Expression of AKT1 Related with Clinicopathological Parameters in Clear Cell Renal Cell Carcinoma
Abstract
1. Introduction
2. Materials and Methods
2.1. Evidence Acquisition via Analysis of Three RNA-Seq Datasets
2.2. Clinical Tissue Samples
2.3. Immunohistochemical Staining and Analysis
2.4. Statistical Analysis
3. Results
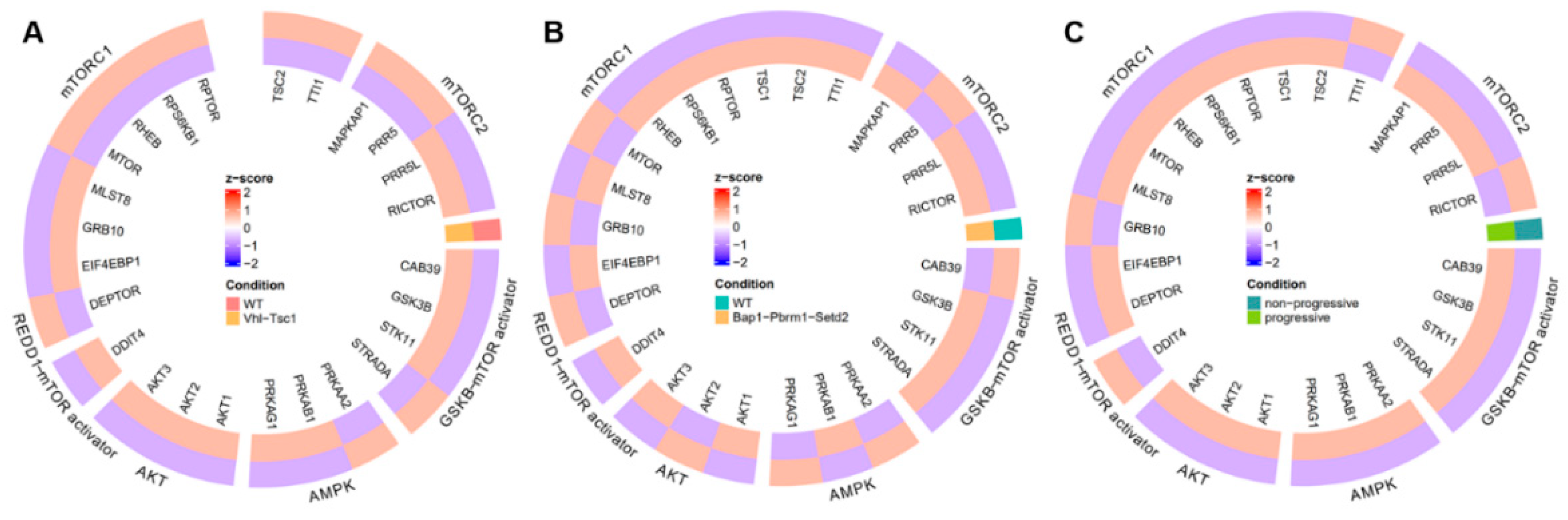
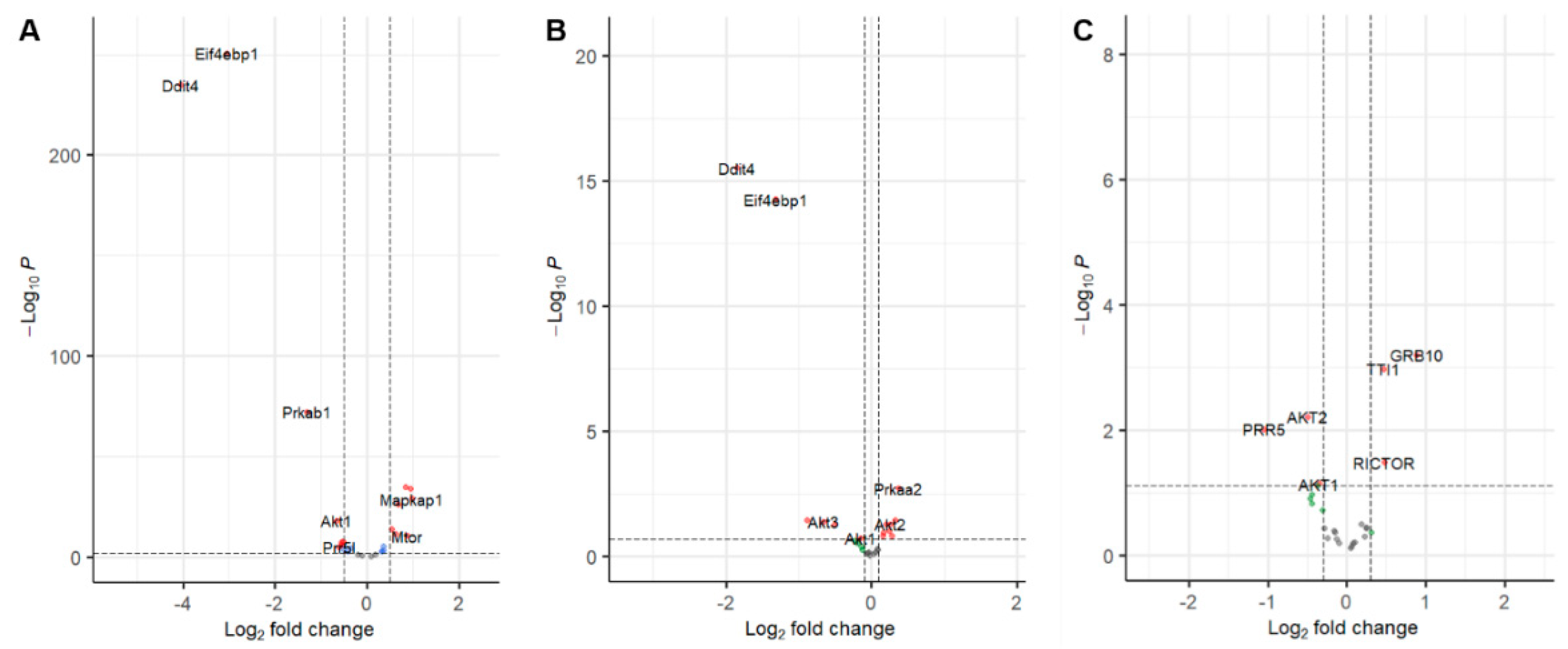
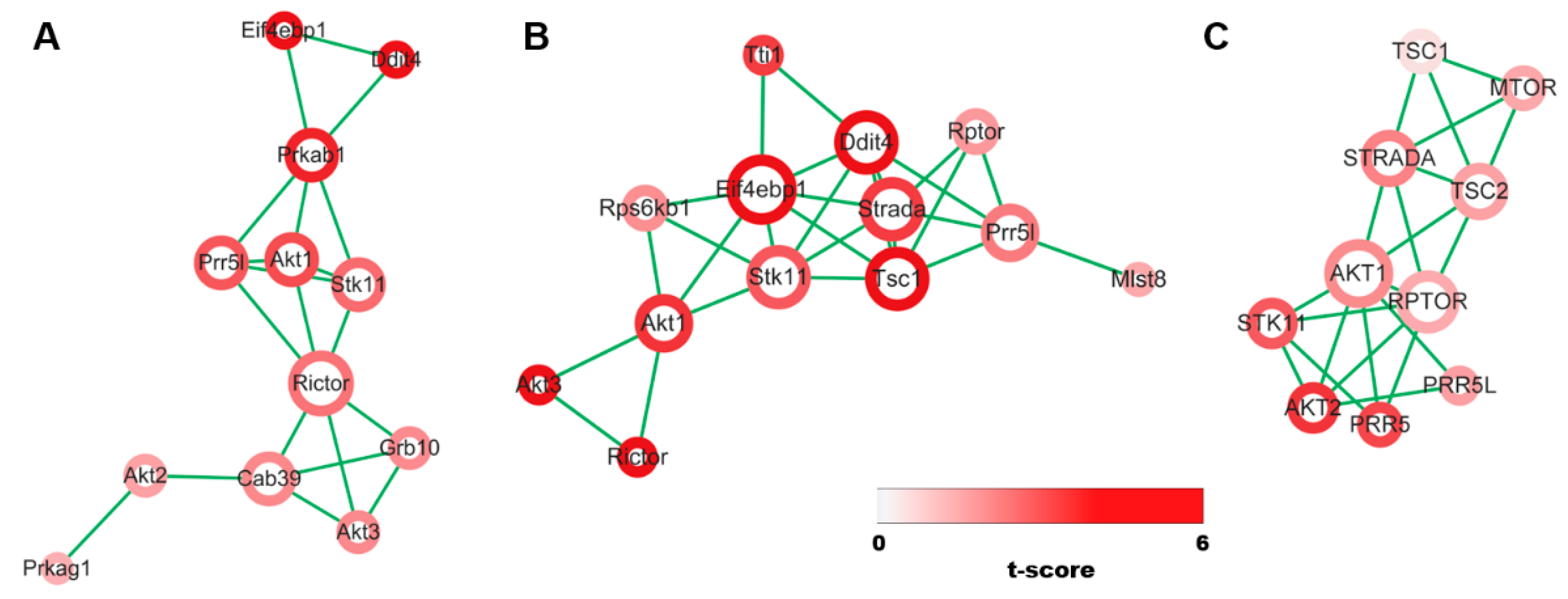
3.1. Evidence Synthesis
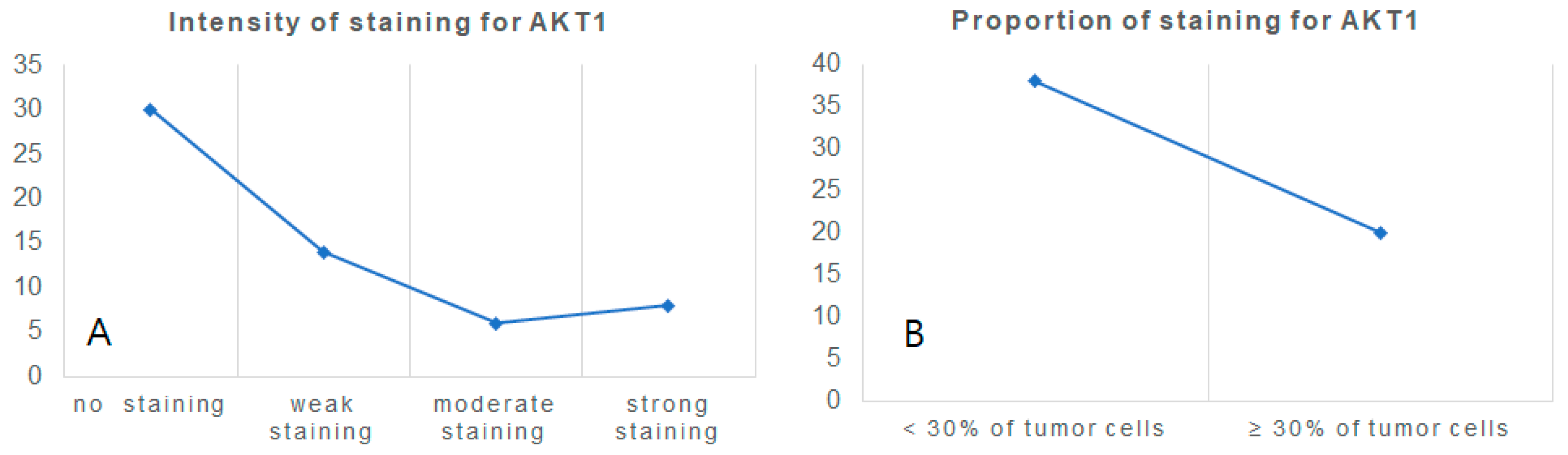
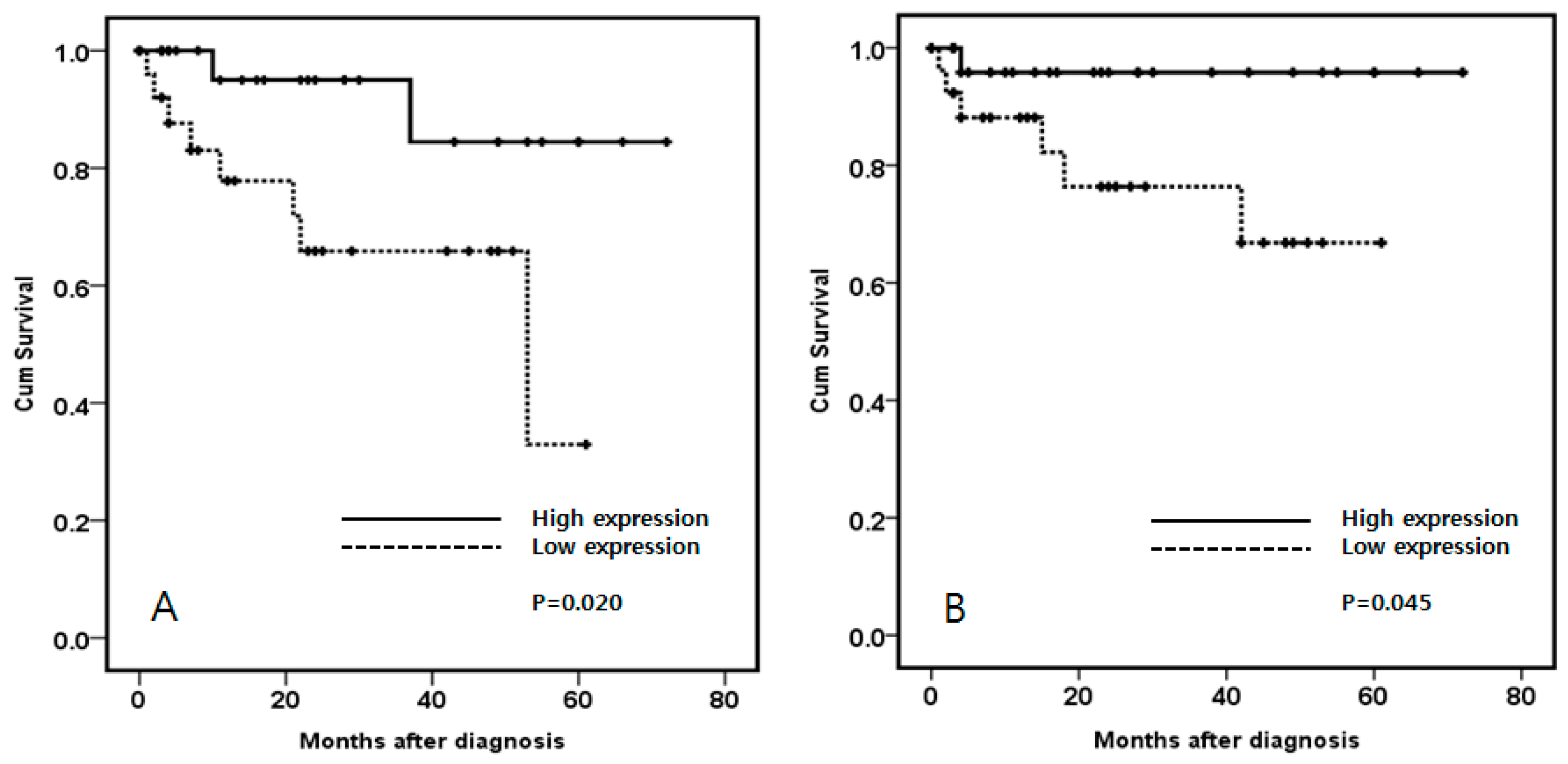
3.2. Specimen Experiment: AKT1 Expression in Clear Cell Renal Cell Carcinoma
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer Statistics. CA Cancer J. Clin. 2017, 67, 7–30. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, L.S.; Linehan, W.M. Genetic predisposition to kidney cancer. Semin. Oncol. 2016, 43, 566–574. [Google Scholar] [CrossRef] [PubMed]
- Kümmerlin, I.P.; Kate, F.J.T.; Wijkstra, H.; De La Rosette, J.J.; Laguna, M.P. Changes in the stage and surgical management of renal tumours during 1995-2005: An analysis of the Dutch national histopathology registry. Br. J. Urol. 2008, 102, 946–951. [Google Scholar] [CrossRef]
- Motzer, R.J.; Russo, P. Systemic Therapy For Renal Cell Carcinoma. J. Urol. 2000, 163, 408–417. [Google Scholar] [CrossRef]
- Chen, W.S.; Xu, P.-Z.; Gottlob, K.; Chen, M.-L.; Sokol, K.; Shiyanova, T.; Roninson, I.; Weng, W.; Suzuki, R.; Tobe, K.; et al. Growth retardation and increased apoptosis in mice with homozygous disruption of the akt1 gene. Genes Dev. 2001, 15, 2203–2208. [Google Scholar] [CrossRef]
- Toker, A. Achieving specificity in Akt signaling in cancer. Adv. Biol. Regul. 2012, 52, 78–87. [Google Scholar] [CrossRef]
- Riggio, M.; Polo, M.L.; Blaustein, M.; Colman-Lerner, A.; Lüthy, I.; Lanari, C.; Novaro, V. PI3K/AKT pathway regulates phosphorylation of steroid receptors, hormone independence and tumor differentiation in breast cancer. Carcinogenesis 2011, 33, 509–518. [Google Scholar] [CrossRef]
- Riggio, M.; Perrone, M.C.; Polo, M.L.; Rodriguez, M.J.; May, M.; Abba, M.; Lanari, C.; Novaro, V. AKT1 and AKT2 isoforms play distinct roles during breast cancer progression through the regulation of specific downstream proteins. Sci. Rep. 2017, 7, 44244. [Google Scholar] [CrossRef]
- Guarnieri, J.W.; Dybas, J.M.; Fazelinia, H.; Kim, M.S.; Frere, J.; Zhang, Y.; Albrecht, Y.S.; Murdock, D.G.; Angelin, A.; Singh, L.N.; et al. Targeted Down Regulation Of Core Mitochondrial Genes During SARS-CoV-2 Infection. bioRxiv 2022. [Google Scholar] [CrossRef]
- Love, M.I.; Huber, W.; Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef] [PubMed]
- Shannon, P.; Markiel, A.; Ozier, O.; Baliga, N.S.; Wang, J.T.; Ramage, D.; Amin, N.; Schwikowski, B.; Ideker, T. Cytoscape: A software environment for integrated models of Biomolecular Interaction Networks. Genome Res. 2003, 13, 2498–2504. [Google Scholar] [CrossRef] [PubMed]
- Edge, S.B.; Compton, C.C. The American Joint Committee on Cancer: The 7th Edition of the AJCC Cancer Staging Manual and the Future of TNM. Ann. Surg. Oncol. 2010, 17, 1471–1474. [Google Scholar] [CrossRef] [PubMed]
- Whitman, M.; Kaplan, D.R.; Schaffhausen, B.; Cantley, L.; Roberts, T.M. Association of phosphatidylinositol kinase activity with polyoma middle-T competent for transformation. Nature 1985, 315, 239–242. [Google Scholar] [CrossRef] [PubMed]
- Samuels, Y.; Wang, Z.; Bardelli, A.; Silliman, N.; Ptak, J.; Szabo, S.; Yan, H.; Gazdar, A.; Powell, S.M.; Riggins, G.J.; et al. High Frequency of Mutations of the PIK3CA Gene in Human Cancers. Science 2004, 304, 554. [Google Scholar] [CrossRef]
- Lee, J.W.; Soung, Y.H.; Kim, S.Y.; Lee, H.W.; Park, W.S.; Nam, S.W.; Kim, S.H.; Lee, J.Y.; Yoo, N.J.; Lee, S.H. PIK3CA gene is frequently mutated in breast carcinomas and hepatocellular carcinomas. Oncogene 2004, 24, 1477–1480. [Google Scholar] [CrossRef]
- Somanath, P.R.; Razorenova, O.V.; Chen, J.; Byzova, T.V. Akt1 in endothelial cell and angiogenesis. Cell Cycle 2006, 5, 512–518. [Google Scholar] [CrossRef]
- Hollander, M.C.; Maier, C.R.; Hobbs, E.A.; Ashmore, A.R.; Linnoila, R.I.; Dennis, P.A. Akt1 deletion prevents lung tumorigenesis by mutant K-ras. Oncogene 2011, 30, 1812–1821. [Google Scholar] [CrossRef]
- Staal, S.P. Molecular cloning of the akt oncogene and its human homologues AKT1 and AKT2: Amplification of AKT1 in a primary human gastric adenocarcinoma. Proc. Natl. Acad. Sci. USA 1987, 84, 5034–5037. [Google Scholar] [CrossRef]
- Chen, L.; Kang, Q.-H.; Chen, Y.; Zhang, Y.-H.; Li, Q.; Xie, S.-Q.; Wang, C.-J. Distinct roles of Akt1 in regulating proliferation, migration and invasion in HepG2 and HCT 116 cells. Oncol. Rep. 2013, 31, 737–744. [Google Scholar] [CrossRef]
- Mancini, M.L.; Lien, E.C.; Toker, A. Oncogenic AKT1(E17K) mutation induces mammary hyperplasia but prevents HER2-driven tumorigenesis. Oncotarget 2016, 7, 17301–17313. [Google Scholar] [CrossRef]
- Phung, T.L.; Du, W.; Xue, Q.; Ayyaswamy, S.; Gerald, D.; Antonello, Z.; Nhek, S.; Perruzzi, C.A.; Acevedo, I.; Ramanna-Valmiki, R.; et al. Akt1 and akt3 exert opposing roles in the regulation of vascular tumor growth. Cancer Res. 2015, 75, 40–50. [Google Scholar] [CrossRef] [PubMed]
- Son, J.Y.; Yoon, S.; Tae, I.H.; Park, Y.J.; De, U.; Jeon, Y.; Park, Y.J.; Rhyu, I.J.; Lee, B.M.; Chung, K.-H.; et al. Novel therapeutic roles of MC-4 in combination with everolimus against advanced renal cell carcinoma by dual targeting of Akt/pyruvate kinase muscle isozyme M2 and mechanistic target of rapamycin complex 1 pathways. Cancer Med. 2018, 7, 5083–5095. [Google Scholar] [CrossRef] [PubMed]
- Ha, Y.-S.; Kim, Y.-Y.; Yu, N.H.; Chun, S.Y.; Choi, S.H.; Lee, J.N.; Kim, B.S.; Yoo, E.S.; Kwon, T.G. Down-regulation of transient receptor potential melastatin member 7 prevents migration and invasion of renal cell carcinoma cells via inactivation of the Src and Akt pathway. Investig. Clin. Urol. 2018, 59, 263–274. [Google Scholar] [CrossRef] [PubMed]
- Nam, H.K.; Jeong, S.R.; Pyo, M.C.; Ha, S.K.; Nam, M.H.; Lee, K.W. Methylglyoxal-Derived Advanced Glycation End Products (AGE4) Promote Cell Proliferation and Survival in Renal Cell Carcinoma Cells through the RAGE/Akt/ERK Signaling Pathways. Biol. Pharm. Bull. 2021, 44, 1697–1706. [Google Scholar] [CrossRef] [PubMed]
- Fan, D.; Liu, Q.; Wu, F.; Liu, N.; Qu, H.; Yuan, Y.; Li, Y.; Gao, H.; Ge, J.; Xu, Y.; et al. Prognostic significance of PI3K/AKT/mTOR signaling pathway members in clear cell renal cell carcinoma. PeerJ 2020, 8, e9261. [Google Scholar] [CrossRef] [PubMed]


| Low Expression of AKT1 (n = 30) | High Expression of AKT1 (n = 28) | p-Value | |
|---|---|---|---|
| Age (mean ± SD) | 60.40 ± 14.122 | 61.00 ± 11.165 | 0.859 |
| Sex (Male: Female) | 20:10 | 18:10 | 0.534 |
| WHO/ISUP grade | 0.040 | ||
| Grade I | 0 | 1 | |
| Grade II | 8 | 16 | |
| Grade III | 19 | 9 | |
| Grade IV | 3 | 2 | |
| T stage | 0.315 | ||
| T1 | 19 | 23 | |
| T2 | 4 | 1 | |
| T3 | 7 | 4 | |
| T4 | 0 | ||
| N stage | 0.526 | ||
| N0 | 28 | 27 | |
| N1 | 2 | 1 | |
| M stage | |||
| M0 | 28 | 27 | 0.526 |
| M1 | 2 | 1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Choi, T.; Yoo, K.H.; Kim, M.S. Expression of AKT1 Related with Clinicopathological Parameters in Clear Cell Renal Cell Carcinoma. Curr. Issues Mol. Biol. 2022, 44, 4921-4929. https://doi.org/10.3390/cimb44100334
Choi T, Yoo KH, Kim MS. Expression of AKT1 Related with Clinicopathological Parameters in Clear Cell Renal Cell Carcinoma. Current Issues in Molecular Biology. 2022; 44(10):4921-4929. https://doi.org/10.3390/cimb44100334
Chicago/Turabian StyleChoi, Taesoo, Koo Han Yoo, and Man S. Kim. 2022. "Expression of AKT1 Related with Clinicopathological Parameters in Clear Cell Renal Cell Carcinoma" Current Issues in Molecular Biology 44, no. 10: 4921-4929. https://doi.org/10.3390/cimb44100334
APA StyleChoi, T., Yoo, K. H., & Kim, M. S. (2022). Expression of AKT1 Related with Clinicopathological Parameters in Clear Cell Renal Cell Carcinoma. Current Issues in Molecular Biology, 44(10), 4921-4929. https://doi.org/10.3390/cimb44100334





