Renal Cell Carcinoma-Infiltrating CD3low Vγ9Vδ1 T Cells Represent Potentially Novel Anti-Tumor Immune Players
Abstract
1. Introduction
2. Materials and Methods
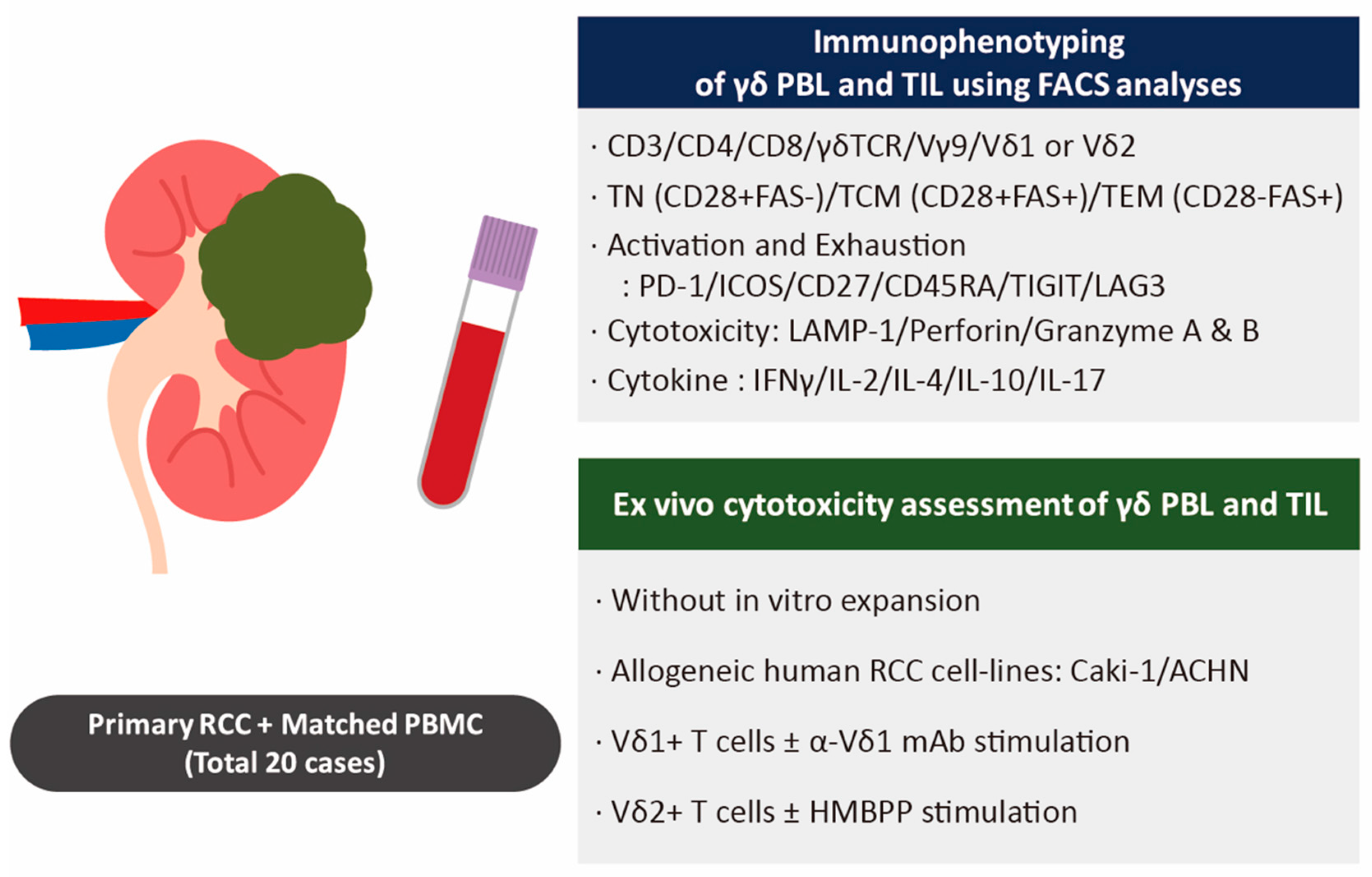
2.1. Patients and Clinical Samples
2.2. Isolation of Tumor-Infiltrating Lymphocytes (TILs) and Matched Peripheral Blood Lymphocytes (PBLs) from Patients with RCC
2.3. Immunophenotyping Using Flow Cytometry of TILs and PBLs from Patients with RCC
2.4. Cytotoxic Assay with Human RCC Cell Lines and γδ T Cells
2.5. Statistical Analysis
3. Results
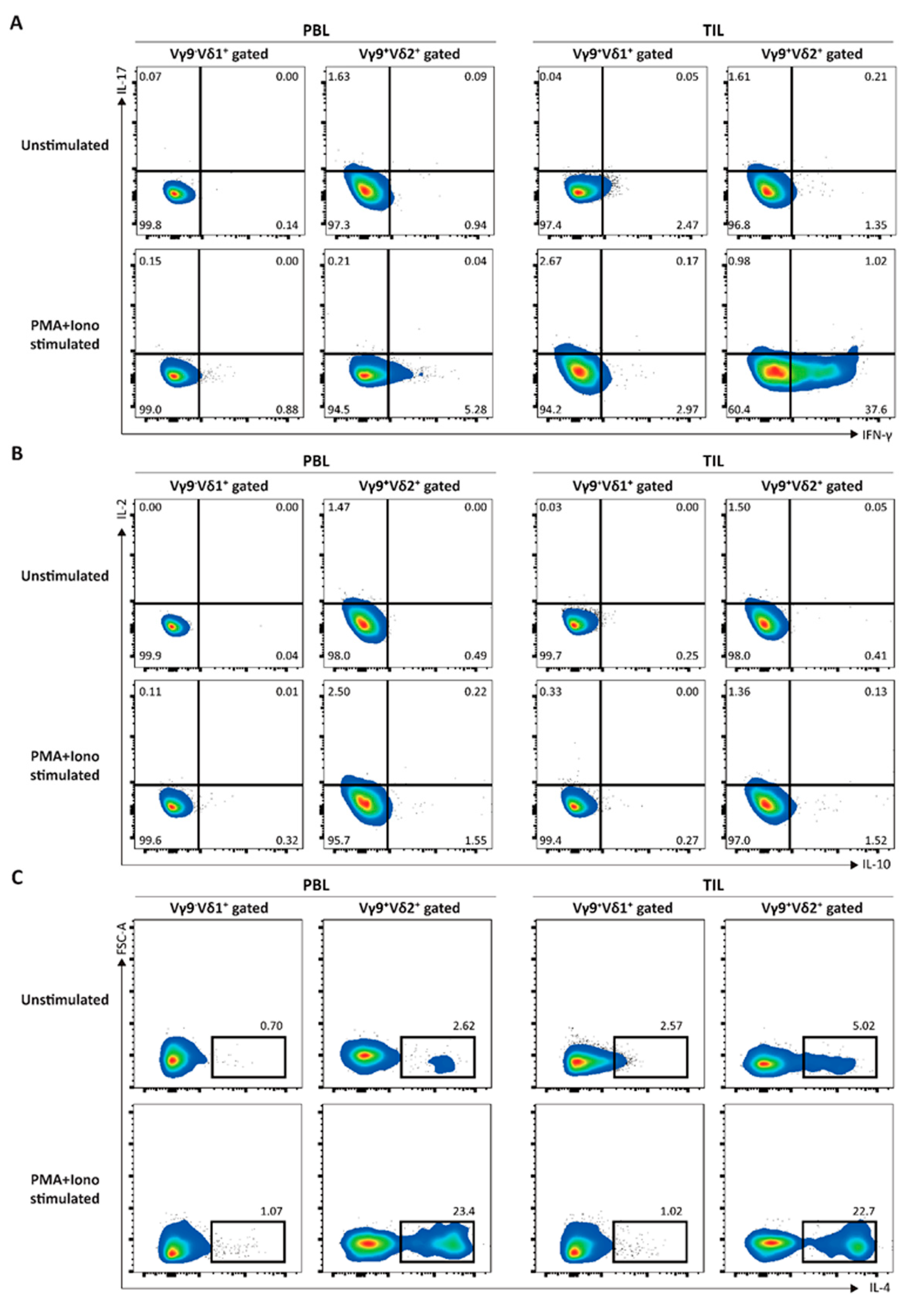
3.1. Elucidation of the Immunological Characteristics of a Novel γδ T Subset in RCC TME
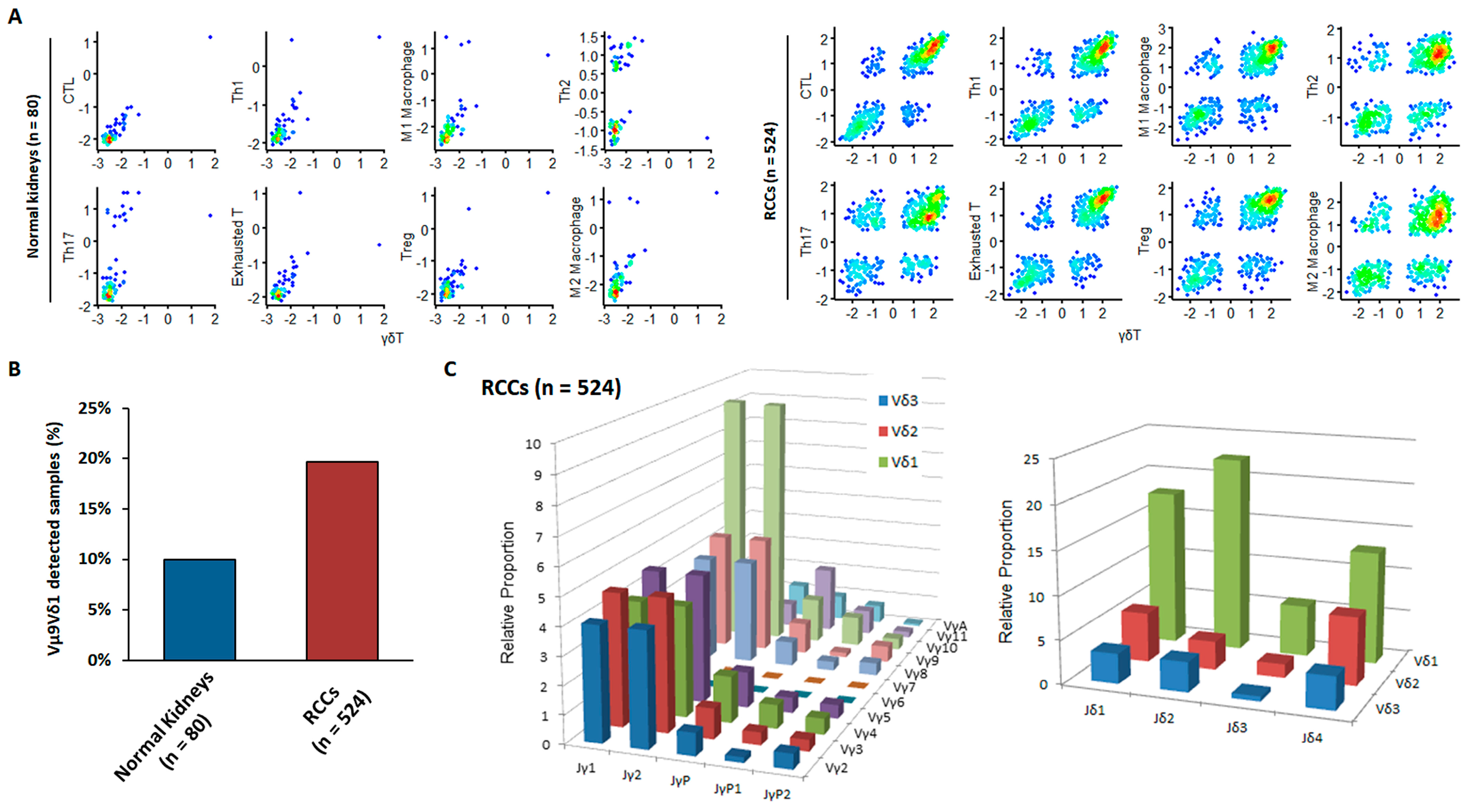
3.2. External Validation of the Anti-Cancer Properties of Newly Detected RCC-Infiltrating Vγ9δ1 T Cells via Bioinformatics Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bhindi, B.; Abel, E.J.; Albiges, L.; Bensalah, K.; Boorjian, S.A.; Daneshmand, S.; Karam, J.A.; Mason, R.J.; Powles, T.; Bex, A. Systematic Review of the Role of Cytoreductive Nephrectomy in the Targeted Therapy Era and Beyond: An Individualized Approach to Metastatic Renal Cell Carcinoma. Eur. Urol. 2019, 75, 111–128. [Google Scholar] [CrossRef]
- Neeman, E.; Zmora, O.; Ben-Eliyahu, S. A new approach to reducing postsurgical cancer recurrence: Perioperative targeting of catecholamines and prostaglandins. Clin. Cancer Res. 2012, 18, 4895–4902. [Google Scholar] [CrossRef] [PubMed]
- Drake, C.G.; Stein, M.N. The Immunobiology of Kidney Cancer. J. Clin. Oncol. 2018, 36, JCO2018792648. [Google Scholar] [CrossRef]
- Zhang, S.; Zhang, E.; Long, J.; Hu, Z.; Peng, J.; Liu, L.; Tang, F.; Li, L.; Ouyang, Y.; Zeng, Z. Immune infiltration in renal cell carcinoma. Cancer Sci. 2019, 110, 1564–1572. [Google Scholar] [CrossRef]
- Yakirevich, E.; Patel, N.R. Tumor mutational burden and immune signatures interplay in renal cell carcinoma. Ann. Transl. Med. 2020, 8, 269. [Google Scholar] [CrossRef] [PubMed]
- Vuong, L.; Kotecha, R.R.; Voss, M.H.; Hakimi, A.A. Tumor Microenvironment Dynamics in Clear-Cell Renal Cell Carcinoma. Cancer Discov. 2019, 9, 1349–1357. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Niu, C.; Cui, J. Gamma-delta (gammadelta) T cells: Friend or foe in cancer development? J. Transl. Med. 2018, 16, 3. [Google Scholar] [CrossRef]
- Pauza, C.D.; Liou, M.L.; Lahusen, T.; Xiao, L.; Lapidus, R.G.; Cairo, C.; Li, H. Gamma Delta T Cell Therapy for Cancer: It Is Good to be Local. Front. Immunol. 2018, 9, 1305. [Google Scholar] [CrossRef]
- Lo Presti, E.; Pizzolato, G.; Corsale, A.M.; Caccamo, N.; Sireci, G.; Dieli, F.; Meraviglia, S. gammadelta T Cells and Tumor Microenvironment: From Immunosurveillance to Tumor Evasion. Front. Immunol. 2018, 9, 1395. [Google Scholar] [CrossRef] [PubMed]
- Davey, M.S.; Willcox, C.R.; Baker, A.T.; Hunter, S.; Willcox, B.E. Recasting Human Vdelta1 Lymphocytes in an Adaptive Role. Trends Immunol. 2018, 39, 446–459. [Google Scholar] [CrossRef]
- Paul, S.; Lal, G. Regulatory and effector functions of gamma-delta (gammadelta) T cells and their therapeutic potential in adoptive cellular therapy for cancer. Int. J. Cancer 2016, 139, 976–985. [Google Scholar] [CrossRef] [PubMed]
- Correia, D.V.; Fogli, M.; Hudspeth, K.; da Silva, M.G.; Mavilio, D.; Silva-Santos, B. Differentiation of human peripheral blood Vdelta1+ T cells expressing the natural cytotoxicity receptor NKp30 for recognition of lymphoid leukemia cells. Blood 2011, 118, 992–1001. [Google Scholar] [CrossRef]
- Mahnke, Y.D.; Brodie, T.M.; Sallusto, F.; Roederer, M.; Lugli, E. The who’s who of T-cell differentiation: Human memory T-cell subsets. Eur. J. Immunol. 2013, 43, 2797–2809. [Google Scholar] [CrossRef] [PubMed]
- Dunne, P.J.; Maher, C.O.; Freeley, M.; Dunne, K.; Petrasca, A.; Orikiiriza, J.; Dunne, M.R.; Reidy, D.; O’Dea, S.; Loy, A.; et al. CD3epsilon Expression Defines Functionally Distinct Subsets of Vdelta1 T Cells in Patients With Human Immunodeficiency Virus Infection. Front. Immunol. 2018, 9, 940. [Google Scholar] [CrossRef]
- Wu, D.; Wu, P.; Qiu, F.; Wei, Q.; Huang, J. Human gammadeltaT-cell subsets and their involvement in tumor immunity. Cell. Mol. Immunol. 2017, 14, 245–253. [Google Scholar] [CrossRef]
- Pawelec, G. Is There a Positive Side to T Cell Exhaustion? Front. Immunol. 2019, 10, 111. [Google Scholar] [CrossRef]
- Lee, H.W.; Chung, Y.S.; Kim, T.J. Heterogeneity of Human gammadelta T Cells and Their Role in Cancer Immunity. Immune Netw. 2020, 20, e5. [Google Scholar] [CrossRef]
- Siegers, G.M.; Lamb, L.S., Jr. Cytotoxic and regulatory properties of circulating Vdelta1+ gammadelta T cells: A new player on the cell therapy field? Mol. Ther. 2014, 22, 1416–1422. [Google Scholar] [CrossRef] [PubMed]
- Muro, R.; Takayanagi, H.; Nitta, T. T cell receptor signaling for gammadeltaT cell development. Inflamm. Regen. 2019, 39, 6. [Google Scholar] [CrossRef]
- El Hentati, F.Z.; Gruy, F.; Iobagiu, C.; Lambert, C. Variability of CD3 membrane expression and T cell activation capacity. Cytometry B Clin. Cytom. 2010, 78, 105–114. [Google Scholar] [CrossRef]
- Davey, M.S.; Willcox, C.R.; Joyce, S.P.; Ladell, K.; Kasatskaya, S.A.; McLaren, J.E.; Hunter, S.; Salim, M.; Mohammed, F.; Price, D.A.; et al. Clonal selection in the human Vdelta1 T cell repertoire indicates gammadelta TCR-dependent adaptive immune surveillance. Nat. Commun. 2017, 8, 14760. [Google Scholar] [CrossRef]
- Chong, T.W.; Goh, F.Y.; Sim, M.Y.; Huang, H.H.; Thike, A.A.; Lim, W.K.; Teh, B.T.; Tan, P.H. CD1d expression in renal cell carcinoma is associated with higher relapse rates, poorer cancer-specific and overall survival. J. Clin. Pathol. 2015, 68, 200–205. [Google Scholar] [CrossRef] [PubMed]
- Adams, E.J.; Gu, S.; Luoma, A.M. Human gamma delta T cells: Evolution and ligand recognition. Cell. Immunol. 2015, 296, 31–40. [Google Scholar] [CrossRef] [PubMed]
- Ito, A.; Levery, S.B.; Saito, S.; Satoh, M.; Hakomori, S. A novel ganglioside isolated from renal cell carcinoma. J. Biol. Chem. 2001, 276, 16695–16703. [Google Scholar] [CrossRef]
- Tsuchida, A.; Senda, M.; Ito, A.; Saito, S.; Kiso, M.; Ando, T.; Harduin-Lepers, A.; Matsuda, A.; Furukawa, K.; Furukawa, K. Roles of GalNAc-disialyl Lactotetraosyl Antigens in Renal Cancer Cells. Sci. Rep. 2018, 8, 7017. [Google Scholar] [CrossRef] [PubMed]



| Pt. | Age | Sex | a Surgery Type | Tumor Type | pT | b Stage | c Grade | Analysis |
|---|---|---|---|---|---|---|---|---|
| #1 | 45 | Male | Radical | Clear cell | pT2a | 2 | Ⅲ | Figure 2 |
| #2 | 82 | Female | Radical | Clear cell | pT1b | 1 | Ⅲ | Figure 2 |
| #3 | 63 | Male | Radical | Clear cell | pT2a | 2 | Ⅱ | Figure 2 |
| #4 | 43 | Female | Radical | Clear cell | pT3a | 3 | Ⅲ | Figure 2 |
| #5 | 72 | Male | Radical | Clear cell | pT3a | 3 | Ⅲ | Figure 2 |
| #6 | 89 | Male | Radical | Clear cell | pT1b | 1 | Ⅲ | Figure 2 |
| #7 | 66 | Male | Radical | Clear cell | pT3a | 3 | Ⅲ | Figure 2 |
| #8 | 61 | Male | Radical | Clear cell | pT3a | 3 | Ⅲ | Figure 2/Figure 3 |
| #9 | 44 | Male | Radical | Clear cell | pT2a | 2 | Ⅳ | Figure 2/Figure 3 |
| #10 | 62 | Female | Partial | Clear cell | pT1a | 1 | Ⅱ | Figure 2 |
| #11 | 72 | Male | Partial | Clear cell | pT3a | 3 | Ⅳ | Figure 2 |
| #12 | 75 | Female | Radical | Clear cell | pT2a | 2 | Ⅱ | Figure 2/Figure 3 |
| #13 | 40 | Male | Radical | Clear cell | pT1b | 1 | Ⅱ | Figure 2/Figure 3/Figure 4 |
| #14 | 56 | Male | Radical | Clear cell | pT1b | 1 | Ⅲ | Figure 2/Figure 3/Figure 4 |
| #15 | 29 | Male | Radical | Chromophobe | pT2a | 2 | Ⅲ | Figure 3/Figure 4 |
| #16 | 58 | Male | Radical | Papillary | pT3a | 3 | Ⅲ | Figure 3/Figure 4 |
| #17 | 61 | Male | Partial | Clear cell | pT1a | 1 | Ⅱ | Figure 5 |
| #18 | 58 | Male | Radical | Clear cell | pT2a | 2 | Ⅲ | Figure 2/Figure 4/Figure 5 |
| #19 | 60 | Male | Partial | Clear cell | pT1a | 1 | Ⅱ | Figure 5 |
| #20 | 62 | Female | Radical | Clear cell | pT3b | 3 | Ⅳ | Figure 5 |
| γδT | Th1 | Th2 | Th17 | Treg | CTL | Exhausted T | M1 Macrophage | M2 Macrophage | ||
|---|---|---|---|---|---|---|---|---|---|---|
| TRGC2 | CD3E | CD3E | CD3E | CD3E | CD3E | CD3E | IL12 | CD40 | ARG1 | EGF |
| TRD | CD4 | CD4 | CD4 | CD4 | CD4 | CD4 | IL23 | IDO1 | ARG2 | CTSA |
| CD3D | TBX21 | GATA3 | RORA | TGFB1 | FASL | PDCD1 | IL12 | KYNU | IL10 | CTSB |
| CD3E | IFNG | IL4 | RORG | FOXP3 | PRF1 | LAG3 | TNF | CCR7 | CD32 | CSTC |
| CD28 | TNF | IL5 | IL17A | IL2RA | GZMA | TIM3 | IL6 | CD45 | CD163 | CTSD |
| KLRK1 | IL2 | IL13 | IL17F | IL10 | GZMB | BTLA | CD86 | CD68 | CD23 | TGFB1 |
| KLRC1 | IL12RB1 | CCL13 | IL21 | CTLA4 | GZMK | CTLA4 | MHCII | CD115 | CD200R1 | TGFB2 |
| KLRC2 | IL12RB2 | CXCL12 | STAT3 | MAF | IFNG | FAS | IL1B | HLA-DR | PD-L2 | TGFB3 |
| KLRC3 | STAT1 | TNF | BATF | MARCO | CD205 | PDL1 | MMP14 | |||
| KLRC4 | iNOS | CD14 | MARCO | MMP19 | ||||||
| KLRD1 | IL12 | CSF1R | MMP9 | |||||||
| CD160 | CD64 | CD206 | CLEC7A | |||||||
| NKG7 | CD80 | IL1RN | WNT7B | |||||||
| GZMB | CXCR10 | IL1R2 | FASL | |||||||
| FASLG | IL23 | IL4R | TNFSF12 | |||||||
| IL18RAP | CXCL9 | CCL4 | TNFSF8 | |||||||
| CCL3 | CXCL10 | CCL13 | CD276 | |||||||
| CCL4 | CXCL11 | CCL20 | VTCN1 | |||||||
| CCL5 | CD86 | CCL17 | MSR1 | |||||||
| XCL1 | IL1A | CCL18 | FN1 | |||||||
| XCL2 | IL1B | CCL22 | IRF4 | |||||||
| IL6 | CCL24 | CD45 | ||||||||
| TNFa | LYVE1 | CD68 | ||||||||
| MHCII | VEGFA | CD115 | ||||||||
| CCL5 | VEGFB | HLA-DR | ||||||||
| IRF5 | VEGFC | CD205 | ||||||||
| IRF1 | VEGFD | CD14 | ||||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, H.W.; Park, C.; Joung, J.-G.; Kang, M.; Chung, Y.S.; Oh, W.J.; Yeom, S.-Y.; Park, W.-Y.; Kim, T.J.; Seo, S.I. Renal Cell Carcinoma-Infiltrating CD3low Vγ9Vδ1 T Cells Represent Potentially Novel Anti-Tumor Immune Players. Curr. Issues Mol. Biol. 2021, 43, 226-239. https://doi.org/10.3390/cimb43010019
Lee HW, Park C, Joung J-G, Kang M, Chung YS, Oh WJ, Yeom S-Y, Park W-Y, Kim TJ, Seo SI. Renal Cell Carcinoma-Infiltrating CD3low Vγ9Vδ1 T Cells Represent Potentially Novel Anti-Tumor Immune Players. Current Issues in Molecular Biology. 2021; 43(1):226-239. https://doi.org/10.3390/cimb43010019
Chicago/Turabian StyleLee, Hye Won, Chanho Park, Je-Gun Joung, Minyong Kang, Yun Shin Chung, Won Joon Oh, Seon-Yong Yeom, Woong-Yang Park, Tae Jin Kim, and Seong Il Seo. 2021. "Renal Cell Carcinoma-Infiltrating CD3low Vγ9Vδ1 T Cells Represent Potentially Novel Anti-Tumor Immune Players" Current Issues in Molecular Biology 43, no. 1: 226-239. https://doi.org/10.3390/cimb43010019
APA StyleLee, H. W., Park, C., Joung, J.-G., Kang, M., Chung, Y. S., Oh, W. J., Yeom, S.-Y., Park, W.-Y., Kim, T. J., & Seo, S. I. (2021). Renal Cell Carcinoma-Infiltrating CD3low Vγ9Vδ1 T Cells Represent Potentially Novel Anti-Tumor Immune Players. Current Issues in Molecular Biology, 43(1), 226-239. https://doi.org/10.3390/cimb43010019






