Screening for Copy Number Variations of the 15q13.3 Hotspot in CHRNA7 Gene and Expression in Patients with Migraines
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Selection of CHRNA7 Gene and CNV
2.3. DNA and RNA Extraction
2.4. Complementary DNA (cDNA) Synthesis with Reverse Transcriptase PCR (RT-PCR)
2.5. Quantitative-Comparative CT (ΔΔCT) Real-Time PCR (qPCR) for Gene Expression
2.6. Copy Number Variation (CNV) Analyses
2.7. Statistical Analyses
3. Results
3.1. Population Data
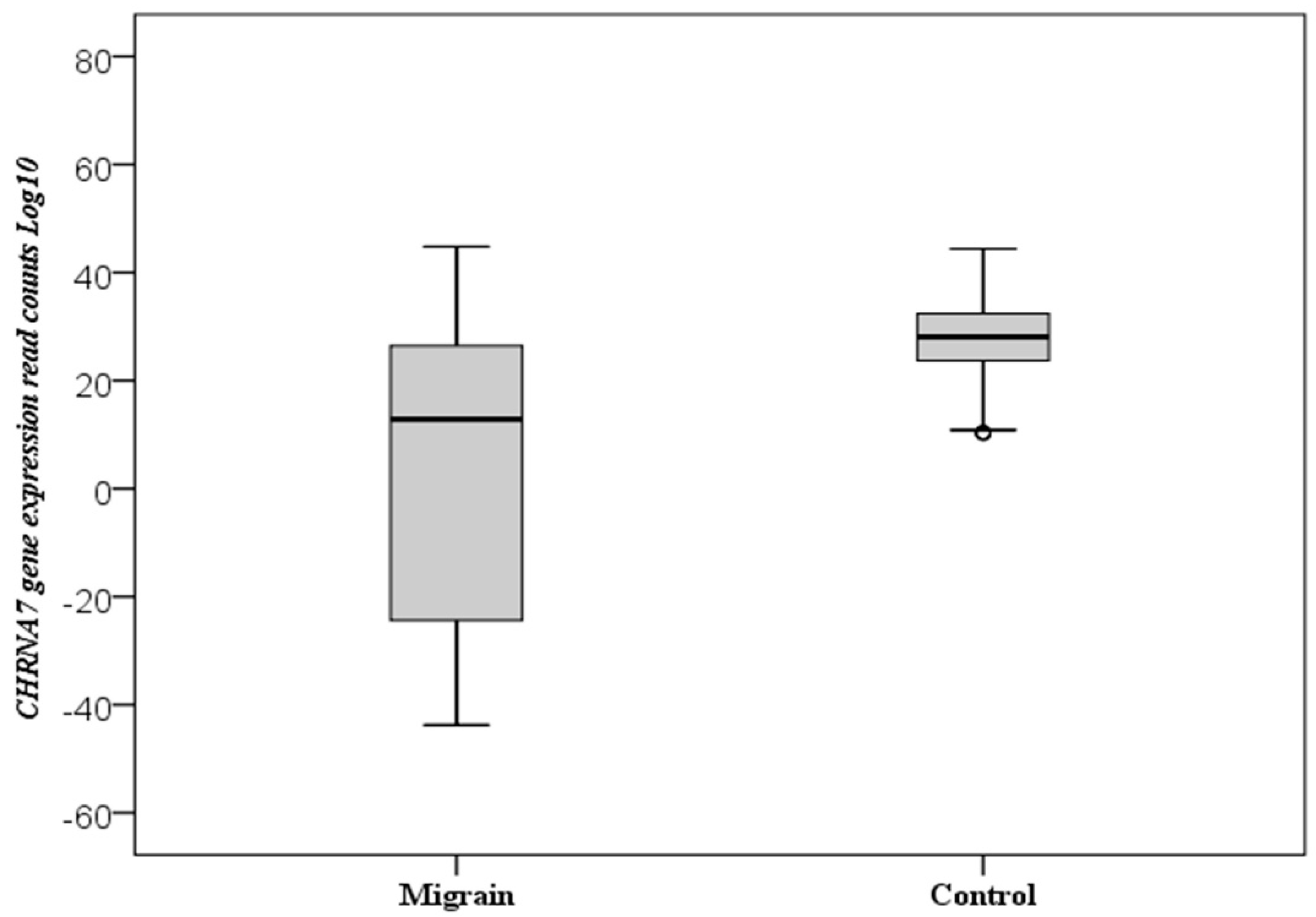
3.2. CHRNA7 Gene Expression
3.3. CNV Measurements of CHRNA7 Gene
3.4. Evaluation of CHRNA7 Gene Expression and Measurements of CNV
3.5. The Clinical Variables and Measurements of CHRNA7 Gene Expression Levels
3.6. The Relationship of CNV with Clinical Variables
3.7. The Receiver Operator Curve (ROC) Analyses
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| α7nAchR | alpha-7 nicotinic receptor |
| ACTB | Beta-actin |
| ADHD | Attention Deficit Hyperactivity Disorder |
| ASDs | Autism spectrum disorder |
| ATP1A2 | ATPase Na+/K+ Transporting Subunit Alpha 2 |
| AUC | Area under the curve |
| BMI | Body Mass Index |
| CACNA1 | Calcium Voltage-Gated Channel Subunit Alpha1 A |
| CHRNA7 | Cholinergic Receptor Nicotinic Alpha 7 Subunit |
| CNV | Copy Number Variation |
| COL4A1 | Collagen type IV alpha 1 |
| CRP | C-Reactive Protein |
| CSNK1D | Casein Kinase 1 Delta |
| E2F1 | E2F Transcription Factor 1 |
| EGFR | Epidermal Growth Factor Receptor |
| ESR | Erythrocyte Sedimentation Rate |
| GWAS | Genome-Wide Association Studies |
| HBG | Hemoglobin |
| HLA | Human Leukocyte Antigen |
| ICHD-3 | The International Classification of Headache Disorders 3rd Edition |
| ID | Mental disability |
| LCR | Low Copy Repeat |
| NAHR | Non-allelic homologous recombination |
| NOTCH3 | Notch Receptor 3 |
| PPP1R3A | Protein Phosphatase 1 Regulatory Subunit 3A |
| PRRT2 | Proline-rich transmembrane protein 2 |
| SCN1A | Sodium Voltage-Gated Channel Alpha Subunit 1 |
| SNP | Single Nucleotide Polymorphism |
| T2DM | Type 2 diabetes mellitus |
| TERT | Telomerase Reverse Transcriptase |
| TREX1 | Three Prime Repair Exonuclease 1 |
| TSPAN8 | Tetraspanin 8 |
| WBC | White Blood Cell |
References
- D’Antona, L.; Matharu, M. Identifying and managing refractory migraine: Barriers and opportunities? J. Headache Pain 2019, 20, 1–11. [Google Scholar] [CrossRef]
- Hoffmann, J.; Recober, A. Migraine and triggers: Post hoc ergo propter hoc? Curr. Pain Headache Rep. 2013, 17, 1–7. [Google Scholar] [CrossRef] [Green Version]
- De Boer, I.; Terwindt, G.M.; van den Maagdenberg, A.M. Genetics of migraine aura: An update. J. Headache Pain 2020, 21, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Yilmaz, G.; Sürer, H.; Inan, L.E.; Coskun, Ö.; Yücel, D. Increased nitrosative and oxidative stress in platelets of migraine patients. Tohoku J. Exp. Med. 2007, 211, 23–30. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sutherland, H.G.; Albury, C.L.; Griffiths, L.R. Advances in genetics of migraine. J. Headache Pain 2019, 20, 1–20. [Google Scholar] [CrossRef]
- Rice, A.M.; McLysaght, A. Dosage-sensitive genes in evolution and disease. BMC Biol. 2017, 15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Piotrowski, A.; Bruder, C.E.; Andersson, R.; de Ståhl, T.D.; Menzel, U.; Sandgren, J.; Poplawski, A.; von Tell, D.; Crasto, C.; Bogdan, A. Somatic mosaicism for copy number variation in differentiated human tissues. Hum. Mutat. 2008, 29, 1118–1124. [Google Scholar] [CrossRef]
- Myers, S.M.; Challman, T.D.; Bernier, R.; Bourgeron, T.; Chung, W.K.; Constantino, J.N.; Eichler, E.E.; Jacquemont, S.; Miller, D.T.; Mitchell, K.J. Insufficient evidence for “autism-specific” genes. Am. J. Hum. Genet. 2020, 106, 587–595. [Google Scholar] [CrossRef]
- Watson, C.T.; Tomas, M.-B.; Sharp, A.J.; Mefford, H.C. The genetics of microdeletion and microduplication syndromes: An update. Annu. Rev. Genom. Hum. Genet. 2014, 15, 215–244. [Google Scholar] [CrossRef] [Green Version]
- Terry, A.V., Jr.; Callahan, P.M. Nicotinic acetylcholine receptor ligands, cognitive function, and preclinical approaches to drug discovery. Nicotine Tob. Res. 2019, 21, 383–394. [Google Scholar] [CrossRef] [PubMed]
- Schaaf, C.P. Nicotinic acetylcholine receptors in human genetic disease. Genet. Med. 2014, 16, 649–656. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Murray, T.A.; Liu, Q.; Whiteaker, P.; Wu, J.; Lukas, R.J. Nicotinic acetylcholine receptor α7 subunits with a C2 cytoplasmic loop yellow fluorescent protein insertion form functional receptors. Acta Pharmacol. Sin. 2009, 30, 828–841. [Google Scholar] [CrossRef] [Green Version]
- Albuquerque, E.X.; Pereira, E.F.; Alkondon, M.; Rogers, S.W. Mammalian nicotinic acetylcholine receptors: From structure to function. Physiol. Rev. 2009, 89, 73–120. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gillentine, M.A.; Schaaf, C.P. The human clinical phenotypes of altered CHRNA7 copy number. Biochem. Pharmacol. 2015, 97, 352–362. [Google Scholar] [CrossRef] [Green Version]
- Flomen, R.H.; Davies, A.F.; Di Forti, M.; La Cascia, C.; Mackie-Ogilvie, C.; Murray, R.; Makoff, A.J. The copy number variant involving part of the α7 nicotinic receptor gene contains a polymorphic inversion. Eur. J. Hum. Genet. 2008, 16, 1364–1371. [Google Scholar] [CrossRef]
- Szafranski, P.; Schaaf, C.P.; Person, R.E.; Gibson, I.B.; Xia, Z.; Mahadevan, S.; Wiszniewska, J.; Bacino, C.A.; Lalani, S.; Potocki, L. Structures and molecular mechanisms for common 15q13. 3 microduplications involving CHRNA7: Benign or pathological? Hum. Mutat. 2010, 31, 840–850. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schrider, D.R.; Hahn, M.W. Gene copy-number polymorphism in nature. Proc. R. Soc. B Biol. Sci. 2010, 277, 3213–3221. [Google Scholar] [CrossRef] [Green Version]
- Gamazon, E.R.; Stranger, B.E. The impact of human copy number variation on gene expression. Brief. Funct. Genom. 2015, 14, 352–357. [Google Scholar] [CrossRef] [Green Version]
- McCarroll, S.A.; Hadnott, T.N.; Perry, G.H.; Sabeti, P.C.; Zody, M.C.; Barrett, J.C.; Dallaire, S.; Gabriel, S.B.; Lee, C.; Daly, M.J. Common deletion polymorphisms in the human genome. Nat. Genet. 2006, 38, 86–92. [Google Scholar] [CrossRef] [PubMed]
- Somerville, M.J.; Mervis, C.B.; Young, E.J.; Seo, E.-J.; Del Campo, M.; Bamforth, S.; Peregrine, E.; Loo, W.; Lilley, M.; Pérez-Jurado, L.A. Severe expressive-language delay related to duplication of the Williams–Beuren locus. N. Engl. J. Med. 2005, 353, 1694–1701. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.A.; Madrid, R.E.; Sperle, K.; Ritterson, C.M.; Hobson, G.M.; Garbern, J.; Lupski, J.R.; Inoue, K. Spastic paraplegia type 2 associated with axonal neuropathy and apparent PLP1 position effect. Ann. Neurol. 2006, 59, 398–403. [Google Scholar] [CrossRef]
- Olesen, J. International classification of headache disorders. Lancet Neurol. 2018, 17, 396–397. [Google Scholar] [CrossRef] [Green Version]
- Liu, Q.; Liu, C.; Jiang, L.; Li, M.; Long, T.; He, W.; Qin, G.; Chen, L.; Zhou, J. α7 nicotinic acetylcholine receptor-mediated anti-inflammatory effect in a chronic migraine rat model via the attenuation of glial cell activation. J. Pain Res. 2018, 11, 1129. [Google Scholar] [CrossRef] [Green Version]
- Abrahams, B.S.; Arking, D.E.; Campbell, D.B.; Mefford, H.C.; Morrow, E.M.; Weiss, L.A.; Menashe, I.; Wadkins, T.; Banerjee-Basu, S.; Packer, A. SFARI Gene 2.0: A community-driven knowledgebase for the autism spectrum disorders (ASDs). Mol. Autism 2013, 4, 1–3. [Google Scholar] [CrossRef] [Green Version]
- Chomczynski, P.; Sacchi, N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal. Biochem. 1987, 162, 156–159. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2− ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Addis, L.; Rosch, R.E.; Valentin, A.; Makoff, A.; Robinson, R.; Everett, K.V.; Nashef, L.; Pal, D.K. Analysis of rare copy number variation in absence epilepsies. Neurol. Genet. 2016, 2. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Di Lorenzo, C.; Grieco, G.S.; Santorelli, F.M. Migraine headache: A review of the molecular genetics of a common disorder. J. Headache Pain 2012, 13, 571–580. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, Y.; Zhu, R.; Xiao, T.; Liu, X. Genetic variants in migraine: A field synopsis and systematic re-analysis of meta-analyses. J. Headache Pain 2020, 21, 1–15. [Google Scholar] [CrossRef]
- Perry, C.J.; Blake, P.; Buettner, C.; Papavassiliou, E.; Schain, A.J.; Bhasin, M.K.; Burstein, R. Upregulation of inflammatory gene transcripts in periosteum of chronic migraineurs: Implications for extracranial origin of headache. Ann. Neurol. 2016, 79, 1000–1013. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bagdas, D.; Wilkerson, J.L.; Kulkarni, A.; Toma, W.; AlSharari, S.; Gul, Z.; Lichtman, A.H.; Papke, R.L.; Thakur, G.A.; Damaj, M.I. The α7 nicotinic receptor dual allosteric agonist and positive allosteric modulator GAT107 reverses nociception in mouse models of inflammatory and neuropathic pain. Br. J. Pharmacol. 2016, 173, 2506–2520. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaur, S.; Ali, A.; Ahmad, U.; Siahbalaei, Y.; Pandey, A.; Singh, B. Role of single nucleotide polymorphisms (SNPs) in common migraine. Egypt. J. Neurol. Psychiatry Neurosurg. 2019, 55, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Kendall, K.M.; Rees, E.; Bracher-Smith, M.; Legge, S.; Riglin, L.; Zammit, S.; O’Donovan, M.C.; Owen, M.J.; Jones, I.; Kirov, G. Association of rare copy number variants with risk of depression. JAMA Psychiatry 2019, 76, 818–825. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sinkus, M.L.; Graw, S.; Freedman, R.; Ross, R.G.; Lester, H.A.; Leonard, S. The human CHRNA7 and CHRFAM7A genes: A review of the genetics, regulation, and function. Neuropharmacology 2015, 96, 274–288. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heywood, J.; Prentice, D. Migraine and hypertension: Is there a relationship? Aust. Fam. Physician 2001, 30, 461. [Google Scholar]
- Eidlitz-Markus, T.; Haimi-Cohen, Y.; Zeharia, A. Association of age at onset of migraine with family history of migraine in children attending a pediatric headache clinic: A retrospective cohort study. Cephalalgia 2015, 35, 722–727. [Google Scholar] [CrossRef] [PubMed]
- Lemos, C.; Castro, M.J.; Barros, J.; Sequeiros, J.; Pereira-Monteiro, J.; Mendonça, D.; Sousa, A. Familial clustering of migraine: Further evidence from a Portuguese study. Headache J. Head Face Pain 2009, 49, 404–411. [Google Scholar] [CrossRef]
- Tsai, C.-K.; Liang, C.-S.; Lin, G.-Y.; Tsai, C.-L.; Lee, J.-T.; Sung, Y.-F.; Lin, Y.-K.; Hung, K.-S.; Chen, W.-L.; Yang, F.-C. Identifying genetic variants for age of migraine onset in a Han Chinese population in Taiwan. J. Headache Pain 2021, 22, 1–10. [Google Scholar] [CrossRef]
- García-Marín, L.M.; Campos, A.I.; Martin, N.G.; Cuéllar-Partida, G.; Rentería, M.E. Phenome-wide analysis highlights putative causal relationships between self-reported migraine and other complex traits. J. Headache Pain 2021, 22, 1–8. [Google Scholar] [CrossRef]
- Leblond, C.S.; Heinrich, J.; Delorme, R.; Proepper, C.; Betancur, C.; Huguet, G.; Konyukh, M.; Chaste, P.; Ey, E.; Rastam, M. Genetic and functional analyses of SHANK2 mutations suggest a multiple hit model of autism spectrum disorders. PLoS Genet. 2012, 8, e1002521. [Google Scholar] [CrossRef] [Green Version]
- Van Den Maagdenberg, A.M.; Nyholt, D.R.; Anttila, V. Novel hypotheses emerging from GWAS in migraine? J. Headache Pain 2019, 20, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Zarrei, M.; MacDonald, J.R.; Merico, D.; Scherer, S.W. A copy number variation map of the human genome. Nat. Rev. Genet. 2015, 16, 172–183. [Google Scholar] [CrossRef]
- Wilkie, A.O. Bad bones, absent smell, selfish testes: The pleiotropic consequences of human FGF receptor mutations. Cytokine Growth Factor Rev. 2005, 16, 187–203. [Google Scholar] [CrossRef]
- Swensen, J.; Keyser, J.; Coffin, C.; Biegel, J.; Viskochil, D.; Williams, M. Familial occurrence of schwannomas and malignant rhabdoid tumour associated with a duplication in SMARCB1. J. Med. Genet. 2009, 46, 68–72. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Valsesia, A.; Stevenson, B.J.; Waterworth, D.; Mooser, V.; Vollenweider, P.; Waeber, G.; Jongeneel, C.V.; Beckmann, J.S.; Kutalik, Z.; Bergmann, S. Identification and validation of copy number variants using SNP genotyping arrays from a large clinical cohort. BMC Genom. 2012, 13, 241. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prabhanjan, M.; Suresh, R.V.; Murthy, M.N.; Ramachandra, N.B. Type 2 diabetes mellitus disease risk genes identified by genome wide copy number variation scan in normal populations. Diabetes Res. Clin. Pract. 2016, 113, 160–170. [Google Scholar] [CrossRef] [PubMed]
- Gillentine, M.A.; Yin, J.; Bajic, A.; Zhang, P.; Cummock, S.; Kim, J.J.; Schaaf, C.P. Functional consequences of CHRNA7 copy-number alterations in induced pluripotent stem cells and neural progenitor cells. Am. J. Hum. Genet. 2017, 101, 874–887. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Liu, J.; Lu, J.; Peng, J.; Juan, L.; Zhu, X.; Li, B.; Wang, Y. Joint detection of copy number variations in parent-offspring trios. Bioinformatics 2016, 32, 1130–1137. [Google Scholar] [CrossRef] [Green Version]
- Kubista, K.E.; Tosakulwong, N.; Wu, Y.; Ryu, E.; Roeder, J.L.; Hecker, L.A.; Baratz, K.H.; Brown, W.L.; Edwards, A.O. Copy number variation in the complement factor H-related genes. Mol. Vis. 2011, 17, 2080–2092. [Google Scholar]

| Characteristics | Control (%) (n = 120) | Migraine (%) (n = 102) |
|---|---|---|
| Age (years) | ||
| 15–20 | 5 (4.2) | 6 (5.8) |
| 21–25 | 16 (13.3) | 11 (10.8) |
| 26–30 | 13 (10.9) | 20 (19.6) |
| 31–35 | 24 (20) | 22 (21.6) |
| 36–40 | 31 (25.8) | 13 (12.7) |
| 41–45 | 22 (18.3) | 14 (13.7) |
| 46–50 | 9 (7.5) | 12 (11.8) |
| 51–55 | 0 | 2 (2) |
| 56–60 | 0 | 2 (2) |
| Gender (n, %) | ||
| Female | 73 (60.8) | 90 (88.2) |
| Male | 47 (39.2) | 12 (11.8) |
| Migraine type | ||
| with aura | NA | 43 (42.2) |
| without aura | 59 (57.8) | |
| Headache frequency (hour) | ||
| less than 12 | NA | 26 (25.5) |
| 12–24 | 35 (34.3) | |
| 24–48 | 29 (28.4) | |
| 48–72 | 12 (11.8) | |
| Site of pain | ||
| unilateral | NA | 52 (51) |
| bilateral | 32 (31.4) | |
| unclear | 18 (17.6) | |
| Location of pain | ||
| from the neck | NA | 42 (41.2) |
| from the temples | 43 (42.2) | |
| no localized | 17 (16.7) | |
| Starting of pain | ||
| generally sudden and severe | NA | 32 (31.4) |
| usually starts mild and becomes severe | 62 (60.8) | |
| not clear | 8 (7.8) | |
| Autonomic symptoms | ||
| (hot flashes, sweating, chills, diarrhea, abdominal pain, nausea, high blood pressure) | ||
| accompanies | NA | 73 (71.6) |
| not accompanies | 29 (28.4) | |
| Phonophobia | ||
| always | NA | 76 (74.5) |
| sometimes | 19 (18.6) | |
| absent | 7 (6.9) | |
| Photophobia | ||
| always | NA | 70 (68.6) |
| sometimes | 26 (25.5) | |
| absent | 6 (5.9) | |
| Severity | ||
| very severe | NA | 45 (44.1) |
| severe | 43 (42.2) | |
| mild | 10 (9.8) | |
| moderate | 4 (3.9) | |
| Frequency of headache | ||
| 2–3 times a week | NA | 32 (31.4) |
| 1 time a week | 23 (22.5) | |
| 1 in 2 weeks | 9 (8.8) | |
| 1–2 times a month | 30 (29.4) | |
| 1–2 times in 2–3 months | 8 (7.8) | |
| MIDAS | ||
| 0–5, little disability | NA | 40 (39.2) |
| 6–10, mild disability | 21 (20.6) | |
| 11–20, moderate disability | 35 (34.3) | |
| 20+, severe disability | 6 (5.9) | |
| Trigger factors | ||
| (irritability, fatigue, insomnia, menstruation, nutritional status, fasting period) | ||
| available | NA | 85 (83.3) |
| not available | 17 (16.7) | |
| Mood | ||
| available | NA | 82 (80.4) |
| not available | 20 (19.6) | |
| Equivalent | ||
| available | NA | 35 (34.3) |
| not available | 67 (65.7) | |
| Alcohol | 6 (5.9) | |
| Cigarette smoke | 24 (23.5) | |
| Use of painkillers | ||
| several or less per month | NA | 40 (39.2) |
| a few a week | 37 (36.3) | |
| a few a day | 23 (22.5) | |
| 4 per day | 2 (2) | |
| Disease history | ||
| no history | 97 (80.8) | 72 (70.6) |
| Hypertension | 7 (5.8) | 5 (4.9) |
| chronic lung disease | 1 (0.8) | 2 (2) |
| Kidney disease | 1 (0.8) | 2 (2) |
| Diabetes | 5 (4.2) | 5 (5) |
| Infections | 0 | 0 |
| Stroke | 0 | 0 |
| Endocrine disease | 3 (2.6) | 6 (5.9) |
| Asthma | 2 (1.7) | 2 (2) |
| Hypertension + chronic lung disease | 2 (1.7) | 0 |
| Hypertension + diabetes | 1 (0.8) | 1 (0.8) |
| Hypertension+ infection | 1 (0.8) | 1 (0.6) |
| Hypertension + endocrine | 0 | 1 (0.8) |
| Unclassified | 0 | 6 (6) |
| Family History | 0 | 9 (8.8) |
| Parameters | Control (%) (n = 120) | Migraine (%) (n = 102) | p-Value |
|---|---|---|---|
| Age | 34.52 ± 7.79 | 34.39 ± 10.03 | 0.703 |
| BMI | 25.11 ± 4.24 | 25.46 ± 4.45 | 0.575 |
| WBC | 8363.84 ± 3008.62 | 7446.13 ± 2730.98 | 0.025 * |
| Urea | 30.35 ± 1.55 | 29.03 ± 12.14 | 0.275 |
| GFR | 103.13 ± 17.93 | 105.58 ± 10.58 | 0.703 |
| ESR | 14.97 ± 8.72 | 19.52 ± 11.73 | 0.007 * |
| CRP | 4.06 ± 2.63 | 3.79 ± 2.62 | 0.466 |
| HBG | 12.86 ± 2.21 | 12.14 ± 2.29 | 0.027 * |
| Gene | Group | n | Mean ± SD | Median | 25th | 75th | OR (%95 CI) | p-Value |
|---|---|---|---|---|---|---|---|---|
| CHRNA7 | Control | 120 | 27.45 ± 8.78 | 28.09 | 10.19 | −44.39 | 1 (reference) | 0.001 * |
| Migraine | 102 | 6.44 ± 27.29 | 12.85 | −43.78 | −44.78 | 0.94 (0.92–0.96) |
| CNV | Groups | OR (95% CI) | p-Value | |
|---|---|---|---|---|
| Control n (%) | Migraine n (%) | |||
| Loss (<2) | 30 (25.0) | 72 (70.6) | 23.66 (9.75–57.41) | 0.001 * |
| Normal (=2) | 69 (57.5) | 7 (6.9) | 1 (Reference) | |
| Gain (>2) | 21 (17.5) | 23 (22.5) | 10.79 (4.06–28.68) | 0.001 * |
| Group | CNV | n | Gene Expression | p-Value | |||
|---|---|---|---|---|---|---|---|
| Mean ± SD | Median | 25th | 75th | ||||
| Control | Loss (<2) | 30 | 27.73 ± 9.96 | 27.99 | 10.43 | 44.39 | 0.981 |
| Normal (=2) | 69 | 27.22 ± 7.67 | 28.2 | 10.33 | 43.97 | ||
| Gain (>2) | 21 | 27.83 ± 10.71 | 27.71 | 10.19 | 43.55 | ||
| Migraine | Loss (<2) | 72 | 6.49 ± 27.94 | 12.82 | −43.78 | 44.78 | 0.940 |
| Normal (=2) | 7 | 5.98 ± 28.2 | 19.05 | −30.08 | 36.99 | ||
| Gain (>2) | 23 | 6.42 ± 26.14 | 12.77 | −43.21 | 43.38 | ||
| Cohorts | n | Mean ± SD | Median | 25th | 75th | p-Value |
|---|---|---|---|---|---|---|
| Cigarette smoke | 0.776 | |||||
| Yes | 24 | 6.36 ± 27.59 | 13.58 | −35.77 | 44.53 | |
| No | 78 | 6.46 ± 27.38 | 12.82 | −43.78 | 44.78 | |
| Diabetes | 0.926 | |||||
| Yes | 6 | 4.78 ± 24.8 | 14.76 | −28.04 | 32.64 | |
| No | 96 | 6.54 ± 27.55 | 12.85 | −43.78 | 44.78 | |
| Hypertension | 0.385 | |||||
| Yes | 7 | 13.84 ± 26.63 | 16.50 | −24.40 | 44.78 | |
| No | 95 | 5.89 ± 27.4 | 12.77 | −43.78 | 44.53 | |
| Migraine attack | 0.336 | |||||
| Yes | 60 | 4.47 ± 27.21 | 11.94 | −43.21 | 44.53 | |
| No | 42 | 9.26 ± 27.48 | 14.83 | −43.78 | 44.78 | |
| Migraine type | 0.085 | |||||
| with aura | 43 | 2.08 ± 25.42 | 12.12 | −43.78 | 41.72 | |
| without aura | 59 | 9.61 ± 28.37 | 15.03 | −43.21 | 44.78 | |
| Headache frequency | 0.682 | |||||
| less than 12 h | 26 | 6.29 ± 24.28 | 13.25 | −30.08 | 41.70 | |
| 12–24 h | 35 | 10.13 ± 28.68 | 17.91 | −43.78 | 44.78 | |
| 24–48 h | 29 | 4.57 ± 30.03 | 12.01 | −43.21 | 44.53 | |
| 48–72 h | 12 | 0.48 ± 23.84 | 11.64 | −30.23 | 41.19 | |
| Site of pain | 0.892 | |||||
| unilateral | 52 | 5.61 ± 27.58 | 12.08 | −43.21 | 44.53 | |
| bilateral | 32 | 6.91 ± 27.89 | 15.57 | −43.78 | 44.78 | |
| unclear | 18 | 8.01 ± 26.81 | 13.58 | −37.80 | 43.71 | |
| Location of pain | 0.204 | |||||
| from the neck | 42 | 10.34 ± 24.49 | 17.57 | −36.3 | 44.53 | |
| from the temples | 43 | 5.76 ± 29.61 | 12.12 | −43.78 | 44.78 | |
| no localized | 17 | −1.49 ± 27.47 | 11.31 | −43.21 | 43.71 | |
| Starting of pain | 0.861 | |||||
| generally sudden and severe | 32 | 5.34 ± 26.33 | 12.57 | −43.17 | 43.71 | |
| usually starts mild and becomes severe | 62 | 6.88 ± 27.52 | 13.66 | −43.78 | 44.78 | |
| Not clear | 8 | 7.44 ± 32.67 | 18.81 | −35.76 | 41.72 | |
| Autonomic symptoms | 0.124 | |||||
| accompanies | 29 | 13.17 ± 24.79 | 17.49 | −36.3 | 44.08 | |
| not accompanies | 73 | 3.76 ± 27.93 | 12.03 | −43.78 | 44.78 | |
| Phonophobia | 0.379 | |||||
| always | 76 | 8.38 ± 25.94 | 13.35 | −43.78 | 44.78 | |
| sometimes | 19 | 3.88 ± 32.04 | 17.49 | −43.21 | 43.71 | |
| absent | 7 | −7.72 ± 27.28 | −23.88 | −32.46 | 32.64 | |
| Photophobia | 0.530 | |||||
| always | 70 | 4.72 ± 27.22 | 12.39 | −43.78 | 44.53 | |
| sometimes | 26 | 10.81 ± 27.66 | 18.03 | −33.48 | 44.78 | |
| absent | 6 | 7.53 ± 29.14 | 17.08 | −32.44 | 38.66 | |
| Severity | 0.752 | |||||
| very severe | 45 | 4.46 ± 29.01 | 12.65 | −43.21 | 44.78 | |
| severe | 43 | 6.26 ± 25.34 | 12.03 | −43.17 | 44.53 | |
| mild | 10 | 11.17 ± 32.80 | 25.35 | −43.78 | 43.88 | |
| moderate | 4 | 18.87 ± 13.28 | 13.25 | 10.31 | 38.66 | |
| Frequency of headache | 0.008 * | |||||
| 2–3 times a week | 32 | −5.93 ± 27.12 | −9.43 | −43.78 | 43.71 | |
| 1 time a week | 23 | 16.42 ± 24.11 | 17.21 | −35.76 | 44.78 | |
| 1 time in 2 weeks | 9 | 8.13 ± 27.62 | 17.49 | −30.23 | 44.08 | |
| 1–2 times a month | 30 | 6.95 ± 26.78 | 15.29 | −41.99 | 43.38 | |
| 1–2 times in 2–3 months | 8 | 23.41 ± 21.4 | 25.77 | −23.49 | 43.88 | |
| MIDAS | 0.260 | |||||
| 0–5, little disability | 40 | 10.65 ± 26.8 | 17.78 | −41.99 | 44.08 | |
| 6–10, mild disability | 21 | 8.55 ± 27.62 | 14.63 | −35.76 | 44.78 | |
| 11–20, moderate disability | 35 | 2.82 ± 26.39 | 11.50 | −43.21 | 43.72 | |
| 20+, severe disability | 6 | −7.94 ± 33.95 | −14.88 | −43.78 | 44.53 | |
| Trigger factors | 0.625 | |||||
| Yes | 85 | 7.23 ± 26.19 | 12.92 | −43.78 | 44.53 | |
| No | 17 | 2.51 ± 32.85 | 11.85 | −43.17 | 44.78 | |
| Mood | 0.153 | |||||
| Yes | 82 | 4.70 ± 27.70 | 12.08 | −43.78 | 44.53 | |
| No | 20 | 13.57 ± 24.91 | 17.57 | −41.99 | 44.78 | |
| Equivalent | 0.891 | |||||
| Yes | 35 | 5.68 ± 28.51 | 14.63 | −43.17 | 44.53 | |
| No | 67 | 6.83 ± 26.84 | 12.71 | −43.78 | 44.78 | |
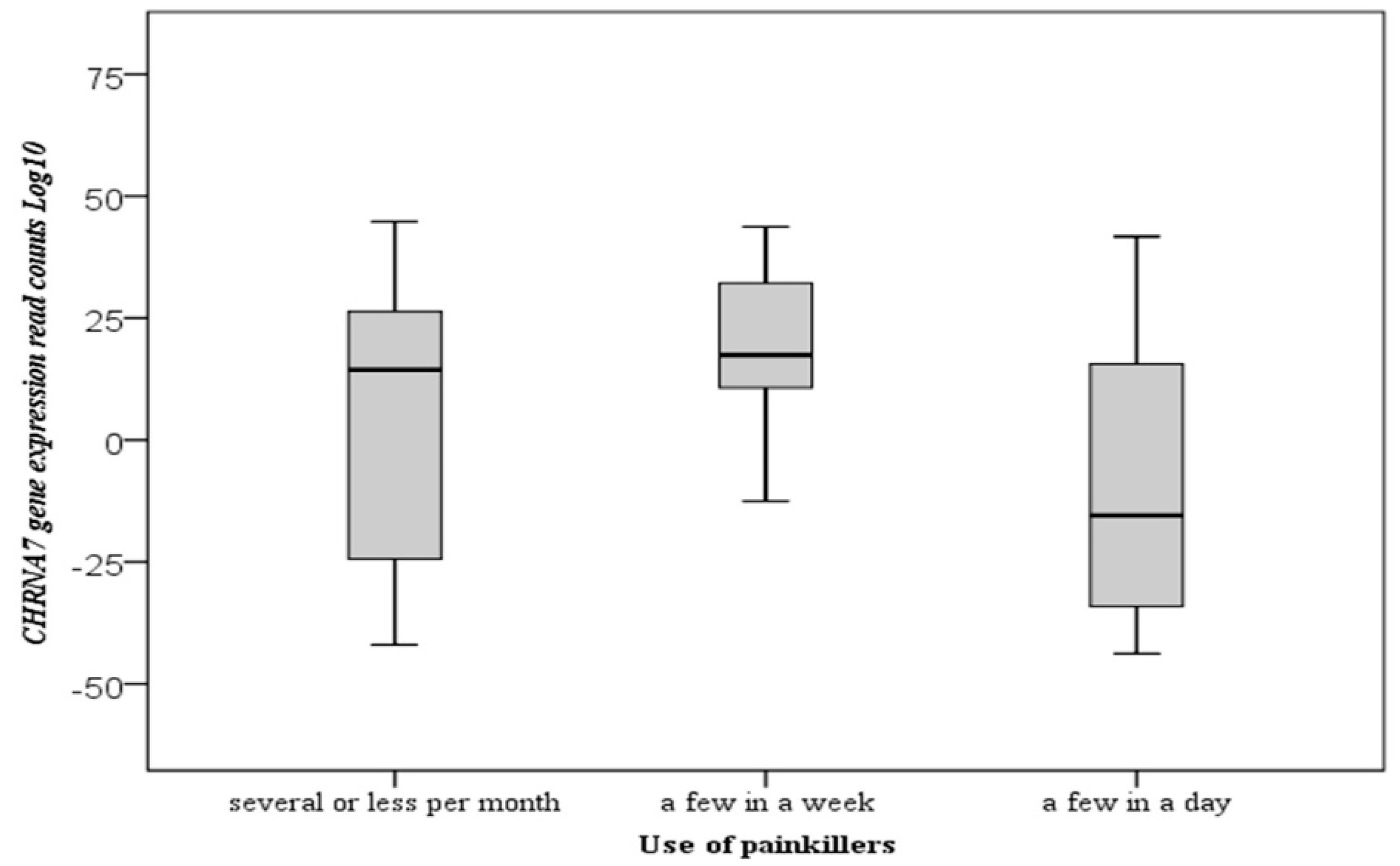
| Use of painkillers | 0.009 * | |||||
| several or less per month | 41 | 6.07 ± 28.07 | 14.39 | −41.99 | 44.78 | |
| a few a week | 38 | 15.72 ± 21.47 | 17.43 | −28.92 | 43.72 | |
| a few a day | 23 | −8.23 ± 28.93 | −15.48 | −43.78 | 41.72 |
| Cohorts | CNV | p-Value | ||
|---|---|---|---|---|
| Loss (<2) n (%) | Normal (= 2) n (%) | Gain (>2) n (%) | ||
| Cigarette smoke | ||||
| Yes | 20 (27.8) | 1 (14.3) | 3 (13.0) | 0.292 |
| No | 52 (72.2) | 6 (85.7) | 20 (87.0) | |
| Diabetes | ||||
| Yes | 4 (5.6) | 2 (28.6) | 0 (0.0) | 0.019 * |
| No | 68 (94.4) | 5 (71.4) | 23 (100.0) | |
| Hypertension | ||||
| Yes | 4 (5.6) | 1 (14.3) | 2 (8.7) | 0.632 |
| No | 68 (94.4) | 6 (85.7) | 21 (91.3) | |
| Migraine attack | 0.491 | |||
| Yes | 40 (55.6) | 4 (57.1) | 16 (69.6) | |
| No | 32 (44.4) | 3 (42.9) | 7 (30.4) | |
| Migraine type | ||||
| with aura | 29 (40.3) | 4 (57.1) | 10 (43.5) | 0.682 |
| without aura | 43 (59.7) | 3 (42.9) | 13 (56.5) | |
| Headache frequency | ||||
| less than 12 h | 20 (27.8) | 2 (28.6) | 4 (17.4) | |
| 12–24 h | 24 (33.3) | 1 (14.3) | 10 (43.5) | 0.681 |
| 24–48 h | 21 (29.2) | 3 (42.9) | 5 (21.7) | |
| 48–72 h | 7 (9.7) | 1 (14.3) | 4 (17.4) | |
| Site of pain | 0.436 | |||
| unilateral | 39 (54.2) | 2 (28.6) | 11 (47.8) | |
| bilateral | 22 (30.6) | 4 (57.1) | 6 (26.1) | |
| unclear | 11 (15.3) | 1 (14.3) | 6 (26.1) | |
| Location of pain | 0.135 | |||
| from the neck | 26 (36.1) | 4 (57.1) | 12 (52.2) | |
| from the temples | 35 (48.6) | 3 (42.9) | 5 (21.7) | |
| no localized | 11 (15.3) | 0 (0.0) | 6 (26.1) | |
| Starting of pain | ||||
| generally sudden and severe | 22 (30.6) | 2 (28.6) | 8 (34.8) | |
| usually starts mild and becomes severe | 44 (61.1) | 5 (71.4) | 13 (56.5) | 0.923 |
| Not clear | 6 (8.3) | 0 (0.0) | 2 (8.7) | |
| Autonomic symptoms | ||||
| accompanies | 18 (25.0) | 2 (28.6) | 9 (39.1) | 0.425 |
| does not accompany | 54 (75.0) | 5 (71.4) | 14 (60.9) | |
| Phonophobia | ||||
| always | 53 (73.6) | 5 (71.4) | 18 (78.3) | |
| sometimes | 15 (20.8) | 0 (0.0) | 4 (17.4) | 0.149 |
| absent | 4 (5.6) | 2 (28.6) | 1 (4.3) | |
| Photophobia | ||||
| always | 48 (66.7) | 3 (42.9) | 19 (82.6) | |
| sometimes | 22 (30.6) | 2 (28.6) | 2 (8.7) | 0.015 * |
| absent | 2 (2.8) | 2 (28.6) | 2 (8.7) | |
| Severity | ||||
| very severe | 31 (43.1) | 4 (57.1) | 10 (43.5) | |
| severe | 30 (41.7) | 3 (42.9) | 10 (43.5) | 0.667 |
| mild | 9 (12.5) | 0 (0.0) | 1 (4.3) | |
| moderate | 2 (2.8) | 0 (0.0) | 2 (8.7) | |
| Frequency of headache | ||||
| 2–3 times a week | 21 (29.2) | 2 (28.6) | 9 (39.1) | |
| 1 time a week | 20 (27.8) | 0 (0.0) | 3 (13.0) | 0.243 |
| 1 time in 2 weeks | 4 (5.6) | 1 (14.3) | 4 (17.4) | |
| 1–2 times a month | 23 (31.9) | 3 (42.9) | 4 (17.4) | |
| 1–2 times in 2–3 months | 4 (5.6) | 1 (14.3) | 3 (13.0) | |
| MIDAS | ||||
| 0–5, little disability | 27 (37.5) | 5 (71.4) | 8 (34.8) | 0.277 |
| 6–10, mild disability | 14 (19.4) | 1 (14.3) | 6 (26.1) | |
| 11–20, moderate disability | 26 (36.1) | 0 (0.0) | 9 (39.1) | |
| 20+, severe disability | 5 (6.9) | 1 (14.3) | 0 (0.0) | |
| Trigger factors | ||||
| Yes | 61 (84.7) | 6 (85.7) | 18 (78.3) | 0.758 |
| No | 11 (15.3) | 1 (14.3) | 5 (21.7) | |
| Mood | ||||
| Yes | 55 (76.4) | 7 (100.0) | 20 (87.0) | 0.216 |
| No | 17 (23.6) | 0 (0.0) | 3 (13.0) | |
| Equivalent | ||||
| Yes | 26 (36.1) | 4 (57.1) | 5 (21.7) | 0.189 |
| No | 46 (63.9) | 3 (42.9) | 18 (78.3) | |
| Use of painkillers | ||||
| several or less per month | 27 (37.5) | 4 (57.1) | 10 (43.5) | 0.821 |
| a few a week | 27 (37.5) | 2 (28.6) | 9 (39.1) | |
| a few a day | 18 (25.0) | 1 (14.3) | 4 (17.4) | |
| Compared Groups | Variation | AUC | 95% CI | p-Value | Specificity (%) | Sensitivity (%) | Criterion |
|---|---|---|---|---|---|---|---|
| Migraine patients vs. Control | CHRNA7 expression | 0.731 | (0.667–0.788) | <0.001 | 80.8 | 66.7 | ≤19.79 |
| CNV | 0.538 | (0.470–0.605) | 0.2563 | 79.0 | 30.4 | >2 | |
| AUC, area under the curve; CI, confidence interval. | |||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Özaltun, M.F.; Geyik, S.; Yılmaz, Ş.G. Screening for Copy Number Variations of the 15q13.3 Hotspot in CHRNA7 Gene and Expression in Patients with Migraines. Curr. Issues Mol. Biol. 2021, 43, 1090-1113. https://doi.org/10.3390/cimb43020078
Özaltun MF, Geyik S, Yılmaz ŞG. Screening for Copy Number Variations of the 15q13.3 Hotspot in CHRNA7 Gene and Expression in Patients with Migraines. Current Issues in Molecular Biology. 2021; 43(2):1090-1113. https://doi.org/10.3390/cimb43020078
Chicago/Turabian StyleÖzaltun, Mehmet Fatih, Sırma Geyik, and Şenay Görücü Yılmaz. 2021. "Screening for Copy Number Variations of the 15q13.3 Hotspot in CHRNA7 Gene and Expression in Patients with Migraines" Current Issues in Molecular Biology 43, no. 2: 1090-1113. https://doi.org/10.3390/cimb43020078
APA StyleÖzaltun, M. F., Geyik, S., & Yılmaz, Ş. G. (2021). Screening for Copy Number Variations of the 15q13.3 Hotspot in CHRNA7 Gene and Expression in Patients with Migraines. Current Issues in Molecular Biology, 43(2), 1090-1113. https://doi.org/10.3390/cimb43020078






