Suppression of Airway Allergic Reactions by a Photocatalytic Filter Using Mouse Model
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals
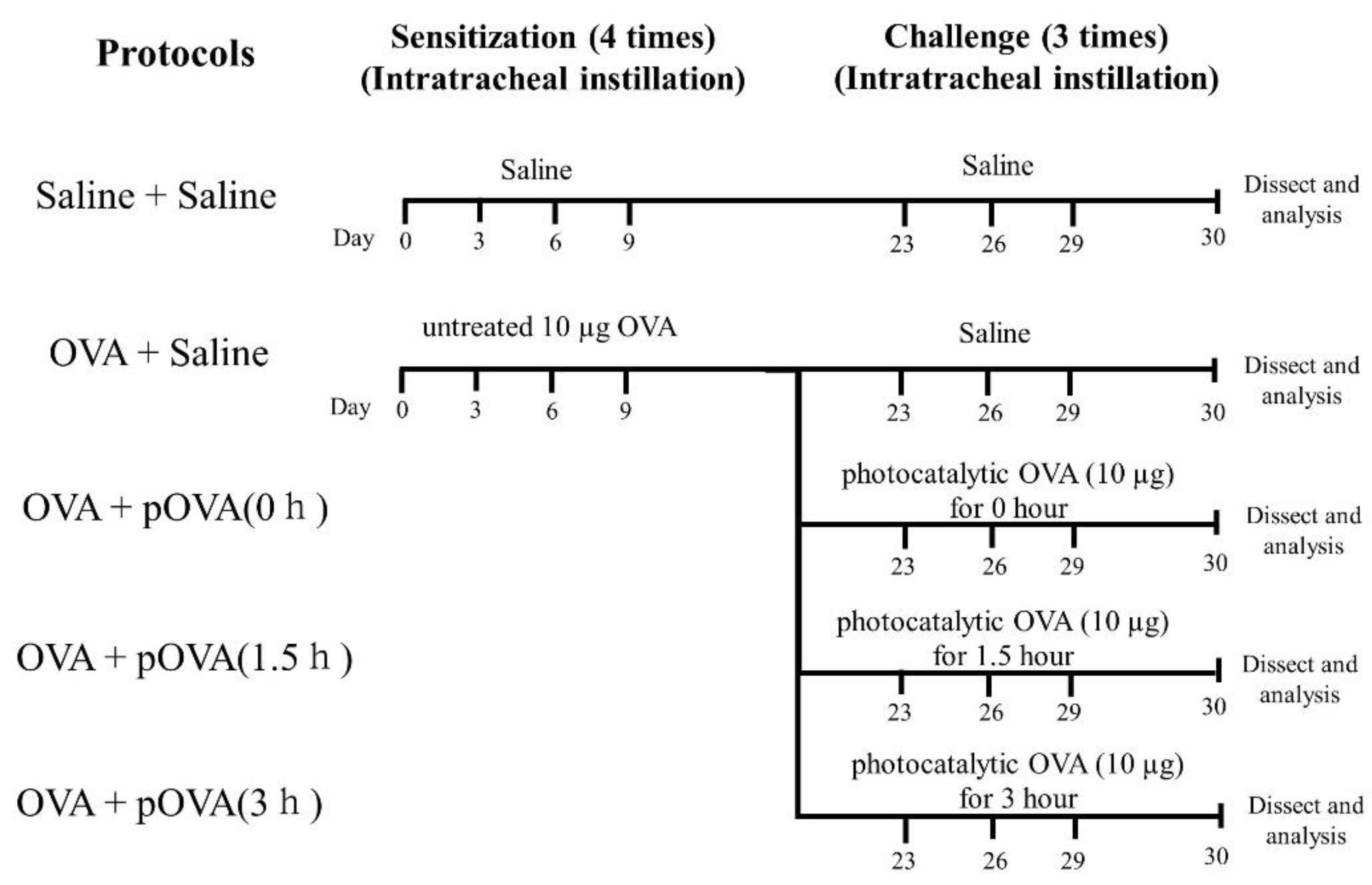
2.2. OVA Sensitization
2.3. OVA with Photocatalytic Treatment
2.4. Instillation of OVA after Photocatalysis
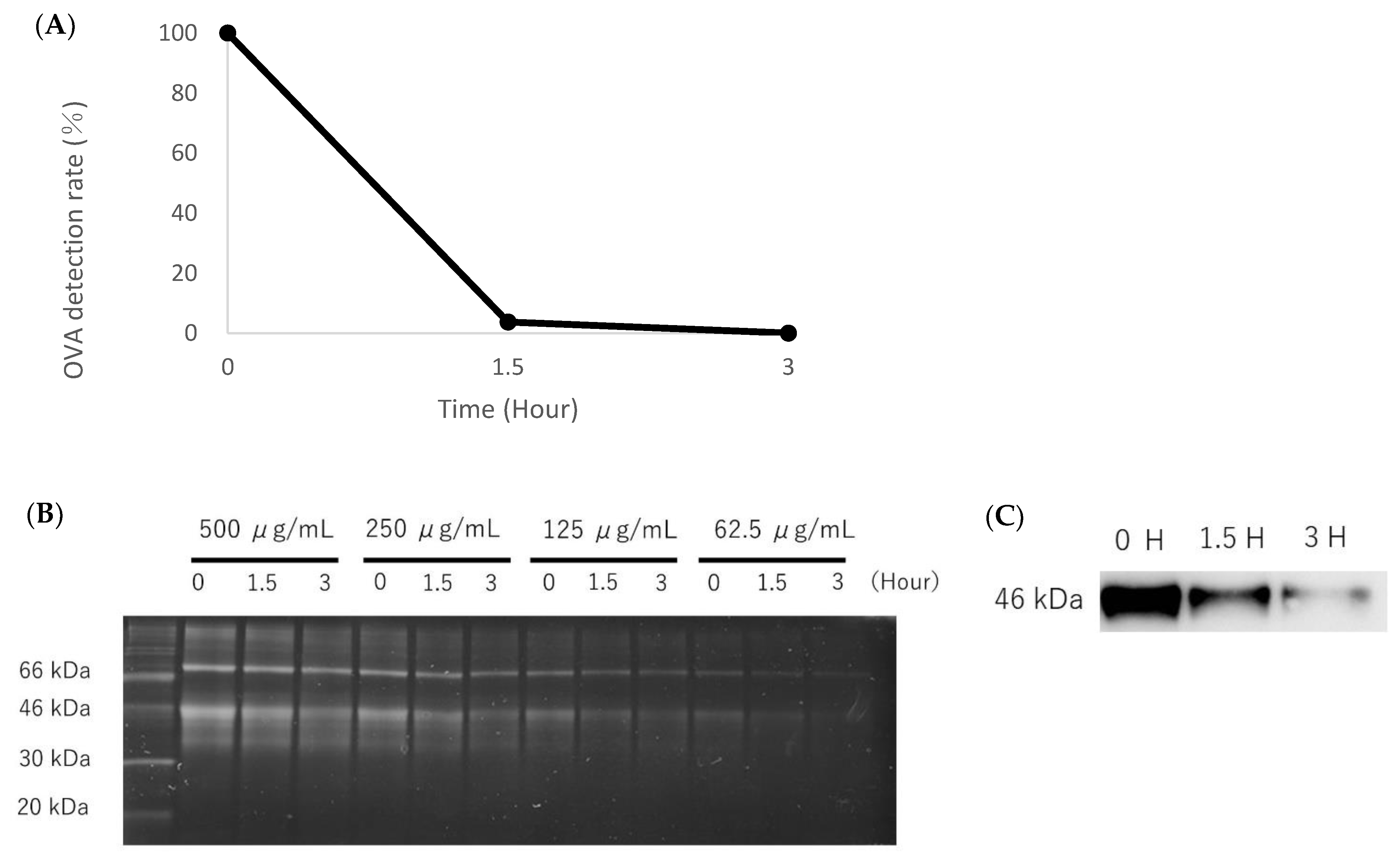
2.5. Analysis of Decomposition of OVA Photocatalysis
2.6. SDS-PAGE and Western Blotting
2.7. Animals following Intratracheal Instillation
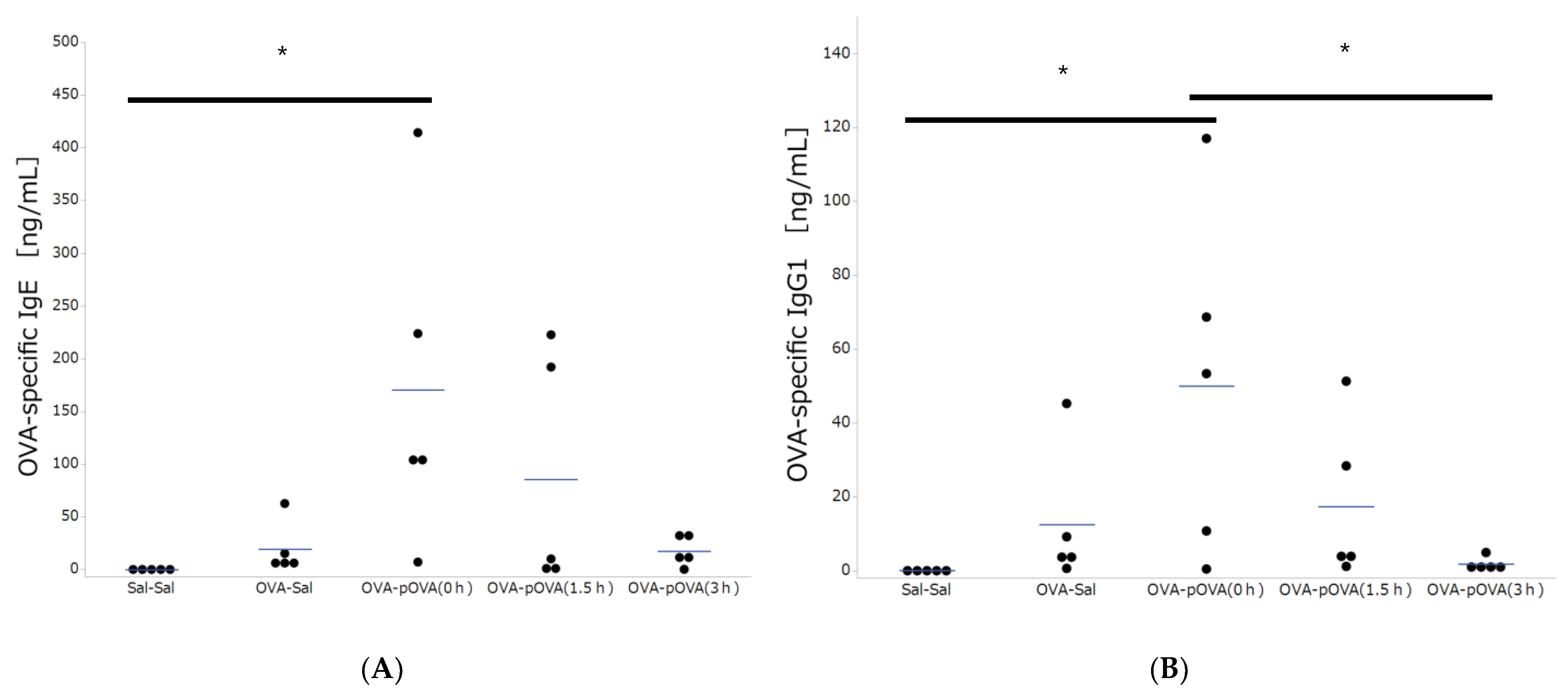
2.8. Analysis of Serum IgE and IgG1
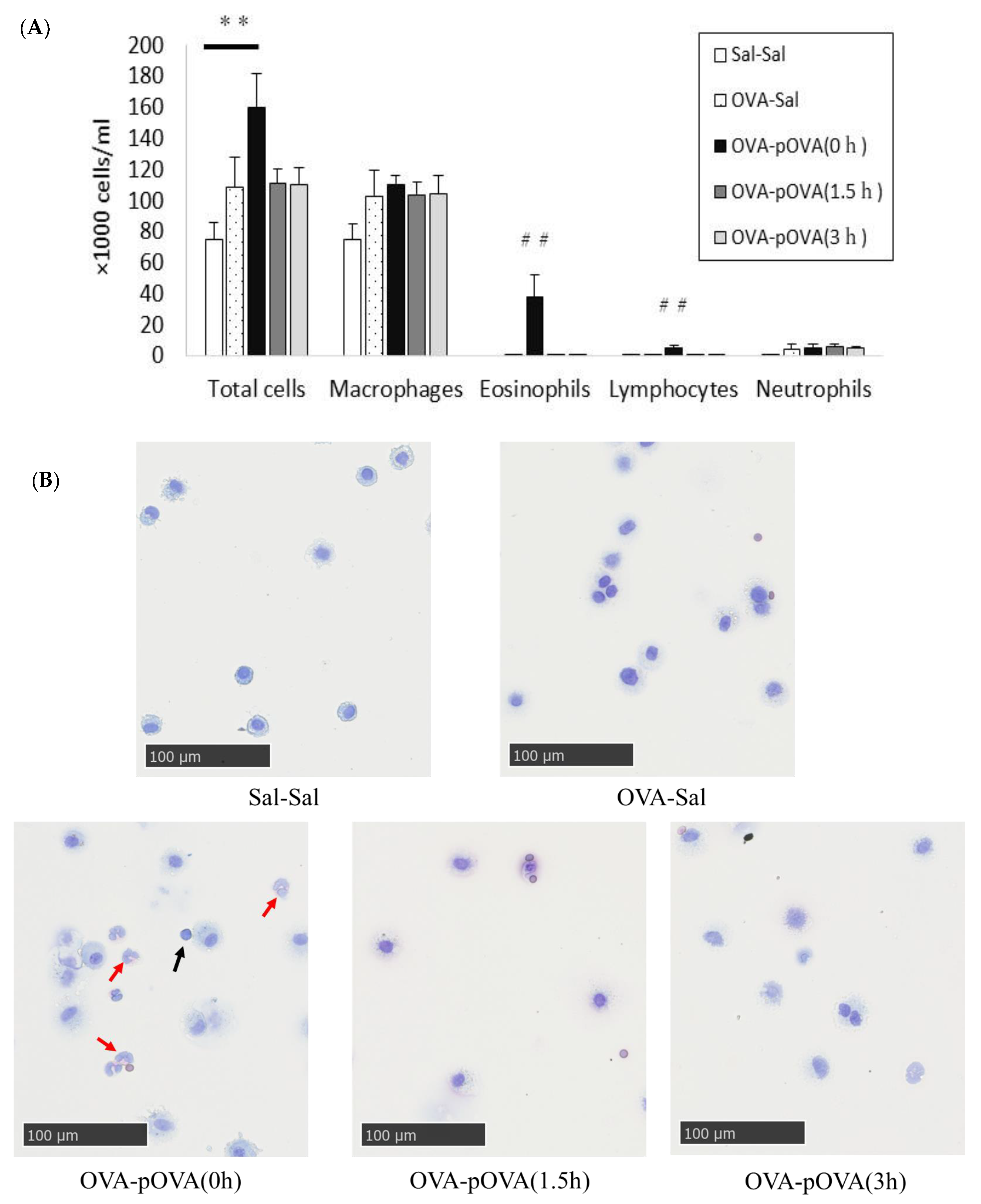
2.9. Histopathology
2.10. Statistical Analysis
3. Results
3.1. Analysis of the Decomposition Ability of OVA
3.2. OVA-Specific IgE and IgG Production
3.3. Cell Analysis in BALF
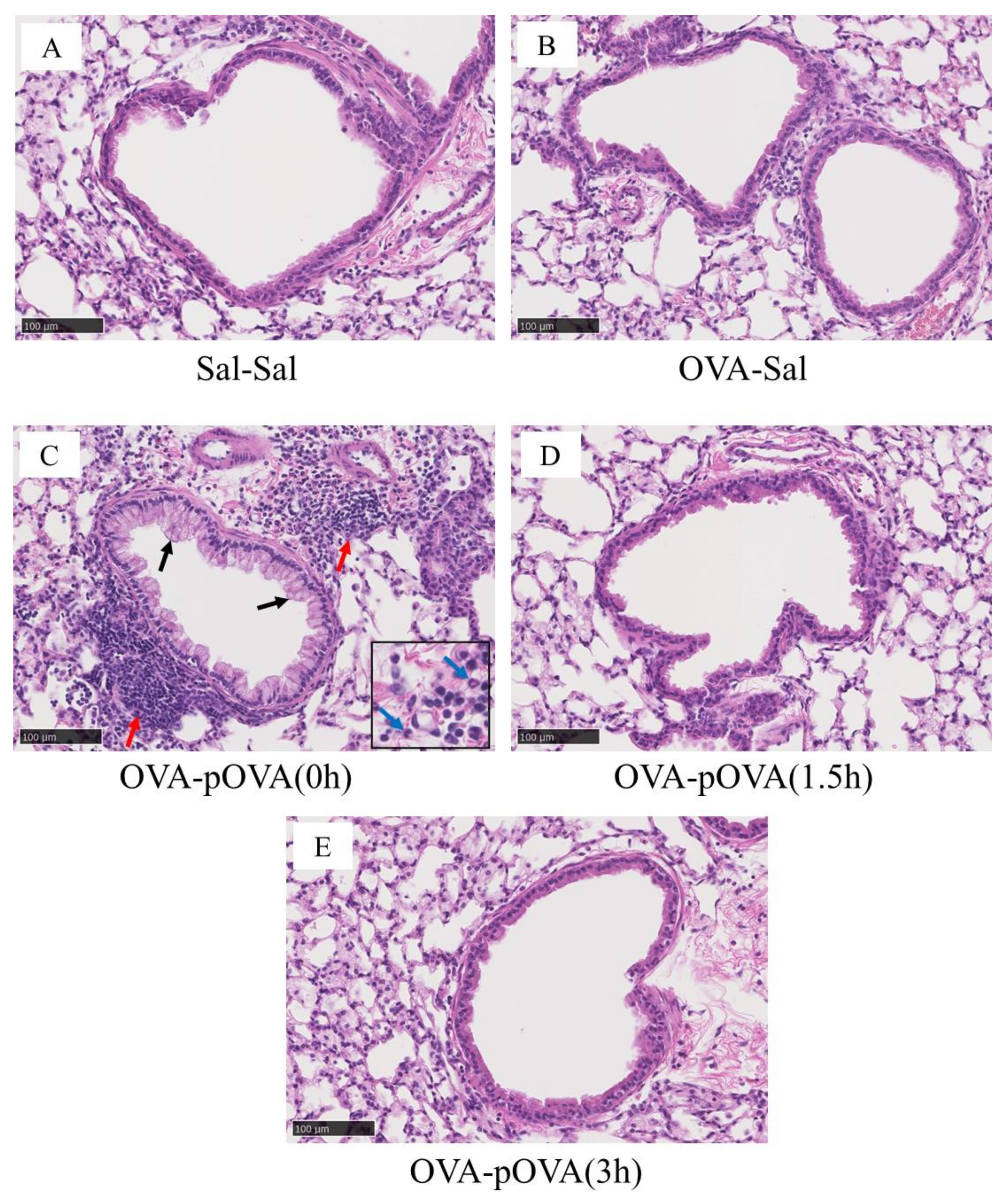
3.4. Pathological Features in the Mouse Lung
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- An, J.; Mikhaylov, A. Russian energy projects in South Africa. J. Energy S. Afr. 2020, 31, 58–64. [Google Scholar] [CrossRef]
- Yakovlev, I.A.; Kabir, L.S.; Nikulina, S.I. Changes in Climate Policies and Financial Strategies of Their Implementation in the EU and Russia. Financ. J. 2021, 13, 11–28. [Google Scholar] [CrossRef]
- Kranina, E.I. China on the Way to Achieving Carbon Neutrality. Financ. J. 2021, 13, 51–61. [Google Scholar] [CrossRef]
- Morimoto, Y.; Khatun, H.; Yee, K.S.W.; Susanto, A.D.; Myong, J.-P.; Tanchuco, J.J.; Sheu, C.-C.; Chau, N.Q.; Azuma, A. Efforts to control air pollution in the Asia-Pacific region. Respirology 2020, 25, 472–474. [Google Scholar] [CrossRef] [PubMed]
- Rohr, A.; McDonald, J. Health effects of carbon-containing particulate matter: Focus on sources and recent research program results. Crit. Rev. Toxicol. 2016, 46, 97–137. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization; Occupational and Environmental Health Team. WHO Air Quality Guidelines for Particulate Matter, Ozone, Nitrogen Dioxide and Sulfur Dioxide: Global Update 2005: Summary of Risk Assessment. Available online: https://apps.who.int/iris/handle/10665/69477 (accessed on 16 February 2021).
- Mukherjee, A.; Agrawal, S. A Global Perspective of Fine Particulate Matter Pollution and Its Health Effects. Rev. Environ. Contam. Toxicol. 2017, 244, 5–51. [Google Scholar] [CrossRef]
- Guarnieri, M.; Balmes, J.R. Outdoor air pollution and asthma. Lancet 2014, 383, 1581–1592. [Google Scholar] [CrossRef] [Green Version]
- Potaczek, D.P.; Harb, H.; Michel, S.; Alhamwe, B.A.; Renz, H.; Tost, J. Epigenetics and allergy: From basic mechanisms to clinical applications. Epigenomics 2017, 9, 539–571. [Google Scholar] [CrossRef]
- Alhamwe, B.A.; Alhamdan, F.; Ruhl, A.; Potaczek, D.P.; Renz, H. The role of epigenetics in allergy and asthma development. Curr. Opin. Allergy Clin. Immunol. 2020, 20, 48–55. [Google Scholar] [CrossRef]
- Cardell, L.-O.; Olsson, P.; Andersson, M.; Welin, K.-O.; Svensson, J.; Tennvall, G.R.; Hellgren, J. TOTALL: High cost of allergic rhinitis—a national Swedish population-based questionnaire study. NPJ Prim. Care Respir. Med. 2016, 26, 15082. [Google Scholar] [CrossRef]
- Fujishima, A.; Honda, K. Electrochemical Photolysis of Water at a Semiconductor Electrode. Nature 1972, 238, 37–38. [Google Scholar] [CrossRef]
- Sapiña, M.; Jimenez-Relinque, E.; Nevshupa, R.; Roman, E.; Castellote, M. Degradation of pollen on nanofunctionalized photocatalytic materials. J. Chem. Technol. Biotechnol. 2017, 92, 210–216. [Google Scholar] [CrossRef]
- Toma, F.-L.; Sokolov, D.; Bertrand, G.; Klein, D.; Coddet, C.; Meunier, C. Comparison of the Photocatalytic Behavior of TiO2 Coatings Elaborated by Different Thermal Spraying Processes. J. Therm. Spray Technol. 2006, 15, 576–581. [Google Scholar] [CrossRef]
- Lee, C.; Choi, H.; Lee, C.; Kim, H. Photocatalytic properties of nano-structured TiO2 plasma sprayed coating. Surf. Coat. Technol. 2003, 173, 192–200. [Google Scholar] [CrossRef]
- Roata, I.C.; Croitoru, C.; Pascu, A.; Stanciu, E.M. Photocatalytic coatings via thermal spraying: A mini-review. AIMS Mater. Sci. 2019, 6, 335–353. [Google Scholar] [CrossRef]
- Jimenez-Relinque, E.; Sapiña, M.; Nevshupa, R.; Roman, E.; Castellote, M. Photocatalytic decomposition of pollen allergenic extracts of Cupressus arizonica and Platanus hybrida. Chem. Eng. J. 2016, 286, 560–570. [Google Scholar] [CrossRef]
- Muszkat, L.; Feigelson, L.; Bir, L.; Muszkat, K. Titanium dioxide photocatalyzed oxidation of proteins in biocontaminated waters. J. Photochem. Photobiol. B Biol. 2001, 60, 32–36. [Google Scholar] [CrossRef]
- Lin, L.; Chai, Y.; Zhao, B.; Wei, W.; He, D.; He, B.; Tang, Q. Photocatalytic oxidation for degradation of VOCs. Open J. Inorg. Chem. 2013, 03, 14–25. [Google Scholar] [CrossRef] [Green Version]
- Dona, D.W.; Suphioglu, C. Egg Allergy: Diagnosis and Immunotherapy. Int. J. Mol. Sci. 2020, 21, 5010. [Google Scholar] [CrossRef]
- Siegel, N.H.A.-H.P.D. Ovalbumin Aeroallergen Exposure-response in Brown Norway Rats. Inhal. Toxicol. 2000, 12, 245–261. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Li, K.; Xu, J. Indoor PM2.5 from coal combustion aggravates ovalbumin-induced asthma-like airway inflammation in BALB/c mice. Am. J. Physiol. Cell. Mol. Physiol. 2019, 317, L29–L38. [Google Scholar] [CrossRef] [PubMed]
- Mlčková, P.; Poláček, M.; Čechová, D.; Marušková, L.; Staňková, I.; Chalupná, P.; Novotná, O.; Julák, J.; Prokešová, L. Intratracheal and intranasal immunization with ovalbumin conjugated with Bacillus firmus as a carrier in mice. Folia Microbiol. 2005, 50, 247–253. [Google Scholar] [CrossRef]
- Jung, K.-H.; Shin, D.; Kim, S.; Min, D.; Kim, W.; Kim, J.; Lee, G.; Bae, H. Intratracheal Ovalbumin Administration Induces Colitis Through the IFN-γ Pathway in Mice. Front. Immunol. 2019, 10, 530. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, R.; Takanezawa, Y.; Sone, Y.; Uraguchi, S.; Sakabe, K.; Kiyono, M. Immunotoxic Effect of Low-Dose Methylmercury Is Negligible in Mouse Models of Ovalbumin or Mite-Induced Th2 Allergy. Biol. Pharm. Bull. 2016, 39, 1353–1358. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hall, S.; Agrawal, D.K. Key mediators in the immunopathogenesis of allergic asthma. Int. Immunopharmacol. 2014, 23, 316–329. [Google Scholar] [CrossRef] [Green Version]
- Walsh, C.J.; Zaihra, T.; Benedetti, A.; Fugère, C.; Olivenstein, R.; Lemière, C.; Hamid, Q.; Martin, J.G. Exacerbation risk in severe asthma is stratified by inflammatory phenotype using longitudinal measures of sputum eosinophils. Clin. Exp. Allergy 2016, 46, 1291–1302. [Google Scholar] [CrossRef] [PubMed]
- Schleich, F.; Brusselle, G.; Louis, R.; Vandenplas, O.; Michils, A.; Pilette, C.; Peche, R.; Manise, M.; Joos, G. Heterogeneity of phenotypes in severe asthmatics. The Belgian Severe Asthma Registry (BSAR). Respir. Med. 2014, 108, 1723–1732. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kay, A.B. Allergy and Allergic Diseases. N. Engl. J. Med. 2001, 344, 30–37. [Google Scholar] [CrossRef] [PubMed]
- Williams, J.; Tjota, M.; Sperling, A. The Contribution of Allergen-Specific IgG to the Development of Th2-Mediated Airway Inflammation. J. Allergy 2012, 2012, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nomura, D.; Furuta, H.; Higuchi, T. Characteristics of the High-Activated Materials Coatings Produced by the HOVF Spraying with Oxygen and Air Mixing Equipment. Available online: https://www.kfjc.co.jp/pdf/14/tsukuru_14_32_36.pdf (accessed on 14 July 2021).
- Hoffmann, M.R.; Martin, S.T.; Choi, W.; Bahnemann, D.W. Environmental Applications of Semiconductor Photocatalysis. Chem. Rev. 1995, 95, 69–96. [Google Scholar] [CrossRef]
- Uchida, K.; Kato, Y.; Kawakishi, S. A novel mechanism for oxidative cleavage of prolyl peptides induced by the hydroxyl radical. Biochem. Biophys. Res. Commun. 1990, 169, 265–271. [Google Scholar] [CrossRef]
- Nishikawa, N.; Kaneco, S.; Katsumata, H.; Suzuki, T.; Ohta, K.; Yamazaki, E.; Masuyama, K.; Hashimoto, T.; Kamiya, K. Photocatalytic degradation of allergens in water with titanium dioxide. Fresenius Environ. Bull. 2007, 16, 310–314. [Google Scholar]

| Physicochemical Properties | Value |
|---|---|
| Primary size | 21 nm |
| Specific surface area (BET) | 50 ± 15 m2/g |
| Crystal structure (Anatase:Rutile) | 4:1 |
| Thickness of TiO2 layer sprayed on the aluminum fiber | 2 μm |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tomonaga, T.; Izumi, H.; Nishida, C.; Kato, K.; Yatera, K.; Kuroda, E.; Morimoto, Y. Suppression of Airway Allergic Reactions by a Photocatalytic Filter Using Mouse Model. Toxics 2022, 10, 40. https://doi.org/10.3390/toxics10010040
Tomonaga T, Izumi H, Nishida C, Kato K, Yatera K, Kuroda E, Morimoto Y. Suppression of Airway Allergic Reactions by a Photocatalytic Filter Using Mouse Model. Toxics. 2022; 10(1):40. https://doi.org/10.3390/toxics10010040
Chicago/Turabian StyleTomonaga, Taisuke, Hiroto Izumi, Chinatsu Nishida, Kaori Kato, Kazuhiro Yatera, Etsushi Kuroda, and Yasuo Morimoto. 2022. "Suppression of Airway Allergic Reactions by a Photocatalytic Filter Using Mouse Model" Toxics 10, no. 1: 40. https://doi.org/10.3390/toxics10010040
APA StyleTomonaga, T., Izumi, H., Nishida, C., Kato, K., Yatera, K., Kuroda, E., & Morimoto, Y. (2022). Suppression of Airway Allergic Reactions by a Photocatalytic Filter Using Mouse Model. Toxics, 10(1), 40. https://doi.org/10.3390/toxics10010040








