Molecular Genetics of Pre-B Acute Lymphoblastic Leukemia Sister Cell Lines during Disease Progression
Abstract
1. Introduction
2. Material and Methods
2.1. Cell Lines
2.2. RNA-Sequencing Analysis
2.3. Whole Exome Sequencing (WES) Analysis
2.4. RT-PCR, Genomic PCR and Sanger Sequencing
2.5. Numerical Aberrations
3. Results
3.1. Mutations and Chromosomal Aberrations
3.2. Subclonal Developments in Sister Cell Lines
4. Discussion
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ferreira, D.; Adega, F.; Chaves, R. The importance of cancer cell lines as in vitro models in cancer methylome analysis and anticancer drugs testing. In Oncogenomics and Cancer Proteomics; Lopez-Camarillo, C., Arechaga-Ocampo, E., Eds.; IntechOpen: London, UK, 2013. [Google Scholar]
- Mirabelli, P.; Coppola, L.; Salvatore, M. Cancer cell lines are useful model systems for medical research. Cancers 2019, 11, 1098. [Google Scholar] [CrossRef]
- Drexler, H.G.; Quentmeier, H. The LL-100 cell lines panel: Tool for molecular leukemia-lymphoma research. Int. J. Mol. Sci. 2020, 21, 5800. [Google Scholar] [CrossRef]
- Waanders, E.; Gu, Z.; Dobson, S.M.; Antic, Z.; Chase Crawford, J.; Ma, X.; Edmonson, M.N.; Payne-Turner, D.; Van de Vorst, M.; Jongmans, M.C.J.; et al. Mutational landscape and patterns of clonal evolution in relapsed pediatric acute lymphoblastic leukemia. Blood Cancer Discov. 2020, 1, 96–111. [Google Scholar] [CrossRef] [PubMed]
- Greaves, M. Evolutionary determinants of cancer. Cancer Discov. 2015, 5, 806–820. [Google Scholar] [CrossRef] [PubMed]
- Ferrando, A.A.; Lopez-Otin, C. Clonal evolution in leukemia. Nat. Med. 2017, 23, 1135–1145. [Google Scholar] [CrossRef]
- Lyne, A.M.; Laplane, L.; Perié, L. To portray clonal evolution in blood cancer, count your stem cells. Blood 2021, 37, 1862–1870. [Google Scholar] [CrossRef]
- Drexler, H.G. Guide to Leukemia-Lymphoma Cell Lines, 2nd ed.; e-Book on CD; Drexler, H.G.: Braunschweig, Germany, 2010. [Google Scholar]
- Dobin, A.; Davies, C.A.; Schlesinger, F.; Drenkow, J.; Zaleski, C.; Jha, S.; Batut, P.; Chaisson, M.; Gingeras, T.R. STAR: Ultrafast universal RNA-seq aligner. Bioinformatics 2013, 29, 15–21. [Google Scholar] [CrossRef]
- Li, H.; Handsaker, B.; Wysoker, A.; Fennell, T.; Ruan, J.; Homer, N.; Marth, G.; Abecasis, G.; Durbin, R. 1000 Genome Project Data Processing Subgroup. The sequence alignment/map format and SAMtools. Bioinformatics 2009, 25, 2078–2079. [Google Scholar] [CrossRef] [PubMed]
- Anders, S.; Pyl, P.T.; Huber, W. HTSeq—A Python framework to work with high-throughput sequencing data. Bioinformatics 2015, 31, 166–169. [Google Scholar] [CrossRef]
- Anders, S.; Huber, W. Differential expression analysis for sequence count data. Genome Biol. 2010, 11, R106. [Google Scholar] [CrossRef] [PubMed]
- Nicorici, D.; Satalan, M.; Edgren, H.; Kangaspeska, S.; Murumagi, A.; Kallioniemi, O.; Virtanen, S.; Kilkku, O. FusionCatcher-a tool for finding somatic fusion genes in paired-end RNA-sequencing data. bioRxiv 2014, 011650. [Google Scholar] [CrossRef]
- Pommerenke, C.; Geffers, R.; Bunk, B.; Bhuju, S.; Eberth, S.; Drexler, H.G.; Quentmeier, H. Enhanced whole exome sequencing by higher DNA insert lengths. BMC Genom. 2016, 17, 399. [Google Scholar] [CrossRef]
- McKenna, A.; Hanna, M.; Banks, E.; Sivachenko, A.; Cibulskis, K.; Kernytsky, A.; Garimella, K.; Altshuler, D.; Gabriel, S.; Daly, M.; et al. The genome analysis toolkit: A MapReduce framework for analyzing next-generation DNA sequencing data. Genome Res. 2010, 20, 1297–1303. [Google Scholar] [CrossRef]
- Koboldt, D.C.; Zhang, Q.; Larson, D.E.; Shen, D.; McLellan, M.D.; Lin, L.; Miller, C.A.; Mardis, E.R.; Ding, L.; Wilson, R.K. VarScan 2: Somatic mutation and copy number alteration discovery in cancer by exome sequencing. Genome Res. 2012, 22, 568–576. [Google Scholar] [CrossRef]
- McLaren, W.; Pritchard, B.; Chen, Y.; Flicek, P.; Cunningham, F. Deriving the consequences of genomic variants with the Ensembl API and SNP effect predictor. Bioinformatics 2010, 26, 2069–2070. [Google Scholar] [CrossRef]
- Shurtleff, S.A.; Buijs, A.; Behm, F.G.; Rubnitz, J.E.; Raimondi, S.C.; Hancock, M.; Chan, G.C.; Pui, C.H.; Grosveld, G.; Downing, J.R. TEL/AML1 fusion resulting from a cryptic t(12;21) is the most common genetic lesion in pediatric ALL and defines a subgroup of patients with an excellent prognosis. Leukemia 1995, 9, 1985–1989. [Google Scholar] [PubMed]
- Mullighan, C.G. Molecular genetics of B-precursor acute lymphoblastic leukemia. J. Clin. Invest 2012, 122, 3407–3415. [Google Scholar] [CrossRef] [PubMed]
- Uphoff, C.C.; MacLeod, R.A.F.; Denkmann, S.A.; Golub, T.R.; Borkhardt, A.; Janssen, J.W.G.; Drexler, H.G. Occurrence of TEL-AML1 fusion resulting from (12;21) translocation in human early B-lineage leukemia cell lines. Leukemia 1997, 11, 441–447. [Google Scholar] [CrossRef]
- Fears, S.; Chakrabarti, S.R.; Nucifora, G.; Rowley, J.D. Differential expression of TCL1 during pre-B-cell acute lymphoblastic leukemia progression. Cancer Genet. Cytogenet. 2002, 135, 110–119. [Google Scholar] [CrossRef]
- Kuiper, R.P.; Schoenmakers, E.F.P.M.; van Reijmersdal, S.V.; Hehir-Kwa, J.Y.; Geurts van Kessel, A.; van Leeuwen, F.N.; Hoogerbrugge, P.M. High-resolution genomic profiling of childhood ALL reveals novel recurrent genetic lesions affecting pathways involved in lymphocyte differentiation and cell cycle progression. Leukemia 2007, 21, 1258–1266. [Google Scholar] [CrossRef]
- Matsuo, Y.; Ariyasu, T.; Ohmoto, E.; Kimura, I.; Minowada, J. Bi-phenotypic t(9;22)-positive leukemia cell lines from a patient with acute leukemia: NALM-20, established at the onset; and NALM-21, NALM-22 and NALM-23, established after relapse. Hum. Cell 1991, 4, 335–338. [Google Scholar] [PubMed]
- Uphoff, C.C.; Habig, S.; Fombonne, S.; Matsuo, Y.; Drexler, H.G. ABL-BCR expression in BCR-ABL-positive human leukemia cell lines. Leuk. Res. 1999, 23, 1055–1060. [Google Scholar] [CrossRef]
- Ariyasu, T.; Matsuo, Y.; Harashima, A.; Nakamura, S.; Takaba, S.; Tsubota, T.; Orita, K. Establishment and characterization of “biphenotypic” acute leukemia cell lines with a variant Ph translocation t(9;22;10) (q34;q11;q22). Hum. Cell 1998, 11, 43–50. [Google Scholar] [PubMed]
- Gu, Z.; Churchman, M.; Roberts, K.; Li, Y.; Liu, Y.; Harvey, R.C.; McCastlain, K.; Reshmi, S.C.; Payne-Turner, D.; Iacobucci, I.; et al. Genomic analyses identify recurrent MEF2D fusions in acute lymphoblastic leukaemia. Nat. Commun. 2016, 7, 13331. [Google Scholar] [CrossRef]
- Goto, H.; Naruto, T.; Tanoshima, R.; Kato, H.; Yokosuka, T.; Yanagimachi, M.; Fujii, H.; Yokota, S.; Komine, H. Chemo-sensitivity in a panel of B-cell precursor acute lymphoblastic leukemia cell lines, YCUB series, derived from children. Leuk. Res. 2009, 33, 1386–1391. [Google Scholar] [CrossRef] [PubMed]
- Mullighan, C.G.; Goorha, S.; Radtke, I.; Miller, C.B.; Coustan-Smith, E.; Dalton, J.D.; Girtman, K.; Mathew, S.; Ma, J.; Pounds, S.B.; et al. Genome-wide analysis of genetic alterations in acute lymphoblastic leukaemia. Nature 2007, 446, 758–764. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Rastogi, P.; Shah, B.; Zhang, L. B lymphoblastic leukemia/lymphoma: New insights into genetics, molecular aberrations, subclassification and targeted therapy. Oncotarget 2017, 8, 66728–66741. [Google Scholar] [CrossRef]
- Tarella, C.; Pregno, P.; Gallo, E.; Caracciolo, D.; Stefani, S.; Barletta, C.; Lanfrancone, L.; Ferrero, D. Establishment from an adult leukemic patient of two novel precursor B cell lines with different growth modality. Leuk. Res. 1990, 14, 177–184. [Google Scholar] [CrossRef]
- Iacobucci, I.; Iraci, N.; Messina, M.; Lonetti, A.; Chiaretti, S.; Valli, E.; Ferrari, A.; Papayannidis, C.; Paolini, F.; Vitale, A.; et al. IKAROS deletions dictate a unique gene expression signature in patients with adult B-cell acute lymphoblastic leukemia. PLoS ONE 2012, 7, e40934. [Google Scholar] [CrossRef] [PubMed]
- Ribera, J.; Zamora, L.; Morgades, M.; Vives, S.; Granada, I.; Montesinos, P.; Gómez-Seguí, I.; Mercadal, S.; Guàrida, R.; Nomdedeu, J.; et al. Molecular profiling refines minimal residual disease-based prognostic assessment in adults with Philadelphia chromosome-negative B-cell precursor acute lymphoblastic leukemia. Genes Chromosomes Cancer 2019, 58, 815–819. [Google Scholar] [CrossRef] [PubMed]
- Stanulla, M.; Cavé, H.; Moorman, A.V. IKZF1 deletions in pediatric acute lymphoblastic leukemia: Still a poor prognostic marker? Blood 2020, 135, 252–260. [Google Scholar] [CrossRef]
- Hof, J.; Krentz, S.; van Schewick, C.; Körner, G.; Shalapour, S.; Rhein, P.; Karawajew, L.; Ludwig, W.D.; Seeger, K.; Henze, G.; et al. Mutations and deletions of the TP53 gene predict nonresponse to treatment and poor outcome in first relapse of childhood acute lymphoblastic leukemia. J. Clin. Oncol. 2011, 29, 3185–3193. [Google Scholar] [CrossRef] [PubMed]
- Quentmeier, H.; Pommerenke, C.; Ammerpohl, O.; Geffers, R.; Hauer, V.; MacLeod, R.A.F.; Nagel, S.; Romani, J.; Rosati, E.; Rosén, A.; et al. Subclones in B-lymphoma cell lines: Isogenic models for the study of gene regulation. Oncotarget 2016, 7, 63456–63465. [Google Scholar] [CrossRef] [PubMed]
- Quentmeier, H.; Amini, R.M.; Berglund, M.; Dirks, W.G.; Ehrentraut, S.; Geffers, R.; MacLeod, R.A.F.; Nagel, S.; Romani, J.; Scherr, M.; et al. U-2932: Two clones in one cell line, a tool for the study of clonal evolution. Leukemia 2013, 27, 1155–1164. [Google Scholar] [CrossRef] [PubMed]
- Ding, J.; Dirks, W.G.; Ehrentraut, S.; Geffers, R.; MacLeod, R.A.F.; Nagel, S.; Pommerenke, C.; Romani, J.; Scherr, M.; Vaas, L.A.I.; et al. BCL6-regulated by AhR/ARNT and wild-type MEF2B-drives expression of germinal center markers MYBL1 and LMO2. Haematologica 2015, 100, 801–809. [Google Scholar] [CrossRef]


| Copy Number | AT-1 (5y) | AT-2 (5y) | NALM-20 (62y) | NALM-21 (63y) | NALM-27 (38y) | NALM-30 (39y) | PC-53 (33y) | PC-53A (34y) | YCUB-4 (7y) | YCUB-4R (7y) |
|---|---|---|---|---|---|---|---|---|---|---|
| BTG1 (12q21.33) | 2n | 2n | 1n | 1n | 2n | 2n | 2n | 2n | 2n | 2n |
| BTLA (3q13.2) | 2n | 2n | 1n | 1n | 1n | 1n | 2n | 2n | 2n | 2n |
| CDKN2A (9p21.3) | 0n | 0n | 0n | 0n | 0n | 0n | 0n | 0n | 0n | 0n |
| CDKN2B (9p21.3) | 0n | 0n | 0n (partial) | 0n (partial) | 0n (partial) | 0n (partial) | 0n | 0n | 0n | 0n |
| ETV6 (12p13.2) | 1n | 1n | 2n | 2n | 2n | 2n | 2n | 2n | 2n | 2n |
| IKZF1 (7p12.2) | 2n | 2n | 0n (partial) | 0n (partial) | 0n (partial) | 0n (partial) | 2n | 2n | 2n | 2n |
| NR3C1 (5q31.3) | 2n | 2n | 2n | 2n | 2n | 2n | 1n | 1n | 2n | 2n |
| PAX5 (9p13.2) | 2n | 2n | 2n | 2n | 2n | 1.5–2n | 2n | 2n | 1n | 1n |
| TP53 (17p13.1) | 2n | 2n | 2n | 2n | 2n | 2n | 1n | 1n | 2n | 2n |
| fusion transcripts | ||||||||||
| BCR-ABL1 | no | no | yes | yes | yes | yes | no | no | no | no |
| ETV6-RUNX1 | yes | yes | no | no | no | no | no | no | no | no |
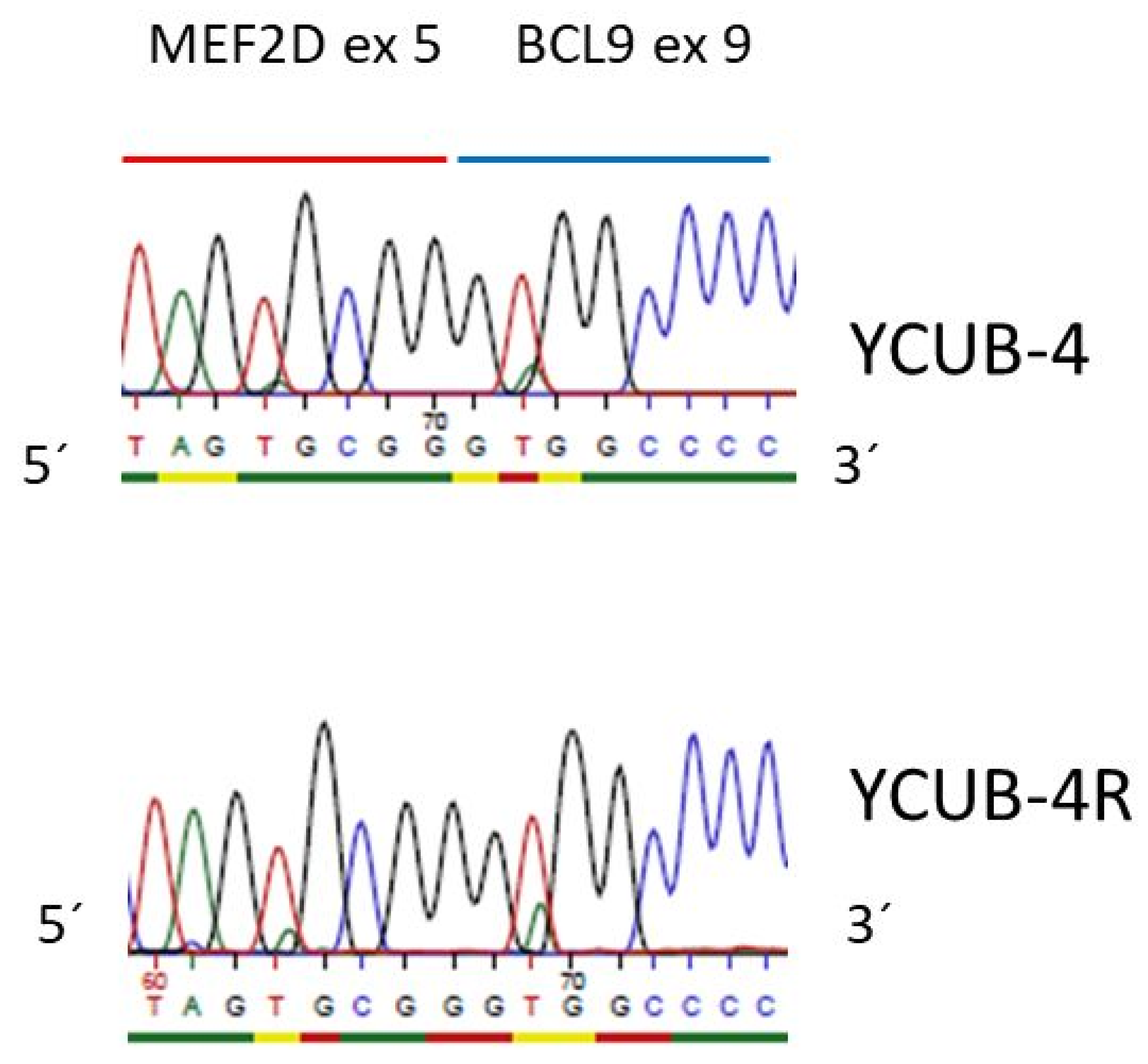
| MEF2D-BCL9 | no | no | no | no | no | no | no | no | yes | yes |
| NSMAF-NUCKS1 | no | no | no | no | no | no | yes | no | no | no |
| RUNX1-ETV6 | yes | yes | no | no | no | no | no | no | no | no |
| point mutations | ||||||||||
| KRAS A146T COSM19404 | 0/1 (85/15) | 0/1 (50/50) | no | no | no | no | no | no | no | no |
| NRAS G12C COSM561 | 0/1 (65/35) | 0/1/ (97/3) | no | no | no | no | no | no | no | no |
| PAX5 R38H COSM5986423 | no | no | no | no | no | no | 0/1 | 0/1 | no | no |
| Cell Line | Clone A KRAS (0/0)/NRAS (0/1) | Clone B KRAS (0/1)/NRAS (0/0) |
|---|---|---|
| AT-1 | 70% | 30% |
| AT-2 | <5% | >95% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Quentmeier, H.; Pommerenke, C.; Drexler, H.G. Molecular Genetics of Pre-B Acute Lymphoblastic Leukemia Sister Cell Lines during Disease Progression. Curr. Issues Mol. Biol. 2021, 43, 2147-2156. https://doi.org/10.3390/cimb43030149
Quentmeier H, Pommerenke C, Drexler HG. Molecular Genetics of Pre-B Acute Lymphoblastic Leukemia Sister Cell Lines during Disease Progression. Current Issues in Molecular Biology. 2021; 43(3):2147-2156. https://doi.org/10.3390/cimb43030149
Chicago/Turabian StyleQuentmeier, Hilmar, Claudia Pommerenke, and Hans G. Drexler. 2021. "Molecular Genetics of Pre-B Acute Lymphoblastic Leukemia Sister Cell Lines during Disease Progression" Current Issues in Molecular Biology 43, no. 3: 2147-2156. https://doi.org/10.3390/cimb43030149
APA StyleQuentmeier, H., Pommerenke, C., & Drexler, H. G. (2021). Molecular Genetics of Pre-B Acute Lymphoblastic Leukemia Sister Cell Lines during Disease Progression. Current Issues in Molecular Biology, 43(3), 2147-2156. https://doi.org/10.3390/cimb43030149







