Effect of Organic Amendments in Soil on Physiological and Biochemical Attributes of Vachellia nilotica and Dalbergia sissoo under Saline Stress
Abstract
1. Introduction
2. Results and Discussion
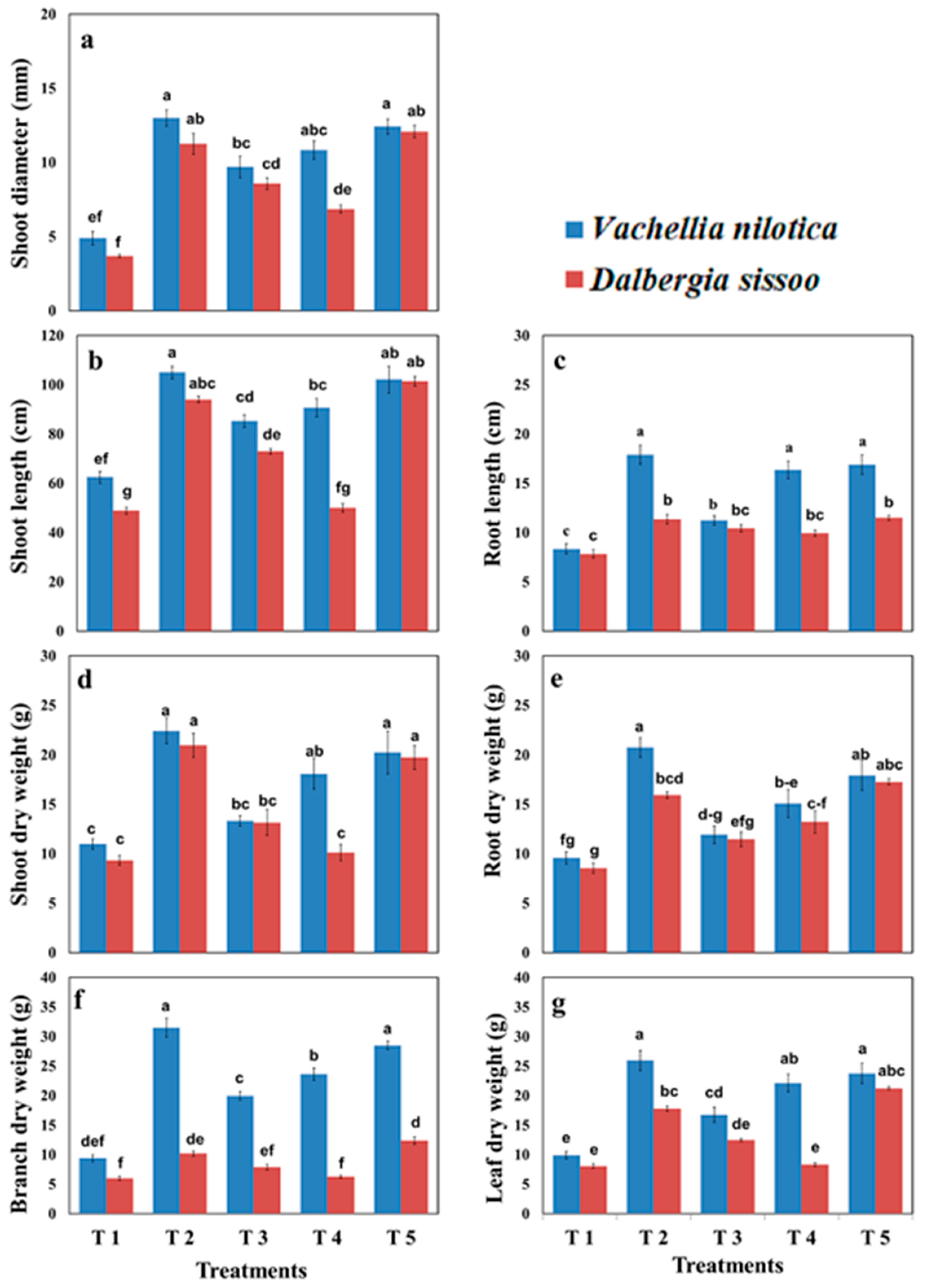
2.1. Growth Attributes
2.2. Physiological Attributes
2.3. Sodium and Potassium Contents in Plants
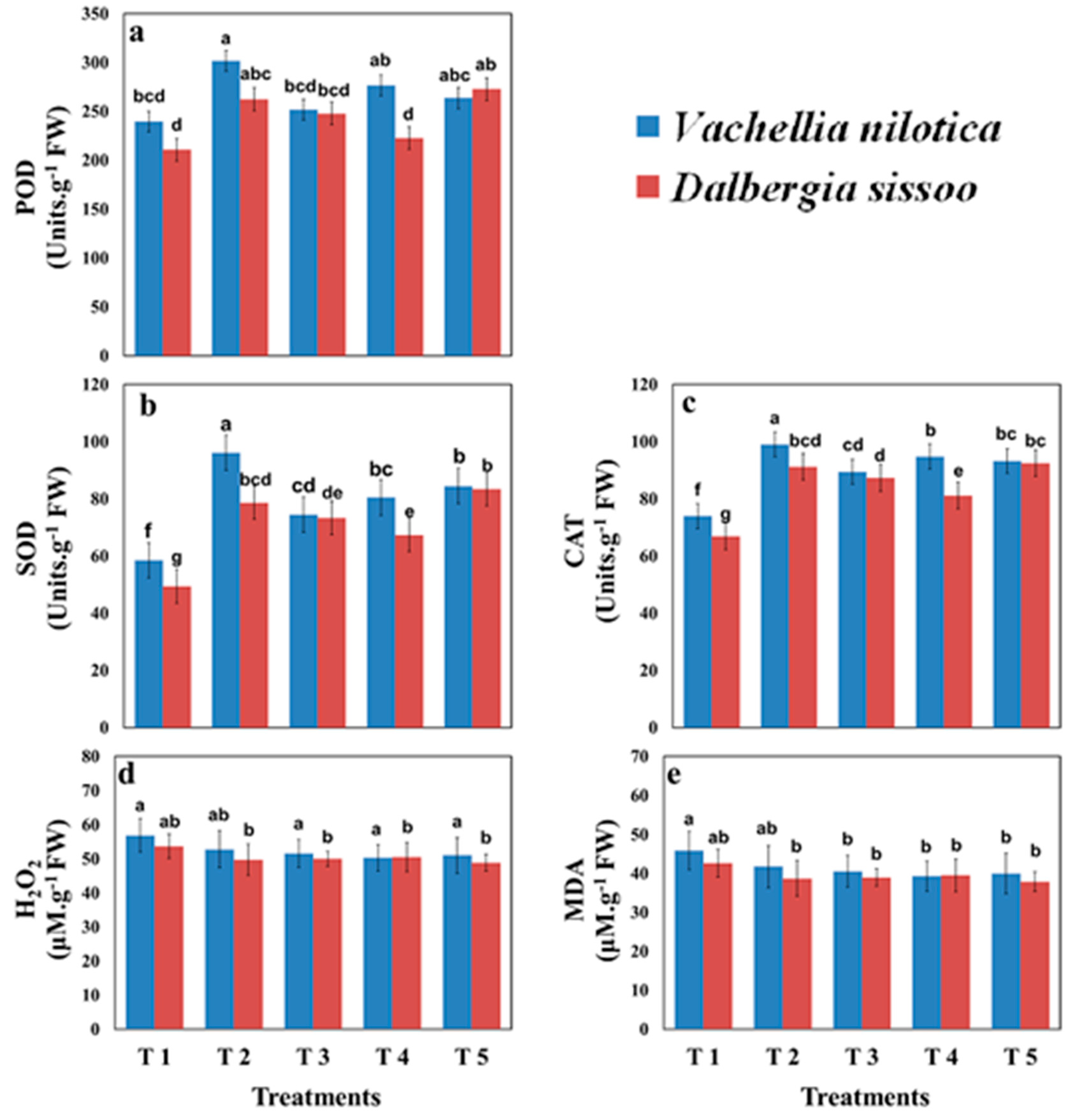
2.4. Biochemical Parameters
2.5. Post-Harvest Soil Characteristics
3. Materials and Methods
4. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dagar, J.C.; Minhas, P.S. Global perspectives on agroforestry for the management of salt-affected soils. In Agroforestry for the Management of Waterlogged Saline Soils and Poor-Quality Waters; Springer: New Dehli, India, 2016; pp. 5–32. [Google Scholar]
- Srivastava, N. Reclamation of saline and sodic soil through phytoremediation. In Environmental Concerns and Sustainable Development; Springer: Singapore, 2020; pp. 279–306. [Google Scholar]
- FAO. FAO Soils Portal. Available online: http://www.fao.org/soils-portal/soil-management/management-of-some-problem-soils/salt-affected-soils/more-information-on-salt-affected-soils/en/ (accessed on 2 December 2021).
- Tripathi, A.D.; Mishra, R.; Maurya, K.K.; Singh, R.B.; Wilson, D.W. Estimates for world population and global food availability for global health. In The Role of Functional Food Security in Global Health; Elsevier: Amsterdam, The Netherlands, 2019; pp. 3–24. [Google Scholar]
- Hossain, S. Present scenario of global salt affected soils, its management and importance of salinity research. Int. Res. J. Biol. Sci. 2019, 1, 1–3. [Google Scholar]
- Mbarki, S.; Sytar, O.; Cerda, A.; Zivcak, M.; Rastogi, A.; He, X.; Zoghlami, A.; Abdelly, C.; Brestic, M. Strategies to mitigate the salt stress effects on photosynthetic apparatus and productivity of crop plants. In Salinity Responses and Tolerance in Plants; Springer International Publishing: Cham, Switzerland, 2018; Volume 1, pp. 85–136. [Google Scholar]
- Gangwar, P.; Singh, R.; Trivedi, M.; Tiwari, R.K. Sodic soil: Management and reclamation strategies. In Environmental Concerns and Sustainable Development; Springer: Singapore, 2020; pp. 175–190. [Google Scholar]
- Herr, D.; Blum, J.; Himes-Cornell, A.; Sutton-Grier, A. An analysis of the potential positive and negative livelihood impacts of coastal carbon offset projects. J. Environ. Manag. 2019, 235, 463–479. [Google Scholar] [CrossRef] [PubMed]
- Yousaf, M.T.B.; Nawaz, M.F.; Zia ur Rehman, M.; Gul, S.; Yasin, G.; Rizwan, M.; Ali, S. Effect of three different types of biochars on eco-physiological response of important agroforestry tree species under salt stress. Int. J. Phytoremediat. 2021, 23, 1412–1422. [Google Scholar] [CrossRef] [PubMed]
- Arora, S. Halotolerant microbes for amelioration of salt-affected soils for sustainable agriculture. In Phyto-Microbiome in Stress Regulation; Springer: Singapore, 2020; pp. 323–343. [Google Scholar]
- George, S.J.; Harper, R.J.; Hobbs, R.J.; Tibbett, M. A sustainable agricultural landscape for Australia: A review of interlacing carbon sequestration, biodiversity and salinity management in agroforestry systems. Agric. Ecosyst. Environ. 2012, 163, 28–36. [Google Scholar] [CrossRef]
- Meir, M.; Zaccai, M.; Raveh, E.; Ben-Asher, J.; Tel-Zur, N. Performance of Ziziphus jujuba trees correlates with tissue mineral content under salinity conditions. Agric. Water Manag. 2014, 142, 47–55. [Google Scholar] [CrossRef]
- Nawaz, M.F.; Yousaf, M.T.B.; Yasin, G.; Gul, S.; Ahmed, I.; Abdullah, M.; Rafay, M.; Tanvir, M.A.; Asif, M.; Afzal, S. Agroforestry status and its role to sequester atmospheric CO2 under semi-arid climatic conditions in Pakistan. Appl. Ecol. Environ. Res. 2018, 16, 645–661. [Google Scholar] [CrossRef]
- Sharma, P.C.; Singh, A. Reviving the productivity of salt-affected lands: Technological options, constraints and research needs. In Research Developments in Saline Agriculture; Springer: Singapore, 2019; pp. 591–627. [Google Scholar]
- Yasin, G.; Nawaz, M.F.; Martin, T.A.; Niazi, N.K.; Gul, S.; Yousaf, M.T. Bin evaluation of agroforestry carbon storage status and potential in irrigated plains of Pakistan. Forests 2019, 10, 640. [Google Scholar] [CrossRef]
- Yousaf, M.T.B.; Nawaz, M.F.; Khawaja, H.F.; Gul, S.; Ali, S.; Ahmad, I.; Rasul, F.; Rizwan, M. Ecophysiological response of early stage Albizia lebbeck to cadmium toxicity and biochar addition. Arab. J. Geosci. 2019, 12, 134. [Google Scholar] [CrossRef]
- Edrisi, S.A.; Tripathi, V.; Chaturvedi, R.K.; Dubey, D.K.; Patel, G.; Abhilash, P.C. Saline soil reclamation index as an efficient tool for assessing restoration progress of saline land. Land Degrad. Dev. 2021, 32, 123–138. [Google Scholar] [CrossRef]
- Behera, L.; Nayak, M.; Patel, D.; Mehta, A.; Sinha, S.; Gunaga, R. Agroforestry practices for physiological amelioration of salt affected soils. J. Plant Stress Physiol. 2015, 1, 13. [Google Scholar] [CrossRef]
- Yousaf, M.T.B.; Nawaz, M.F.; Zia, M.; Rasul, F.; Tanvir, M.A. Ecophysiological response of early stage Eucalyptus camaldulensis to biochar and other organic amendments under salt stress. Pak. J. Agric. Sci. 2021, 58, 999–1006. [Google Scholar] [CrossRef]
- Abbas, G.; Saqib, M.; Akhtar, J.; Basra, S.M.A. Salinity tolerance potential of two acacia species at early seedling stage. Pak. J. Agric. Sci. 2013, 50, 683–688. [Google Scholar]
- Kaur, I.; Jadhav, S.K.; Tiwari, K.L.; Quraishi, A. Lead tolerance and its accumulation by a tree legume: Dalbergia sissoo DC. Bull. Environ. Contam. Toxicol. 2018, 101, 506–513. [Google Scholar] [CrossRef]
- Devi, P.; Singh, S. Promila phytochemical and pharmacological profiling of Dalbergia sissoo Roxb. Stem. J. Pharmacogn. Phytochem. 2017, 6, 2483–2486. [Google Scholar]
- Amadou, I.; Soulé, M.; Salé, A. An overview on the importance of Acacia nilotica (L.) willd. ex del.: A review. Asian J. Res. Agric. For. 2020, 5, 12–18. [Google Scholar] [CrossRef]
- Khan, T.F.; Salma, M.U.; Hossain, S.A. Impacts of different sources of biochar on plant growth characteristics. Am. J. Plant Sci. 2018, 9, 1922–1934. [Google Scholar] [CrossRef][Green Version]
- Vibha, J.B.; Shekhawat, N.S.; Mehandru, P.; Dinesh, R. Rapid multiplication of Dalbergia sissoo Roxb.: A timber yielding tree legume through axillary shoot proliferation and ex vitro rooting. Physiol. Mol. Biol. Plants 2014, 20, 81–87. [Google Scholar] [CrossRef] [PubMed]
- Devi, S.; Nandwal, A.S.; Angrish, R.; Arya, S.S.; Kumar, N.; Sharma, S.K. Phytoremediation potential of some halophytic species for soil salinity. Int. J. Phytoremediat. 2016, 18, 693–696. [Google Scholar] [CrossRef] [PubMed]
- Prabakaran, K.; Li, J.; Anandkumar, A.; Leng, Z.; Zou, C.B.; Du, D. Managing environmental contamination through phytoremediation by invasive plants: A review. Ecol. Eng. 2019, 138, 28–37. [Google Scholar] [CrossRef]
- Awan, A.R.; Mahmood, K. Tree plantation in problem soils. In Text Book of Applied Forestry; University of Agriculture: Faisalabad, Pakistan, 2017; pp. 140–159. [Google Scholar]
- Banyal, R.; Rajkumar; Kumar, M.; Yadav, R.K.; Dagar, J.C. Agroforestry for rehabilitation and sustenance of saline ecologies. In Agroforestry; Springer: Singapore, 2017; pp. 413–454. [Google Scholar]
- Verma, P.; Kumar, M.; Mishra, G.; Sahoo, D. Multivariate analysis of fatty acid and biochemical constitutes of seaweeds to characterize their potential as bioresource for biofuel and fine chemicals. Bioresour. Technol. 2017, 226, 132–144. [Google Scholar] [CrossRef] [PubMed]
- Verma, E.; Singh, S.; Niveshika; Mishra, A.K. Salinity-induced oxidative stress-mediated change in fatty acids composition of cyanobacterium Synechococcus sp. PCC7942. Int. J. Environ. Sci. Technol. 2019, 16, 875–886. [Google Scholar] [CrossRef]
- Omar, Z.; Bouajila, A.; Bouajila, J.; Rahmani, R.; Besser, H.; Hamed, Y. Spectroscopic and chromatographic investigation of soil organic matter composition for different agrosystems from arid saline soils from Southeastern Tunisia. Arab. J. Geosci. 2020, 13, 524. [Google Scholar] [CrossRef]
- Mekuria, W.; Noble, A. The role of biochar in ameliorating disturbed soils and sequestering soil carbon in tropical agricultural production systems. Appl. Environ. Soil Sci. 2013, 2013, 354965. [Google Scholar] [CrossRef]
- Oo, A.N.; Iwai, C.B.; Saenjan, P. Soil properties and maize growth in saline and nonsaline soils using cassava-industrial waste compost and vermicompost with or without earthworms. Land Degrad. Dev. 2015, 26, 300–310. [Google Scholar] [CrossRef]
- Tully, K.L.; McAskill, C. Promoting soil health in organically managed systems: A review. Org. Agric. 2020, 10, 339–358. [Google Scholar] [CrossRef]
- Zhu, H.; Yang, J.; Yao, R.; Wang, X.; Xie, W.; Zhu, W.; Liu, X.; Cao, Y.; Tao, J. Interactive effects of soil amendments (biochar and gypsum) and salinity on ammonia volatilization in coastal saline soil. Catena 2020, 190, 104527. [Google Scholar] [CrossRef]
- Nawab, J.; Ghani, J.; Khan, S.; Xiaoping, W. Minimizing the risk to human health due to the ingestion of arsenic and toxic metals in vegetables by the application of biochar, farmyard manure and peat moss. J. Environ. Manag. 2018, 214, 172–183. [Google Scholar] [CrossRef] [PubMed]
- Jabeen, N.; Ahmad, R. Growth response and nitrogen metabolism of sunflower (Helianthus annuus L.) to vermicompost and biogas slurry under salinity stress. J. Plant Nutr. 2017, 40, 104–114. [Google Scholar] [CrossRef]
- Tejada, M.; Garcia, C.; Gonzalez, J.L.; Hernandez, M.T. Use of organic amendment as a strategy for saline soil remediation: Influence on the physical, chemical and biological properties of soil. Soil Biol. Biochem. 2006, 38, 1413–1421. [Google Scholar] [CrossRef]
- Awasthi, M.K.; Sarsaiya, S.; Wainaina, S.; Rajendran, K.; Kumar, S.; Quan, W.; Duan, Y.; Awasthi, S.K.; Chen, H.; Pandey, A.; et al. A critical review of organic manure biorefinery models toward sustainable circular bioeconomy: Technological challenges, advancements, innovations, and future perspectives. Renew. Sustain. Energy Rev. 2019, 111, 115–131. [Google Scholar] [CrossRef]
- Wang, L.; Sun, X.; Li, S.; Zhang, T.; Zhang, W.; Zhai, P. Application of organic amendments to a coastal saline soil in North China: Effects on soil physical and chemical properties and tree growth. PLoS ONE 2014, 9, e89185. [Google Scholar] [CrossRef]
- Wan, D.; Wu, L.; Liu, Y.; Zhao, H.; Fu, J.; Xiao, S. Adsorption of low concentration perchlorate from aqueous solution onto modified cow dung biochar: Effective utilization of cow dung, an agricultural waste. Sci. Total Environ. 2018, 636, 1396–1407. [Google Scholar] [CrossRef]
- Yuan, P.; Wang, J.; Pan, Y.; Shen, B.; Wu, C. Review of biochar for the management of contaminated soil: Preparation, application and prospect. Sci. Total Environ. 2019, 659, 473–490. [Google Scholar] [CrossRef]
- He, K.; He, G.; Wang, C.; Zhang, H.; Xu, Y.; Wang, S.; Kong, Y.; Zhou, G.; Hu, R. Biochar amendment ameliorates soil properties and promotes Miscanthus growth in a coastal saline-alkali soil. Appl. Soil Ecol. 2020, 155, 103674. [Google Scholar] [CrossRef]
- Cabot, C.; Sibole, J.V.; Barceló, J.; Poschenrieder, C. Lessons from crop plants struggling with salinity. Plant Sci. 2014, 226, 2–13. [Google Scholar] [CrossRef] [PubMed]
- Gupta, B.; Huang, B. Mechanism of salinity tolerance in plants: Physiological, biochemical, and molecular characterization. Int. J. Genom. 2014, 2014, 701596. [Google Scholar] [CrossRef] [PubMed]
- Koubouris, G.C.; Tzortzakis, N.; Kourgialas, N.N.; Darioti, M.; Metzidakis, I. Growth, photosynthesis and pollen performance in saline water treated olive plants under high temperature. Int. J. Plant Biol. 2015, 6, 6038. [Google Scholar] [CrossRef]
- Roy, S.J.; Negrão, S.; Tester, M. Salt resistant crop plants. Curr. Opin. Biotechnol. 2014, 26, 115–124. [Google Scholar] [CrossRef]
- Bandingan, K.; Tanah, P.; Ke Atas, B.; Gandum, T.; Pengeluaran, D.; Salin-Sodik, T.; Zia-Ur-Rehman, M.; Rizwan, M.; Sabir, M. Comparative effects of different soil conditioners on wheat growth and yield grown in saline-sodic soils. Sains Malays. 2016, 45, 339–346. [Google Scholar]
- Khaliq, R.; Tita, O.; Zafar, Z.U. Possible assessment of salt tolerance in ocimum basilicum by chlorophyll fluorescence. Sci. Pap. Ser. Manag. Econ. Eng. Agric. Rural Dev. 2017, 17, 175–182. [Google Scholar]
- Mohamed, A.K.S.H.; Qayyum, M.F.; Abdel-Hadi, A.M.; Rehman, R.A.; Ali, S.; Rizwan, M. Interactive effect of salinity and silver nanoparticles on photosynthetic and biochemical parameters of wheat. Arch. Agron. Soil Sci. 2017, 63, 1736–1747. [Google Scholar] [CrossRef]
- Parvaiz, A.; Satyawati, S. Salt stress and phyto-biochemical responses of plants—A review. Plant Soil Environ. 2008, 54, 89–99. [Google Scholar] [CrossRef]
- Tewari, P.; Saxena, A.K.; Rao, O.P. Effect of sodicity and salinity on seedling growth of two early successional agroforestry tree species. Trop. Ecol. 2006, 47, 125–132. [Google Scholar]
- Soliveres, S.; Monerris, J.; Cortina, J. Irrigation, organic fertilization and species successional stage modulate the response of woody seedlings to herbaceous competition in a semi-arid quarry restoration. Appl. Veg. Sci. 2012, 15, 175–186. [Google Scholar] [CrossRef]
- Gaur, A.; Adholeya, A. Diverse response of five ornamental plant species to mixed indigenous and single isolate arbuscular-mycorrhizal inocula in marginal soil amended with organic matter. J. Plant Nutr. 2005, 28, 707–723. [Google Scholar] [CrossRef]
- Wortman, S.E.; Holmes, A.A.; Miernicki, E.; Knoche, K.; Pittelkow, C.M. First-season crop yield response to organic soil amendments: A meta-analysis. Agron. J. 2017, 109, 1210–1217. [Google Scholar] [CrossRef]
- Somerville, P.D.; Farrell, C.; May, P.B.; Livesley, S.J. Tree water use strategies and soil type determine growth responses to biochar and compost organic amendments. Soil Tillage Res. 2019, 192, 12–21. [Google Scholar] [CrossRef]
- Akhtar, S.S.; Andersen, M.N.; Liu, F. Residual effects of biochar on improving growth, physiology and yield of wheat under salt stress. Agric. Water Manag. 2015, 158, 61–68. [Google Scholar] [CrossRef]
- Lashari, M.S.; Ye, Y.; Ji, H.; Li, L.; Kibue, G.W.; Lu, H.; Zheng, J.; Pan, G. Biochar-manure compost in conjunction with pyroligneous solution alleviated salt stress and improved leaf bioactivity of maize in a saline soil from central China: A 2-year field experiment. J. Sci. Food Agric. 2015, 95, 1321–1327. [Google Scholar] [CrossRef] [PubMed]
- Oram, N.J.; van de Voorde, T.F.J.; Ouwehand, G.-J.; Bezemer, T.M.; Mommer, L.; Jeffery, S.; van Groenigen, J.W. Soil amendment with biochar increases the competitive ability of legumes via increased potassium availability. Agric. Ecosyst. Environ. 2014, 191, 92–98. [Google Scholar] [CrossRef]
- Abrishamkesh, S.; Gorji, M.; Asadi, H.; Bagheri-Marandi, G.H.; Pourbabaee, A.A. Effects of rice husk biochar application on the properties of alkaline soil and lentil growth. Plant Soil Environ. 2016, 61, 475–482. [Google Scholar] [CrossRef]
- Liew, R.K.; Chai, C.; Yek, P.N.Y.; Phang, X.Y.; Chong, M.Y.; Nam, W.L.; Su, M.H.; Lam, W.H.; Ma, N.L.; Lam, S.S. Innovative production of highly porous carbon for industrial effluent remediation via microwave vacuum pyrolysis plus sodium-potassium hydroxide mixture activation. J. Clean. Prod. 2019, 208, 1436–1445. [Google Scholar] [CrossRef]
- Chowdhury, S.; Bhusan, D.; Hashem, M.A.; Hoque, M.A. Organic amendments for mitigating soil salinity in rice. Res. Agric. Livest. Fish. 2019, 6, 11–17. [Google Scholar] [CrossRef]
- Nagasawa, K.; Wang, B.; Nishiya, K.; Ushijima, K.; Zhu, Q.; Fukushima, M.; Ichijo, T. Effects of humic acids derived from lignite and cattle manure on antioxidant enzymatic activities of barley root. J. Environ. Sci. Health Part B 2016, 51, 81–89. [Google Scholar] [CrossRef]
- Turan, V. Potential of pistachio shell biochar and dicalcium phosphate combination to reduce Pb speciation in spinach, improved soil enzymatic activities, plant nutritional quality, and antioxidant defense system. Chemosphere 2020, 245, 125611. [Google Scholar] [CrossRef] [PubMed]
- Shahkolaie, S.S.; Baranimotlagh, M.; Dordipour, E.; Khormali, F. Effects of inorganic and organic amendments on physiological parameters and antioxidant enzymes activities in Zea mays L. from a cadmium-contaminated calcareous soil. S. Afr. J. Bot. 2020, 128, 132–140. [Google Scholar] [CrossRef]
- Ramzani, P.M.A.; Coyne, M.S.; Anjum, S.; Khan, W.-D.; Iqbal, M. In Situ immobilization of Cd by organic amendments and their effect on antioxidant enzyme defense mechanism in mung bean (Vigna radiata L.) seedlings. Plant Physiol. Biochem. 2017, 118, 561–570. [Google Scholar] [CrossRef]
- Elshaikh, N.A.; Zhipeng, L.; Dongli, S.; Timm, L.C. Increasing the okra salt threshold value with biochar amendments. J. Plant Interact. 2018, 13, 51–63. [Google Scholar] [CrossRef]
- Rezaie, N.; Razzaghi, F.; Sepaskhah, A.R. Different levels of irrigation water salinity and biochar influence on faba bean yield, water productivity, and ions uptake. Commun. Soil Sci. Plant Anal. 2019, 50, 611–626. [Google Scholar] [CrossRef]
- Tanure, M.M.C.; da Costa, L.M.; Huiz, H.A.; Fernandes, R.B.A.; Cecon, P.R.; Pereira Junior, J.D.; da Luz, J.M.R. Soil water retention, physiological characteristics, and growth of maize plants in response to biochar application to soil. Soil Tillage Res. 2019, 192, 164–173. [Google Scholar] [CrossRef]
- Meena, M.D.; Yadav, R.K.; Narjary, B.; Yadav, G.; Jat, H.S.; Sheoran, P.; Meena, M.K.; Antil, R.S.; Meena, B.L.; Singh, H.V.; et al. Municipal solid waste (MSW): Strategies to improve salt affected soil sustainability: A review. Waste Manag. 2019, 84, 38–53. [Google Scholar] [CrossRef]
- Bhadha, J.H.; Capasso, J.M.; Khatiwada, R.; Swanson, S. Raising Soil Organic Matter Content to Improve Water Holding Capacity; UF/IFAS: Gainesville, FL, USA, 2017; pp. 1–5. [Google Scholar]
- Cesarano, G.; De Filippis, F.; La Storia, A.; Scala, F.; Bonanomi, G. Organic amendment type and application frequency affect crop yields, soil fertility and microbiome composition. Appl. Soil Ecol. 2017, 120, 254–264. [Google Scholar] [CrossRef]
- Mi, W.; Wu, L.; Brookes, P.C.; Liu, Y.; Zhang, X.; Yang, X. Changes in soil organic carbon fractions under integrated management systems in a low-productivity paddy soil given different organic amendments and chemical fertilizers. Soil Tillage Res. 2016, 163, 64–70. [Google Scholar] [CrossRef]
- Niazi, N.K.; Bibi, I.; Shahid, M.; Ok, Y.S.; Shaheen, S.M.; Rinklebe, J.; Wang, H.; Murtaza, B.; Islam, E.; Farrakh Nawaz, M.; et al. Arsenic removal by Japanese oak wood biochar in aqueous solutions and well water: Investigating arsenic fate using integrated spectroscopic and microscopic techniques. Sci. Total Environ. 2018, 621, 1642–1651. [Google Scholar] [CrossRef]
- Saifullah; Dahlawi, S.; Naeem, A.; Rengel, Z.; Naidu, R. Biochar application for the remediation of salt-affected soils: Challenges and opportunities. Sci. Total Environ. 2018, 625, 320–335. [Google Scholar] [CrossRef]
- Trivedi, P.; Singh, K.; Pankaj, U.; Verma, S.K.; Verma, R.K.; Patra, D.D. Effect of organic amendments and microbial application on sodic soil properties and growth of an aromatic crop. Ecol. Eng. 2017, 102, 127–136. [Google Scholar] [CrossRef]
- Diacono, M.; Montemurro, F. Effectiveness of organic wastes as fertilizers and amendments in salt-affected soils. Agriculture 2015, 5, 221–230. [Google Scholar] [CrossRef]
- Cherubin, M.R.; Chavarro-Bermeo, J.P.; Silva-Olaya, A.M. Agroforestry systems improve soil physical quality in northwestern Colombian Amazon. Agrofor. Syst. 2019, 93, 1741–1753. [Google Scholar] [CrossRef]
- Yazdanpanah, N.; Mahmoodabadi, M.; Cerdà, A. The impact of organic amendments on soil hydrology, structure and microbial respiration in semiarid lands. Geoderma 2016, 266, 58–65. [Google Scholar] [CrossRef]
- Luo, X.; Liu, G.; Xia, Y.; Chen, L.; Jiang, Z.; Zheng, H.; Wang, Z. Use of biochar-compost to improve properties and productivity of the degraded coastal soil in the Yellow River Delta, China. J. Soils Sediments 2017, 17, 780–789. [Google Scholar] [CrossRef]
- Leogrande, R.; Vitti, C. Use of organic amendments to reclaim saline and sodic soils: A review. Arid. Land Res. Manag. 2019, 33, 1–21. [Google Scholar] [CrossRef]
- Ali, S.; Rizwan, M.; Qayyum, M.F.; Ok, Y.S.; Ibrahim, M.; Riaz, M.; Arif, M.S.; Hafeez, F.; Al-Wabel, M.I.; Shahzad, A.N. Biochar soil amendment on alleviation of drought and salt stress in plants: A critical review. Environ. Sci. Pollut. Res. 2017, 24, 12700–12712. [Google Scholar] [CrossRef] [PubMed]
- Drake, J.A.; Cavagnaro, T.R.; Cunningham, S.C.; Jackson, W.R.; Patti, A.F. Does biochar improve establishment of tree seedlings in saline sodic soils? Land Degrad. Dev. 2016, 27, 52–59. [Google Scholar] [CrossRef]
- Wu, Y.; Xu, G.; Shao, H.B. Furfural and its biochar improve the general properties of a saline soil. Solid Earth 2014, 5, 665–671. [Google Scholar] [CrossRef]
- Sun, H.; Lu, H.; Chu, L.; Shao, H.; Shi, W. Biochar applied with appropriate rates can reduce N leaching, keep N retention and not increase NH3 volatilization in a coastal saline soil. Sci. Total Environ. 2017, 575, 820–825. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Zou, W.; Chen, J.; Chen, H.; Yu, Z.; Huang, J.; Tang, H.; Wei, X.; Gao, B. Biochar amendment improves crop production in problem soils: A review. J. Environ. Manag. 2019, 232, 8–21. [Google Scholar] [CrossRef] [PubMed]
- Zheng, H.; Wang, X.; Chen, L.; Wang, Z.; Xia, Y.; Zhang, Y.; Wang, H.; Luo, X.; Xing, B. Enhanced growth of halophyte plants in biochar-amended coastal soil: Roles of nutrient availability and rhizosphere microbial modulation. Plant. Cell Environ. 2018, 41, 517–532. [Google Scholar] [CrossRef]
- Husien, A. Effect of biochar, farmyard manure and nitrogen fertilizers on soil chemical properties in Sinana District, South Eastern Oromia, Ethiopia. Int. J. Appl. Agric. Sci. 2017, 3, 148. [Google Scholar] [CrossRef]
- McGeorge, W.T. Diagnosis and improvement of saline and alkaline soils. Soil Sci. Soc. Am. J. 1954, 18, 348. [Google Scholar] [CrossRef]
- Bouyoucos, G.J. Hydrometer method improved for making particle size analyses of soils 1. Agron. J. 1962, 54, 464–465. [Google Scholar] [CrossRef]
- Sikora, L.J.; Moore-Kucera, J. Soil Test Methods from the Southeastern United States; University of Georgia College of Agricultural & Environmental Sciences: Athens, GA, USA, 2014; Volume 419, ISBN 9798624955. [Google Scholar]
- Ur Rahman, S.; Xuebin, Q.; Yasin, G.; Cheng, H.; Mehmood, F.; Zain, M.; Shehzad, M.; Ahmad, M.I.; Riaz, L.; Rahim, A.; et al. Role of silicon on root morphological characters of wheat (Triticum aestivum L.) plants grown under Cd-contaminated nutrient solution. Acta Physiol. Plant. 2021, 43, 60. [Google Scholar] [CrossRef]
- Rahman, S.U.; Xuebin, Q.; Riaz, L.; Yasin, G.; Shah, A.N.; Shahzad, U.; Jahan, M.S.; Ditta, A.; Bashir, M.A.; Rehim, A.; et al. The interactive effect of pH variation and cadmium stress on wheat (Triticum aestivum L.) growth, physiological and biochemical parameters. PLoS ONE 2021, 16, e253798. [Google Scholar] [CrossRef] [PubMed]
- Ur Rahman, S.; Xuebin, Q.; Kamran, M.; Yasin, G.; Cheng, H.; Rehim, A.; Riaz, L.; Rizwan, M.; Ali, S.; Alsahli, A.A.; et al. Silicon elevated cadmium tolerance in wheat (Triticum aestivum L.) by endorsing nutrients uptake and antioxidative defense mechanisms in the leaves. Plant Physiol. Biochem. 2021, 166, 148–159. [Google Scholar] [CrossRef] [PubMed]
- Ur Rahman, S.; Xuebin, Q.; Zhao, Z.; Du, Z.; Imtiaz, M.; Mehmood, F.; Hongfei, L.; Hussain, B.; Ashraf, M.N. Alleviatory effects of Silicon on the morphology, physiology, and antioxidative mechanisms of wheat (Triticum aestivum L.) roots under cadmium stress in acidic nutrient solutions. Sci. Rep. 2021, 11, 1958. [Google Scholar] [CrossRef] [PubMed]
- Shafeeq-ur-Rahman; Xuebin, Q.; Yatao, X.; Ahmad, M.I.; Shehzad, M.; Zain, M. Silicon and its application methods improve physiological traits and antioxidants in Triticum aestivum (L.) under cadmium stress. J. Soil Sci. Plant Nutr. 2020, 20, 1110–1121. [Google Scholar] [CrossRef]
- Aebi, H. Catalase in vitro. In Methods in Enzymology; Elsevier: Amsterdam, The Netherlands, 1984; pp. 121–126. [Google Scholar]
- Ur Rehman, M.Z.; Rizwan, M.; Rauf, A.; Ayub, M.A.; Ali, S.; Qayyum, M.F.; Waris, A.A.; Naeem, A.; Sanaullah, M. Split application of silicon in cadmium (Cd) spiked alkaline soil plays a vital role in decreasing Cd accumulation in rice (Oryza sativa L.) grains. Chemosphere 2019, 226, 454–462. [Google Scholar] [CrossRef]


| Parameters | T1 | T2 | T3 | T4 | T5 | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| SP 1 | SP 2 | SP 1 | SP 2 | SP 1 | SP 2 | SP 1 | SP 2 | SP 1 | SP 2 | |
| pH | 7.77a ± 0.04 | 7.77a ± 0.04 | 7.87a ± 0.04 | 7.88a ± 0.04 | 7.87a ± 0.04 | 7.89a ± 0.04 | 7.85a ± 0.04 | 7.87a ± 0.04 | 7.84a ± 0.04 | 7.84a ± 0.04 |
| EC (dS/m) | 8.71b ± 0.2 | 12.4a ± 0.2 | 3.94de ± 0.2 | 8.42b ± 0.2 | 6.83c ± 0.2 | 8.75b ± 0.2 | 4.13de ± 0.2 | 8.15b ± 0.2 | 3.57e ± 0.2 | 4.53e ± 0.2 |
| TSS(mmolc/L) | 87.10b ± 2.1 | 124.1a ± 2.1 | 39.3de ± 2.1 | 84.2b ± 2.1 | 68.3c ± 2.1 | 87.5b ± 2.1 | 41.3de ± 2.1 | 81.5b ± 2.1 | 35.7e ± 2.1 | 45.2e ± 2.1 |
| HCO3−(mmolc/L) | 4.44a ± 0.3 | 4.51a ± 0.3 | 3.2b ± 0.3 | 3.5ab ± 0.3 | 3.6b ± 0.3 | 3.7ab ± 0.3 | 3.2ab ± 0.3 | 3.8ab ± 0.3 | 3.2b ± 0.3 | 3.5ab ± 0.3 |
| Cl− (mmolc/L) | 51.0b ± 1.7 | 60.0a ± 1.7 | 19.3fg ± 1.7 | 28.6cde ± 1.7 | 31.3cd ± 1.7 | 34.6cd ± 1.7 | 22.6efg ± 1.7 | 27.3de ± 1.7 | 17.3g ± 1.7 | 25.4def ± 1.7 |
| Ca2+ + Mg2+ (mmolc/L) | 3.7 c ± 0.7 | 3.83bc ± 0.7 | 7.5a ± 0.7 | 7.5a ± 0.7 | 7.1a ± 0.7 | 6.6a ± 0.7 | 6.4ab ± 0.7 | 6.6ab ± 0.7 | 5.0abc ± 0.7 | 5.2abc ± 0.7 |
| Na+ (mmolc/L) | 51.0b ± 1.2 | 62.33a ± 1.2 | 22.3fg ± 1.2 | 35.0d ± 1.2 | 28.0e ± 1.2 | 37.3cd ± 1.2 | 25.0e ± 1.2 | 40.3c ± 1.2 | 18.5h ± 1.2 | 19.9gh ± 1.2 |
| SAR | 37.4b ± 1.3 | 45.5a ± 1.3 | 11.7e ± 1.3 | 18.0cd ± 1.3 | 14.9de ± 1.3 | 20.6c ± 1.3 | 13.9de ± 1.3 | 22.1c ± 1.3 | 11.6e ± 1.3 | 12.3e ± 1.3 |
| K+(mmolc/L) | 6.5c ± 0.4 | 6.0c ± 0.4 | 11.3a ± 0.4 | 11.4a ± 0.4 | 10.3ab ± 0.4 | 10.4ab ± 0.4 | 9.5b ± 0.4 | 9.4 b ± 0.4 | 11.6a ± 0.4 | 12.0a ± 0.4 |
| OM (%) | 0.51e ± 0.1 | 0.50e ± 0.1 | 0.69bc ± 0.1 | 0.66cd ± 0.1 | 0.64cd ± 0.1 | 0.63d ± 0.1 | 0.62d ± 0.1 | 0.63d ± 0.1 | 0.77a ± 0.1 | 0.74ab ± 0.1 |
| Saturation (%) | 34.5d ± 0.47 | 34.5d ± 0.5 | 39.6b ± 0.5 | 38.8b ± 0.5 | 37.6c ± 0.5 | 37.6c ± 0.5 | 38.5bc ± 0.5 | 38.5bc ± 0.5 | 45.4a ± 0.5 | 45.3a ± 0.5 |
| Month | Average Max. Temp. (°C) | Average Min. Temp. (°C) | Precipitation (mm) | Sunshine Duration (Hours) | ET₀ (mm) |
|---|---|---|---|---|---|
| March | 27.3 | 14.2 | 16.2 | 07.2 | 02.7 |
| April | 37.7 | 20.9 | 28.3 | 09.2 | 05.2 |
| May | 41.1 | 26.0 | 10.1 | 10.4 | 05.7 |
| June | 39.8 | 27.3 | 41.6 | 09.38 | 05.3 |
| July | 38.5 | 28.9 | 117.2 | 07.0 | 04.0 |
| August | 38.1 | 28.6 | 66 | 07.9 | 03.8 |
| Parameters | Soil | FYM | PM | SL | FYMB |
|---|---|---|---|---|---|
| pH | 8.5 ± 0.3 | 5.8 ± 0.2 | 6.3 ± 0.3 | 6.0 ± 0.3 | 7.0 ± 0.2 |
| EC (dS/m) | 20.5 ± 9 | 6.55 ± 2.5 | 7.31 ± 3.2 | 6.82 ± 3.8 | 2.08 ± 0.34 |
| TSS (mg/kg) | 205 ± 20 | 65 ± 5.8 | 73.1 ± 10.2 | 68.2 ± 8.4 | 20.8 ± 4.2 |
| CO32− (mg/kg) | 10 ± 2 | (-) | (-) | (-) | (-) |
| HCO3− (mg/kg) | 30 ± 4 | 10 ± 3.2 | 6 ± 3.8 | 12 ± 4.5 | 8 ± 2.5 |
| Cl− (mg.kg−1) | 140 ± 19 | 8.0 ± 2.2 | 5 ± 3.2 | 5 ± 3.2 | 4 ± 2.1 |
| Ca2+ + Mg2+ (mg/kg) | 12 ± 4.5 | 7.3 ± 4.2 | 6.3 ± 3.1 | 6.9 ± 2.5 | 13.2 ± 2.2 |
| Na+ (mg/kg) | 160 ± 15.2 | 11.6 ± 1.8 | 15.2 ± 3.4 | 13.4 ± 2.5 | 3.4 ± 0.8 |
| K+ (mg/kg) | 47 ± 7 | 9.2 ± 2.7 | 8.8 ± 2.5 | 8.9 ± 2.9 | 7.2 ± 2.2 |
| OM (%) | 0.64 ± 0.03 | 50.8 ± 2.4 | 67.2 ± 5.5 | 65.4 ± 4.3 | 95.4 ± 3.6 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Talha Bin Yousaf, M.; Farrakh Nawaz, M.; Yasin, G.; Ahmad, I.; Gul, S.; Ijaz, M.; Zia-ur-Rehman, M.; Qi, X.; Ur Rahman, S. Effect of Organic Amendments in Soil on Physiological and Biochemical Attributes of Vachellia nilotica and Dalbergia sissoo under Saline Stress. Plants 2022, 11, 228. https://doi.org/10.3390/plants11020228
Talha Bin Yousaf M, Farrakh Nawaz M, Yasin G, Ahmad I, Gul S, Ijaz M, Zia-ur-Rehman M, Qi X, Ur Rahman S. Effect of Organic Amendments in Soil on Physiological and Biochemical Attributes of Vachellia nilotica and Dalbergia sissoo under Saline Stress. Plants. 2022; 11(2):228. https://doi.org/10.3390/plants11020228
Chicago/Turabian StyleTalha Bin Yousaf, Muhammad, Muhammad Farrakh Nawaz, Ghulam Yasin, Irfan Ahmad, Sadaf Gul, Muhammad Ijaz, Muhammad Zia-ur-Rehman, Xuebin Qi, and Shafeeq Ur Rahman. 2022. "Effect of Organic Amendments in Soil on Physiological and Biochemical Attributes of Vachellia nilotica and Dalbergia sissoo under Saline Stress" Plants 11, no. 2: 228. https://doi.org/10.3390/plants11020228
APA StyleTalha Bin Yousaf, M., Farrakh Nawaz, M., Yasin, G., Ahmad, I., Gul, S., Ijaz, M., Zia-ur-Rehman, M., Qi, X., & Ur Rahman, S. (2022). Effect of Organic Amendments in Soil on Physiological and Biochemical Attributes of Vachellia nilotica and Dalbergia sissoo under Saline Stress. Plants, 11(2), 228. https://doi.org/10.3390/plants11020228








