Effect of Bombyx mori on the Liver Protection of Non-Alcoholic Fatty Liver Disease Based on In Vitro and In Vivo Models
Abstract
1. Introduction
2. Materials and Methods
2.1. SW Powder Preparation for Metabolite Analysis
2.2. Gas Chromatography-Time of Flight-Mass Spectrometry Analysis (GC-TOF-MS)
2.3. Ultrahigh Performance Liquid Chromatography-Linear Trap Quadrupole-Orbitrap-Mass Spectrometry (UHPLC-LTQ-Orbitrap-MS/MS) Analysis
2.4. Cell Culture and Free Fatty Acid (FFA) Treatment
2.5. Cell Cytotoxicity Detection and Oil Red O Staining
2.6. Experimental Animals and Diets
2.7. Blood and Tissue Sample Preparation and Histological Analysis
2.8. Biochemical Analysis of Liver Tissues
2.9. Quantification of Gene Expression Using Real-Time PCR
2.10. Immunoblot Analysis
2.11. Fecal Microbiota Analysis
2.12. Data Analysis
3. Results
3.1. Metabolites Identified through GC-TOF-MS and UHPLC-LTQ-Orbitrap-MS Analyses
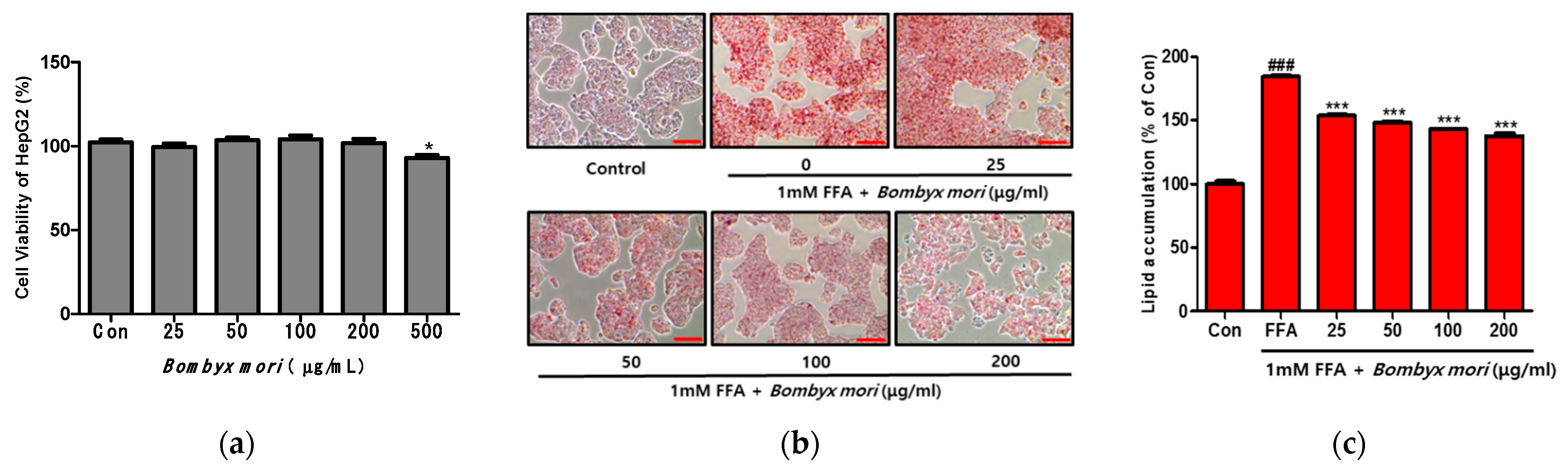
3.2. Effects of SW on Cell Viability and FFA-Induced Lipid Accumulation in HepG2 Cells
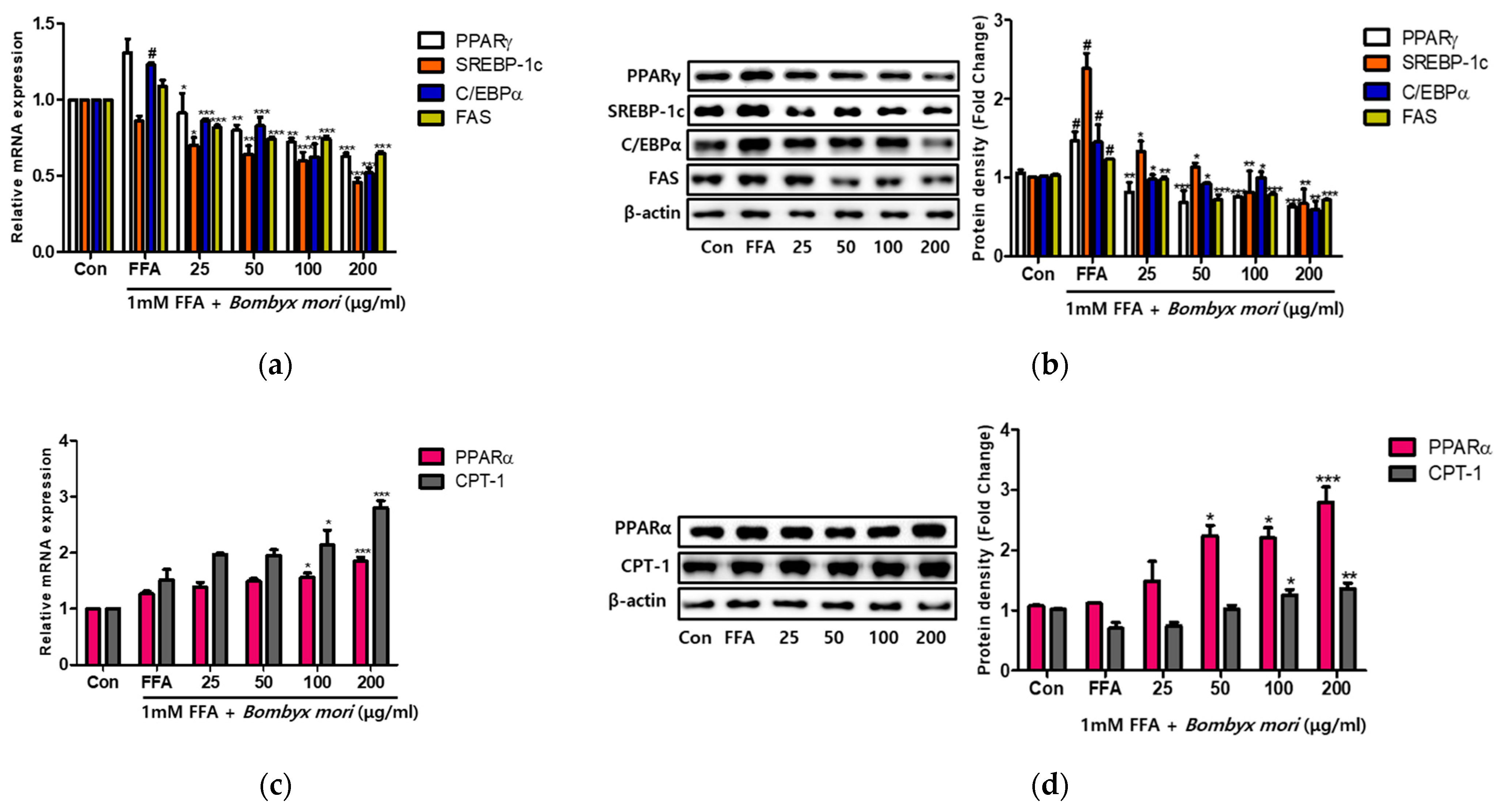
3.3. Effects of SW on Lipogenesis and Fatty Acid Oxidation in FFA-Induced Steatosis
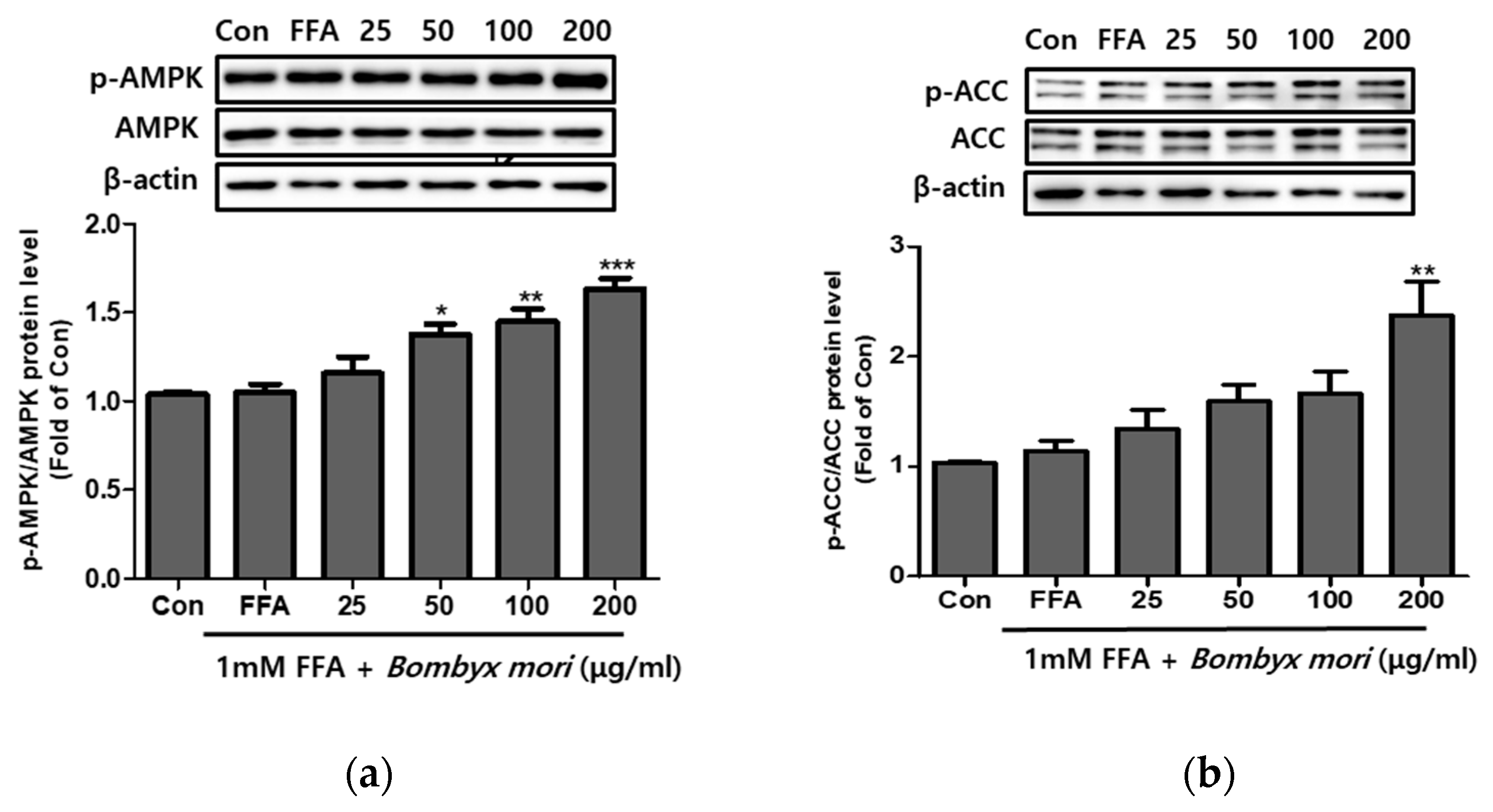
3.4. Effects of SW on the Expression of Phosphorylation of AMPK and ACC in FFA-Induced Steatosis
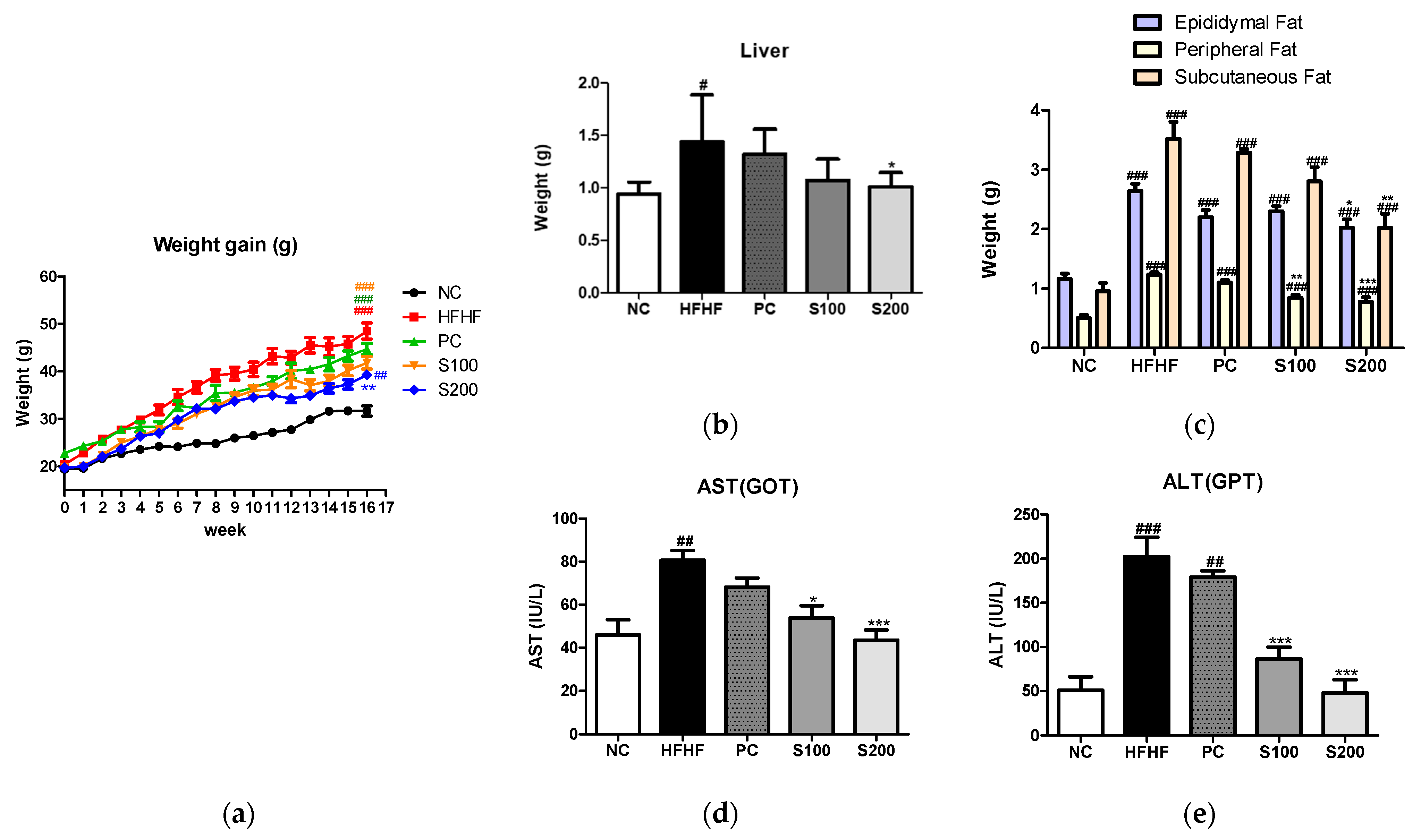
3.5. Effects of SW on Body and Organ Weights in NAFLD-Induced Mice
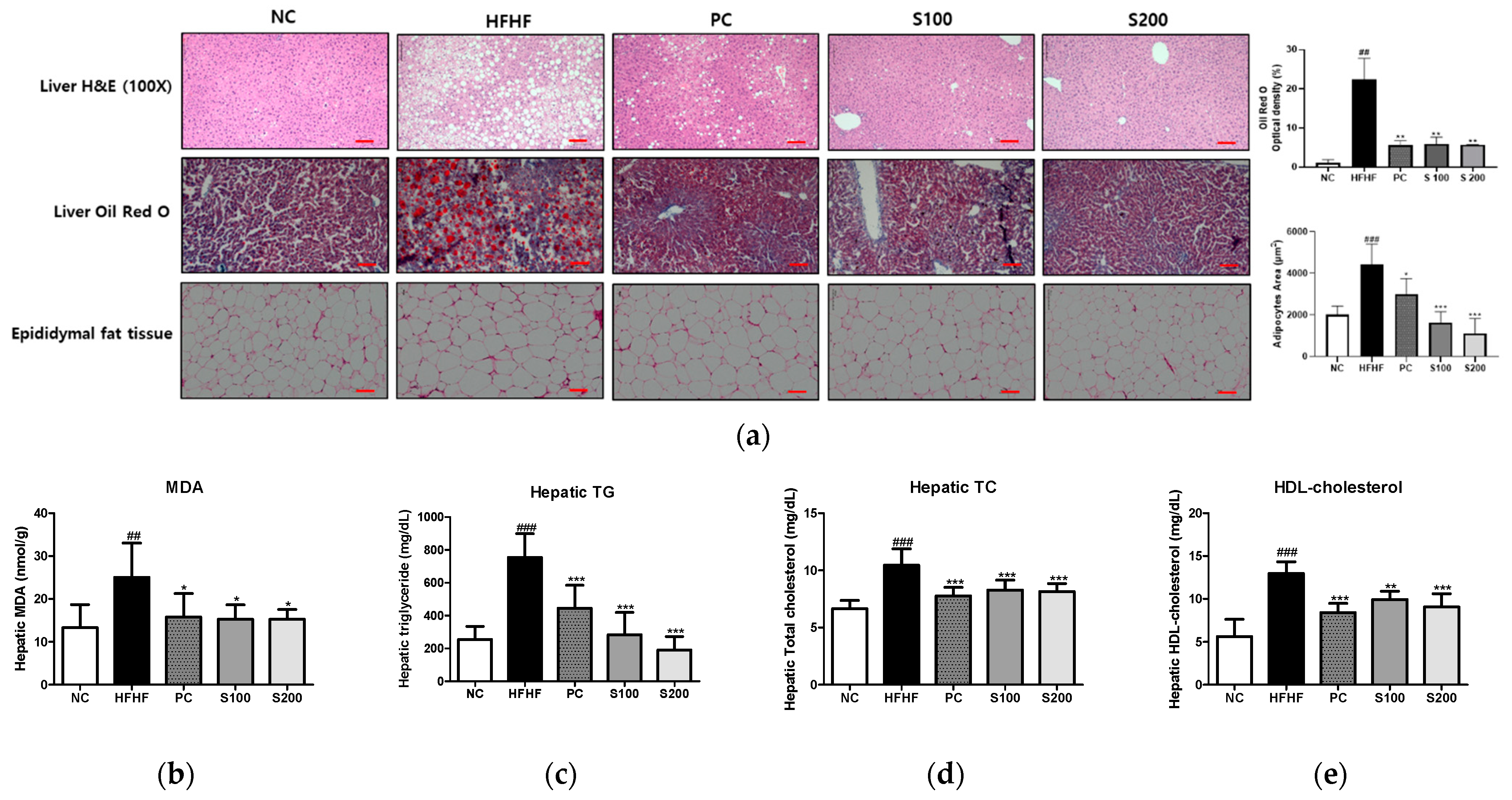
3.6. Effects of SW on Hepatic Steatosis in NAFLD-Induced Mice
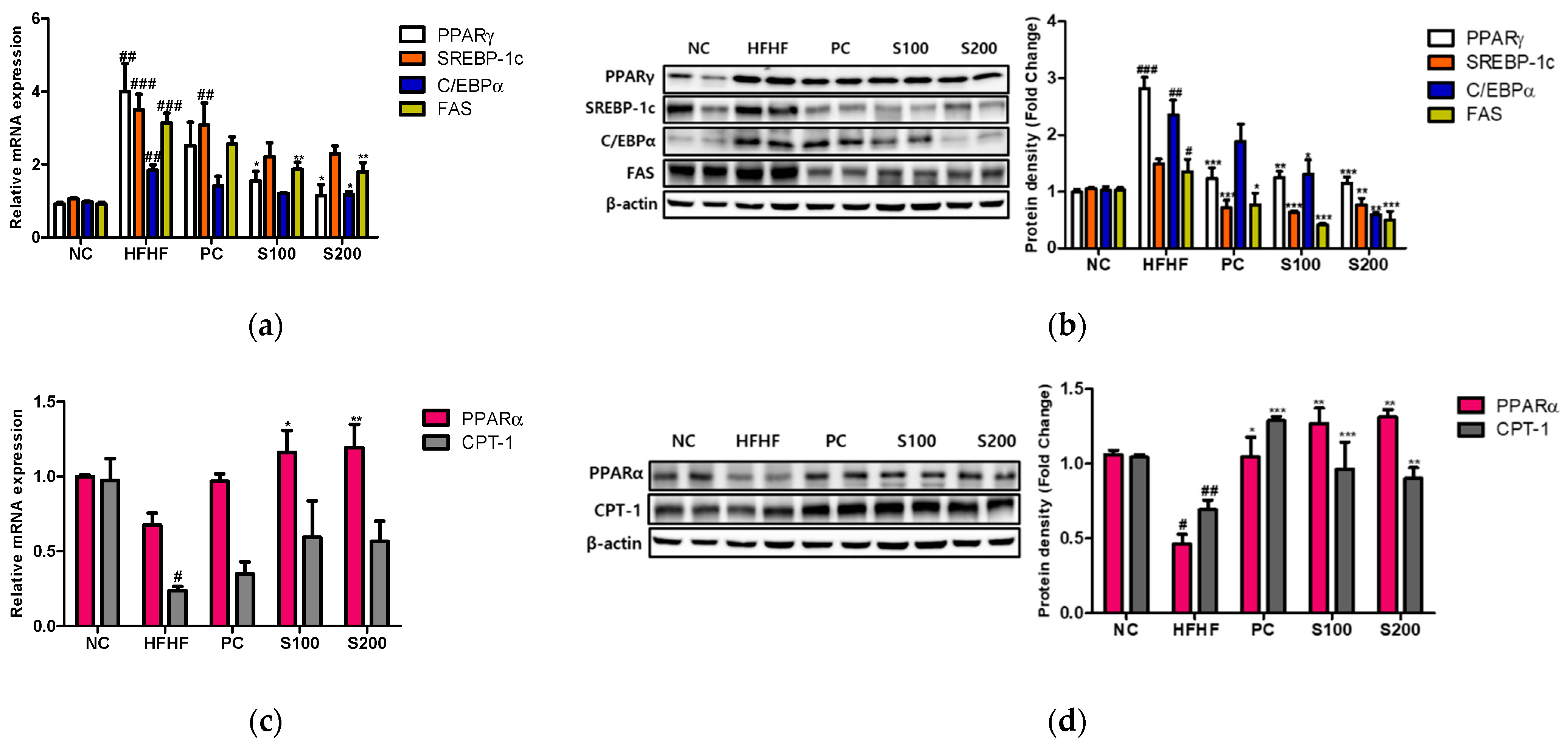
3.7. Effects of SW on Hepatic Lipid Gene Expression and Fatty Acid Oxidation in NAFLD-Induced Mice
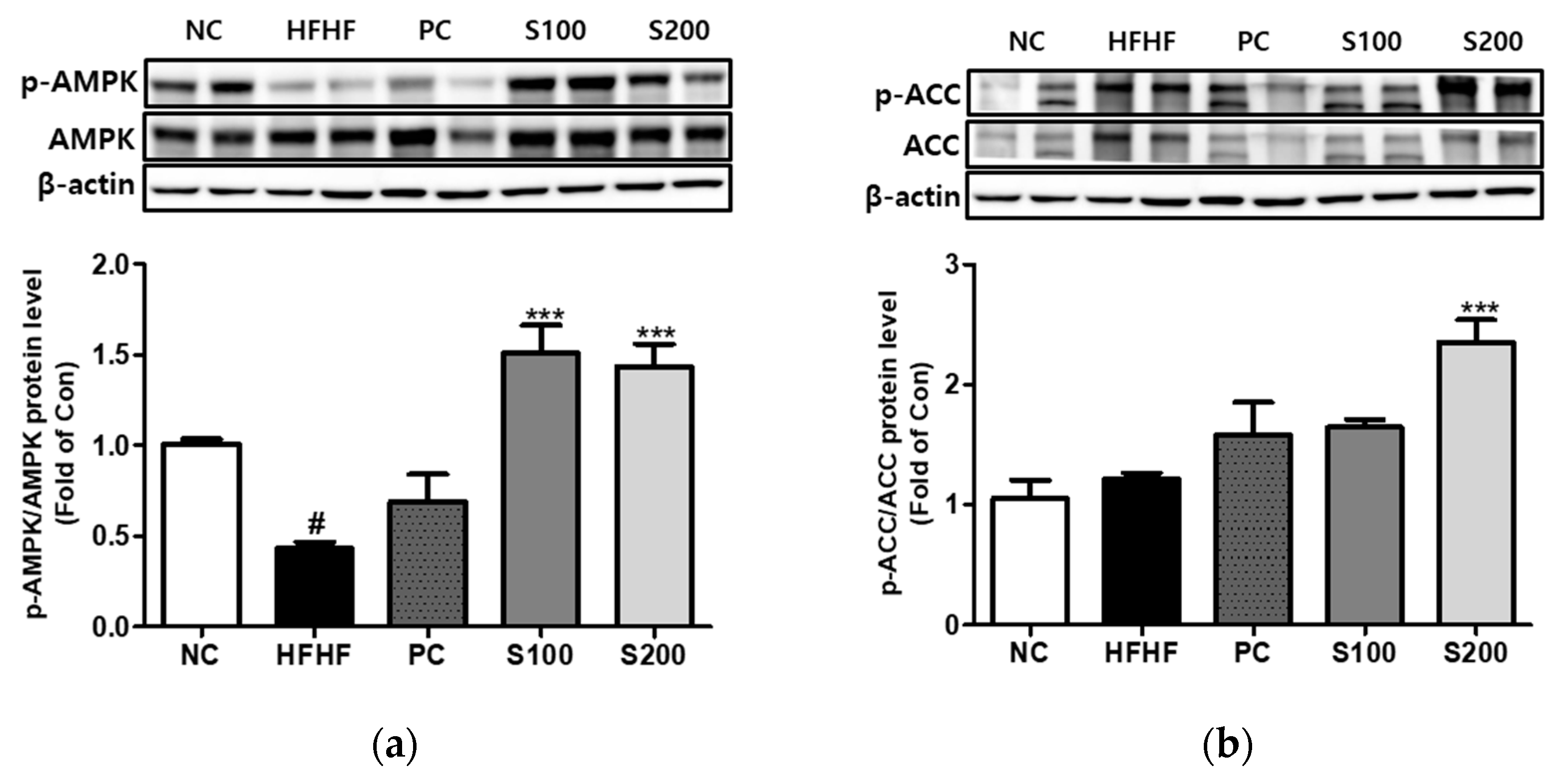
3.8. Effects of SW on the Expression of Phosphorylation of AMPK and ACC in NAFLD-Induced Mice
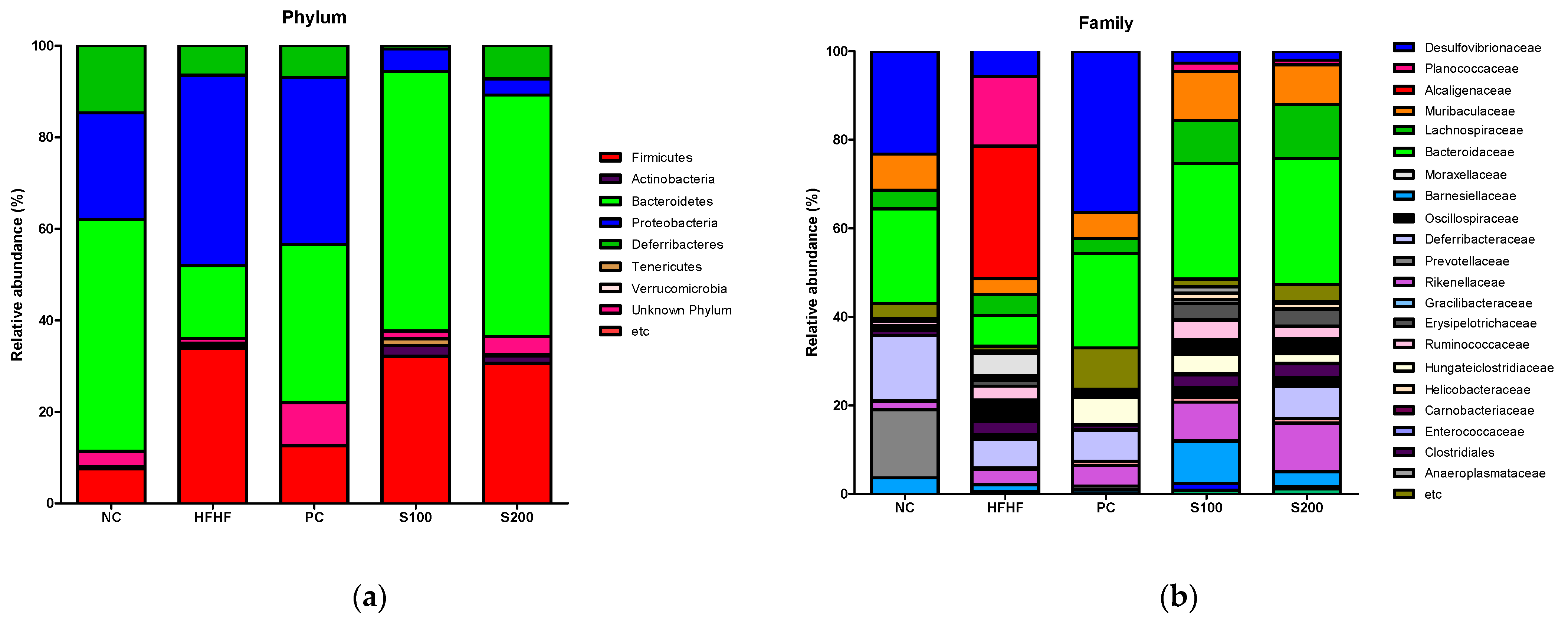
3.9. Effects of SW on the Composition of Fecal Microbiota
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Adams, L.A.; Lymp, J.F.; St Sauver, J.; Sanderson, S.O.; Lindor, K.D.; Feldstein, A.; Angulo, P. The natural history of nonalcoholic fatty liver disease: A population-based cohort study. Gastroenterology 2005, 129, 113–121. [Google Scholar] [CrossRef] [PubMed]
- Dietrich, P.; Hellerbrand, C. Non-alcoholic fatty liver disease, obesity and the metabolic syndrome. Best Pract. Res. Clin. Gastroenterol. 2014, 28, 637–653. [Google Scholar] [CrossRef]
- Angulo, P. Nonalcoholic Fatty Liver Disease. N. Engl. J. Med. 2002, 346, 1221–1231. [Google Scholar] [CrossRef]
- Kawano, Y.; Cohen, D.E. Mechanisms of hepatic triglyceride accumulation in non-alcoholic fatty liver disease. J. Gastroenterol 2013, 48, 434–441. [Google Scholar] [CrossRef]
- Liu, Q.; Bengmark, S.; Qu, S. The role of hepatic fat accumulation in pathogenesis of non-alcoholic fatty liver disease (NAFLD). Lipids Health Dis. 2010, 9, 42. [Google Scholar] [CrossRef] [PubMed]
- Matteoni, C.A.; Younossi, Z.M.; Gramlich, T.; Boparai, N.; Liu, Y.C.; McCullough, A.J. Nonalcoholic fatty liver disease: A spectrum of clinical and pathological severity. Gastroenterology 1999, 116, 1413–1419. [Google Scholar] [CrossRef]
- Konerman, M.A.; Jones, J.C.; Harrison, S.A. Pharmacotherapy for NASH: Current and emerging. J. Hepatol. 2018, 68, 362–375. [Google Scholar] [CrossRef]
- Bukkens, S.G.F. The nutritional value of edible insects. Ecol. Food Nutr. 1997, 36, 287–319. [Google Scholar] [CrossRef]
- van Huis, A. Edible insects are the future? Proc. Nutr. Soc. 2016, 75, 294–305. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Wang, B. Biodegradation of silk biomaterials. Int. J. Mol. Sci. 2009, 10, 1514–1524. [Google Scholar] [CrossRef]
- Mitsuhashi, J. Insects as traditionalfoods in Japan. Ecol. Food Nutr. 1997, 36, 187–199. [Google Scholar] [CrossRef]
- Suzuki, S. Honzo-Komoku (Translation); Shunyodo Pub. Co.: Tokyo, Japan, 1976; Volume 10, p. 649. [Google Scholar]
- Miyake, T. Surveys on food and medicinal insects in Japan. Agric. Exp. Stn. 1919, 31, 203. [Google Scholar]
- Raju, S. Utilization of sericultural By-products—A Chinese example. Indian Silk 1996, 35, 19–20. [Google Scholar]
- Hirabayashi, K.; Takei, M.; Kanbe, A. Manufacture of Silk Proteins as Food Additives. Jpn Kokai Tokkyo Koho JP 08 19372 [96 19372], 23 January 1996. [Google Scholar]
- Ramakanth; Raman, K. Cocoon Pelade for better health. Indian Silk 1997, 35, 35. [Google Scholar]
- Kanebo Ltd. Tar from Silkworm Pupa. Jpn Tokkyo Koho JP 80 27,885, 24 July 1980. [Google Scholar]
- Koul, S.; Dhar, A.; Bindroo, B.B. Industrial utilization of sericultural resources in China. Pop. Sci. 1994, 3, 29–34. [Google Scholar]
- Singh, K.P.; Jayasomu, R.S. Bombyx mori—A Review of its Potential as a Medicinal Insect. Pharm. Biol. 2002, 40, 28–32. [Google Scholar] [CrossRef]
- Huis, A.; Van Itterbeeck, J.; Klunder, H.; Mertens, E.; Halloran, A.; Muir, G.; Vantomme, P. Edible Insects: Future Prospects for Food and Feed Security; Food and Agriculture Organiation of the United Nations (FAO): Rome, Italy, 2013. [Google Scholar]
- Ryu, K.S.; Lee, H.S.; Chung, S.H.; Lee, H.S. An Activity of Lowering Blood—Glucose Levels Accoring to Preparative Conditions of Silkworm Powder. J. Sericult. Entomol. Sci. 1997, 39, 79–85. [Google Scholar]
- Han, J.; Inoue, S.; Isoda, H. Effects of silkworm powder on glucose absorption by human intestinal epithelial cell line Caco-2. J. Nat. Med. 2007, 61, 387–390. [Google Scholar] [CrossRef]
- Kamei, Y.; Miura, S.; Suganami, T.; Akaike, F.; Kanai, S.; Sugita, S.; Katsumata, A.; Aburatani, H.; Unterman, T.G.; Ezaki, O.; et al. Regulation of SREBP1c gene expression in skeletal muscle: Role of retinoid X receptor/liver X receptor and forkhead-O1 transcription factor. Endocrinology 2008, 149, 2293–2305. [Google Scholar] [CrossRef]
- Mao, J.; DeMayo, F.J.; Li, H.; Abu-Elheiga, L.; Gu, Z.; Shaikenov, T.E.; Kordari, P.; Chirala, S.S.; Heird, W.C.; Wakil, S.J. Liver-specific deletion of acetyl-CoA carboxylase 1 reduces hepatic triglyceride accumulation without affecting glucose homeostasis. Proc. Natl. Acad. Sci. USA 2006, 103, 8552–8557. [Google Scholar] [CrossRef]
- Montagner, A.; Polizzi, A.; Fouché, E.; Ducheix, S.; Lippi, Y.; Lasserre, F.; Barquissau, V.; Régnier, M.; Lukowicz, C.; Benhamed, F.; et al. Liver PPARα is crucial for whole-body fatty acid homeostasis and is protective against NAFLD. Gut 2016, 65, 1202–1214. [Google Scholar] [CrossRef]
- Menendez, J.A.; Lupu, R. Fatty acid synthase and the lipogenic phenotype in cancer pathogenesis. Nat. Rev. Cancer 2007, 7, 763–777. [Google Scholar] [CrossRef]
- McGarry, J.D.; Brown, N.F. The Mitochondrial Carnitine Palmitoyltransferase System—From Concept to Molecular Analysis. Eur. J. Biochem. 1997, 244, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Gregoire, F.M.; Smas, C.M.; Sul, H.S. Understanding adipocyte differentiation. Physiol. Rev. 1998, 78, 783–809. [Google Scholar] [CrossRef]
- Herzig, S.; Shaw, R.J. AMPK: Guardian of metabolism and mitochondrial homeostasis. Nat. Rev. Mol. Cell Biol. 2018, 19, 121–135. [Google Scholar] [CrossRef] [PubMed]
- Viollet, B.; Foretz, M.; Guigas, B.; Horman, S.; Dentin, R.; Bertrand, L.; Hue, L.; Andreelli, F. Activation of AMP-activated protein kinase in the liver: A new strategy for the management of metabolic hepatic disorders. J. Physiol. 2006, 574, 41–53. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wang, X.; Chen, Q.; Hou, Y.; Xia, Q.; Zhao, P. Metabolomics Analysis of the Larval Head of the Silkworm, Bombyx mori. Int. J. Mol. Sci. 2016, 17, 1460. [Google Scholar] [CrossRef] [PubMed]
- Alkhouri, N. NASH and NAFLD: Emerging drugs, therapeutic targets and translational and clinical challenges. Expert Opin. Investig. Drugs 2020, 29, 87. [Google Scholar] [CrossRef] [PubMed]
- Younossi, Z.M. Non-alcoholic fatty liver disease’ A global public health perspective. J. Hepatol. 2019, 70, 531–544. [Google Scholar] [CrossRef]
- Verkerk, M.C.; Tramper, J.; van Trijp, J.C.; Martens, D.E. Insect cells for human food. Biotechnol. Adv. 2007, 25, 198–202. [Google Scholar] [CrossRef] [PubMed]
- Park, M.; Yoo, J.-H.; Lee, Y.-S.; Park, E.-J.; Lee, H.-J. Ameliorative effects of black ginseng on nonalcoholic fatty liver disease in free fatty acid–induced HepG2 cells and high-fat/high-fructose diet-fed mice. J. Ginseng. Res. 2020, 44, 350–361. [Google Scholar] [CrossRef] [PubMed]
- Younossi, Z.; Anstee, Q.M.; Marietti, M.; Hardy, T.; Henry, L.; Eslam, M.; George, J.; Bugianesi, E. Global burden of NAFLD and NASH: Trends, predictions, risk factors and prevention. Nat. Rev. Gastroenterol. Hepatol. 2018, 15, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Im, A.R.; Yang, W.-K.; Park, Y.-C.; Kim, S.H.; Chae, S. Hepatoprotective Effects of Insect Extracts in an Animal Model of Nonalcoholic Fatty Liver Disease. Nutrients 2018, 10, 735. [Google Scholar] [CrossRef]
- Mlcek, J.; Rop, O.; Borkovcova, M.; Bednarova, M. A Comprehensive Look at the Possibilities of Edible Insects as Food in Europe—A Review. Pol. J. Food Nutr. Sci. 2014, 64, 147–157. [Google Scholar] [CrossRef]
- Hong, K.-S.; Yun, S.-M.; Cho, J.-M.; Lee, D.-Y.; Ji, S.-D.; Son, J.-G.; Kim, E.-H. Silkworm (Bombyx mori) powder supplementation alleviates alcoholic fatty liver disease in rats. J. Funct. Foods 2018, 43, 29–36. [Google Scholar] [CrossRef]
- Ji, S.-D.; Kim, N.-S.; Lee, J.-Y.; Kim, M.-J.; Kweon, H.; Sung, G.; Kang, P.-D.; Kim, K.-Y. Development of processing technology for edible mature silkworm. J. Sericult. Entomol. Sci. 2015, 53, 38–43. [Google Scholar]
- Mardinoglu, A.; Agren, R.; Kampf, C.; Asplund, A.; Uhlen, M.; Nielsen, J. Genome-scale metabolic modelling of hepatocytes reveals serine deficiency in patients with non-alcoholic fatty liver disease. Nat. Commun. 2014, 5, 3083. [Google Scholar] [CrossRef]
- Hodson, L.; Gunn, P.J. The regulation of hepatic fatty acid synthesis and partitioning: The effect of nutritional state. Nat. Rev. Endocrinol. 2019, 15, 689–700. [Google Scholar] [CrossRef]
- Papandreou, D.; Andreou, E. Role of diet on non-alcoholic fatty liver disease: An updated narrative review. World J. Hepatol. 2015, 7, 575–582. [Google Scholar] [CrossRef]
- Miquilena-Colina, M.E.; Lima-Cabello, E.; Sánchez-Campos, S.; García-Mediavilla, M.V.; Fernández-Bermejo, M.; Lozano-Rodríguez, T.; Vargas-Castrillón, J.; Buqué, X.; Ochoa, B.; Aspichueta, P.; et al. Hepatic fatty acid translocase CD36 upregulation is associated with insulin resistance, hyperinsulinaemia and increased steatosis in non-alcoholic steatohepatitis and chronic hepatitis C. Gut 2011, 60, 1394–1402. [Google Scholar] [CrossRef]
- Koonen, D.P.; Jacobs, R.L.; Febbraio, M.; Young, M.E.; Soltys, C.L.; Ong, H.; Vance, D.E.; Dyck, J.R. Increased hepatic CD36 expression contributes to dyslipidemia associated with diet-induced obesity. Diabetes 2007, 56, 2863–2871. [Google Scholar] [CrossRef]
- Eaton, S.; Bartlett, K.; Pourfarzam, M. Mammalian mitochondrial beta-oxidation. Biochem. J. 1996, 320 Pt 2, 345–357. [Google Scholar] [CrossRef]
- Bruce, C.R.; Hoy, A.J.; Turner, N.; Watt, M.J.; Allen, T.L.; Carpenter, K.; Cooney, G.J.; Febbraio, M.A.; Kraegen, E.W. Overexpression of carnitine palmitoyltransferase-1 in skeletal muscle is sufficient to enhance fatty acid oxidation and improve high-fat diet-induced insulin resistance. Diabetes 2009, 58, 550–558. [Google Scholar] [CrossRef]
- Brocker, C.N.; Patel, D.P.; Velenosi, T.J.; Kim, D.; Yan, T.; Yue, J.; Li, G.; Krausz, K.W.; Gonzalez, F.J. Extrahepatic PPARα modulates fatty acid oxidation and attenuates fasting-induced hepatosteatosis in mice. J. Lipid Res. 2018, 59, 2140–2152. [Google Scholar] [CrossRef]
- Xie, G.; Yin, S.; Zhang, Z.; Qi, D.; Wang, X.; Kim, D.; Yagai, T.; Brocker, C.N.; Wang, Y.; Gonzalez, F.J.; et al. Hepatocyte Peroxisome Proliferator-Activated Receptor α Enhances Liver Regeneration after Partial Hepatectomy in Mice. Am. J. Pathol 2019, 189, 272–282. [Google Scholar] [CrossRef]
- Galic, S.; Loh, K.; Murray-Segal, L.; Steinberg, G.R.; Andrews, Z.B.; Kemp, B.E. AMPK signaling to acetyl-CoA carboxylase is required for fasting- and cold-induced appetite but not thermogenesis. eLife 2018, 7, e32656. [Google Scholar] [CrossRef] [PubMed]
- Garcia, D.; Shaw, R.J. AMPK: Mechanisms of Cellular Energy Sensing and Restoration of Metabolic Balance. Mol. Cell 2017, 66, 789–800. [Google Scholar] [CrossRef]
- Smith, B.K.; Marcinko, K.; Desjardins, E.M.; Lally, J.S.; Ford, R.J.; Steinberg, G.R. Treatment of nonalcoholic fatty liver disease: Role of AMPK. Am. J. Physiol. Endocrinol. Metab. 2016, 311, E730–E740. [Google Scholar] [CrossRef]
- Shimano, H.; Sato, R. SREBP-regulated lipid metabolism: Convergent physiology—divergent pathophysiology. Nat. Rev. Endocrinol. 2017, 13, 710–730. [Google Scholar] [CrossRef]
- Li, Y.; Xu, S.; Mihaylova, M.M.; Zheng, B.; Hou, X.; Jiang, B.; Park, O.; Luo, Z.; Lefai, E.; Shyy, J.Y.J.; et al. AMPK phosphorylates and inhibits SREBP activity to attenuate hepatic steatosis and atherosclerosis in diet-induced insulin-resistant mice. Cell Metab. 2011, 13, 376–388. [Google Scholar] [CrossRef] [PubMed]
- Mouzaki, M.; Comelli, E.M.; Arendt, B.M.; Bonengel, J.; Fung, S.K.; Fischer, S.E.; McGilvray, I.D.; Allard, J.P. Intestinal microbiota in patients with nonalcoholic fatty liver disease. Hepatology 2013, 58, 120–127. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; You, L.J.; Huang, Q.; Fu, X.; Zhang, B.; Liu, R.H.; Li, C. Modulation of gut microbiota by mulberry fruit polysaccharide treatment of obese diabetic db/db mice. Food Funct. 2018, 9, 3732–3742. [Google Scholar] [CrossRef] [PubMed]
- Seo, B.; Jeon, K.; Moon, S.; Lee, K.; Kim, W.K.; Jeong, H.; Cha, K.H.; Lim, M.Y.; Kang, W.; Kweon, M.N.; et al. Roseburia spp. Abundance Associates with Alcohol Consumption in Humans and Its Administration Ameliorates Alcoholic Fatty Liver in Mice. Cell Host Microbe 2020, 27, 25–40.e6. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, M.; Kang, C.; Lee, H.-J. Effect of Bombyx mori on the Liver Protection of Non-Alcoholic Fatty Liver Disease Based on In Vitro and In Vivo Models. Curr. Issues Mol. Biol. 2021, 43, 21-35. https://doi.org/10.3390/cimb43010003
Park M, Kang C, Lee H-J. Effect of Bombyx mori on the Liver Protection of Non-Alcoholic Fatty Liver Disease Based on In Vitro and In Vivo Models. Current Issues in Molecular Biology. 2021; 43(1):21-35. https://doi.org/10.3390/cimb43010003
Chicago/Turabian StylePark, Miey, Chaewon Kang, and Hae-Jeung Lee. 2021. "Effect of Bombyx mori on the Liver Protection of Non-Alcoholic Fatty Liver Disease Based on In Vitro and In Vivo Models" Current Issues in Molecular Biology 43, no. 1: 21-35. https://doi.org/10.3390/cimb43010003
APA StylePark, M., Kang, C., & Lee, H.-J. (2021). Effect of Bombyx mori on the Liver Protection of Non-Alcoholic Fatty Liver Disease Based on In Vitro and In Vivo Models. Current Issues in Molecular Biology, 43(1), 21-35. https://doi.org/10.3390/cimb43010003






