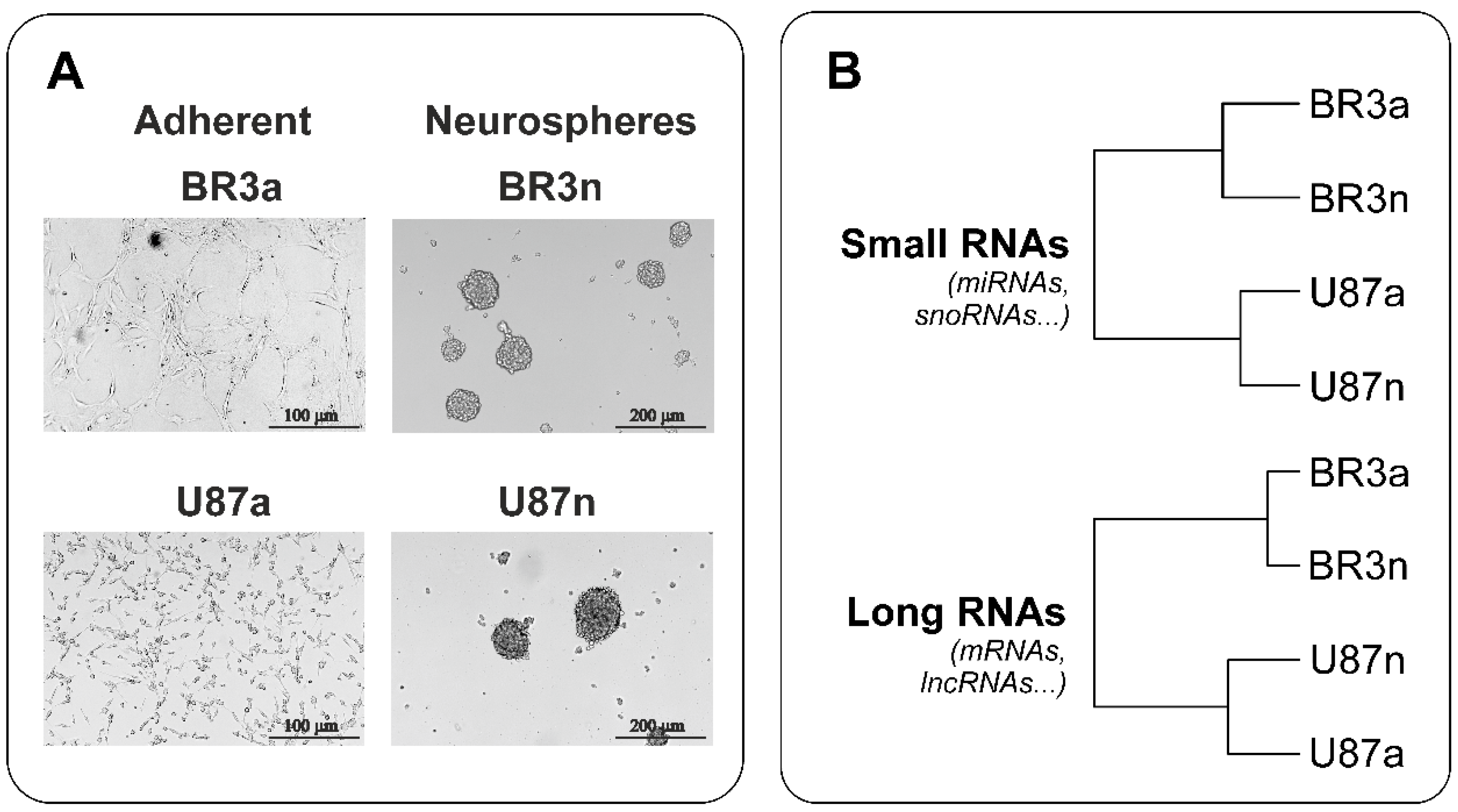
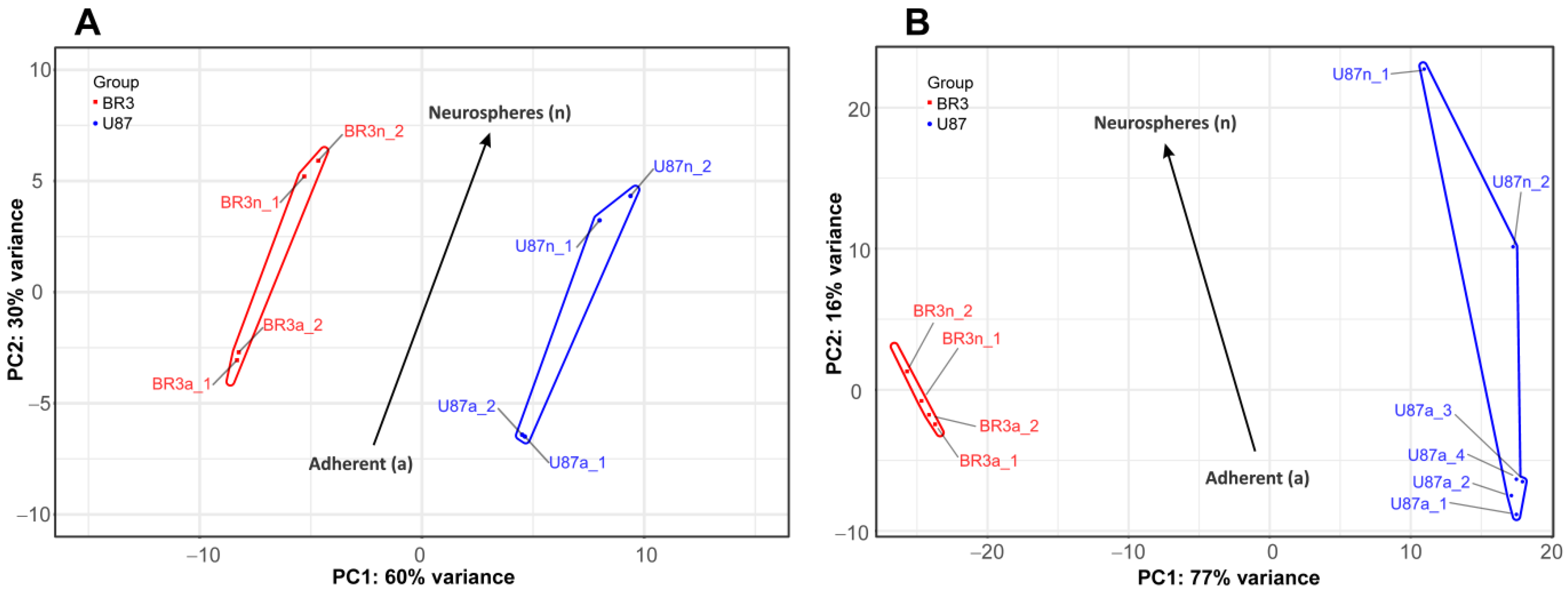

| Cell Culture | Histological Characteristic | Culture Conditions * | NGS-Library ** | Number of Replicates | Number of NGS-Sequencing Reads (106) |
|---|---|---|---|---|---|
| BR3 | GBM | MN | BR3a | 2 | 19.55 |
| MN | mBR3a | 2 | 20.23 | ||
| NS | BR3n | 2 | 19.58 | ||
| NS | mBR3n | 2 | 9.42 | ||
| U87 *** | GBM | MN | U87a | 4 | 46.35 |
| MN | mU87a | 2 | 23.08 | ||
| NS | U87n | 2 | 22.95 | ||
| NS | mU87n | 2 | 22.64 |
| Term | BR3 | U87 MG | Common Genes * | ||
|---|---|---|---|---|---|
| p Value | Adj. p Value | p Value | Adj. p Value | ||
| Upregulated | |||||
| EMT ** | 4.63 × 10−8 | 1.57 × 10−6 | 3.62 × 10−9 | 5.70 × 10−8 | ITGA2; PMEPA1; SAT1; TIMP1; MSX1; TGM2 |
| KRAS Signaling Up | 1.56 × 10−4 | 2.65 × 10−3 | 6.88 × 10−8 | 4.82 × 10−7 | ITGA2; TRIB2 |
| TNF-alpha Signaling via NF-kB | 4.44 × 10−3 | 3.02 × 10−2 | 1.14 × 10−24 | 5.59 × 10−23 | PMEPA1; SAT1 |
| Hypoxia | 4.44 × 10−3 | 3.02 × 10−2 | 1.61 × 10−8 | 1.58 × 10−7 | TGM2; ERRFI1; TGFB3 |
| Apoptosis | 8.00 × 10−3 | 3.89 × 10−2 | 4.66 × 10−9 | 5.70 × 10−8 | SAT1; TIMP1 |
| Angiogenesis | 3.09 × 10−2 | 1.17 × 10−1 *** | 1.95 × 10−7 | 1.19 × 10−6 | TIMP1; MSX1 |
| Downregulated | |||||
| mTORC1 Signaling | 1.99 × 10−5 | 2.19 × 10−4 | 9.43 × 10−3 | 4.93 × 10−2 | -- |
| EMT ** | 2.37 × 10−3 | 1.31 × 10−2 | 1.09 × 10−5 | 3.21 × 10−4 | GREM1; FLNA |
| Glycolysis | 2.37 × 10−3 | 1.31 × 10−2 | 9.43 × 10−3 | 4.93 × 10−2 | -- |
| HedgehogSignaling | 4.56 × 10−3 | 2.01 × 10−2 | 1.65 × 10−2 | 7.76 × 10−2 | ADGRG1; ETS2 |
| UV Response Dn | 7.67 × 10−3 | 2.81 × 10−2 | 6.47 × 10−3 | 4.93 × 10−2 | -- |
| PI3K/AKT/mTOR Signaling | 3.50 × 10−2 | 1.10 × 10−1 *** | 2.41 × 10−3 | 2.27 × 10−2 | -- |
| miRNA | Enrichr | miRNet | ||
|---|---|---|---|---|
| mRNA-Targets | Library * | mRNA-Targets | Literature ** | |
| Downregulated miRNAs—Upregulated mRNAs*** | ||||
| hsa-miR-130b-5p | BDKRB2 | miRTarBase_2017 | BDKRB2; SPRY4 | 19536157, tarbase |
| hsa-miR-25-5p | SPRY4 | miRTarBase_2017 | SPRY4 | 26701625 |
| hsa-mir-335-3p | -- | -- | ERRFI1 | tarbase |
| hsa-mir-339-5p | -- | -- | TGM2 | tarbase |
| Upregulated miRNAs—Downregulated mRNAs*** | ||||
| hsa-mir-139-5p | -- | -- | RTN4 | tarbase |
| hsa-mir-148a-3p | -- | -- | FLNA; RTN4 | tarbase, tarbase |
| hsa-miR-192-5p | SH3BP4 | miRTarBase_2017 | SH3BP4 | 19074876 |
| hsa-miR-218-5p | DNPEP; ETS2; FLNA | miRTarBase_2017 | DNPEP; ETS2; FLNA; RTN4 | 23212916; 20371350; 23212916; tarbase |
| hsa-miR-34a-5p | RTN4 | miRTarBase_2017 | ETS2; MICALL1; RTN4 | tarbase; tarbase; 21566225|20371350 |
| hsa-miR-381-3p | GREM1 | miRTarBase_2017 | GREM1 | 23824327 |
| mRNA | miRNA | Pearson’s R2 | GBM Subtypes * | Kaplan–Meier Survival Curve 1Half vs. 2Half Comparison p-val | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| mRNA | miRNA | ||||||||||||
| C | M | P | N | C | M | P | N | ||||||
| DNPEP | Down | miR-218 | Up | 0.78 | C,M,N,U | 0.220 | 0 ** | 0.198 | 0.396 | 0.617 | 0.614 | 0.406 | 0.768 |
| ERRFI1 | Up | miR-335 | Down | 0.75 | C,M,N,U | 0.673 | 0.063 | 0.222 | 0.026 | 0.405 | 0.825 | 0.674 | 0.268 |
| ETS2 | Down | miR-218 | Up | 0.51 | M,N,P,U | 0.626 | 0.251 | 0.619 | 0.239 | 0.617 | 0.614 | 0.406 | 0.768 |
| ETS2 | Down | miR-34a | Up | 0.51 | M,N,P,U | 0.626 | 0.251 | 0.619 | 0.239 | 0.982 | 0.972 | 0 | 0.333 |
| FLNA | Down | miR-218 | Up | 0.91 | C,N,P,U | 0.353 | 0.241 | 0.343 | 0.130 | 0.617 | 0.614 | 0.406 | 0.768 |
| GREM | Down | miR-381 | Up | 0.97 | M,N,P,U | 0.576 | 0.297 | 0.057 | 0.969 | 0.362 | 0.865 | 0.723 | 0.181 |
| RTN4 | Down | miR-148a | Up | 0.98 | M,N,P,U | 0.226 | 0.817 | 0.452 | 0.369 | 0.442 | 0.097 | 0 | 0.014 |
| RTN4 | Down | miR-34a | Up | 0.73 | C,M,N,P,U | 0.226 | 0.817 | 0.452 | 0.369 | 0.982 | 0.972 | 0 | 0.333 |
| SH3BP4 | Down | miR-192 | Up | 0.65 | C,M,N,U | 0.460 | 0.926 | 0.588 | 0.737 | 0.986 | 0.446 | 0.900 | 0.425 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dymova, M.A.; Vasileva, N.S.; Kuligina, E.V.; Savinovskaya, Y.I.; Zinchenko, N.D.; Ageenko, A.B.; Mishinov, S.V.; Stepanov, G.A.; Richter, V.A.; Semenov, D.V. MicroRNA and mRNA Expression Changes in Glioblastoma Cells Cultivated under Conditions of Neurosphere Formation. Curr. Issues Mol. Biol. 2022, 44, 5294-5311. https://doi.org/10.3390/cimb44110360
Dymova MA, Vasileva NS, Kuligina EV, Savinovskaya YI, Zinchenko ND, Ageenko AB, Mishinov SV, Stepanov GA, Richter VA, Semenov DV. MicroRNA and mRNA Expression Changes in Glioblastoma Cells Cultivated under Conditions of Neurosphere Formation. Current Issues in Molecular Biology. 2022; 44(11):5294-5311. https://doi.org/10.3390/cimb44110360
Chicago/Turabian StyleDymova, Maya A., Natalia S. Vasileva, Elena V. Kuligina, Yulya I. Savinovskaya, Nikita D. Zinchenko, Alisa B. Ageenko, Sergey V. Mishinov, Grigory A. Stepanov, Vladimir A. Richter, and Dmitry V. Semenov. 2022. "MicroRNA and mRNA Expression Changes in Glioblastoma Cells Cultivated under Conditions of Neurosphere Formation" Current Issues in Molecular Biology 44, no. 11: 5294-5311. https://doi.org/10.3390/cimb44110360
APA StyleDymova, M. A., Vasileva, N. S., Kuligina, E. V., Savinovskaya, Y. I., Zinchenko, N. D., Ageenko, A. B., Mishinov, S. V., Stepanov, G. A., Richter, V. A., & Semenov, D. V. (2022). MicroRNA and mRNA Expression Changes in Glioblastoma Cells Cultivated under Conditions of Neurosphere Formation. Current Issues in Molecular Biology, 44(11), 5294-5311. https://doi.org/10.3390/cimb44110360






