Possibility of SARS-CoV-2 Infection in the Metastatic Microenvironment of Cancer
Abstract
:1. Introduction
2. Materials and Methods
2.1. Case Selection for Immunohistochemical Staining
2.2. Antibodies and Immunohistochemistry (IHC)
2.3. Ethical Approval and Consent to Participate
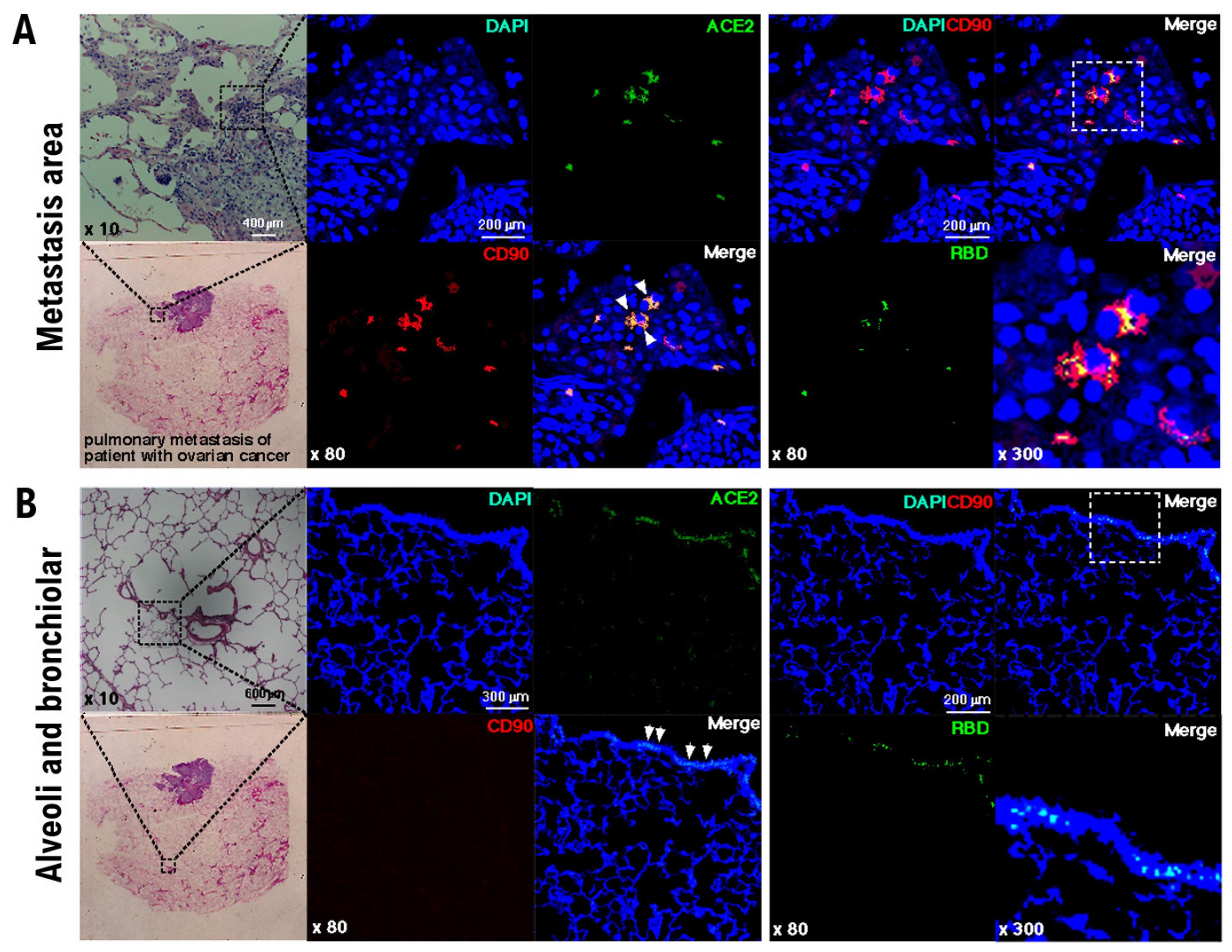
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kuderer, N.M.; Choueiri, T.K.; Shah, D.P.; Shyr, Y.; Rubinstein, S.M.; Rivera, D.R.; Shete, S.; Hsu, C.Y.; Desai, A.; de Lima Lopes, G., Jr.; et al. Clinical impact of COVID-19 on patients with cancer (CCC19): A cohort study. Lancet 2020, 395, 1907–1918. [Google Scholar] [CrossRef]
- Moris, D.; Tsilimigras, D.I.; Schizas, D. Cancer and COVID-19. Lancet 2020, 396, 1066. [Google Scholar] [CrossRef]
- Kudo, K.; Ichihara, E. COVID-19 Pneumonia. Gan Kagaku Ryoho 2020, 47, 1657–1661. [Google Scholar]
- Cesaro, S.; Giacchino, M.; Fioredda, F.; Barone, A.; Battisti, L.; Bezzio, S.; Frenos, S.; De Santis, R.; Livadiotti, S.; Marinello, S.; et al. Guidelines on vaccinations in paediatric haematology and oncology patients. Biomed. Res. Int. 2014, 2014, 707691. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salahudeen, A.A.; Choi, S.S.; Rustagi, A.; Zhu, J.; van Unen, V.; Sean, M.; Flynn, R.A.; Margalef-Català, M.; Santos, A.J.; Ju, J.; et al. Progenitor identification and SARS-CoV-2 infection in human distal lung organoids. Nature 2020, 588, 670–675. [Google Scholar] [CrossRef] [PubMed]
- Katsura, H.; Sontake, V.; Tata, A.; Kobayashi, Y.; Edwards, C.E.; Heaton, B.E.; Konkimalla, A.; Asakura, T.; Mikami, Y.; Fritch, E.J.; et al. Human Lung Stem Cell-Based Alveolospheres Provide Insights into SARS-CoV-2-Mediated Interferon Responses and Pneumocyte Dysfunction. Cell Stem Cell 2020, 27, 890–904.e8. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, T.; Sano, K.; Aburatani, H.; Yaegashi, N.; Konishi, I. Initialization of epithelial cells by tumor cells in a metastatic microenvironment. Oncogene 2020, 39, 2638–2640. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, T.; Sano, K.; Ichimura, T.; Kanai, Y.; Zharhary, D.; Aburatani, H.; Yaegashi, N.; Konishi, I. Characteristics of Leiomyosarcoma: Induction of Hematogenous Metastasis by Isolated Uterine Mesenchymal Tumor Stem-like Cells. Anticancer Res. 2020, 40, 1255–1265. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Horiuchi, A.; Hayashi, T.; Kikuchi, N.; Hayashi, A.; Fuseya, C.; Shiozawa, T.; Konishi, I. Hypoxia upregulates ovarian cancer invasiveness via the binding of HIF-1α to a hypoxia-induced, methylation-free hypoxia response element of S100A4 gene. Int. J. Cancer 2012, 131, 1755–1767. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ombrato, L.; Nolan, E.; Kurelac, I.; Mavousian, A.; Bridgeman, V.L.; Heinze, I.; Chakravarty, P.; Horswell, S.; Gonzalez-Gualda, E.; Matacchione, G.; et al. Metastatic-niche labelling reveals parenchymal cells with stem features. Nature 2019, 572, 603–608. [Google Scholar] [CrossRef] [PubMed]
- Yao, X.H.; He, Z.C.; Li, T.Y.; Zhang, H.R.; Wang, Y.; Mou, H.; Guo, Q.; Yu, S.C.; Ding, Y.; Liu, X.; et al. Pathological evidence for residual SARS-CoV-2 in pulmonary tissues of a ready-for-discharge patient. Cell Res. 2020, 30, 541–543. [Google Scholar] [CrossRef] [PubMed]
- Shiozawa, Y.; Pedersen, E.A.; Havens, A.M.; Jung, Y.; Mishra, A.; Joseph, J.; Kim, J.K.; Patel, L.R.; Ying, C.; Ziegler, A.M.; et al. Human prostate cancer metastases target the hematopoietic stem cell niche to establish footholds in mouse bone marrow. J. Clin. Investig. 2011, 121, 1298–1312. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Asselin-Labat, M.L. Cells tagged near an early spread of cancer. Nature 2019, 572, 589–590. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Link, T.; Kuhlmann, J.D.; Kobelt, D.; Herrmann, P.; Vassileva, Y.D.; Kramer, M.; Frank, K.; Göckenjan, M.; Wimberger, P.; Stein, U. Clinical relevance of circulating MACC1 and S100A4 transcripts for ovarian cancer. Mol. Oncol. 2019, 13, 1268–1279. [Google Scholar] [CrossRef] [PubMed]
- Maděrka, M.; Pilka, R.; Neubert, D.; Hambálek, J. New serum tumor markers S100, TFF3 and AIF-1 and their possible use in oncogynecology. Ceska Gynekol. 2019, 84, 303–308. [Google Scholar] [PubMed]
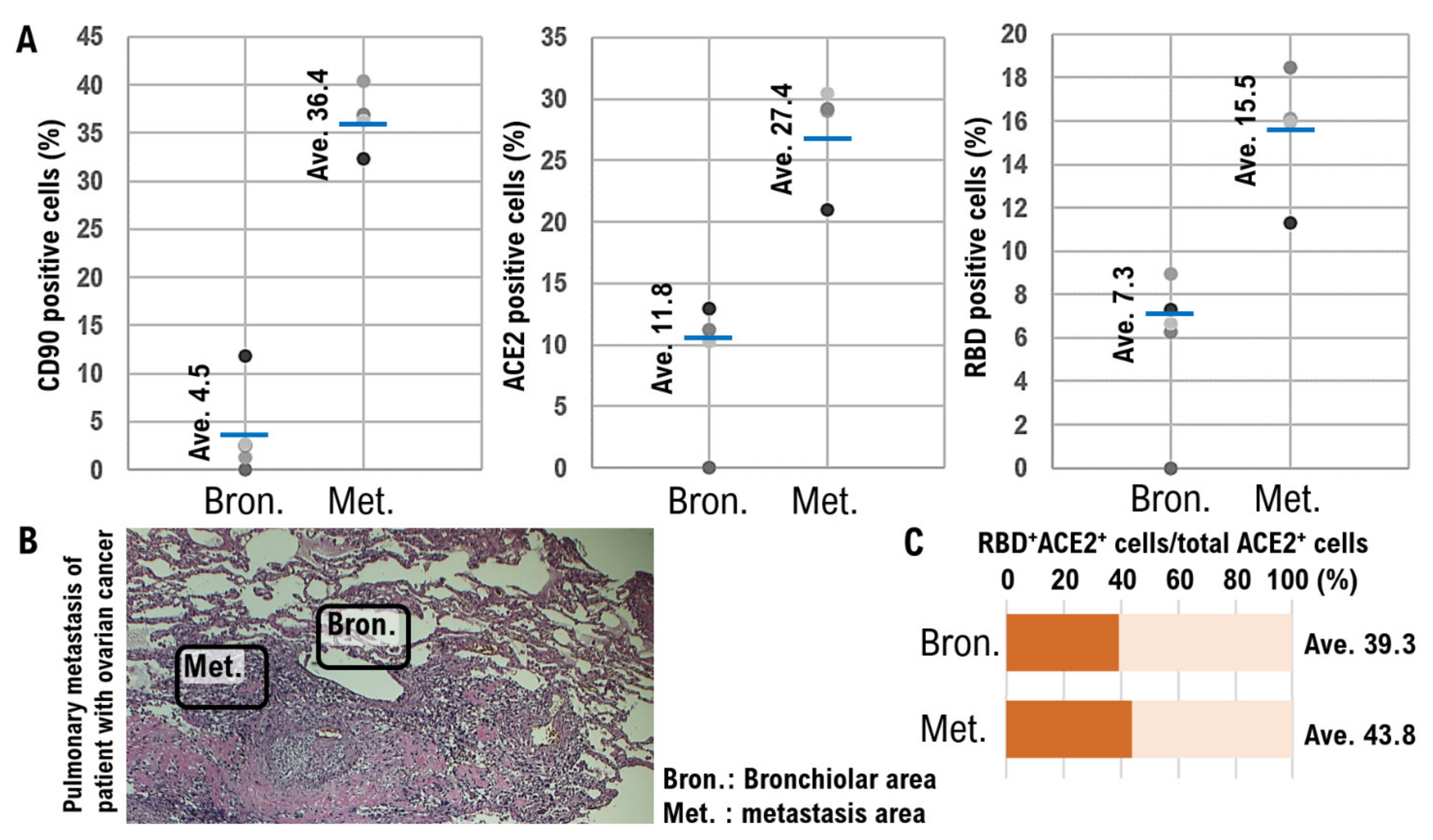
| Patient No. | Age Range | Age at Surgery (Years) | Histological Type | FIGO Stage | Grade | No. of Lung Metastatic Lesions | Pulmonary Metastatic Niche | Vital Status | |
| CD90* (%) | ACE2* (%) | ||||||||
| 1 | 40 s | 40–45 | HG serous | IVA | 3 | Single | 36.43 | 27.42 | Alive |
| 2 | 50 s | 50–55 | HG serous | IVA | 3 | Single | 33.87 | 18.93 | Alive |
| 3 | 50 s | 50–55 | HG serous | IVA | 3 | Multiple | 38.32 | 29.38 | Deceased |
| 4 | 40 s | 45–50 | HG serous | IVB | 3 | Multiple | 32.67 | 28.05 | Alive |
| Normal alveolar and bronchiolar areas | |||||||||
| Patient No. | Normal Alveoli | Normal Bronchioles | |||||||
| CD90* (%) | ACE2* (%) | CD90* (%) | ACE2* (%) | ||||||
| 1 | 4.53 | 11.82 | 3.23 | 20.67 | 4.53 | 11.82 | 3.23 | 20.67 | |
| 2 | 3.91 | 12.57 | 3.18 | 21.46 | 3.91 | 12.57 | 3.18 | 21.46 | |
| 3 | 4.34 | 12.71 | 3.45 | 22.05 | 4.34 | 12.71 | 3.45 | 22.05 | |
| 4 | 4.08 | 13.43 | 2.98 | 21.92 | 4.08 | 13.43 | 2.98 | 21.92 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hayashi, T.; Sano, K.; Konishi, I. Possibility of SARS-CoV-2 Infection in the Metastatic Microenvironment of Cancer. Curr. Issues Mol. Biol. 2022, 44, 233-241. https://doi.org/10.3390/cimb44010017
Hayashi T, Sano K, Konishi I. Possibility of SARS-CoV-2 Infection in the Metastatic Microenvironment of Cancer. Current Issues in Molecular Biology. 2022; 44(1):233-241. https://doi.org/10.3390/cimb44010017
Chicago/Turabian StyleHayashi, Takuma, Kenji Sano, and Ikuo Konishi. 2022. "Possibility of SARS-CoV-2 Infection in the Metastatic Microenvironment of Cancer" Current Issues in Molecular Biology 44, no. 1: 233-241. https://doi.org/10.3390/cimb44010017
APA StyleHayashi, T., Sano, K., & Konishi, I. (2022). Possibility of SARS-CoV-2 Infection in the Metastatic Microenvironment of Cancer. Current Issues in Molecular Biology, 44(1), 233-241. https://doi.org/10.3390/cimb44010017







