Comparison of Extracorporeal Shockwave Therapy with Non-Steroid Anti-Inflammatory Drugs and Intra-Articular Hyaluronic Acid Injection for Early Osteoarthritis of the Knees
Abstract
:1. Introduction
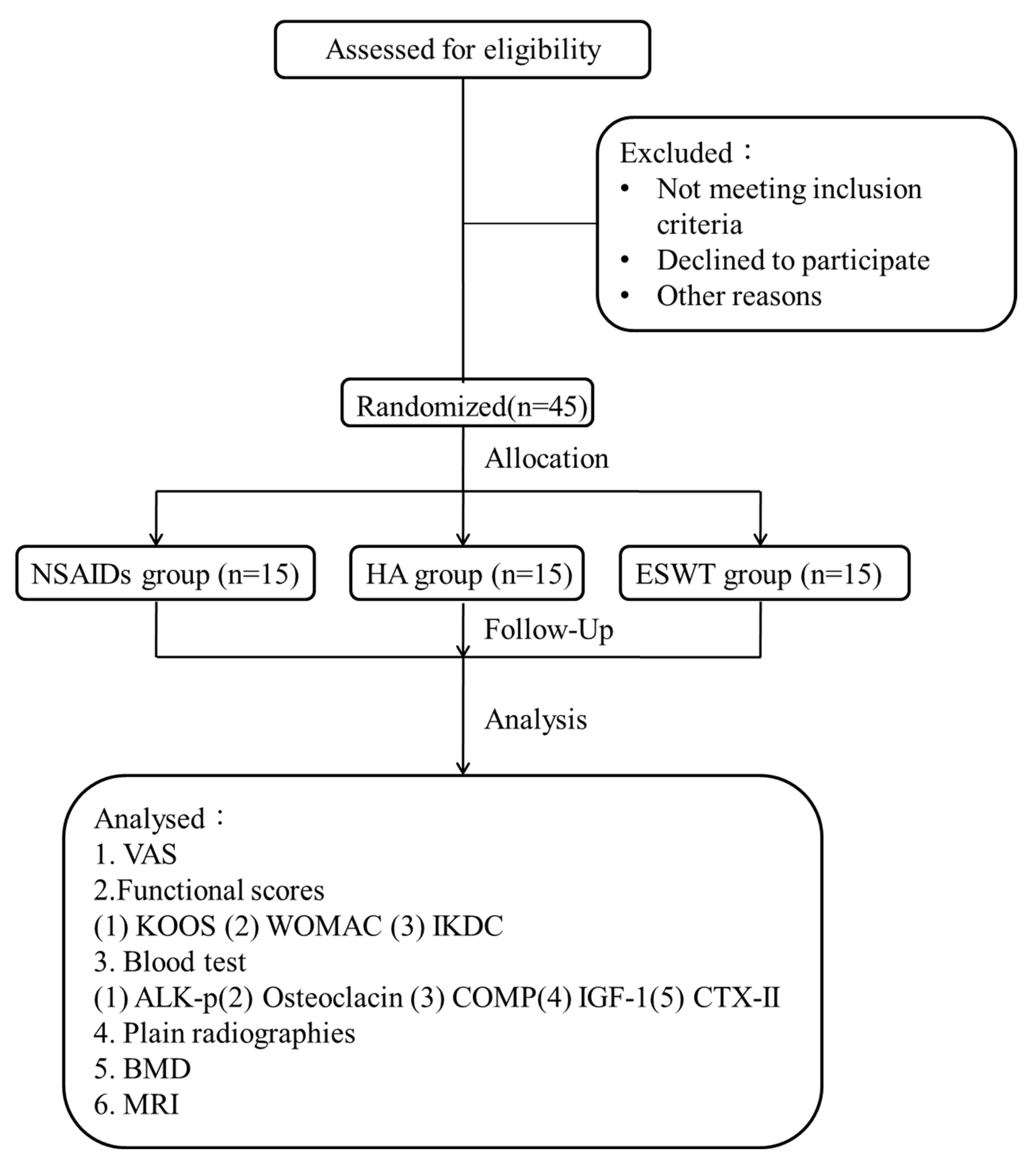
2. Materials and Methods
2.1. The Study Design
2.2. The Patients
2.3. Enzyme-Linked Immunosorbent Assay
2.4. The Functional Scores
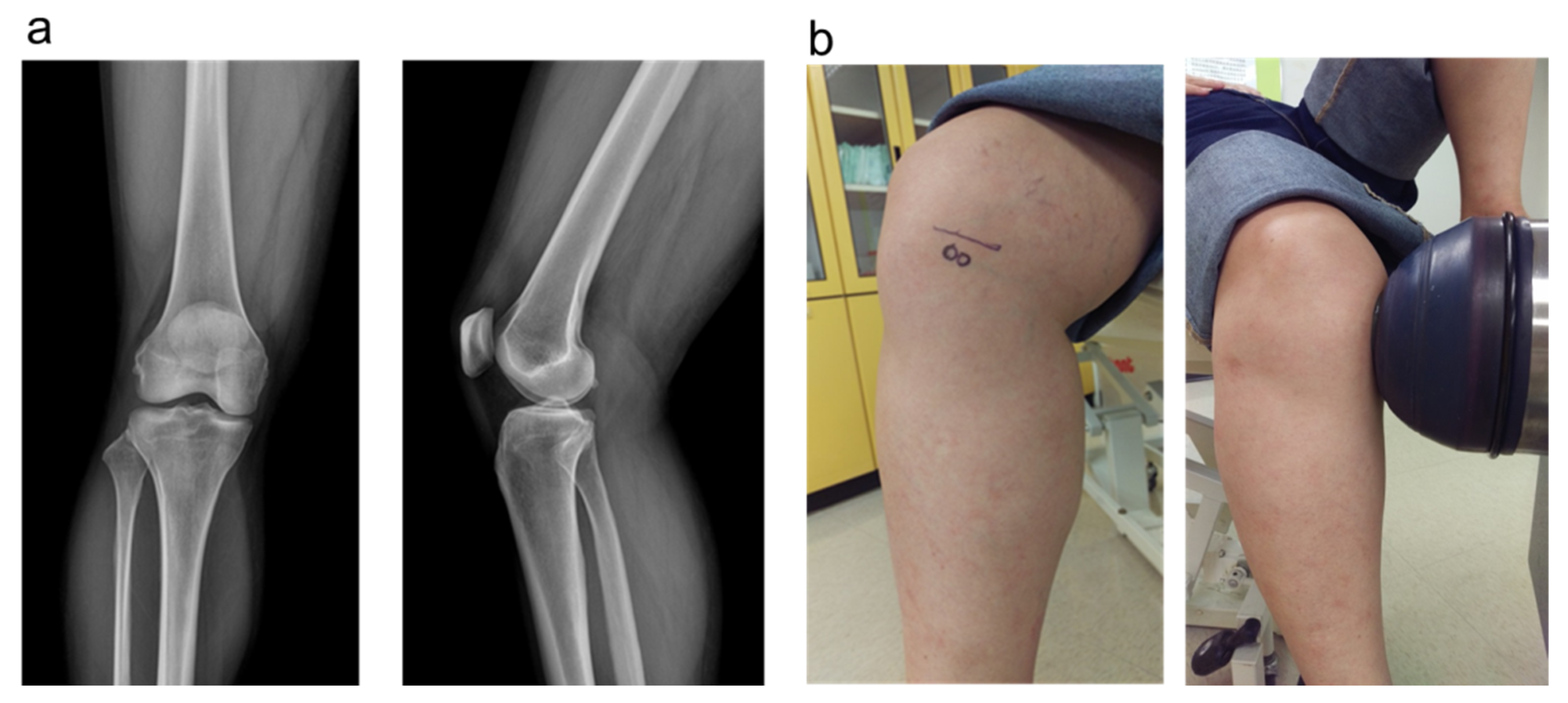
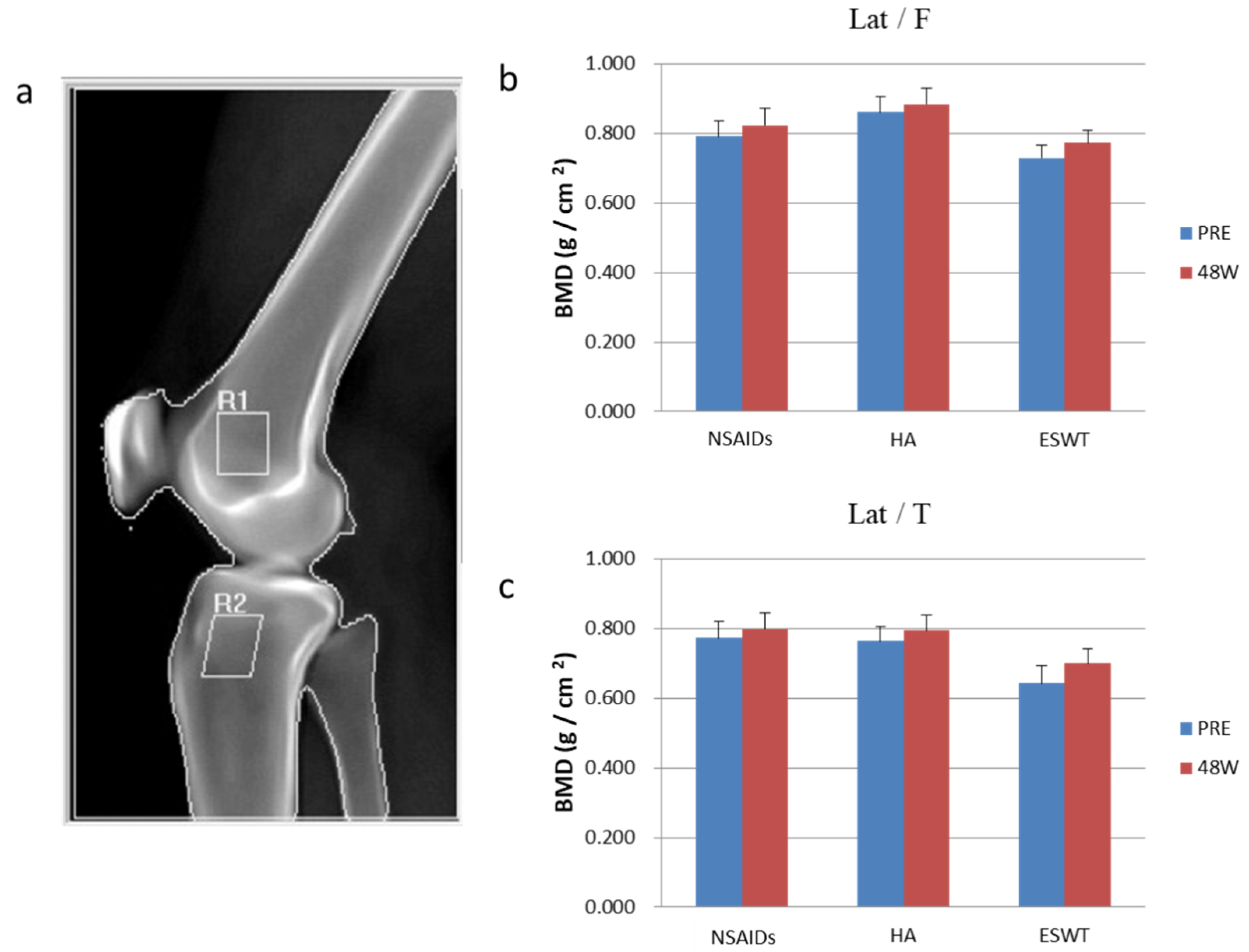
2.5. The Plain Radiographies and MRI
2.6. Shockwave Application
2.7. Statistical Analysis
3. Results
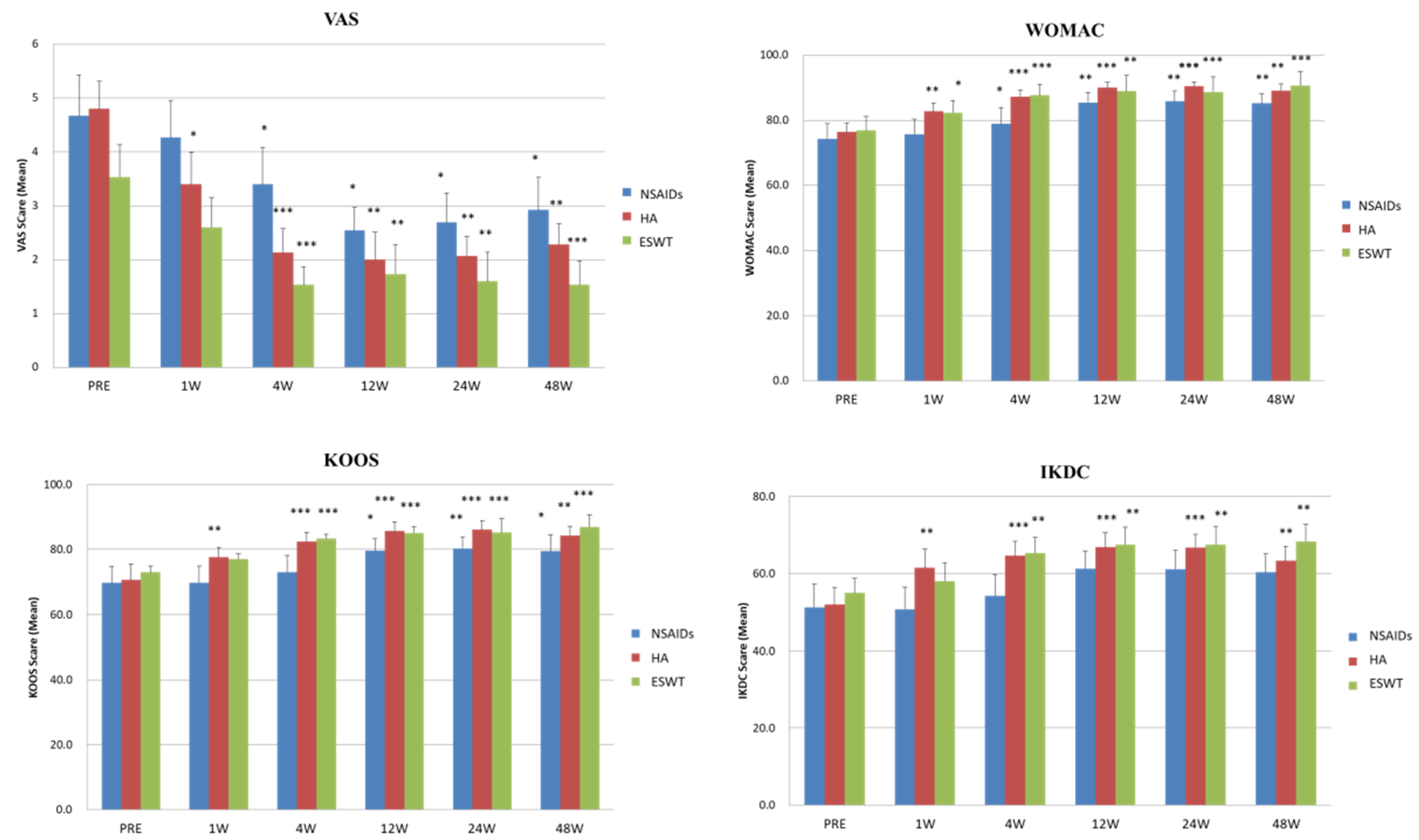
3.1. Comparison of the VAS and Functional Scores before and after NSAIDs, HA, and ESWT Treatments
3.2. The Time Chasing and Tendency of VAS and Functional Scores after NSAIDs, HA, and ESWT Treatments
3.3. The Results of Enzyme-Linked Immunosorbent Assay
3.4. The Analysis of Dual-Energy X-ray Absorptiometry and MRI
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cui, A.; Li, H.; Wang, D.; Zhong, J.; Chen, Y.; Lu, H. Global, regional prevalence, incidence and risk factors of knee osteoarthritis in population-based studies. EClinicalMedicine 2020, 29–30, 100587. [Google Scholar] [CrossRef] [PubMed]
- Ding, J.; Niu, X.; Su, Y.; Li, X. Expression of synovial fluid biomarkers in patients with knee osteoarthritis and meniscus injury. Exp. Ther. Med. 2017, 14, 1609–1613. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rim, Y.A.; Ju, J.H. The Role of Fibrosis in Osteoarthritis Progression. Life 2020, 11, 3. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.-H.; Hsu, C.-C.; Hsu, S.-L.; Chou, W.-Y.; Wu, Y.-N.; Kuo, C.-E.A.; Hsu, T.-C.; Shiu, L.-Y.; Jhan, S.-W. Adipose-Derived Mesenchymal Stem Cells-Conditioned Medium Modulates the Expression of Inflammation Induced Bone Morphogenetic Protein-2, -5 and -6 as Well as Compared with Shockwave Therapy on Rat Knee Osteoarthritis. Biomedicines 2021, 9, 1399. [Google Scholar] [CrossRef] [PubMed]
- Michael, J.W.; Schlüter-Brust, K.U.; Eysel, P. The epidemiology, etiology, diagnosis, and treatment of osteoarthritis of the knee. Dtsch. Arztebl. Int. 2010, 107, 152–162. [Google Scholar] [CrossRef]
- Hochberg, M.C.; Altman, R.D.; April, K.T.; Benkhalti, M.; Guyatt, G.; McGowan, J.; Towheed, T.; Welch, V.; Wells, G.; Tugwell, P. American College of Rheumatology 2012 recommendations for the use of nonpharmacologic and pharmacologic therapies in osteoarthritis of the hand, hip, and knee. Arthritis Care Res. 2012, 64, 465–474. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Szwedowski, D.; Szczepanek, J.; Paczesny, Ł.; Zabrzyński, J.; Gagat, M.; Mobasheri, A.; Jeka, S. The Effect of Platelet-Rich Plasma on the Intra-Articular Microenvironment in Knee Osteoarthritis. Int. J. Mol. Sci. 2021, 22, 5492. [Google Scholar] [CrossRef]
- Szwedowski, D.; Dallo, I.; Irlandini, E.; Gobbi, A. Osteo-core Plasty: A Minimally Invasive Approach for Subchondral Bone Marrow Lesions of the Knee. Arthrosc. Tech. 2020, 9, e1773–e1777. [Google Scholar] [CrossRef]
- Gupta, R.C.; Lall, R.; Srivastava, A.; Sinha, A. Hyaluronic Acid: Molecular Mechanisms and Therapeutic Trajectory. Front. Vet. Sci. 2019, 6, 192. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vincent, P. Intra-articular hyaluronic acid in knee osteoarthritis: Clinical data for a product family (ARTHRUM), with comparative meta-analyses. Curr. Ther. Res. 2021, 95, 100637. [Google Scholar] [CrossRef]
- Hu, Y.; Chen, X.; Wang, S.; Jing, Y.; Su, J. Subchondral bone microenvironment in osteoarthritis and pain. Bone Res. 2021, 9, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Stewart, H.L.; Kawcak, C.E. The Importance of Subchondral Bone in the Pathophysiology of Osteoarthritis. Front. Vet. Sci. 2018, 5, 178. [Google Scholar] [CrossRef] [PubMed]
- Driban, J.B.; Harkey, M.S.; Barbe, M.F.; Ward, R.J.; MacKay, J.W.; Davis, J.E.; Lu, B.; Price, L.L.; Eaton, C.B.; Lo, G.H.; et al. Risk factors and the natural history of accelerated knee osteoarthritis: A narrative review. BMC Musculoskelet. Disord. 2020, 21. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.J.; Weng, L.H.; Ko, J.Y.; Wang, J.W.; Chen, J.M.; Sun, Y.C.; Yang, Y.J. Extracorporeal shockwave shows regression of osteoarthritis of the knee in rats. J. Surg. Res. 2011, 171, 601–608. [Google Scholar] [CrossRef]
- Wang, H.-J.; Cheng, J.-H.; Chuang, Y.-C. Potential applications of low-energy shock waves in functional urology. Int. J. Urol. 2017, 24, 573–581. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, C.J. An overview of shock wave therapy in musculoskeletal disorders. Chang Gung Med. J. 2003, 26, 220–232. [Google Scholar] [PubMed]
- Wang, C.J.; Hsu, S.L.; Weng, L.H.; Sun, Y.C.; Wang, F.S. Extracorporeal shockwave therapy shows a number of treatment related chondroprotective effect in osteoarthritis of the knee in rats. BMC Musculoskelet. Disord. 2013, 14, 44. [Google Scholar] [CrossRef] [Green Version]
- Dahlberg, J.; Fitch, G.; Evans, R.B.; McClure, S.R.; Conzemius, M. The evaluation of extracorporeal shockwave therapy in naturally occurring osteoarthritis of the stifle joint in dogs. Vet. Comp. Orthop. Traumatol. 2005, 18, 147–152. [Google Scholar] [PubMed]
- Frisbie, D.D.; Kawcak, C.E.; McIlwraith, C.W. Evaluation of the effect of extracorporeal shock wave treatment on experimentally induced osteoarthritis in middle carpal joints of horses. Am. J. Vet. Res. 2009, 70, 449–454. [Google Scholar] [CrossRef] [PubMed]
- Mueller, M.; Bockstahler, B.; Skalicky, M.; Mlacnik, E.; Lorinson, D. Effects of radial shockwave therapy on the limb function of dogs with hip osteoarthritis. Vet. Rec. 2007, 160, 762–765. [Google Scholar] [CrossRef]
- Ochiai, N.; Ohtori, S.; Sasho, T.; Nakagawa, K.; Takahashi, K.; Takahashi, N.; Murata, R.; Takahashi, K.; Moriya, H.; Wada, Y.; et al. Extracorporeal shock wave therapy improves motor dysfunction and pain originating from knee osteoarthritis in rats. Osteoarthr. Cartil. 2007, 15, 1093–1096. [Google Scholar] [CrossRef] [Green Version]
- Revenaugh, M.S. Extracorporeal shock wave therapy for treatment of osteoarthritis in the horse: Clinical applications. Vet. Clin. Equine Pract. 2005, 21, 609–625. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.J.; Cheng, J.H.; Chou, W.Y.; Hsu, S.L.; Chen, J.H.; Huang, C.Y. Changes of articular cartilage and subchondral bone after extracorporeal shockwave therapy in osteoarthritis of the knee. Int. J. Med. Sci. 2017, 14, 213–223. [Google Scholar] [CrossRef] [Green Version]
- Kellgren, J.H.; Lawrence, J.S. Radiological assessment of osteo-arthrosis. Ann. Rheum. Dis. 1957, 16, 494–502. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Collins, N.J.; Misra, D.; Felson, D.T.; Crossley, K.M.; Roos, E.M. Measures of knee function: International Knee Documentation Committee (IKDC) Subjective Knee Evaluation Form, Knee Injury and Osteoarthritis Outcome Score (KOOS), Knee Injury and Osteoarthritis Outcome Score Physical Function Short Form (KOOS-PS), Knee Outcome Survey Activities of Daily Living Scale (KOS-ADL), Lysholm Knee Scoring Scale, Oxford Knee Score (OKS), Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC), Activity Rating Scale (ARS), and Tegner Activity Score (TAS). Arthritis Care Res. 2011, 63 (Suppl. S11), S208–S228. [Google Scholar] [CrossRef] [Green Version]
- Chiba, K.; Uetani, M.; Kido, Y.; Ito, M.; Okazaki, N.; Taguchi, K.; Shindo, H. Osteoporotic changes of subchondral trabecular bone in osteoarthritis of the knee: A 3-T MRI study. Osteoporos. Int. 2012, 23, 589–597. [Google Scholar] [CrossRef] [PubMed]
- Schneider, E.; Lo, G.H.; Sloane, G.; Fanella, L.; Hunter, D.J.; Eaton, C.B.; McAlindon, T.E. Magnetic resonance imaging evaluation of weight-bearing subchondral trabecular bone in the knee. Skelet. Radiol. 2011, 40, 95–103. [Google Scholar] [CrossRef] [Green Version]
- Peterfy, C.G.; Guermazi, A.; Zaim, S.; Tirman, P.F.; Miaux, Y.; White, D.; Kothari, M.; Lu, Y.; Fye, K.; Zhao, S.; et al. Whole-Organ Magnetic Resonance Imaging Score (WORMS) of the knee in osteoarthritis. Osteoarthr. Cartil. 2004, 12, 177–190. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, C.J.; Weng, L.H.; Ko, J.Y.; Sun, Y.C.; Yang, Y.J.; Wang, F.S. Extracorporeal shockwave therapy shows chondroprotective effects in osteoarthritic rat knee. Arch. Orthop. Trauma Surg. 2011, 131, 1153–1158. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Jing, R.; Shi, Z.; Zhao, B.; Ai, Q.; Xing, G. Efficacy of extracorporeal shockwave therapy for knee osteoarthritis: A randomized controlled trial. J. Surg. Res. 2013, 185, 661–666. [Google Scholar] [CrossRef]
- Li, W.; Pan, Y.; Yang, Q.; Guo, Z.G.; Yue, Q.; Meng, Q.G. Extracorporeal shockwave therapy for the treatment of knee osteoarthritis: A retrospective study. Medicine 2018, 97, e11418. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Z.; Liu, B.; Liu, G.; Chen, J.; Li, Y.; Chen, J.; Liu, X.; Hu, Y. A Randomized Controlled Trial on the Effects of Low-Dose Extracorporeal Shockwave Therapy in Patients with Knee Osteoarthritis. Arch. Phys. Med. Rehabil. 2019, 100, 1695–1702. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Uysal, A.; Yildizgoren, M.T.; Guler, H.; Turhanoglu, A.D. Effects of radial extracorporeal shock wave therapy on clinical variables and isokinetic performance in patients with knee osteoarthritis: A prospective, randomized, single-blind and controlled trial. Int. Orthop. 2020, 44, 1311–1319. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.; Gao, F.; Han, J.; Mao, T.; Sun, W.; Wang, B.; Guo, W.; Cheng, L.; Li, Z. Extracorporeal shock wave treatment can normalize painful bone marrow edema in knee osteoarthritis: A comparative historical cohort study. Medicine 2018, 97, e9796. [Google Scholar] [CrossRef]
- Xu, Y.; Wu, K.; Liu, Y.; Geng, H.; Zhang, H.; Liu, S.; Qu, H.; Xing, G. The effect of extracorporeal shock wave therapy on the treatment of moderate to severe knee osteoarthritis and cartilage lesion. Medicine 2019, 98, e15523. [Google Scholar] [CrossRef]
- Lee, J.K.; Lee, B.Y.; Shin, W.Y.; An, M.J.; Jung, K.I.; Yoon, S.R. Effect of Extracorporeal Shockwave Therapy Versus Intra-articular Injections of Hyaluronic Acid for the Treatment of Knee Osteoarthritis. Ann. Rehabil. Med. 2017, 41, 828–835. [Google Scholar] [CrossRef] [Green Version]
- Sedrak, P.; Hache, P.; Horner, N.S.; Ayeni, O.R.; Adili, A.; Khan, M. Differential characteristics and management of pseudoseptic arthritis following hyaluronic acid injection is a rare complication: A systematic review. J. Isakos 2021, 6, 94–101. [Google Scholar] [CrossRef]
- Ong, K.L.; Runa, M.; Xiao, Z.; Ngai, W.; Lau, E.; Altman, R.D. Severe Acute Localized Reactions Following Intra-Articular Hyaluronic Acid Injections in Knee Osteoarthritis. Cartilage 2020, 13, 1474S–1486S. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.C.; Huang, H.T.; Huang, P.J.; Liu, Z.M.; Shih, C.L. Efficacy and Safety of Extracorporeal Shockwave Therapy for Treatment of Knee Osteoarthritis: A Systematic Review and Meta-analysis. Pain Med. 2020, 21, 822–835. [Google Scholar] [CrossRef] [PubMed]
- Moya, D.; Ramón, S.; Schaden, W.; Wang, C.J.; Guiloff, L.; Cheng, J.H. The Role of Extracorporeal Shockwave Treatment in Musculoskeletal Disorders. J. Bone Joint Surg. Am. 2018, 100, 251–263. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, C.J.; Huang, C.Y.; Hsu, S.L.; Chen, J.H.; Cheng, J.H. Extracorporeal shockwave therapy in osteoporotic osteoarthritis of the knee in rats: An experiment in animals. Arthritis Res. Ther. 2014, 16, R139. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| NSAIDs | HA | ESWT | Overall | |
|---|---|---|---|---|
| Patient numbers/Knees | 15/17 | 15/17 | 15/16 | 45/50 |
| Average age | 49 | 52 | 54 | 51 |
| (Range) | (30–63) | (33–64) | (40–60) | (30–64) |
| Gender (Male/Female) | 1/14 | 2/13 | 1/14 | 4/41 |
| Side | ||||
| Right/Left | 5/8 | 6/7 | 9/5 | 20/20 |
| Bilateral knees | 2 | 2 | 1 | 5 |
| NSAIDs | HA | ESWT | p-Value *b | p-Value *c | p-Value *d | |
|---|---|---|---|---|---|---|
| VAS score | ||||||
| Before treatment | 4.7 ± 0.8 | 4.8 ± 0.5 | 3.5 ± 0.6 | |||
| After treatment | 2.9 ± 0.6 | 2.3 ± 0.4 | 1.5 ± 0.4 | 0.614 | 0.015 * | 0.015 * |
| p-value *a | 0.037 * | 0.005 * | 0.001 * | |||
| KOOS score | ||||||
| Before treatment | 69.7 ± 5.1 | 70.6 ± 2.8 | 73.0 ± 4.1 | |||
| After treatment | 79.4 ± 3.6 | 84.4 ± 2.2 | 87.0 ± 4.2 | 0.254 | 0.042 * | 0.005 * |
| p-value *a | 0.028 * | 0.006 * | 0.001 * | |||
| WOMAC score | ||||||
| Before treatment | 74.3 ± 4.6 | 76.4 ± 2.7 | 76.9 ± 4.2 | |||
| After treatment | 85.3 ± 3.0 | 89.2 ± 1.9 | 90.7 ± 4.0 | 0.224 | 0.023 * | 0.006 * |
| p-value *a | 0.005 * | 0.006 * | 0.001 * | |||
| IKDC score | ||||||
| Before treatment | 51.3 ± 6.0 | 51.9 ± 4.4 | 55.0 ± 3.8 | |||
| After treatment | 60.3 ± 4.8 | 63.3 ± 3.8 | 68.4 ± 4.4 | 0.697 | 0.325 | 0.24 |
| p-value *a | 0.136 | 0.005 * | 0.005 * |
| NSAIDs | HA | ESWT | p-Value *1 | p-Value *2 | p-Value *3 | |
|---|---|---|---|---|---|---|
| ALK-P | ||||||
| Before treatment | 63.7 | 67.0 | 65.1 | |||
| After treatment | 64.6 | 68.4 | 65.3 | 0.367 | 0.298 | 0.362 |
| p-value * | 0.371 | 0.298 | 0.450 | |||
| Osteoclacin | ||||||
| Before treatment | 5.0 | 4.4 | 5.0 | |||
| After treatment | 4.2 | 4.0 | 3.9 | 0.286 | 0.896 | 0.394 |
| p-value * | 0.221 | 0.397 | 0.001 * | |||
| COMP | ||||||
| Before treatment | 165.1 | 185.8 | 173.7 | |||
| After treatment | 191.1 | 209.8 | 174.2 | 0.028 | 0.107 | 0.357 |
| p-value * | 0.061 | 0.024 * | 0.484 | |||
| IGF-1 | ||||||
| Before treatment | 134.2 | 123.8 | 116.0 | |||
| After treatment | 125.3 | 111.8 | 120.2 | 0.217 | 0.206 | 0.389 |
| p-value * | 0.122 | 0.077 | 0.212 | |||
| CTX-II | ||||||
| Before treatment | 0.9 | 1.3 | 1.3 | |||
| After treatment | 0.8 | 1.1 | 1.2 | 0.058 | 0.76 | 0.102 |
| p-value * | 0.345 | 0.397 | 0.65 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jhan, S.-W.; Wang, C.-J.; Wu, K.-T.; Siu, K.-K.; Ko, J.-Y.; Huang, W.-C.; Chou, W.-Y.; Cheng, J.-H. Comparison of Extracorporeal Shockwave Therapy with Non-Steroid Anti-Inflammatory Drugs and Intra-Articular Hyaluronic Acid Injection for Early Osteoarthritis of the Knees. Biomedicines 2022, 10, 202. https://doi.org/10.3390/biomedicines10020202
Jhan S-W, Wang C-J, Wu K-T, Siu K-K, Ko J-Y, Huang W-C, Chou W-Y, Cheng J-H. Comparison of Extracorporeal Shockwave Therapy with Non-Steroid Anti-Inflammatory Drugs and Intra-Articular Hyaluronic Acid Injection for Early Osteoarthritis of the Knees. Biomedicines. 2022; 10(2):202. https://doi.org/10.3390/biomedicines10020202
Chicago/Turabian StyleJhan, Shun-Wun, Ching-Jen Wang, Kuan-Ting Wu, Ka-Kit Siu, Jih-Yang Ko, Wen-Chiung Huang, Wen-Yi Chou, and Jai-Hong Cheng. 2022. "Comparison of Extracorporeal Shockwave Therapy with Non-Steroid Anti-Inflammatory Drugs and Intra-Articular Hyaluronic Acid Injection for Early Osteoarthritis of the Knees" Biomedicines 10, no. 2: 202. https://doi.org/10.3390/biomedicines10020202
APA StyleJhan, S.-W., Wang, C.-J., Wu, K.-T., Siu, K.-K., Ko, J.-Y., Huang, W.-C., Chou, W.-Y., & Cheng, J.-H. (2022). Comparison of Extracorporeal Shockwave Therapy with Non-Steroid Anti-Inflammatory Drugs and Intra-Articular Hyaluronic Acid Injection for Early Osteoarthritis of the Knees. Biomedicines, 10(2), 202. https://doi.org/10.3390/biomedicines10020202








