Effect of Metformin and Simvastatin in Inhibiting Proadipogenic Transcription Factors
Abstract
1. Introduction
2. Materials and Methods
2.1. Drugs and Reagents
2.2. Cell Culture and 3T3-L1 Cell Differentiation
2.3. Cell Viability Assay
2.4. Oil Red O Lipid Staining
2.5. Gene Expression Analysis
2.6. Protein Extraction and Western Blot
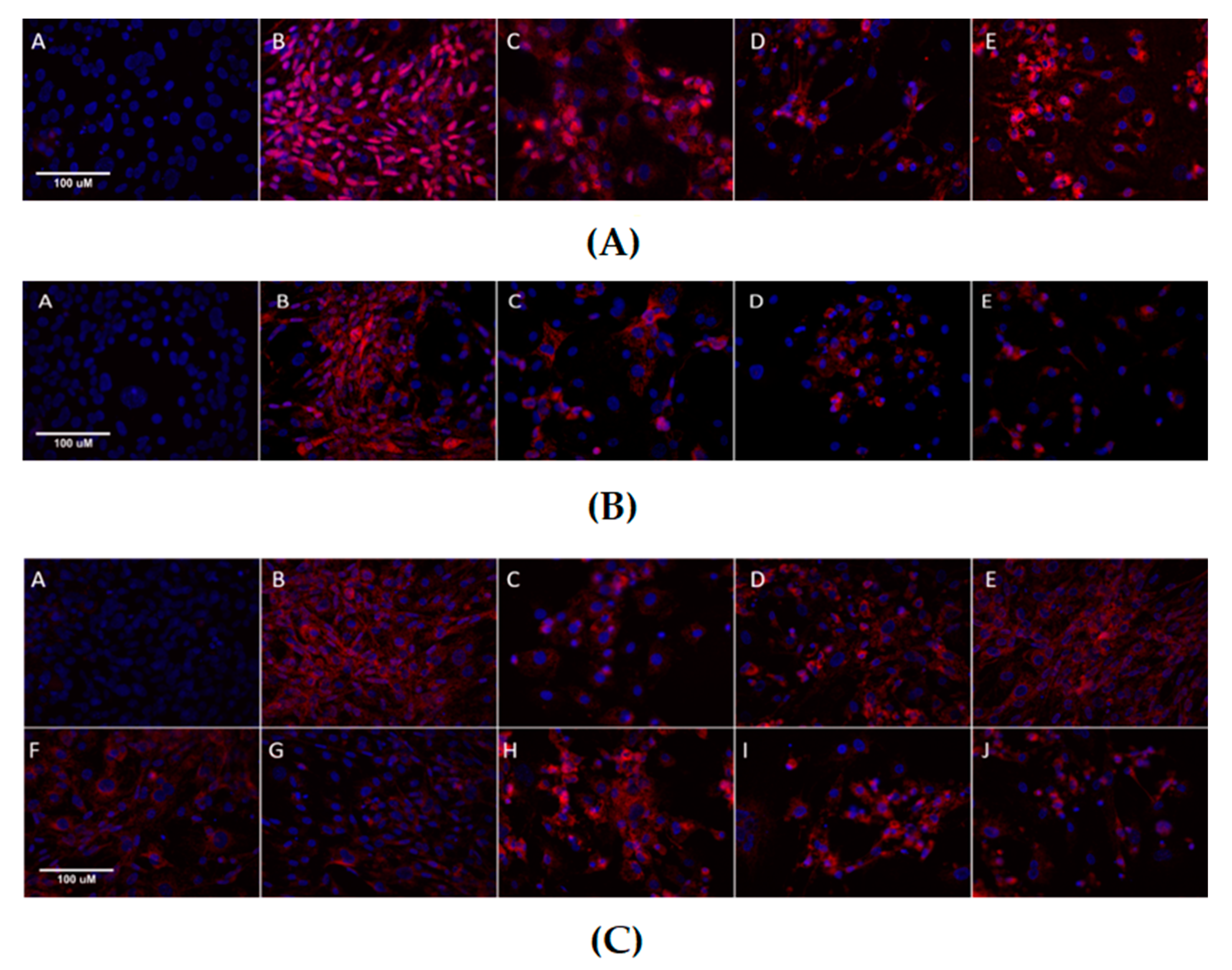
2.7. Immunocytochemistry and Immunofluorescent Staining
2.8. Statistical Analysis
3. Results
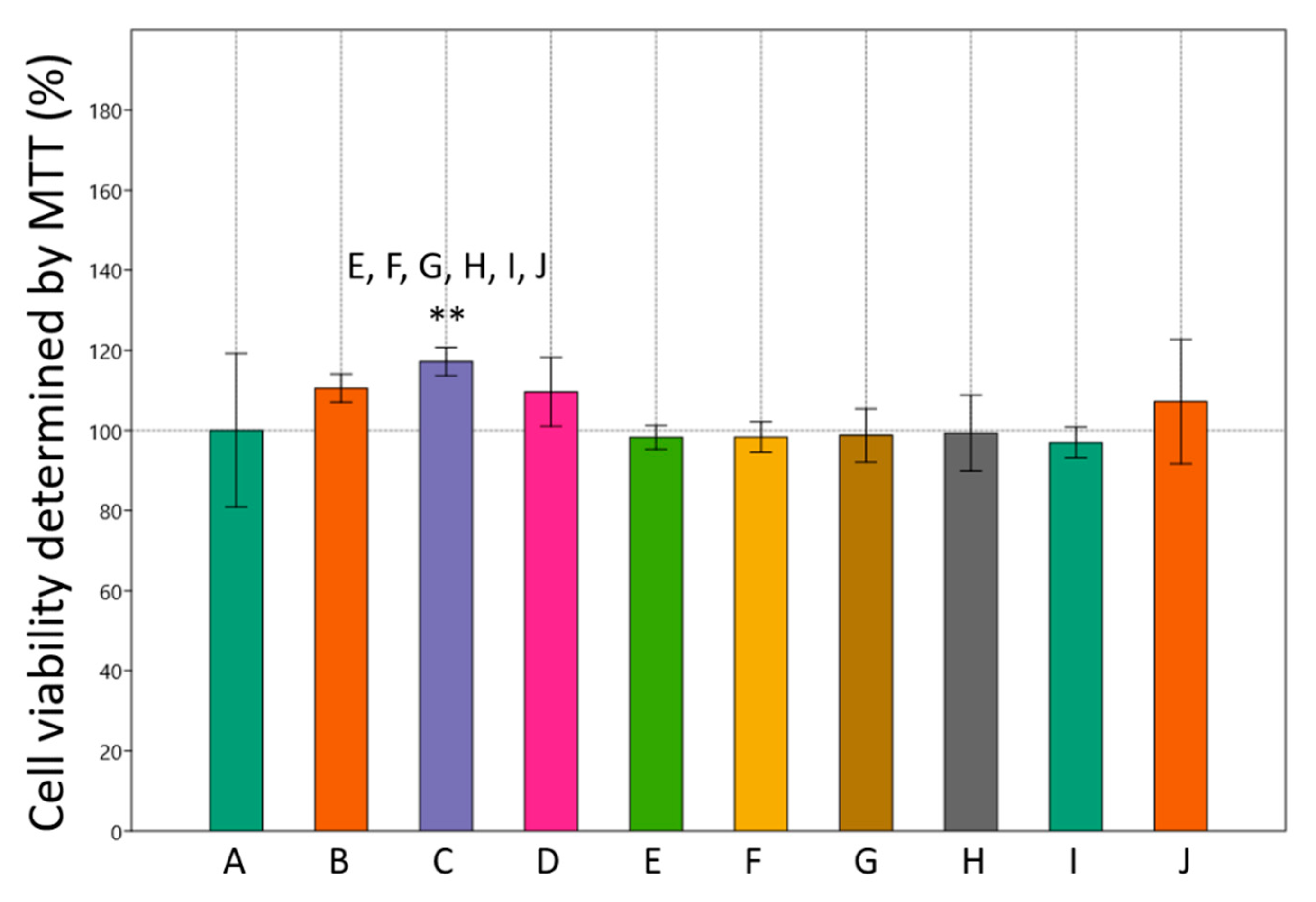
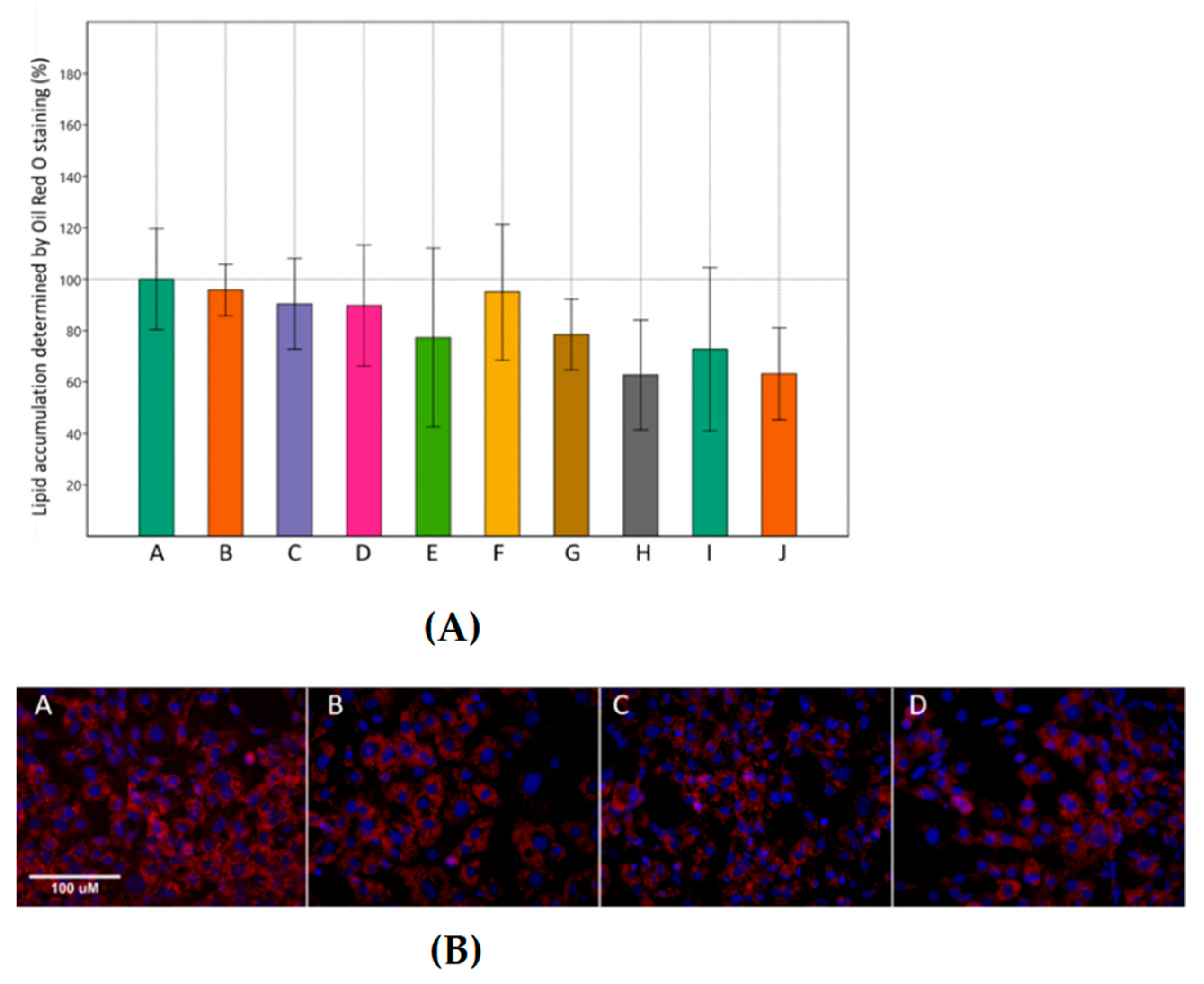
3.1. Effects of Metformin and Simvastatin on Cell Viability and Lipid Accumulation
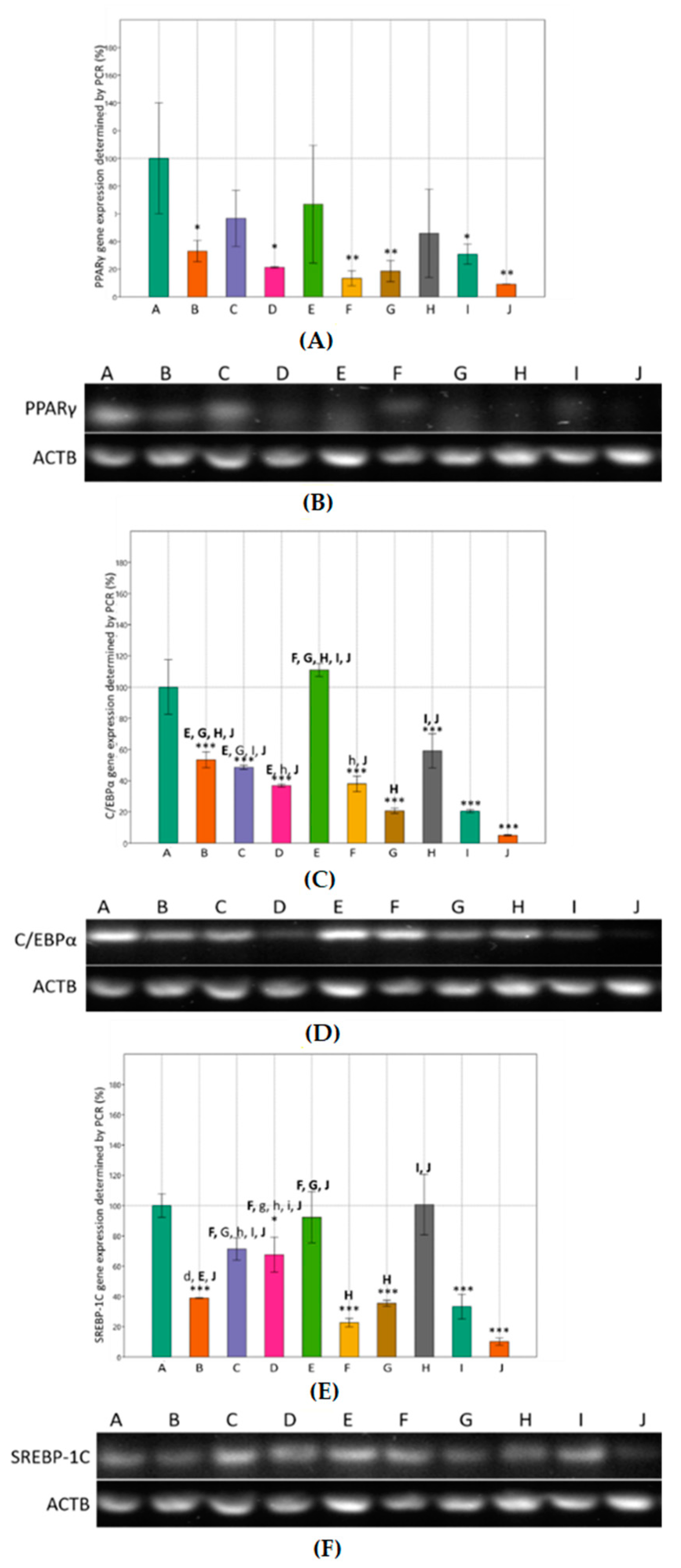
3.2. Effects of Metformin and Simvastatin on Expression of Adipogenic Genes
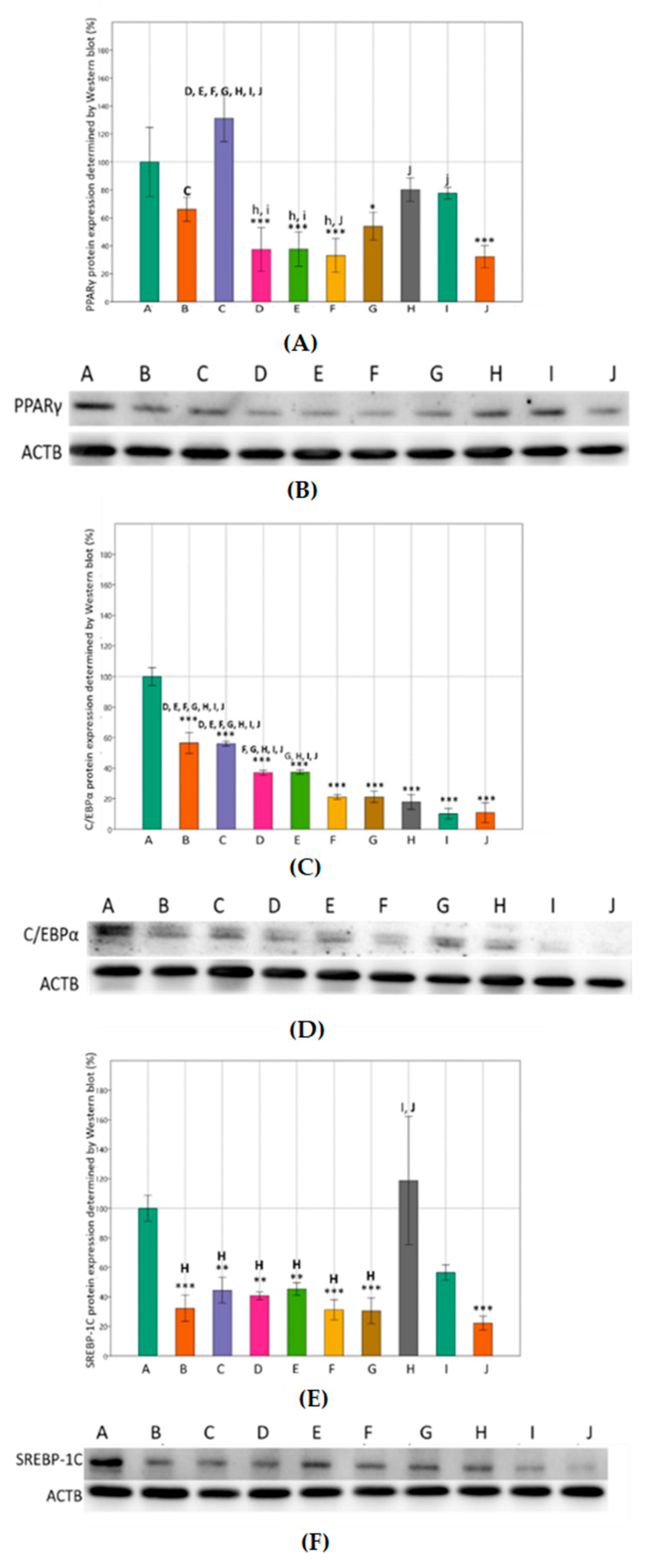
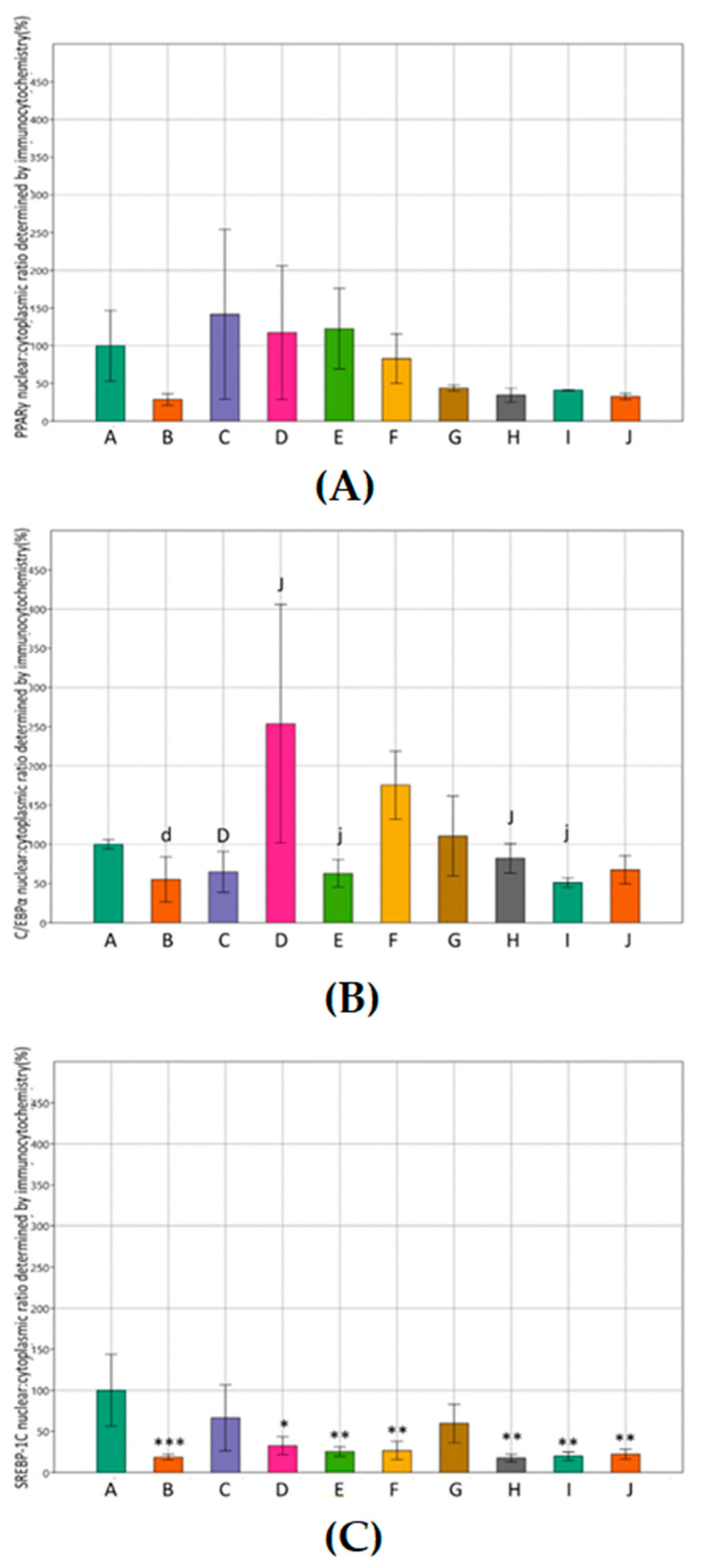
3.3. Effects of Metformin and Simvastatin on Protein Expression and Immunoreactivity of Adipogenic Transcription Factors
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dias, S.; Paredes, S.; Ribeiro, L. Drugs Involved in Dyslipidemia and Obesity Treatment: Focus on Adipose Tissue. Int. J. Endocrinol. 2018, 2018, 2637418. [Google Scholar] [CrossRef]
- Chen, D.; Wang, Y.; Wu, K.; Wang, X. Dual Effects of Metformin on Adipogenic Differentiation of 3T3-L1 Preadipocyte in AMPK-Dependent and Independent Manners. Int. J. Mol. Sci. 2018, 19, 1547. [Google Scholar] [CrossRef]
- Bielczyk-Maczynska, E. White Adipocyte Plasticity in Physiology and Disease. Cells 2019, 8, 1507. [Google Scholar] [CrossRef] [PubMed]
- Oliviera, M.; Mathias, L.S.; Sibio, M.T.; Noronha-Matos, J.B.; Costa, M.A.; Nogueira, C.R.; Correia-de-Sa, P. Pitfalls and challenges of the purinergic signaling cascade in obesity. Biochem. Pharmacol. 2020, 182, 114214. [Google Scholar] [CrossRef] [PubMed]
- Jackson, R.M.; Griesel, B.A.; Gurley, J.M.; Szweda, L.I.; Olson, A.L. Glucose availability controls adipogenesis in mouse 3T3-L1 adipocytes via up-regulation of nicotinamide metabolism. J. Biol. Chem. 2017, 292, 18556–18564. [Google Scholar] [CrossRef] [PubMed]
- Janderova, L.; McNeil, M.; Murrel, A.N.; Mynatt, R.L.; Smith, S.R. Human Mesenchymal Stem Cells as an in Vitro Model for Human Adipogenesis. Obes. Res. 2003, 11, 65–74. [Google Scholar] [CrossRef]
- Dave, S.; Kaur, N.J.; Nanduri, R.; Dkhar, H.K.; Kumar, A.; Gupta, P. Inhibition of adipogenesis and induction of apoptosis and lipolysis by stem bromelain in 3T3-L1 adipocytes. PLoS ONE 2012, 7, e30831. [Google Scholar] [CrossRef]
- Zebisch, K.; Voigt, V.; Wabitsch, M.; Brandsch, M. Protocol for effective differentiation of 3T3-L1 cells to adipocytes. Anal. Biochem. 2012, 425, 88–90. [Google Scholar] [CrossRef]
- Qi, T.; Chen, Y.; Li, H.; Pei, Y.; Woo, S.L.; Guo, X.; Zhao, J.; Qian, X.; Awika, J.; Huo, Y.; et al. A Role for PFKFB3/iPFK2 in Metformin Suppression of Adipocyte Inflammatory Responses. J. Mol. Endocrinol. 2017, 59, 49–59. [Google Scholar] [CrossRef]
- Campbell, J.M.; Bellman, S.M.; Stephenson, M.D.; Lisy, K. Metformin reduces all-cause mortality and diseases of ageing independent of its effect on diabetes control: A systematic review and meta-analysis. Ageing Res. Rev. 2017, 40, 31–44. [Google Scholar] [CrossRef]
- Salvatore, T.; Pafundi, P.C.; Morgillo, F.; Di Liello, R.; Galiero, R.; Nevola, R.; Marfella, R.; Monaco, L.; Rinaldi, L.; Adinolfi, L.E.; et al. Metformin: An old drug against old age and associated morbidities. Diabetes Res. Clin. Pract. 2020, 160, 108025. [Google Scholar] [CrossRef] [PubMed]
- Morgillo, F.; Fasano, M.; Della Corte, C.M.; Sasso, F.C.; Papaccio, F.; Viscardi, G.; Esposito, G.; Di Liello, R.; Normanno, N.; Capuano, A.; et al. Results of the safety run-in part of the METAL (METformin in Advanced Lung cancer) study: A multicentre, open-label phase I-II study of metformin with erlotinib in second-line therapy of patients with stage IV non-small-cell lung cancer. ESMO Open 2017, 2, e000132. [Google Scholar] [CrossRef]
- De Aguiar, L.G.K.; Kraemer-Aguiar, L.G.; Bahia, L.R.; Villela, N.; Laflor, C.; Sicuro, F.; Wiernsperger, N.F.; Bottino, D.; Bouskela, E. Metformin Improves Endothelial Vascular Reactivity in First-Degree Relatives of Type 2 Diabetic Patients With Metabolic Syndrome and Normal Glucose Tolerance. Diabetes Care 2006, 29, 1083–1089. [Google Scholar] [CrossRef] [PubMed]
- Salvatore, T.; Pafundi, P.C.; Galiero, R.; Rinaldi, L.; Caturano, A.; Vetrano, E.; Aprea, C.; Albanese, G.; Di Martino, A.; Ricozzi, C.; et al. Can Metformin Exert as an Active Drug on Endothelial Dysfunction in Diabetic Subjects? Biomedicines 2021, 9, 3. [Google Scholar] [CrossRef]
- Rojas, L.B.; Gomes, M.B. Metformin: An old but still the best treatment for type 2 diabetes. Diabetol. Metab. Syndr. 2013, 5, 6. [Google Scholar] [CrossRef] [PubMed]
- Kain, V.; Kapadia, B.; Misra, P.; Saxena, U. Simvastatin may induce insulin resistance through a novel fatty acid mediated cholesterol independent mechanism. Sci. Rep. 2015, 5, 13823. [Google Scholar] [CrossRef]
- Bulcão, C.; Giuffrida, F.M.; Ribeiro-Filho, F.F.; Ferreira, S.R. Are the beneficial cardiovascular effects of simvastatin and metformin also associated with a hormone-dependent mechanism improving insulin sensitivity? Braz. J. Med. Biol. Res. 2007, 40, 229–235. [Google Scholar] [CrossRef][Green Version]
- Kim, B.H.; Han, S.; Lee, H.; Park, C.H.; Chung, Y.M.; Shin, K.; Lee, H.G.; Ye, S.K. Metformin enhances the anti-adipogenic effects of atorvastatin via modulation of STAT3 and TGF-β/Smad3 signaling. Biochem. Biophys. Res. Commun. 2015, 456, 173–178. [Google Scholar] [CrossRef]
- Schmitz, G.; Drobnik, W. Pharmacogenomics and pharmacogenetics of cholesterol-lowering therapy. Clin. Chem. Lab. Med. 2003, 41, 581–589. [Google Scholar] [CrossRef]
- Zjalić, M.; Mustapić, M.; Glumac, Z.; Prološčić, I.; Blažetić, S.; Vuković, A.; Masud, M.; Billah, M.; Khan, A.; Šegota, S.; et al. Construction of AC/DC magnetic syringe device for stimulated drug release, injection and ejection of nanocarriers and testing cytotoxicity in vitro. MethodsX 2021, 8, 101321. [Google Scholar] [CrossRef]
- Hammer, Ø.; Harper, D.A.T.; Ryan, P.D. Past: Paleontological statistics software package for education and data analysis. Palaeontol. Electron. 2001, 4, 4. [Google Scholar]
- Lilja, J.J.; Kivistö, K.T.; Neuvonen, P.J. Grapefruit juice-simvastatin interaction: Effect on serum concentrations of simvastatin, simvastatin acid, and HMG-CoA reductase inhibitors. Clin. Pharmacol. Ther. 1998, 64, 477–483. [Google Scholar] [CrossRef]
- van Stee, M.F.; de Graaf, A.A.; Groen, A.K. Actions of metformin and statins on lipid and glucose metabolism and possible benefit of combination therapy. Cardiovasc. Diabetol. 2018, 17, 94. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.H.; Lin, M.C.; Muo, C.H.; Yeh, S.Y.; Sung, F.C.; Kao, C.H. Combination Therapy of Metformin and Statin May Decrease Hepatocellular Carcinoma Among Diabetic Patients in Asia. Medicine 2015, 94, e1013. [Google Scholar] [CrossRef] [PubMed]
- Simu, Y.S.; Ahn, S.; Castro-Aceituno, V.; Yang, D.C. Ginsenoside Rg5: Rk1 Exerts an Anti-obesity Effect on 3T3-L1 Cell Line by the Downregulation of PPARγ and CEBPα. Iranian. J. Biotech. 2017, 15, e1517. [Google Scholar] [CrossRef]
- Zhang, J.; Tang, H.; Zhang, Y.; Deng, R.; Shao, L.; Liu, Y.; Li, F.; Wang, X.; Zhou, L. Identification of suitable reference genes for quantitative RT-PCR during 3T3-L1 adipocyte differentiation. Int. J. Mol. Med. 2014, 33, 1209–1218. [Google Scholar] [CrossRef] [PubMed]
- Alexandre, K.B.; Smit, A.M.; Gray, I.P.; Crowther, N.J. Metformin inhibits intracellular lipid accumulation in the murine pre-adipocyte cell line, 3T3-L1. Diabetes Obes. Metab. 2008, 10, 688–690. [Google Scholar] [CrossRef] [PubMed]
- Maeda, T.; Horiuchi, N. Simvastatin suppresses leptin expression in 3T3-L1 adipocytes via activation of the cyclic AMP-PKA pathway induced by inhibition of protein prenylation. J. Biochem. 2009, 145, 771–781. [Google Scholar] [CrossRef]
- Nakata, M.; Nagasaka, S.; Kusaka, I.; Matsuoka, H.; Ishibashi, S.; Yada, T. Effects of statins on the adipocyte maturation and expression of glucose transporter 4 (SLC2A4): Implications in glycaemic control. Diabetologia 2006, 49, 1881–1892. [Google Scholar] [CrossRef]
- Ahmad, B.; Serpell, C.J.; Fong, I.L.; Wong, E.H. Molecular Mechanisms of Adipogenesis: The Anti-adipogenic Role of AMP-Activated Protein Kinase. Front. Mol. Biosci. 2020, 7, 76. [Google Scholar] [CrossRef]
- Moreno-Navarrete, J.M.; Fernández-Real, J.M. Adipocyte Differentiation. In Adipose Tissue Biology; Symonds, M.E., Ed.; Springer: New York, NY, USA, 2012; Volume VI, pp. 17–38. [Google Scholar]
- Chang, E.; Kim, C.Y. Natural Products and Obesity: A Focus on the Regulation of Mitotic Clonal Expansion during Adipogenesis. Molecules 2019, 24, 1157. [Google Scholar] [CrossRef]
- Gao, Y.; Xue, J.; Li, X.; Jia, Y.; Hu, J. Metformin regulates osteoblast and adipocyte differentiation of rat mesenchymal stem cells. J. Pharm. Pharmacol. 2008, 60, 1695–1700. [Google Scholar] [CrossRef]
- Kim, E.K.; Lee, S.H.; Jhun, J.Y.; Byun, J.K.; Jeong, J.H.; Lee, S.Y.; Kim, J.K.; Choi, J.Y.; Cho, M.L. Metformin Prevents Fatty Liver and Improves Balance of White/Brown Adipose in an Obesity Mouse Model by Inducing FGF21. Mediators Inflamm. 2016, 2016, 5813030. [Google Scholar] [CrossRef]
- Tebbe, C.; Chhina, J.; Dar, S.A.; Sarigiannis, K.; Giri, S.; Munkarah, A.R.; Rattan, R. Metformin limits the adipocyte tumor-promoting effect on ovarian cancer. Oncotarget 2014, 5, 4746–4764. [Google Scholar] [CrossRef] [PubMed]
- Zhou, G.; Myers, R.; Li, Y.; Chen, Y.; Shen, X.; Fenyk-Melody, J.; Wu, M.; Ventre, J.; Doebber, T.; Fujii, N.; et al. Role of AMP-activated protein kinase in mechanism of metformin action. J. Clin. Investig. 2001, 108, 1167–1174. [Google Scholar] [CrossRef] [PubMed]
- Elfakhani, M.; Torabi, S.; Hussein, D.; Mills, N.; Verbeck, G.F.; Mo, H. Mevalonate deprivation mediates the impact of lovastatin on the differentiation of murine 3T3-F442A preadipocytes. Exp. Biol. Med. 2014, 239, 293–301. [Google Scholar] [CrossRef]
- Singh, P.; Zhang, Y.; Sharma, P.; Covassin, N.; Soucek, F.; Friedman, P.A.; Somers, V.K. Statins decrease leptin expression in human white adipocytes. Physiol. Rep. 2018, 6, e13566. [Google Scholar] [CrossRef] [PubMed]
- Redondo-Morata, L.; Lea Sanford, R.; Andersen, O.S.; Scheuring, S. Effect of Statins on the Nanomechanical Properties of Supported Lipid Bilayers. Biophys J. 2016, 111, 363–372. [Google Scholar] [CrossRef]
- Umemoto, T.; Fujuiki, Y. Ligand-dependent nucleo-cytoplasmic shuttling of peroxisome proliferator-activated receptors PPARa and PPARγ. Genes Cells 2012, 17, 576–596. [Google Scholar] [CrossRef] [PubMed]
- Björkhem-Bergman, L.; Lindh, J.D.; Bergman, P. What is a relevant statin concentration in cell experiments claiming pleiotropic effects? Br. J. Clin. Pharmacol. 2011, 72, 164–165. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jakab, J.; Zjalić, M.; Mikšić, Š.; Tušek, I.; Ćosić, V.; Volarić, N.; Nakić, D.; Včev, A.; Miškić, B. Effect of Metformin and Simvastatin in Inhibiting Proadipogenic Transcription Factors. Curr. Issues Mol. Biol. 2021, 43, 2082-2097. https://doi.org/10.3390/cimb43030144
Jakab J, Zjalić M, Mikšić Š, Tušek I, Ćosić V, Volarić N, Nakić D, Včev A, Miškić B. Effect of Metformin and Simvastatin in Inhibiting Proadipogenic Transcription Factors. Current Issues in Molecular Biology. 2021; 43(3):2082-2097. https://doi.org/10.3390/cimb43030144
Chicago/Turabian StyleJakab, Jelena, Milorad Zjalić, Štefica Mikšić, Ivan Tušek, Vesna Ćosić, Nikola Volarić, Dario Nakić, Aleksandar Včev, and Blaženka Miškić. 2021. "Effect of Metformin and Simvastatin in Inhibiting Proadipogenic Transcription Factors" Current Issues in Molecular Biology 43, no. 3: 2082-2097. https://doi.org/10.3390/cimb43030144
APA StyleJakab, J., Zjalić, M., Mikšić, Š., Tušek, I., Ćosić, V., Volarić, N., Nakić, D., Včev, A., & Miškić, B. (2021). Effect of Metformin and Simvastatin in Inhibiting Proadipogenic Transcription Factors. Current Issues in Molecular Biology, 43(3), 2082-2097. https://doi.org/10.3390/cimb43030144





