Anti-Inflammatory Effects of Resveratrol on Human Retinal Pigment Cells and a Myopia Animal Model
Abstract
:1. Introduction
2. Material and Methods
2.1. Cell Culture
2.2. Cell Viability Test
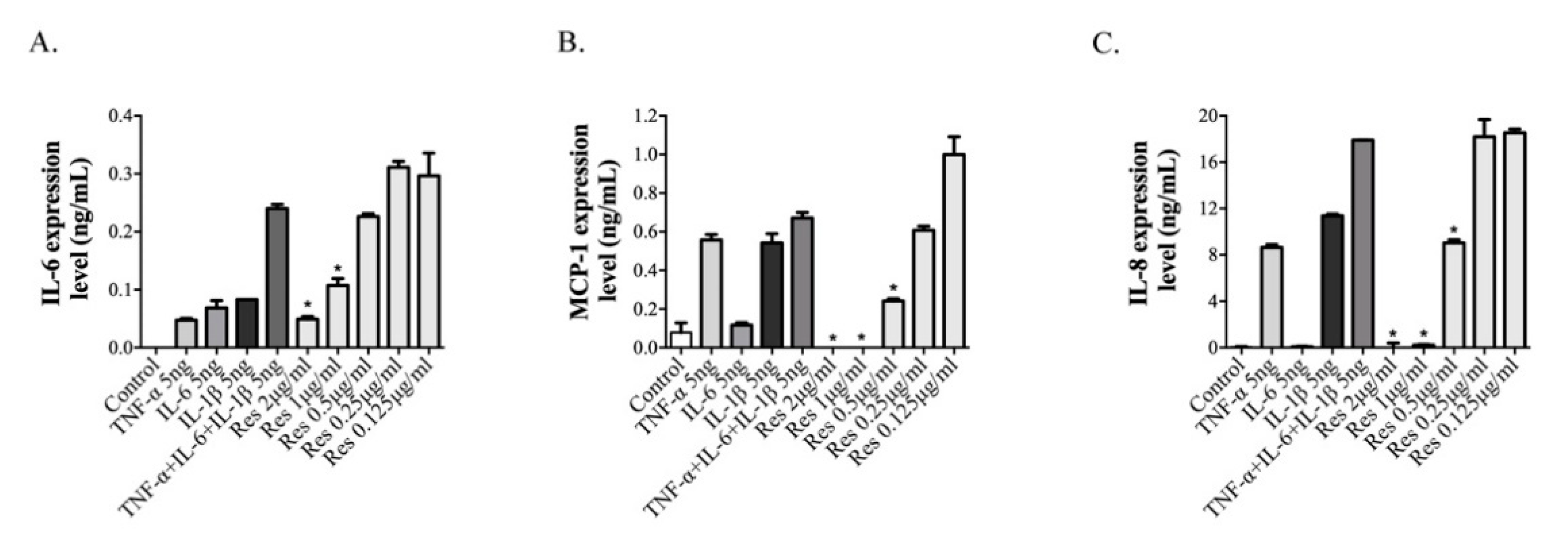
2.3. Enzyme-Linked Immunosorbent Assay (ELISA)
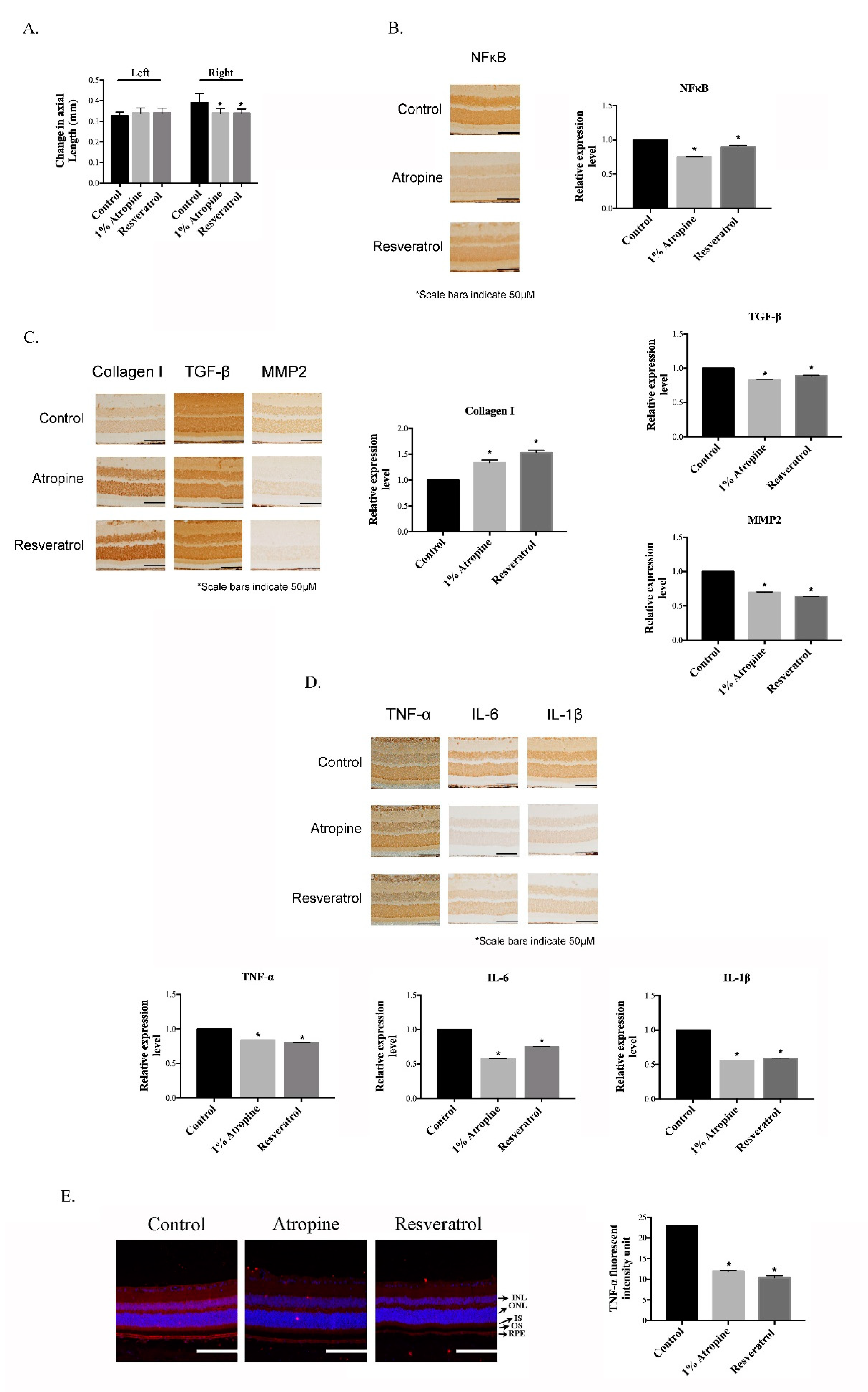
2.4. Animals and MFD Induction
2.5. Preparation of Resveratrol Solution
2.6. Immunohistochemical and Immunofluorescence Analyses
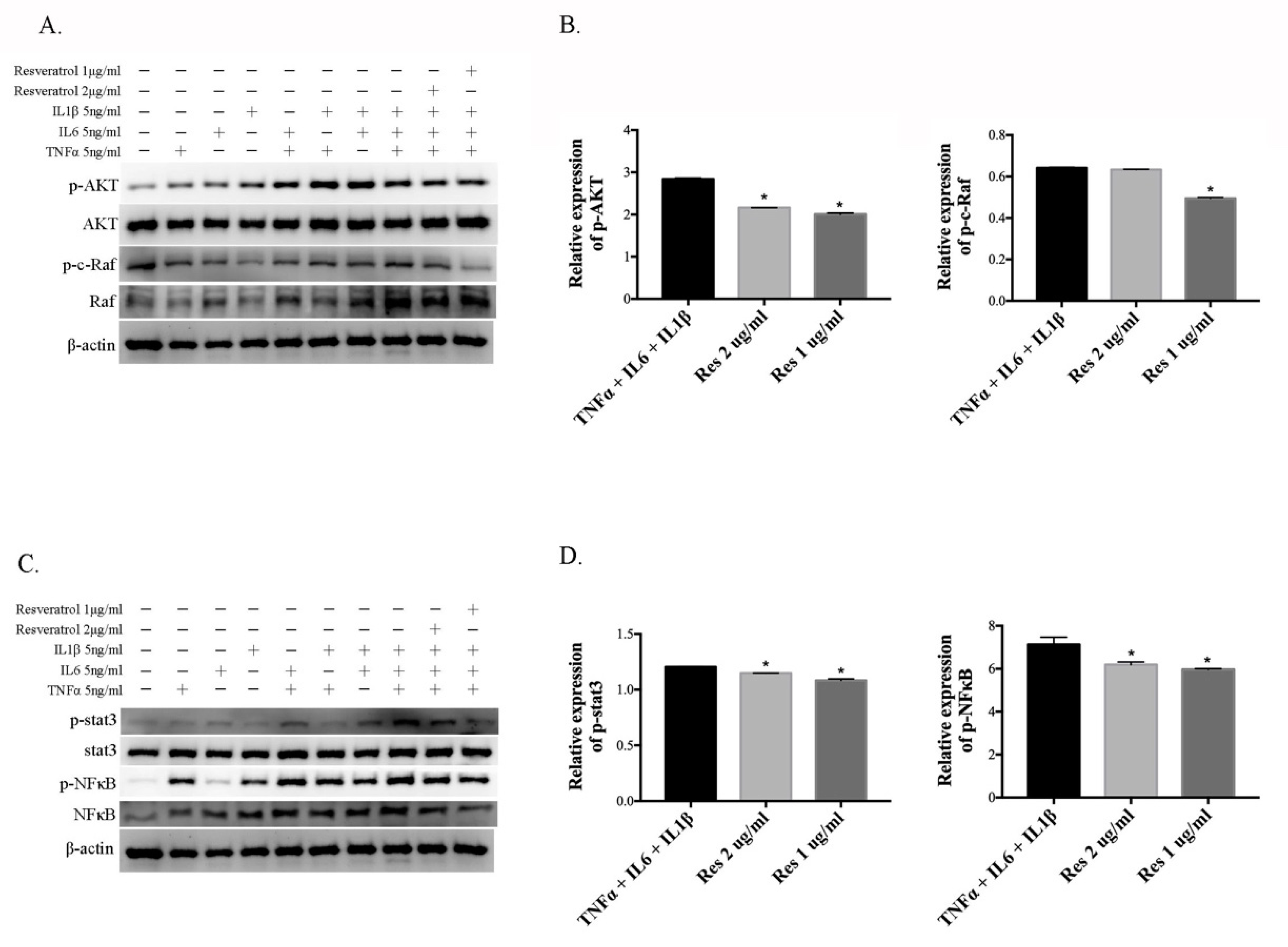
2.7. Western Blotting Analysis
2.8. Statistical Analysis
3. Results
3.1. Resveratrol Inhibits the Progression of Myopia by Suppressing Inflammation in the MFD Animal Model
3.2. Resveratrol Shows No Significant Effect on the Viability of ARPE-19 Cells
3.3. Resveratrol Inhibits the Inflammatory Effects of ARPE-19 Cells
3.4. Resveratrol Reduces the Activation of Inflammatory Cytokine-Induced AKT, c-Raf, Stat3, and NFκB in ARPE-19 Cells
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Resnikoff, S.; Pascolini, D.; Mariotti, S.P.; Pokharel, G.P. Global magnitude of visual impairment caused by uncorrected refractive errors in 2004. Bull. World Health Organ. 2008, 86, 63–70. [Google Scholar] [CrossRef]
- McBrien, N.A.; Millodot, M. A biometric investigation of late onset myopic eyes. Acta Ophthalmol. 1987, 65, 461–468. [Google Scholar] [CrossRef]
- McBrien, N.A.; Lawlor, P.; Gentle, A. Scleral remodeling during the development of and recovery from axial myopia in the tree shrew. Investig. Ophthalmol. Vis. Sci. 2000, 41, 3713–3719. [Google Scholar]
- Christensen, A.M.; Wallman, J. Evidence that increased scleral growth underlies visual deprivation myopia in chicks. Investig. Ophthalmol. Vis. Sci. 1991, 32, 2143–2150. [Google Scholar]
- Norton, T.T.; Rada, J.A. Reduced extracellular matrix in mammalian sclera with induced myopia. Vis. Res. 1995, 35, 1271–1281. [Google Scholar] [CrossRef]
- She, M.; Li, B.; Li, T.; Hu, Q.; Zhou, X. Modulation of the ERK1/2-MMP-2 pathway in the sclera of guinea pigs following induction of myopia by flickering light. Exp. Ther. Med. 2021, 21, 371. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Tang, Z.; Xue, R.; Singh, G.K.; Lv, Y.; Shi, K.; Cai, K.; Deng, L.; Yang, L. TGF-beta1 promoted MMP-2 mediated wound healing of anterior cruciate ligament fibroblasts through NF-kappaB. Connect Tiss. Res. 2011, 52, 218–225. [Google Scholar] [CrossRef] [PubMed]
- Guggenheim, J.A.; McBrien, N.A. Form-deprivation myopia induces activation of scleral matrix metalloproteinase-2 in tree shrew. Investig. Ophthalmol. Vis. Sci. 1996, 37, 1380–1395. [Google Scholar]
- Lin, H.J.; Wei, C.C.; Chang, C.Y.; Chen, T.H.; Hsu, Y.A.; Hsieh, Y.C.; Chen, H.J.; Wan, L. Role of Chronic Inflammation in Myopia Progression: Clinical Evidence and Experimental Validation. EBioMedicine 2016, 10, 269–281. [Google Scholar] [CrossRef] [Green Version]
- Huang, F.C.; Kuo, H.C.; Huang, Y.H.; Yu, H.R.; Li, S.C.; Kuo, H.C. Anti-inflammatory effect of resveratrol in human coronary arterial endothelial cells via induction of autophagy: Implication for the treatment of Kawasaki disease. BMC Pharmacol. Toxicol. 2017, 18, 3. [Google Scholar] [CrossRef] [Green Version]
- Galiniak, S.; Aebisher, D.; Bartusik-Aebisher, D. Health benefits of resveratrol administration. Acta Biochim. Pol. 2019, 66, 13–21. [Google Scholar] [CrossRef] [Green Version]
- Zhou, Z.X.; Mou, S.F.; Chen, X.Q.; Gong, L.L.; Ge, W.S. Anti-inflammatory activity of resveratrol prevents inflammation by inhibiting NFkappaB in animal models of acute pharyngitis. Mol. Med. Rep. 2018, 17, 1269–1274. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deng, Z.Y.; Hu, M.M.; Xin, Y.F.; Gang, C. Resveratrol alleviates vascular inflammatory injury by inhibiting inflammasome activation in rats with hypercholesterolemia and vitamin D2 treatment. Inflamm. Res. 2015, 64, 321–332. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Xia, N.; Hasselwander, S.; Daiber, A. Resveratrol and Vascular Function. Int. J. Mol. Sci. 2019, 20, 2155. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, S.; Jin, Y.; Choi, Y.; Park, T. Resveratrol exerts anti-obesity effects via mechanisms involving down-regulation of adipogenic and inflammatory processes in mice. Biochem. Pharmacol. 2011, 81, 1343–1351. [Google Scholar] [CrossRef]
- Orlandi, I.; Stamerra, G.; Strippoli, M.; Vai, M. During yeast chronological aging resveratrol supplementation results in a short-lived phenotype Sir2-dependent. Redox. Biol. 2017, 12, 745–754. [Google Scholar] [CrossRef]
- Pyo, I.S.; Yun, S.; Yoon, Y.E.; Choi, J.W.; Lee, S.J. Mechanisms of Aging and the Preventive Effects of Resveratrol on Age-Related Diseases. Molecules 2020, 25, 4649. [Google Scholar] [CrossRef] [PubMed]
- Lancon, A.; Frazzi, R.; Latruffe, N. Anti-Oxidant, Anti-Inflammatory and Anti-Angiogenic Properties of Resveratrol in Ocular Diseases. Molecules 2016, 21, 304. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.A.J.; Kaushik, R.S.; Dwivedi, C. Effects of resveratrol, an important component of red wine, on intestinal cancer development. Int. J. Wine Res. 2009, I, 147–153. [Google Scholar]
- Malaguarnera, L. Influence of Resveratrol on the Immune Response. Nutrients 2019, 11, 946. [Google Scholar] [CrossRef] [Green Version]
- Ku, K.L.; Chang, P.S.; Cheng, Y.C.; Lien, C.Y. Production of stilbenoids from the callus of Arachis hypogaea: A novel source of the anticancer compound piceatannol. J. Agric. Food Chem. 2005, 53, 3877–3881. [Google Scholar] [CrossRef]
- Qiao-Grider, Y.; Hung, L.F.; Kee, C.S.; Ramamirtham, R.; Smith, E.L., 3rd. Recovery from form-deprivation myopia in rhesus monkeys. Investig. Ophthalmol. Vis. Sci. 2004, 45, 3361–3372. [Google Scholar] [CrossRef] [Green Version]
- Lin, T.; Grimes, P.A.; Stone, R.A. Expansion of the retinal pigment epithelium in experimental myopia. Vis. Res. 1993, 33, 1881–1885. [Google Scholar] [CrossRef]
- Szende, B.; Tyihak, E.; Kiraly-Veghely, Z. Dose-dependent effect of resveratrol on proliferation and apoptosis in endothelial and tumor cell cultures. Exp. Mol. Med. 2000, 32, 88–92. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nonomura, S.; Kanagawa, H.; Makimoto, A. Chemical Constituents of Polygonaceous Plants. I. Studies on the Components of Ko-J O-Kon. (Polygonum cuspidatum Sieb. Et Zucc.). Yakugaku Zasshi 1963, 83, 988–990. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chedea, V.S.; Vicas, S.I.; Sticozzi, C.; Pessina, F.; Frosini, M.; Maioli, E.; Valacchi, G. Resveratrol: From diet to topical usage. Food Funct. 2017, 8, 3879–3892. [Google Scholar] [CrossRef] [PubMed]
- Romero-Pérez, A.I.; Lamuela-Raventós, R.M.; Waterhouse, A.L.; Torre-Boronat, M.C.d.l. Levels of cis- and trans-Resveratrol and Their Glucosides in White and Rosé Vitis vinifera Wines from Spain. J. Agric. Food Chem. 1996, 44, 2124–2128. [Google Scholar] [CrossRef]
- Hubbard, B.P.; Sinclair, D.A. Small molecule SIRT1 activators for the treatment of aging and age-related diseases. Trends Pharmacol. Sci. 2014, 35, 146–154. [Google Scholar] [CrossRef] [Green Version]
- Wu, Y.; Pang, Y.; Wei, W.; Shao, A.; Deng, C.; Li, X.; Chang, H.; Hu, P.; Liu, X.; Zhang, X. Resveratrol protects retinal ganglion cell axons through regulation of the SIRT1-JNK pathway. Exp. Eye Res. 2020, 200, 108249. [Google Scholar] [CrossRef]
- Wang, S.; Wang, Z.; Yang, S.; Yin, T.; Zhang, Y.; Qin, Y.; Weinreb, R.N.; Sun, X. Tissue Distribution of trans-Resveratrol and Its Metabolites after Oral Administration in Human Eyes. J. Ophthalmol. 2017, 2017, 4052094. [Google Scholar] [CrossRef] [Green Version]
- Baur, J.A.; Pearson, K.J.; Price, N.L.; Jamieson, H.A.; Lerin, C.; Kalra, A.; Prabhu, V.V.; Allard, J.S.; Lopez-Lluch, G.; Lewis, K.; et al. Resveratrol improves health and survival of mice on a high-calorie diet. Nature 2006, 444, 337–342. [Google Scholar] [CrossRef]
- Rauf, A.; Imran, M.; Suleria, H.A.R.; Ahmad, B.; Peters, D.G.; Mubarak, M.S. A comprehensive review of the health perspectives of resveratrol. Food Funct. 2017, 8, 4284–4305. [Google Scholar] [CrossRef]
- Gupta, P.K.; DiPette, D.J.; Supowit, S.C. Protective effect of resveratrol against pressure overload-induced heart failure. Food Sci. Nutr. 2014, 2, 218–229. [Google Scholar] [CrossRef] [PubMed]
- McCracken, J.L.; Veeranki, S.P.; Ameredes, B.T.; Calhoun, W.J. Diagnosis and Management of Asthma in Adults: A Review. JAMA 2017, 318, 279–290. [Google Scholar] [CrossRef] [PubMed]
- Royce, S.G.; Dang, W.; Yuan, G.; Tran, J.; El Osta, A.; Karagiannis, T.C.; Tang, M.L. Resveratrol has protective effects against airway remodeling and airway hyperreactivity in a murine model of allergic airways disease. Pathobiol. Aging Age Relat. Dis. 2011, 1. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.; Kim, S.; Kwon, O.K.; Oh, S.R.; Lee, H.K.; Ahn, K. Anti-inflammatory and anti-asthmatic effects of resveratrol, a polyphenolic stilbene, in a mouse model of allergic asthma. Int. Immunopharmacol. 2009, 9, 418–424. [Google Scholar] [CrossRef]
- Bi, X.L.; Yang, J.Y.; Dong, Y.X.; Wang, J.M.; Cui, Y.H.; Ikeshima, T.; Zhao, Y.Q.; Wu, C.F. Resveratrol inhibits nitric oxide and TNF-alpha production by lipopolysaccharide-activated microglia. Int. Immunopharmacol. 2005, 5, 185–193. [Google Scholar] [CrossRef] [PubMed]
- Nagai, N.; Kubota, S.; Tsubota, K.; Ozawa, Y. Resveratrol prevents the development of choroidal neovascularization by modulating AMP-activated protein kinase in macrophages and other cell types. J. Nutr. Biochem. 2014, 25, 1218–1225. [Google Scholar] [CrossRef] [Green Version]
- Capiralla, H.; Vingtdeux, V.; Zhao, H.; Sankowski, R.; Al-Abed, Y.; Davies, P.; Marambaud, P. Resveratrol mitigates lipopolysaccharide- and Abeta-mediated microglial inflammation by inhibiting the TLR4/NF-kappaB/STAT signaling cascade. J. Neurochem. 2012, 120, 461–472. [Google Scholar] [CrossRef] [Green Version]
- Meng, T.; Xiao, D.; Muhammed, A.; Deng, J.; Chen, L.; He, J. Anti-Inflammatory Action and Mechanisms of Resveratrol. Molecules 2021, 26, 229. [Google Scholar] [CrossRef]
- Adamus, G.; Webb, S.; Shiraga, S.; Duvoisin, R.M. Anti-recoverin antibodies induce an increase in intracellular calcium, leading to apoptosis in retinal cells. J. Autoimmun. 2006, 26, 146–153. [Google Scholar] [CrossRef] [PubMed]
- Shetty, R.; Subramani, M.; Murugeswari, P.; Anandula, V.R.; Matalia, H.; Jayadev, C.; Ghosh, A.; Das, D. Resveratrol Rescues Human Corneal Epithelial Cells Cultured in Hyperosmolar Conditions: Potential for Dry Eye Disease Treatment. Cornea 2020, 39, 1520–1532. [Google Scholar] [CrossRef] [PubMed]
- Subramani, M.; Ponnalagu, M.; Krishna, L.; Jeyabalan, N.; Chevour, P.; Sharma, A.; Jayadev, C.; Shetty, R.; Begum, N.; Archunan, G.; et al. Resveratrol reverses the adverse effects of bevacizumab on cultured ARPE-19 cells. Sci. Rep. 2017, 7, 12242. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Y.; Wildsoet, C.F. RPE and Choroid Mechanisms Underlying Ocular Growth and Myopia. Prog. Mol. Biol. Transl. Sci. 2015, 134, 221–240. [Google Scholar] [CrossRef] [Green Version]
- Inamori, Y.; Ota, M.; Inoko, H.; Okada, E.; Nishizaki, R.; Shiota, T.; Mok, J.; Oka, A.; Ohno, S.; Mizuki, N. The COL1A1 gene and high myopia susceptibility in Japanese. Hum. Genet. 2007, 122, 151–157. [Google Scholar] [CrossRef] [Green Version]
- Rada, J.A.; Brenza, H.L. Increased latent gelatinase activity in the sclera of visually deprived chicks. Investig. Ophthalmol. Vis. Sci. 1995, 36, 1555–1565. [Google Scholar]
- Wei, C.C.; Kung, Y.J.; Chen, C.S.; Chang, C.Y.; Lin, C.J.; Tien, P.T.; Chang, H.Y.; Chen, H.J.; Huang, Y.S.; Lin, H.J.; et al. Allergic Conjunctivitis-induced Retinal Inflammation Promotes Myopia Progression. EBioMedicine 2018, 28, 274–286. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pascolini, D.; Mariotti, S.P. Global estimates of visual impairment: 2010. Br. J. Ophthalmol. 2012, 96, 614–618. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Strauss, O. The retinal pigment epithelium in visual function. Physiol. Rev. 2005, 85, 845–881. [Google Scholar] [CrossRef] [Green Version]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hsu, Y.-A.; Chen, C.-S.; Wang, Y.-C.; Lin, E.-S.; Chang, C.-Y.; Chen, J.J.-Y.; Wu, M.-Y.; Lin, H.-J.; Wan, L. Anti-Inflammatory Effects of Resveratrol on Human Retinal Pigment Cells and a Myopia Animal Model. Curr. Issues Mol. Biol. 2021, 43, 716-727. https://doi.org/10.3390/cimb43020052
Hsu Y-A, Chen C-S, Wang Y-C, Lin E-S, Chang C-Y, Chen JJ-Y, Wu M-Y, Lin H-J, Wan L. Anti-Inflammatory Effects of Resveratrol on Human Retinal Pigment Cells and a Myopia Animal Model. Current Issues in Molecular Biology. 2021; 43(2):716-727. https://doi.org/10.3390/cimb43020052
Chicago/Turabian StyleHsu, Yu-An, Chih-Sheng Chen, Yao-Chien Wang, En-Shyh Lin, Ching-Yao Chang, Jamie Jiin-Yi Chen, Ming-Yen Wu, Hui-Ju Lin, and Lei Wan. 2021. "Anti-Inflammatory Effects of Resveratrol on Human Retinal Pigment Cells and a Myopia Animal Model" Current Issues in Molecular Biology 43, no. 2: 716-727. https://doi.org/10.3390/cimb43020052
APA StyleHsu, Y.-A., Chen, C.-S., Wang, Y.-C., Lin, E.-S., Chang, C.-Y., Chen, J. J.-Y., Wu, M.-Y., Lin, H.-J., & Wan, L. (2021). Anti-Inflammatory Effects of Resveratrol on Human Retinal Pigment Cells and a Myopia Animal Model. Current Issues in Molecular Biology, 43(2), 716-727. https://doi.org/10.3390/cimb43020052





