Effect of Cannabidiolic Acid, N-Trans-Caffeoyltyramine and Cannabisin B from Hemp Seeds on microRNA Expression in Human Neural Cells
Abstract
1. Introduction
2. Materials and Methods
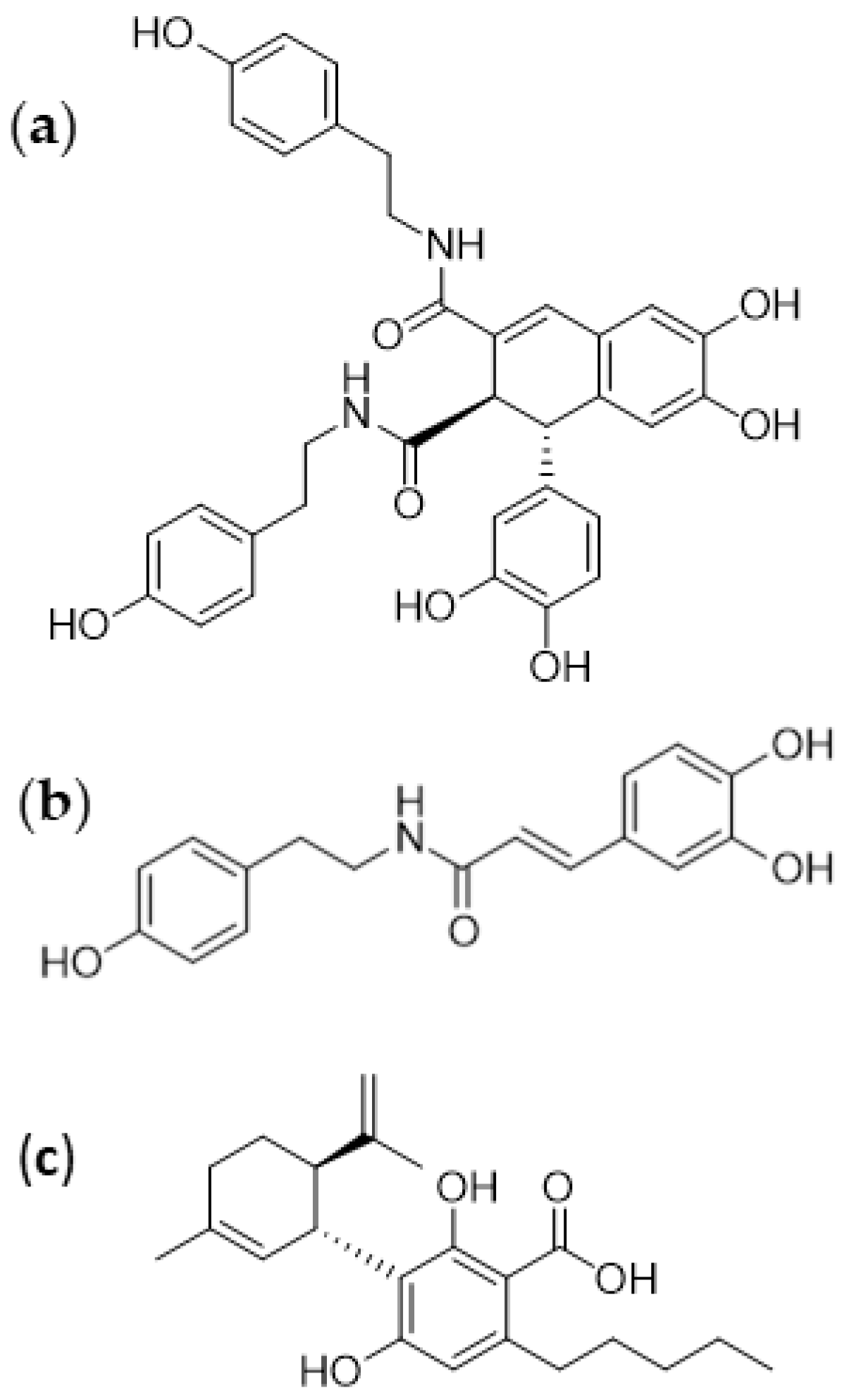
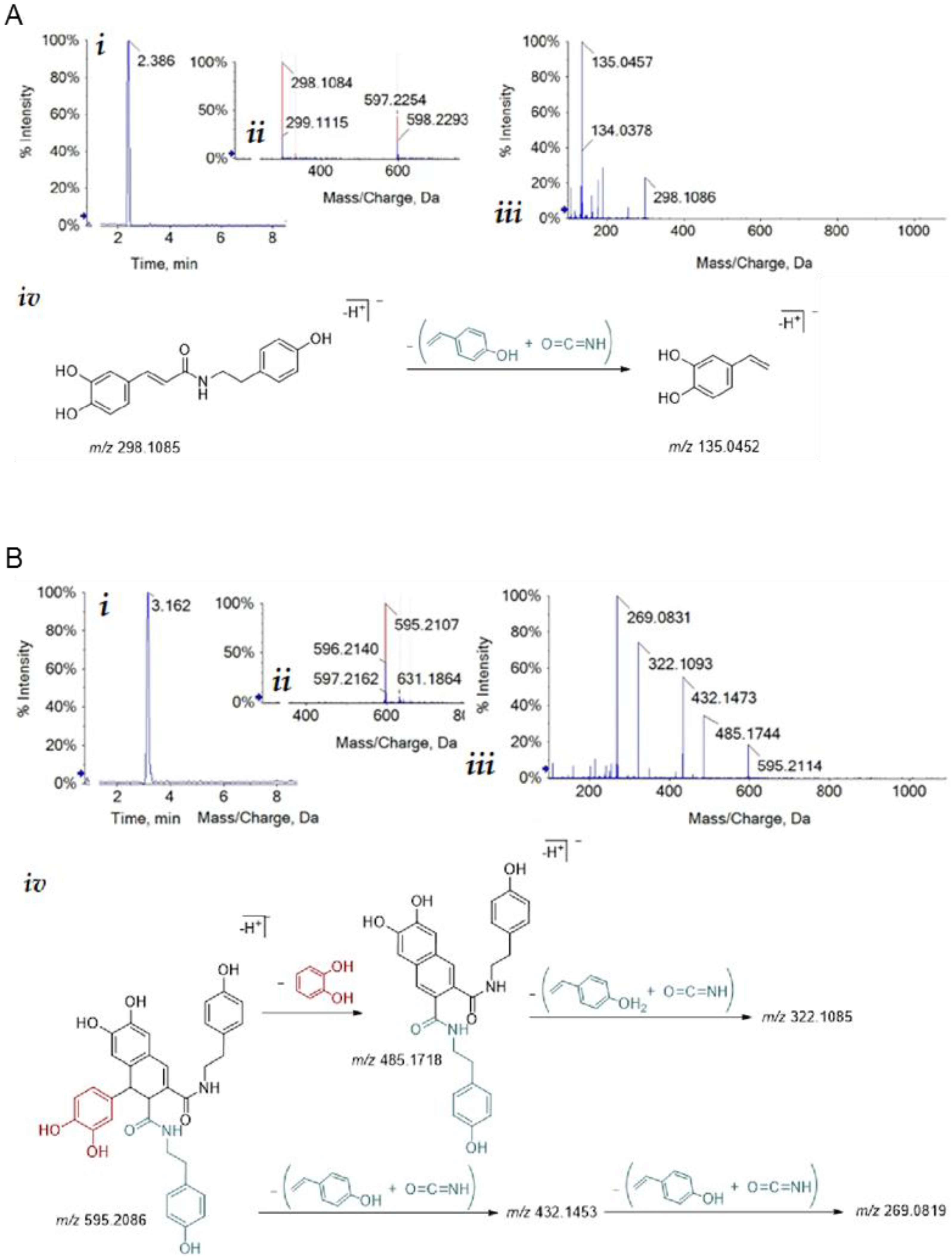
2.1. Isolation of Pure Compounds from Hemp Seeds
2.2. Cell Cultures
2.3. MTT Assay
2.4. RNA Isolation, Sequencing and Data Analysis
3. Results
3.1. Isolation of Bioactive Compounds from Hemp Seeds
3.2. Cytotoxicity Assays
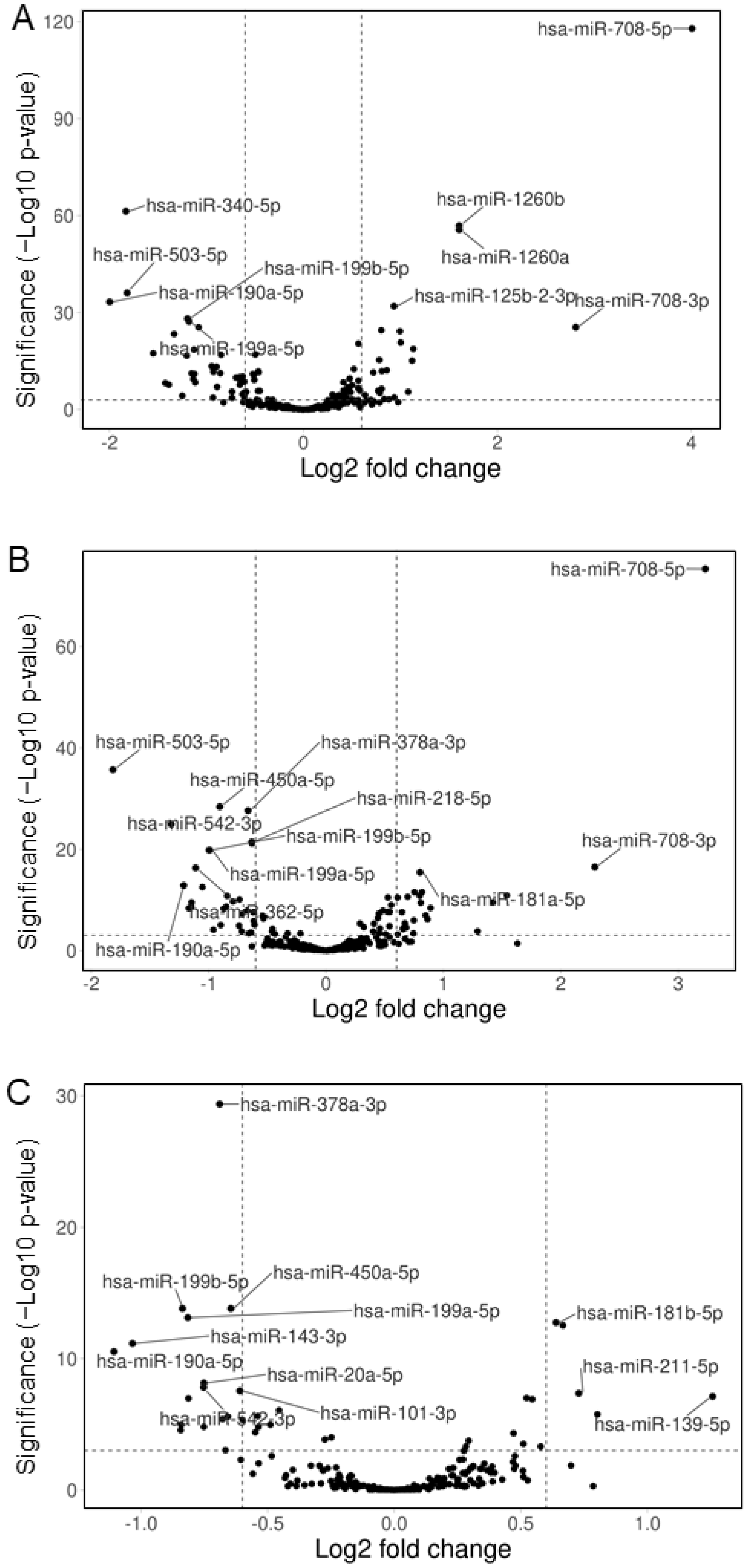
3.3. Hemp Seed Metabolites Change the microRNA Expression Profiles of Neural Cells
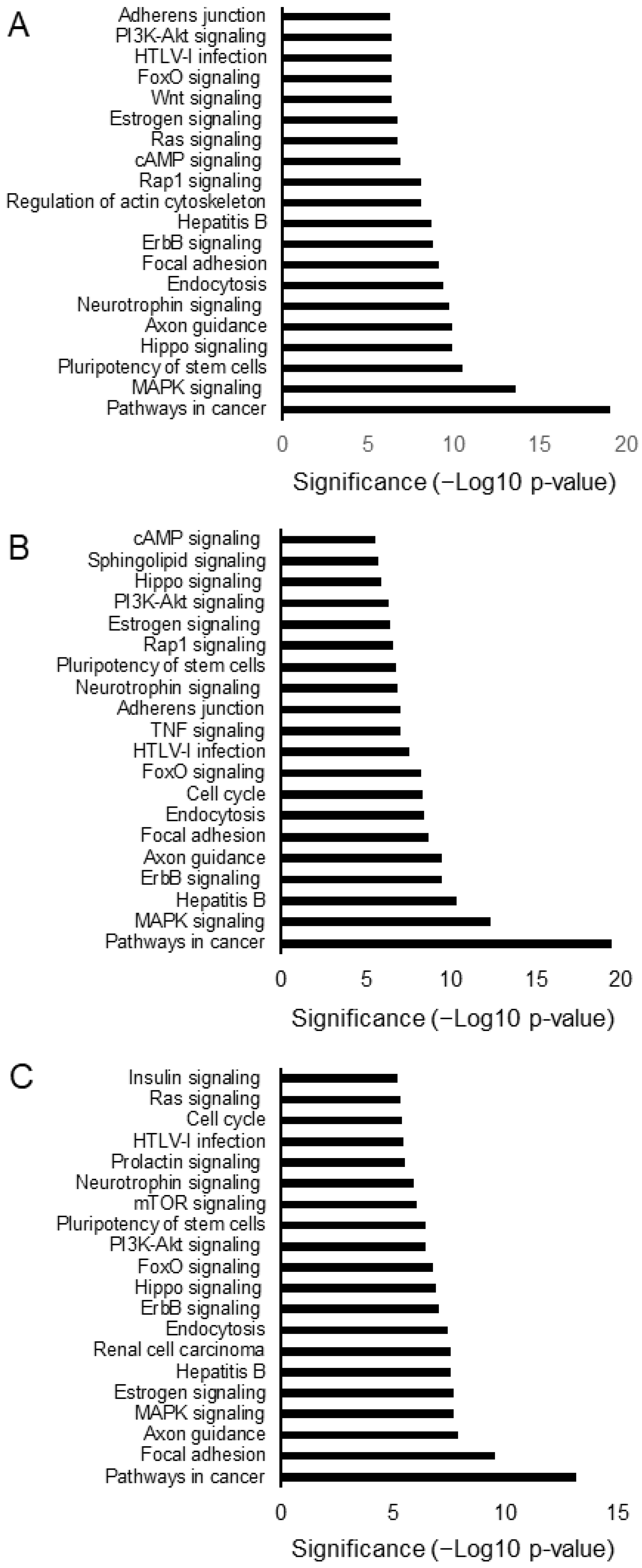
3.4. Comparison of the Effects of the Three Specialized Hemp Seed Metabolites
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lei, P.; Ayton, S.; Bush, A.I. The Essential Elements of Alzheimer’s Disease. J. Biol. Chem. 2021, 296, 100105. [Google Scholar] [CrossRef]
- Wei, W.; Wang, Z.-Y.; Ma, L.-N.; Zhang, T.-T.; Cao, Y.; Li, H. MicroRNAs in Alzheimer’s Disease: Function and Potential Applications as Diagnostic Biomarkers. Front. Mol. Neurosci. 2020, 13, 160. [Google Scholar] [CrossRef]
- Kou, X.; Chen, D.; Chen, N. The Regulation of MicroRNAs in Alzheimer’s Disease. Front. Neurol. 2020, 11, 288. [Google Scholar] [CrossRef]
- Tsarbopoulos, A. Alzheimer’s Disease: Exploring Nature’s “medicinal Chest” for New Therapeutic Agents. Biomol. Concepts 2020, 11, 201–208. [Google Scholar] [CrossRef]
- Wang, Q.; Dong, X.; Zhang, R.; Zhao, C. Flavonoids with Potential Anti-Amyloidogenic Effects as Therapeutic Drugs for Treating Alzheimer’s Disease. J. Alzheimer’s Dis. 2021, 84, 505–533. [Google Scholar] [CrossRef] [PubMed]
- Gregory, J.; Vengalasetti, Y.V.; Bredesen, D.E.; Rao, R.V. Neuroprotective Herbs for the Management of Alzheimer’s Disease. Biomolecules 2021, 11, 543. [Google Scholar] [CrossRef]
- Abate, G.; Uberti, D.; Tambaro, S. Potential and Limits of Cannabinoids in Alzheimer’s Disease Therapy. Biology 2021, 10, 542. [Google Scholar] [CrossRef]
- Pérez-Olives, C.; Rivas-Santisteban, R.; Lillo, J.; Navarro, G.; Franco, R. Recent Advances in the Potential of Cannabinoids for Neuroprotection in Alzheimer’s, Parkinson’s, and Huntington’s Diseases. Adv. Exp. Med. Biol. 2021, 1264, 81–92. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Liu, Y.; Tian, D.; Tian, L.; Ju, X.; Qi, L.; Wang, Y.; Liang, C. Overview of Cannabidiol (CBD) and Its Analogues: Structures, Biological Activities, and Neuroprotective Mechanisms in Epilepsy and Alzheimer’s Disease. Eur. J. Med. Chem. 2020, 192, 112163. [Google Scholar] [CrossRef] [PubMed]
- Olatunji, O.J.; Chen, H.; Zhou, Y. Neuroprotective Effect of Trans-N-Caffeoyltyramine from Lycium Chinense against H2O2 Induced Cytotoxicity in PC12 Cells by Attenuating Oxidative Stress. Biomed. Pharm. 2017, 93, 895–902. [Google Scholar] [CrossRef]
- Jiang, Y.; Yu, L.; Wang, M.-H. N-Trans-Feruloyltyramine Inhibits LPS-Induced NO and PGE2 Production in RAW 264.7 Macrophages: Involvement of AP-1 and MAP Kinase Signalling Pathways. Chem. Biol. Interact. 2015, 235, 56–62. [Google Scholar] [CrossRef] [PubMed]
- Thangnipon, W.; Suwanna, N.; Kitiyanant, N.; Soi-Ampornkul, R.; Tuchinda, P.; Munyoo, B.; Nobsathian, S. Protective Role of N-Trans-Feruloyltyramine against β-Amyloid Peptide-Induced Neurotoxicity in Rat Cultured Cortical Neurons. Neurosci. Lett. 2012, 513, 229–232. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Rimando, A.M.; Levenson, A.S. Resveratrol and Pterostilbene as a MicroRNA-Mediated Chemopreventive and Therapeutic Strategy in Prostate Cancer. Ann. N. Y. Acad. Sci. 2017, 1403, 15–26. [Google Scholar] [CrossRef]
- Sayeed, M.A.; Bracci, M.; Lucarini, G.; Lazzarini, R.; Di Primio, R.; Santarelli, L. Regulation of MicroRNA Using Promising Dietary Phytochemicals: Possible Preventive and Treatment Option of Malignant Mesothelioma. Biomed. Pharmacother. 2017, 94, 1197–1224. [Google Scholar] [CrossRef]
- Kang, H. MicroRNA-Mediated Health-Promoting Effects of Phytochemicals. Int. J. Mol. Sci. 2019, 20, 2535. [Google Scholar] [CrossRef]
- Kim, D.H.; Khan, H.; Ullah, H.; Hassan, S.T.S.; Šmejkal, K.; Efferth, T.; Mahomoodally, M.F.; Xu, S.; Habtemariam, S.; Filosa, R.; et al. MicroRNA Targeting by Quercetin in Cancer Treatment and Chemoprotection. Pharmacol. Res. 2019, 147, 104346. [Google Scholar] [CrossRef]
- Zappavigna, S.; Vanacore, D.; Lama, S.; Potenza, N.; Russo, A.; Ferranti, P.; Dallio, M.; Federico, A.; Loguercio, C.; Sperlongano, P.; et al. Silybin-Induced Apoptosis Occurs in Parallel to the Increase of Ceramides Synthesis and MiRNAs Secretion in Human Hepatocarcinoma Cells. Int. J. Mol. Sci. 2019, 20, 2190. [Google Scholar] [CrossRef]
- Mathers, J.C. Nutrigenomics in the Modern Era. Proc. Nutr. Soc. 2017, 76, 265–275. [Google Scholar] [CrossRef]
- Crescente, G.; Piccolella, S.; Esposito, A.; Scognamiglio, M.; Fiorentino, A.; Pacifico, S. Chemical Composition and Nutraceutical Properties of Hempseed: An Ancient Food with Actual Functional Value. Phytochem. Rev. 2018, 17, 733–749. [Google Scholar] [CrossRef]
- Faugno, S.; Piccolella, S.; Sannino, M.; Principio, L.; Crescente, G.; Baldi, G.M.; Fiorentino, N.; Pacifico, S. Can Agronomic Practices and Cold-Pressing Extraction Parameters Affect Phenols and Polyphenols Content in Hempseed Oils? Ind. Crops Prod. 2019, 130, 511–519. [Google Scholar] [CrossRef]
- Piccolella, S.; Formato, M.; Pecoraro, M.T.; Crescente, G.; Pacifico, S. Discrimination of CBD-, THC- and CBC-Type Acid Cannabinoids through Diagnostic Ions by UHPLC-HR-MS/MS in Negative Ion Mode. J. Pharm. Biomed. Anal. 2021, 201, 114125. [Google Scholar] [CrossRef] [PubMed]
- Formato, M.; Crescente, G.; Scognamiglio, M.; Fiorentino, A.; Pecoraro, M.T.; Piccolella, S.; Catauro, M.; Pacifico, S. Cannabidiolic Acid, a Still Overlooked Bioactive Compound: An Introductory Review and Preliminary Research. Molecules 2020, 25, 2638. [Google Scholar] [CrossRef]
- Aparicio-Puerta, E.; Lebrón, R.; Rueda, A.; Gómez-Martín, C.; Giannoukakos, S.; Jaspez, D.; Medina, J.M.; Zubkovic, A.; Jurak, I.; Fromm, B.; et al. SRNAbench and SRNAtoolbox 2019: Intuitive Fast Small RNA Profiling and Differential Expression. Nucleic. Acids Res. 2019, 47, W530–W535. [Google Scholar] [CrossRef]
- Love, M.I.; Huber, W.; Anders, S. Moderated Estimation of Fold Change and Dispersion for RNA-Seq Data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef] [PubMed]
- Nigro, E.; Pecoraro, M.T.; Formato, M.; Piccolella, S.; Ragucci, S.; Mallardo, M.; Russo, R.; Di Maro, A.; Daniele, A.; Pacifico, S. Cannabidiolic Acid in Hemp Seed Oil Table Spoon and Beyond. Molecules 2022, 27, 2566. [Google Scholar] [CrossRef]
- Nigro, E.; Crescente, G.; Formato, M.; Pecoraro, M.T.; Mallardo, M.; Piccolella, S.; Daniele, A.; Pacifico, S. Hempseed Lignanamides Rich-Fraction: Chemical Investigation and Cytotoxicity towards U-87 Glioblastoma Cells. Molecules 2020, 25, 1049. [Google Scholar] [CrossRef]
- Goedhart, J.; Luijsterburg, M.S. VolcaNoseR Is a Web App for Creating, Exploring, Labeling and Sharing Volcano Plots. Sci. Rep. 2020, 10, 20560. [Google Scholar] [CrossRef]
- Sticht, C.; De La Torre, C.; Parveen, A.; Gretz, N. MiRWalk: An Online Resource for Prediction of MicroRNA Binding Sites. PLoS ONE 2018, 13, e0206239. [Google Scholar] [CrossRef]
- Huang, D.W.; Sherman, B.T.; Lempicki, R.A. Systematic and Integrative Analysis of Large Gene Lists Using DAVID Bioinformatics Resources. Nat. Protoc. 2009, 4, 44–57. [Google Scholar] [CrossRef] [PubMed]
- McIlwraith, E.K.; Lieu, C.V.; Belsham, D.D. Bisphenol A Induces MiR-708-5p through an ER Stress-Mediated Mechanism Altering Neuronatin and Neuropeptide Y Expression in Hypothalamic Neuronal Models. Mol. Cell Endocrinol. 2022, 539, 111480. [Google Scholar] [CrossRef] [PubMed]
- Burgos, K.; Malenica, I.; Metpally, R.; Courtright, A.; Rakela, B.; Beach, T.; Shill, H.; Adler, C.; Sabbagh, M.; Villa, S.; et al. Profiles of Extracellular MiRNA in Cerebrospinal Fluid and Serum from Patients with Alzheimer’s and Parkinson’s Diseases Correlate with Disease Status and Features of Pathology. PLoS ONE 2014, 9, e94839. [Google Scholar] [CrossRef]
- Song, D.; Li, G.; Hong, Y.; Zhang, P.; Zhu, J.; Yang, L.; Huang, J. MiR-199a Decreases Neuritin Expression Involved in the Development of Alzheimer’s Disease in APP/PS1 Mice. Int. J. Mol. Med. 2020, 46, 384–396. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Yuan, X.; Bai, J.; Han, R.; Li, Z.; Zhang, H.; Xiu, R. MicroRNA-181a Protects against Pericyte Apoptosis via Directly Targeting FOXO1: Implication for Ameliorated Cognitive Deficits in APP/PS1 Mice. Aging 2019, 11, 6120–6133. [Google Scholar] [CrossRef] [PubMed]
- Serpente, M.; Fenoglio, C.; D’Anca, M.; Arcaro, M.; Sorrentino, F.; Visconte, C.; Arighi, A.; Fumagalli, G.G.; Porretti, L.; Cattaneo, A.; et al. MiRNA Profiling in Plasma Neural-Derived Small Extracellular Vesicles from Patients with Alzheimer’s Disease. Cells 2020, 9, 1443. [Google Scholar] [CrossRef] [PubMed]
- Cheng, L.; Doecke, J.D.; Sharples, R.A.; Villemagne, V.L.; Fowler, C.J.; Rembach, A.; Martins, R.N.; Rowe, C.C.; Macaulay, S.L.; Masters, C.L.; et al. Prognostic Serum MiRNA Biomarkers Associated with Alzheimer’s Disease Shows Concordance with Neuropsychological and Neuroimaging Assessment. Mol. Psychiatry 2015, 20, 1188–1196. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.; Jia, N.; Li, R.; Zhang, Z.; Zhong, Y.; Han, K. MiR-143-3p Inhibition Promotes Neuronal Survival in an Alzheimer’s Disease Cell Model by Targeting Neuregulin-1. Folia Neuropathol. 2020, 58, 10–21. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Di Palo, A.; Siniscalchi, C.; Crescente, G.; De Leo, I.; Fiorentino, A.; Pacifico, S.; Russo, A.; Potenza, N. Effect of Cannabidiolic Acid, N-Trans-Caffeoyltyramine and Cannabisin B from Hemp Seeds on microRNA Expression in Human Neural Cells. Curr. Issues Mol. Biol. 2022, 44, 5106-5116. https://doi.org/10.3390/cimb44100347
Di Palo A, Siniscalchi C, Crescente G, De Leo I, Fiorentino A, Pacifico S, Russo A, Potenza N. Effect of Cannabidiolic Acid, N-Trans-Caffeoyltyramine and Cannabisin B from Hemp Seeds on microRNA Expression in Human Neural Cells. Current Issues in Molecular Biology. 2022; 44(10):5106-5116. https://doi.org/10.3390/cimb44100347
Chicago/Turabian StyleDi Palo, Armando, Chiara Siniscalchi, Giuseppina Crescente, Ilenia De Leo, Antonio Fiorentino, Severina Pacifico, Aniello Russo, and Nicoletta Potenza. 2022. "Effect of Cannabidiolic Acid, N-Trans-Caffeoyltyramine and Cannabisin B from Hemp Seeds on microRNA Expression in Human Neural Cells" Current Issues in Molecular Biology 44, no. 10: 5106-5116. https://doi.org/10.3390/cimb44100347
APA StyleDi Palo, A., Siniscalchi, C., Crescente, G., De Leo, I., Fiorentino, A., Pacifico, S., Russo, A., & Potenza, N. (2022). Effect of Cannabidiolic Acid, N-Trans-Caffeoyltyramine and Cannabisin B from Hemp Seeds on microRNA Expression in Human Neural Cells. Current Issues in Molecular Biology, 44(10), 5106-5116. https://doi.org/10.3390/cimb44100347









