Fabrication and Evaluation of Quercetin Nanoemulsion: A Delivery System with Improved Bioavailability and Therapeutic Efficacy in Diabetes Mellitus
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials Employed
2.2. Solubility Assessment of Que
2.3. Screening of Surfactants
2.4. Screening of Cosurfactants
2.5. Effect of Surfactant and Cosurfactant (Smix) Mass Ratio
2.6. Preparation of Nanoemulsion
2.7. Optimization of Nanoemulsion by Box–Behnken Design (BBD)
2.8. Determination of Droplet Size, PDI, and Zeta Potential
2.9. Determination of Entrapment Efficiency (% EE)
2.10. Effect of Storage Temperature on Droplet Size and PDI
2.11. Morphological Characterization by Transmission Electron Microscope (TEM)
2.12. Animals
2.13. In Vivo Pharmacokinetics Study
2.14. In Vivo Antidiabetic Study
2.14.1. Induction of Diabetes and Animal Grouping
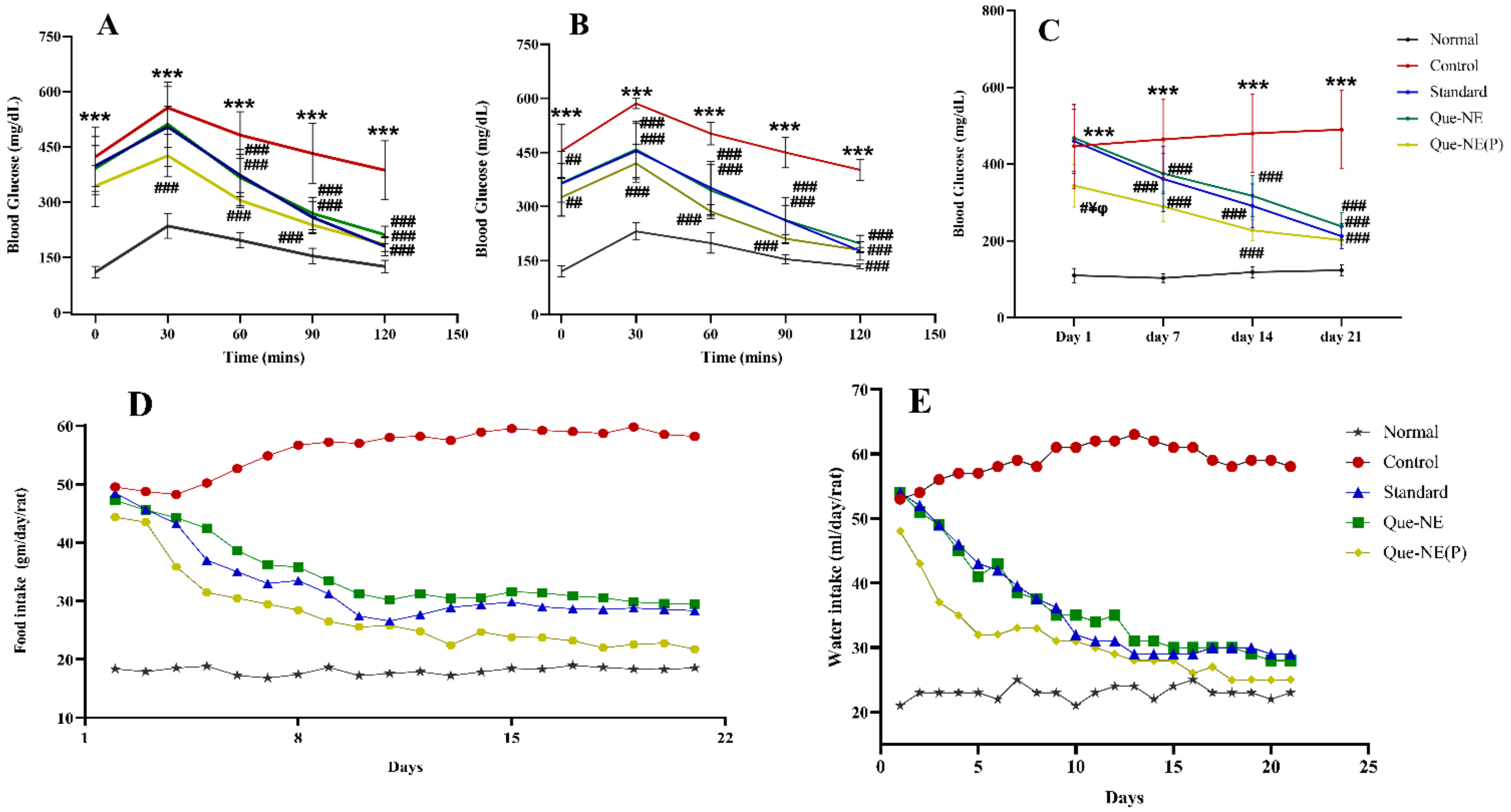
2.14.2. Measurement of Blood Glucose Level (BGL), Food Intake, and Water Consumption
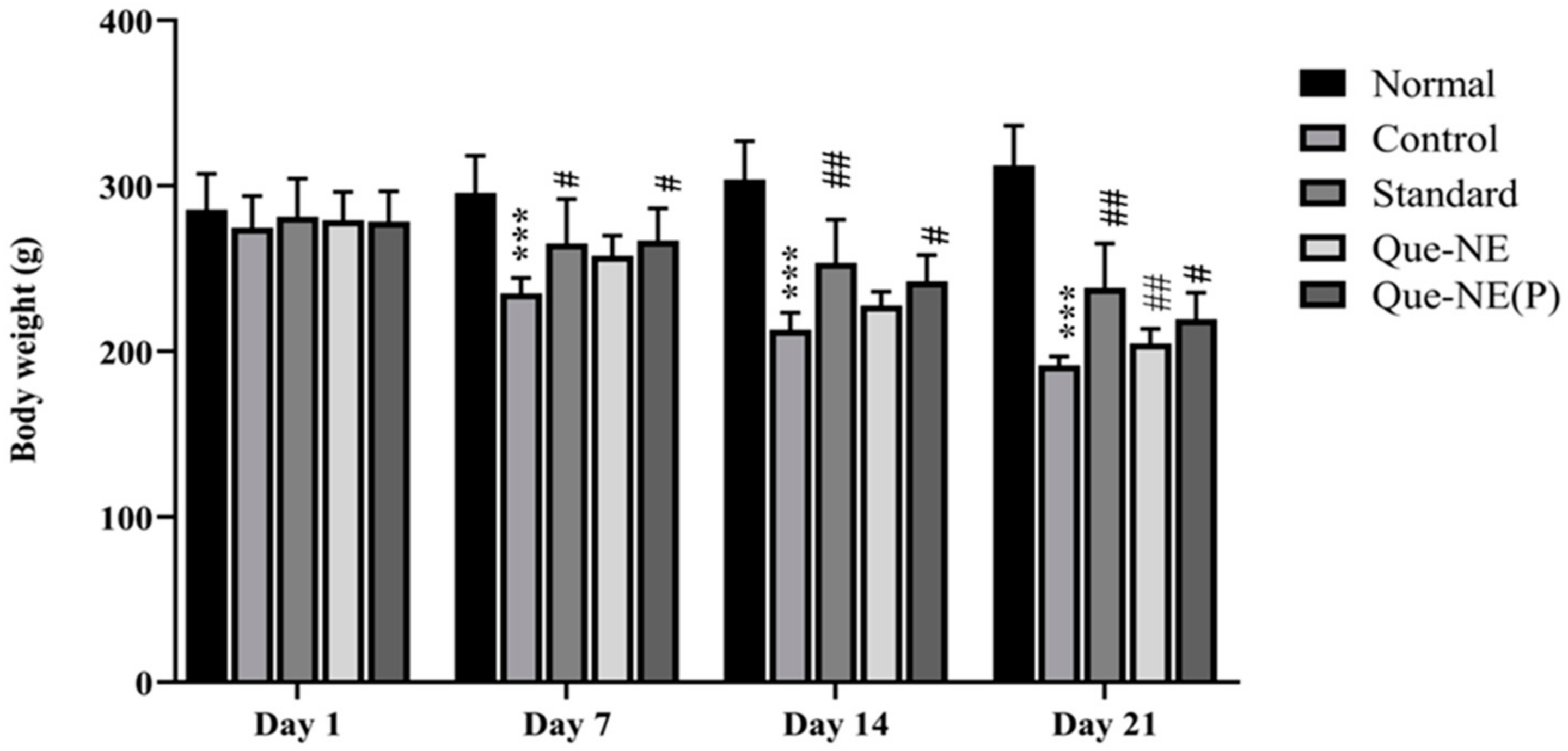
2.14.3. Measurement of Bodyweight (BW)
2.14.4. Oral Glucose Tolerance Test (OGTT)
2.14.5. Histopathological Analysis of Pancreas and Liver Tissues
3. Results and Discussion
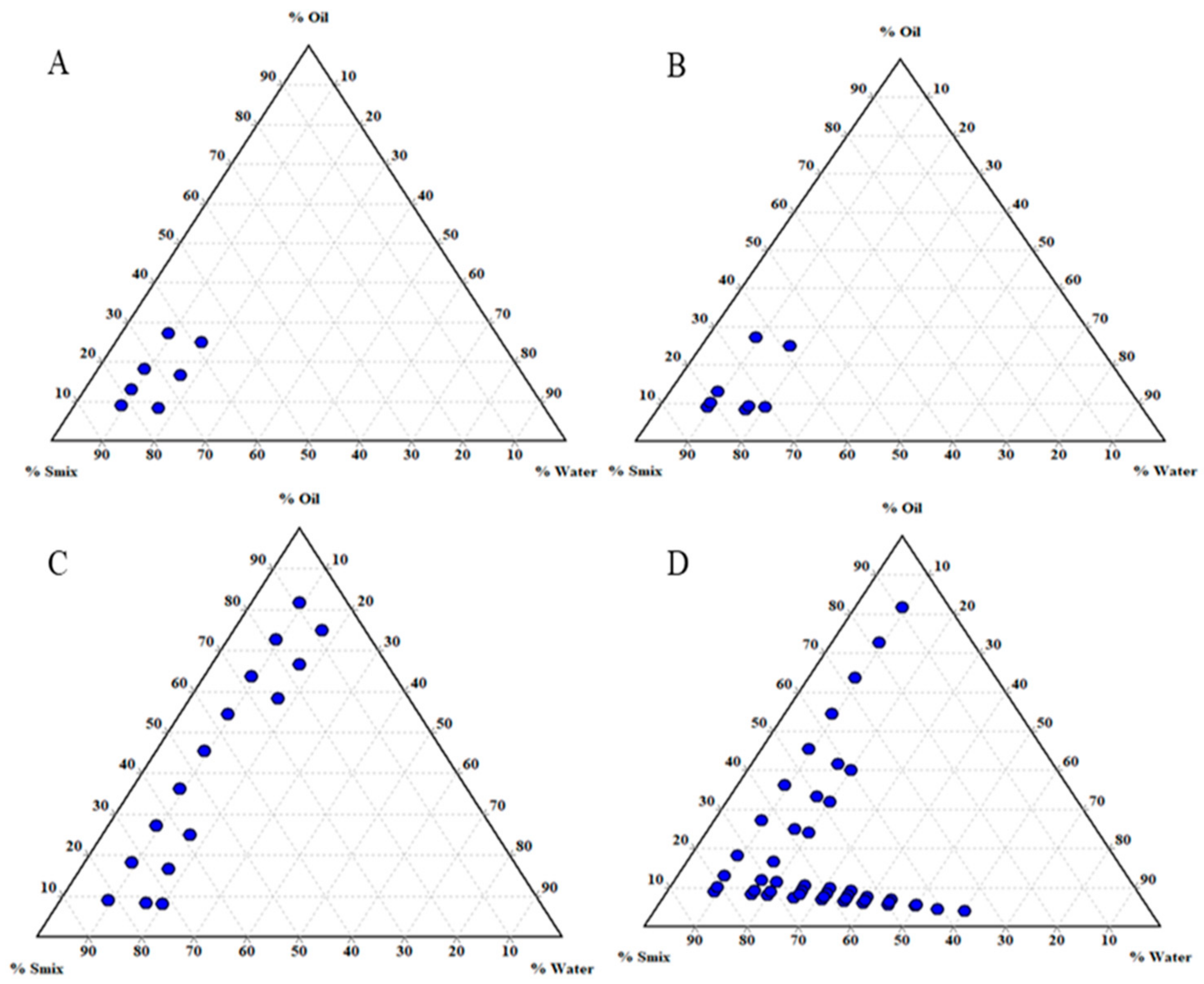
3.1. Screening of Nanoemulsion Components
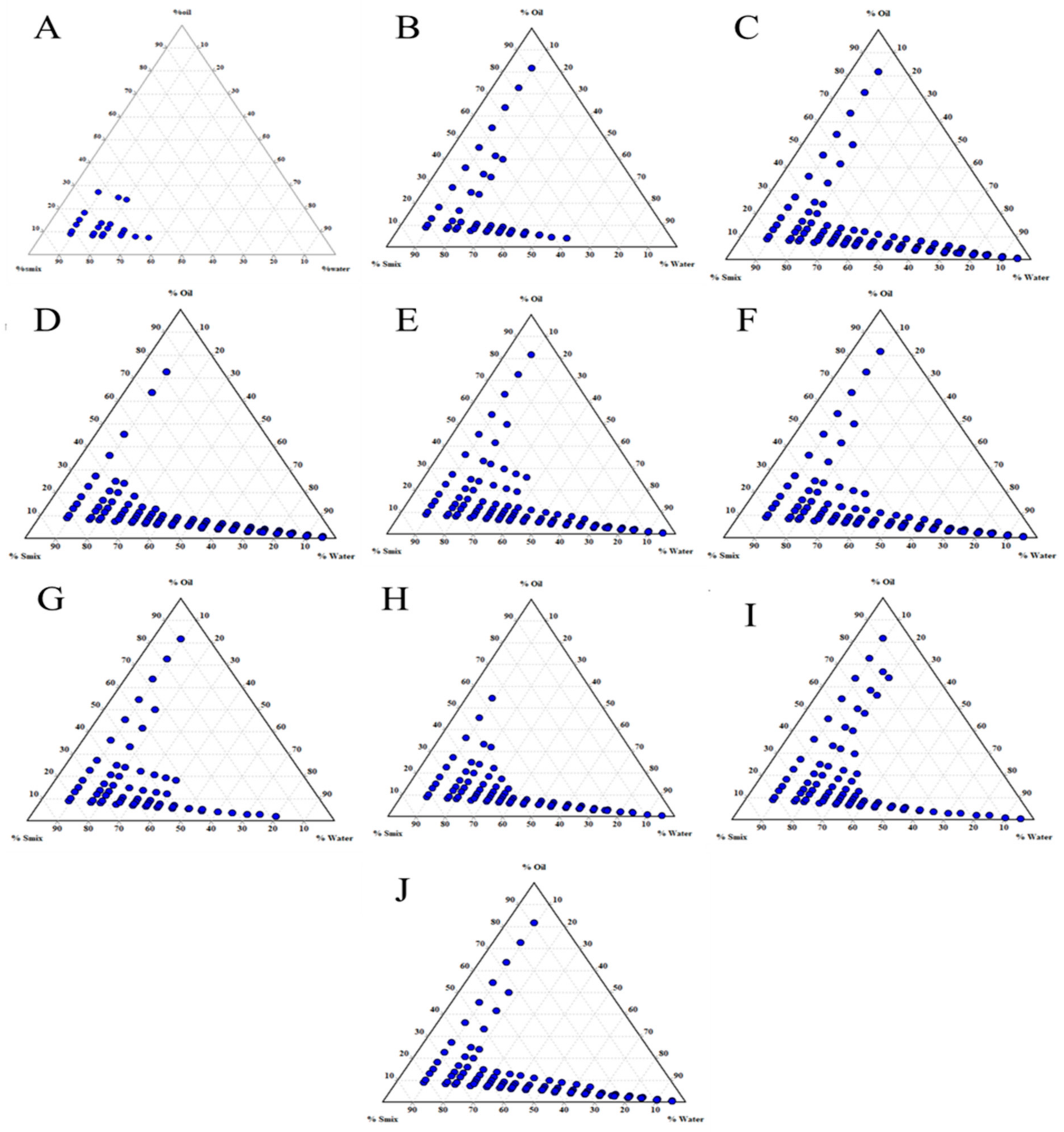
3.2. Effect of Smix Mass Ratio
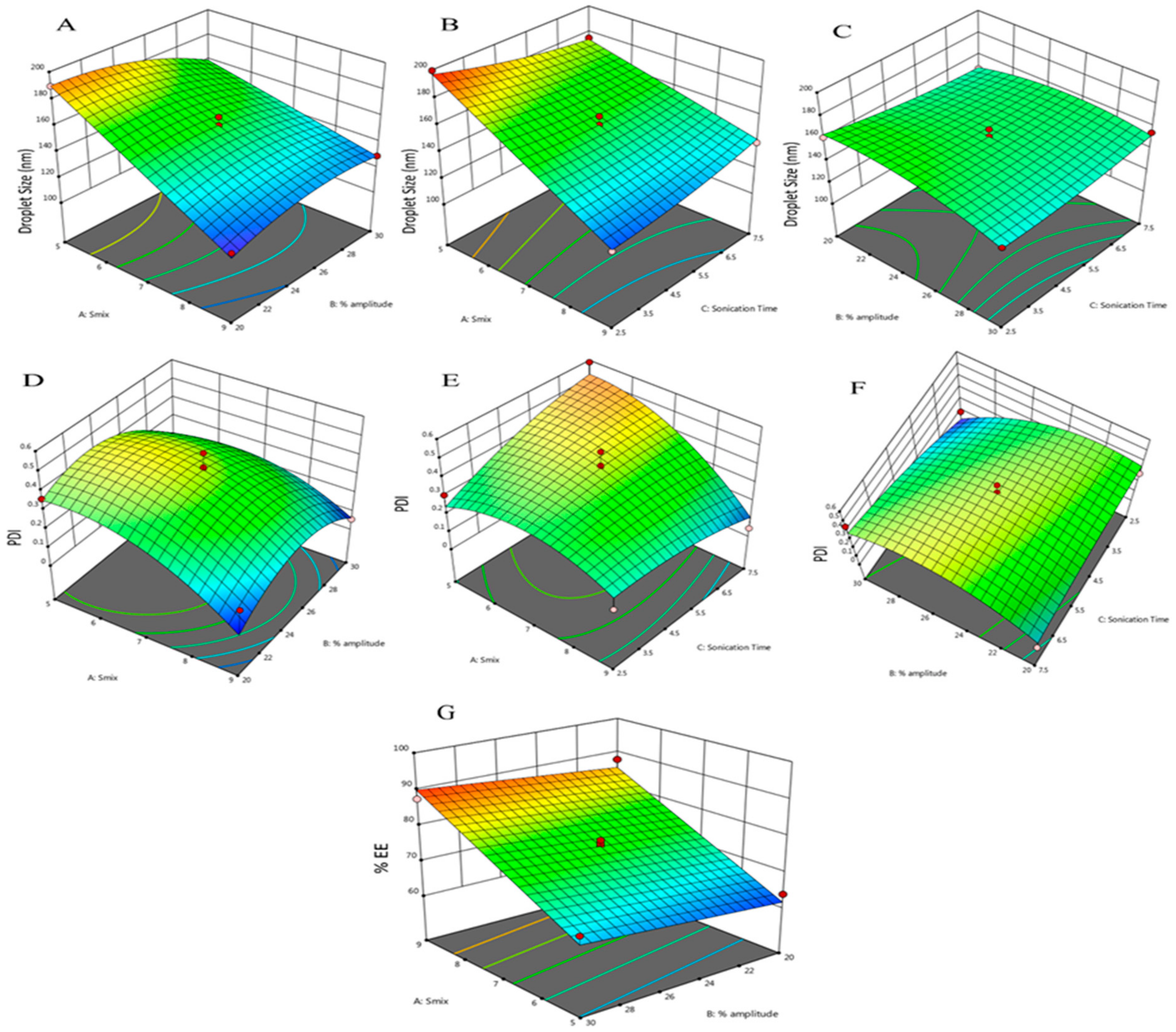
3.3. Optimization of Nanoemulsion by BBD
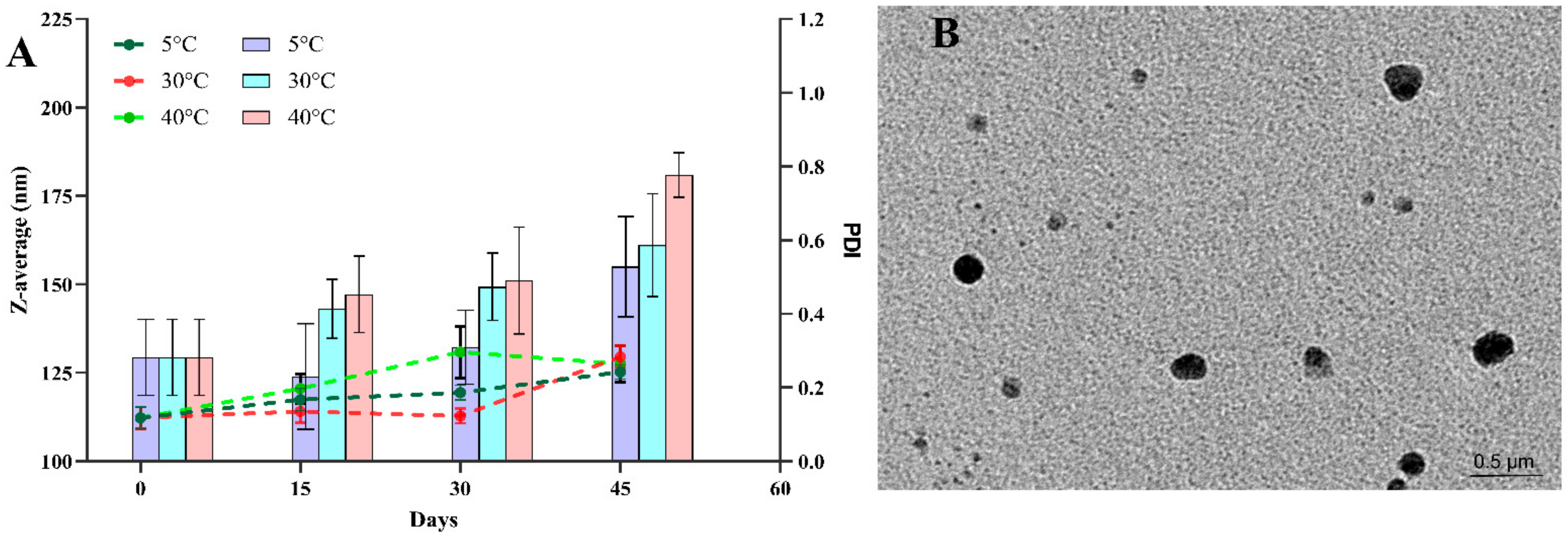
3.4. Effect of Storage Conditions on Droplet Size and %EE
3.5. Morphological Characterization by Transmission Electron Microscopy (TEM)
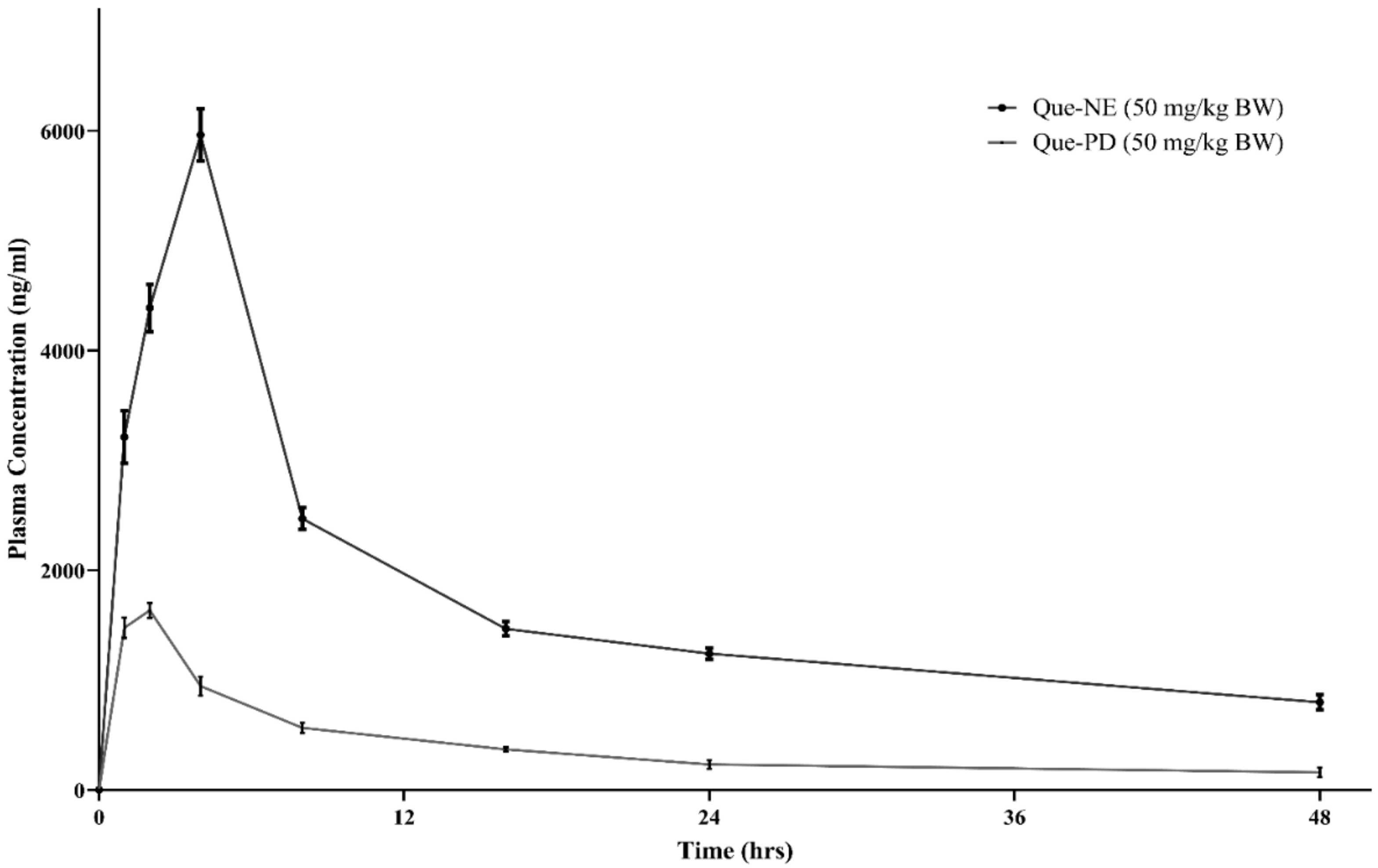
3.6. In Vivo Pharmacokinetic Studies
3.7. Effect of Que-NE and Que-NE (P) on Blood Glucose Level (BGL), Food Intake, and Water Consumption
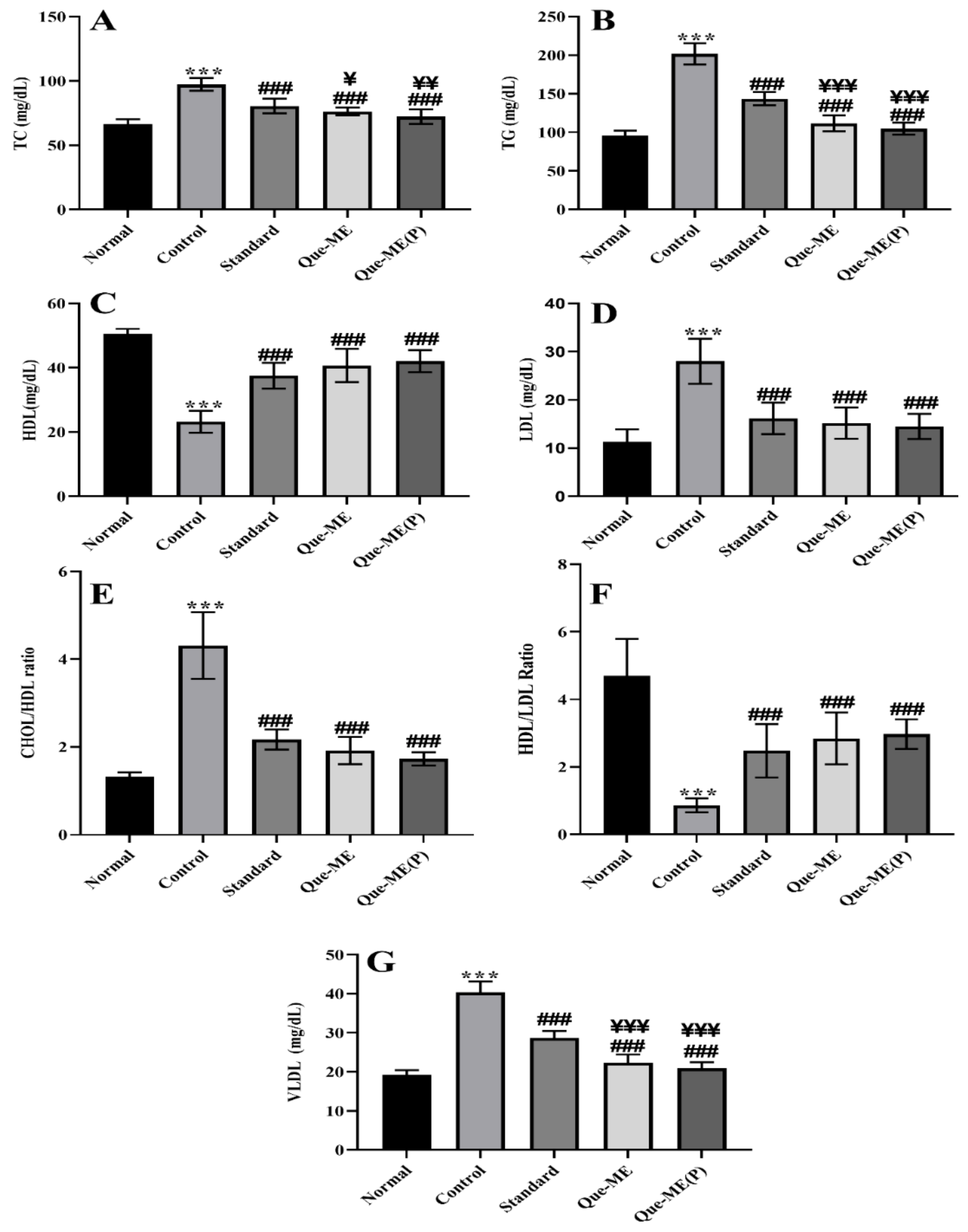
3.8. Effect of Que-NE and Que-NE (P) Treatment on Serum Lipid Level
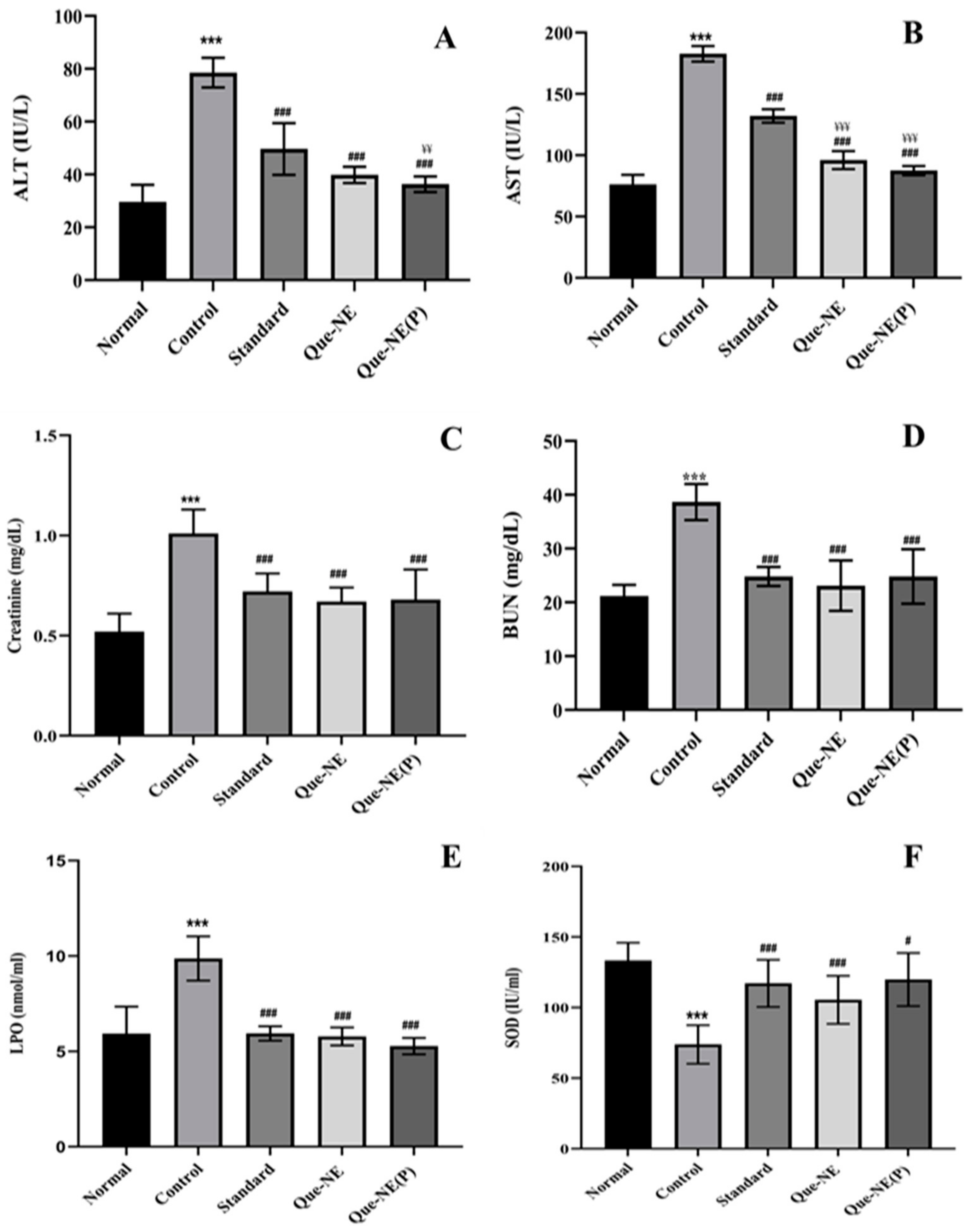
3.9. Effect of Que-NE and Que-NE (P) on Tissue Injury and Oxidative Stress Markers
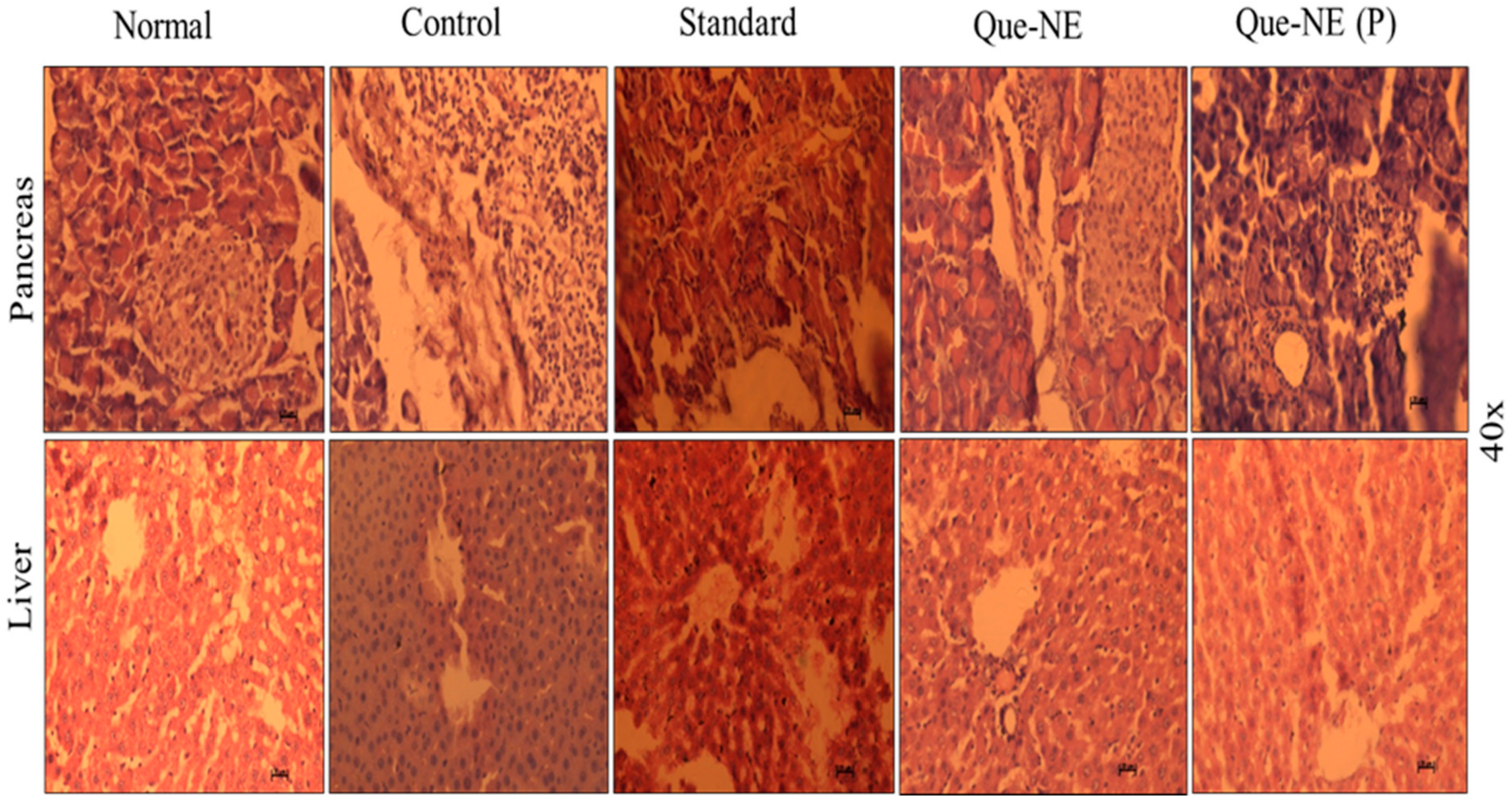
3.10. Histopathological Analysis of Liver and Pancreatic Tissue
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Davies, M.J.; Forni, L.G.; Willson, R.L. Vitamin E analogue Trolox C. E.s.r. and pulse-radiolysis studies of free-radical reactions. Biochem. J. 1988, 255, 513–522. [Google Scholar]
- Shi, G.J.; Li, Y.; Cao, Q.H.; Wu, H.X.; Tang, X.Y.; Gao, X.H.; Yu, J.Q.; Chen, Z.; Yang, Y. In vitro and in vivo evidence that quercetin protects against diabetes and its complications: A systematic review of the literature. Biomed. Pharmacother. 2019, 109, 1085–1099. [Google Scholar] [CrossRef]
- Riva, A.; Ronchi, M.; Petrangolini, G.; Bosisio, S.; Allegrini, P. Improved Oral Absorption of Quercetin from Quercetin Phytosome®, a New Delivery System Based on Food Grade Lecithin. Eur. J. Drug Metab. Pharmacokinet. 2019, 44, 169–177. [Google Scholar] [CrossRef]
- Feeney, O.M.; Crum, M.F.; McEvoy, C.L.; Trevaskis, N.L.; Williams, H.D.; Pouton, C.W.; Charman, W.N.; Bergström, C.A.S.; Porter, C.J.H. 50 years of oral lipid-based formulations: Provenance, progress and future perspectives. Adv. Drug Deliv. Rev. 2016, 101, 167–194. [Google Scholar] [CrossRef]
- Kumar, M.; Bishnoi, R.S.; Shukla, A.K.; Jain, C.P. Techniques for formulation of nanoemulsion drug delivery system: A review. Prev. Nutr. Food Sci. 2019, 24, 225–234. [Google Scholar] [CrossRef]
- Kabri, T.; Arab-tehrany, E.; Belhaj, N.; Linder, M. Physico-chemical characterization of nano- emulsions in cosmetic matrix enriched on omega-3. J. Nanobiotechnol. 2011, 9, 41. [Google Scholar] [CrossRef]
- Komaiko, J.S.; Mcclements, D.J. Formation of Food-Grade Nanoemulsions Using Low-Energy Preparation Methods: A Review of Available Methods. Compr. Rev. Food Sci. Food Saf. 2016, 15, 331–352. [Google Scholar] [CrossRef]
- Aswathanarayan, J.B.; Vittal, R.R. Nanoemulsions and Their Potential Applications in Food Industry. Front. Sustain. Food Syst. 2019, 3, 95. [Google Scholar] [CrossRef]
- Matough, F.A.; Budin, S.B.; Hamid, Z.A.; Alwahaibi, N.; Mohamed, J. The role of oxidative stress and antioxidants in diabetic complications. Sultan Qaboos Univ. Med. J. 2012, 12, 556–569. [Google Scholar] [CrossRef]
- Ahmed, S.; Gull, A.; Alam, M.; Aqil, M.; Sultana, Y. Ultrasonically tailored, chemically engineered and “QbD” enabled fabrication of agomelatine nanoemulsion; optimization, characterization, ex-vivo permeation and stability study. Ultrason. Sonochem. 2018, 41, 213–226. [Google Scholar] [CrossRef]
- Qian, C.; McClements, D.J. Formation of nanoemulsions stabilized by model food-grade emulsifiers using high-pressure homogenization: Factors affecting particle size. Food Hydrocoll. 2011, 25, 1000–1008. [Google Scholar] [CrossRef]
- Son, H.Y.; Lee, M.S.; Chang, E.; Kim, S.Y.; Kang, B.; Ko, H.; Kim, I.H.; Zhong, Q.; Jo, Y.H.; Kim, C.T.; et al. Formulation and characterization of quercetin-loaded oil in water nanoemulsion and evaluation of hypocholesterolemic activity in rats. Nutrients 2019, 11, 244. [Google Scholar] [CrossRef]
- Gokhale, J.P.; Mahajan, H.S.; Surana, S.S. Quercetin loaded nanoemulsion-based gel for rheumatoid arthritis: In vivo and in vitro studies. Biomed. Pharmacother. 2019, 112, 108622. [Google Scholar] [CrossRef]
- Lotfi, M.; Kazemi, S.; Ebrahimpour, A.; Shirafkan, F.; Pirzadeh, M.; Hosseini, M.; Moghadamnia, A.A. Protective Effect of Quercetin Nanoemulsion on 5-Fluorouracil-Induced Oral Mucositis in Mice. J. Oncol. 2021, 2021, 5598230. [Google Scholar] [CrossRef]
- Honary, S.; Ebrahimi, P.; Nikbakht, M. Optimization of finasteride nano-emulsion preparation using chemometric approach. Trop. J. Pharm. Res. 2013, 12, 457–460. [Google Scholar] [CrossRef]
- Ni, S.; Hu, C.; Sun, R.; Zhao, G.; Xia, Q. Nanoemulsions-Based Delivery Systems for Encapsulation of Quercetin: Preparation, Characterization, and Cytotoxicity Studies. J. Food Process Eng. 2017, 40, e12374. [Google Scholar] [CrossRef]
- Myers, R.H.; Montgomery, D.C.; Anderson-Cook, C.M. Response Surface Methodology Process and Product Optimization Using Designed Experiments, 3rd ed.; Wiley: Hoboken, NJ, USA, 2009; ISBN 9780470174463. [Google Scholar]
- Pongsumpun, P.; Iwamoto, S.; Siripatrawan, U. Response surface methodology for optimization of cinnamon essential oil nanoemulsion with improved stability and antifungal activity. Ultrason. Sonochem. 2020, 60, 104604. [Google Scholar] [CrossRef]
- Li, Y.; Fabiano-tixier, A.S.; Tomao, V.; Cravotto, G.; Chemat, F. Ultrasonics Sonochemistry Green ultrasound-assisted extraction of carotenoids based on the bio-refinery concept using sunflower oil as an alternative solvent. Ultrason. Sonochem. 2013, 20, 12–18. [Google Scholar] [CrossRef]
- Tran, T.H.; Guo, Y.; Song, D.; Bruno, R.S.; Lu, X. Quercetin-containing self-nanoemulsifying drug delivery system for improving oral bioavailability. J. Pharm. Sci. 2014, 103, 840–852. [Google Scholar] [CrossRef]
- Azeem, A.; Rizwan, M.; Ahmad, F.J.; Iqbal, Z.; Khar, R.K.; Aqil, M.; Talegaonkar, S. Nanoemulsion components screening and selection: A technical note. AAPS PharmSciTech 2009, 10, 69–76. [Google Scholar] [CrossRef]
- Syed, H.K.; Peh, K.K. Identification of phases of various oil, surfactant/co-surfactants and water system by ternary phase diagram. Acta Pol. Pharm.—Drug Res. 2014, 71, 301–309. [Google Scholar]
- Chen, H.; Chang, X.; Weng, T.; Zhao, X.; Gao, Z. A study of microemulsion systems for transdermal delivery of triptolide. J. Control. Release 2004, 98, 427–436. [Google Scholar] [CrossRef]
- Abbas, S.; Bashari, M.; Akhtar, W.; Li, W.W.; Zhang, X. Process optimization of ultrasound-assisted curcumin nanoemulsions stabilized by OSA-modified starch. Ultrason. Sonochem. 2014, 21, 1265–1274. [Google Scholar] [CrossRef]
- Kumar, S.; Lather, V.; Pandita, D. Stability indicating simplified HPLC method for simultaneous analysis of resveratrol and quercetin in nanoparticles and human plasma. Food Chem. 2016, 197, 959–964. [Google Scholar] [CrossRef]
- Ke, Z.; Hou, X.; Jia, X. bin Design and optimization of self-nanoemulsifying drug delivery systems for improved bioavailability of cyclovirobuxine D. Drug Des. Devel. Ther. 2016, 10, 2049–2060. [Google Scholar] [CrossRef]
- Yang, D.K.; Kang, H.S. Anti-diabetic effect of cotreatment with quercetin and resveratrol in streptozotocin-induced diabetic rats. Biomol. Ther. 2018, 26, 130–138. [Google Scholar] [CrossRef]
- Friedewald, W.T.; Levy, R.I.; Fredrickson, D.S. Estimation of the Concentration of Low-Density Lipoprotein Cholesterol in Plasma, Without Use of the Preparative Ultracentrifuge. Clin. Chem. 1972, 18, 499–502. [Google Scholar] [CrossRef]
- Wu, J.; Yan, L.J. Streptozotocin-induced type 1 diabetes in rodents as a model for studying mitochondrial mechanisms of diabetic β cell glucotoxicity. Diabetes Metab. Syndr. Obes. Targets Ther. 2015, 8, 181–188. [Google Scholar] [CrossRef]
- Noeman, S.A.; Hamooda, H.E.; Baalash, A.A. Biochemical study of oxidative stress markers in the liver, kidney and heart of high fat diet induced obesity in rats. Diabetol. Metab. Syndr. 2011, 3, 17. [Google Scholar] [CrossRef]
- Kawakami, K.; Yoshikawa, T.; Hayashi, T.; Nishihara, Y.; Masuda, K. Microemulsion formulation for enhanced absorption of poorly soluble drugs: II. In vivo study. J. Control. Release 2002, 81, 75–82. [Google Scholar] [CrossRef]
- Eaimtrakarn, S.; Rama Prasad, Y.V.; Ohno, T.; Konishi, T.; Yoshikawa, Y.; Shibata, N.; Takada, K. Absorption enhancing effect of Labrasol on the intenstinal absorption of insulin in rats. J. Drug Target. 2002, 10, 255–260. [Google Scholar] [CrossRef]
- Wang-Fischer, Y.; Garyantes, T. Improving the reliability and utility of streptozotocin-induced rat diabetic model. J. Diabetes Res. 2018, 2018, 8054073. [Google Scholar] [CrossRef]
- Elsheikh, M.A.; Elnaggar, Y.S.R.; Gohar, E.Y.; Abdallah, O.Y. Nanoemulsion liquid preconcentrates for raloxifene hydrochloride: Optimization and in vivo appraisal. Int. J. Nanomed. 2012, 7, 3787–3802. [Google Scholar] [CrossRef]
- Thakore, S.; Patel, R.; Patel, M. Nanoemulsion or Microemulsion?—Understanding the Differences and Similarities. Pharma Rev. 2014, 136–142. [Google Scholar]
- Mazonde, P.; Khamanga, S.M.M.; Walker, R.B. Design, optimization, manufacture and characterization of Efavirenz-loaded flaxseed oil nanoemulsions. Pharmaceutics 2020, 12, 797. [Google Scholar] [CrossRef]
- Kale, S.N.; Deore, S.L. Emulsion micro emulsion and nano emulsion: A review. Syst. Rev. Pharm. 2016, 8, 39–47. [Google Scholar] [CrossRef]
- Carpenter, J.; Saharan, V.K. Ultrasonic assisted formation and stability of mustard oil in water nanoemulsion: Effect of process parameters and their optimization. Ultrason. Sonochem. 2017, 35, 422–430. [Google Scholar] [CrossRef]
- Taylor, P.; Homayoonfal, M.; Khodaiyan, F.; Mousavi, S.M.; Homayoonfal, M.; Khodaiyan, F.; Mousavi, S.M. Walnut Oil Nanoemulsion: Optimization of the Emulsion Capacity, Cloudiness, Density, and Surface Tension. J. Dispers. Sci. Technol. 2014, 35, 725–733. [Google Scholar] [CrossRef]
- D’Souza, S. A Review of In Vitro Drug Release Test Methods for Nano-Sized Dosage Forms. Adv. Pharm. 2014, 2014, 304757. [Google Scholar] [CrossRef]
- Hou, J.; Han, M.; Wang, J. Manipulation of surface charges of oil droplets and carbonate rocks to improve oil recovery. Sci. Rep. 2021, 11, 14518. [Google Scholar] [CrossRef]
- Shah, B.M.; Misra, M.; Shishoo, C.J.; Padh, H. Nose to brain microemulsion-based drug delivery system of rivastigmine: Formulation and ex-vivo characterization. Drug Deliv. 2015, 22, 918–930. [Google Scholar] [CrossRef]
- Lv, L.; Liu, C.; Li, Z.; Song, F.; Li, G.; Huang, X. Pharmacokinetics of Quercetin-Loaded Methoxy Poly(ethylene glycol)-b-poly(L-lactic acid) Micelle after Oral Administration in Rats. Biomed Res. Int. 2017, 2017, 1750895. [Google Scholar] [CrossRef]
- Li, H.L.; Zhao, X.B.; Ma, Y.K.; Zhai, G.X.; Li, L.B.; Lou, H.X. Enhancement of gastrointestinal absorption of quercetin by solid lipid nanoparticles. J. Control. Release 2009, 133, 238–244. [Google Scholar] [CrossRef]
- Hussain, N.; Jaitley, V.; Florence, A.T. Recent advances in the understanding of uptake of microparticulates across the gastrointestinal lymphatics. Adv. Drug Deliv. Rev. 2001, 50, 107–142. [Google Scholar] [CrossRef]
- Venkatesan, N.; Uchino, K.; Amagase, K.; Ito, Y.; Shibata, N.; Takada, K. Gastro-intestinal patch system for the delivery of erythropoietin. J. Control. Release 2006, 111, 19–26. [Google Scholar] [CrossRef]
- Lim, S.J.; Lee, M.K.; Kim, C.K. Altered chemical and biological activities of all-trans retinoic acid incorporated in solid lipid nanoparticle powders. J. Control. Release 2004, 100, 53–61. [Google Scholar] [CrossRef]
- Salehi, B.; Machin, L.; Monzote, L.; Sharifi-Rad, J.; Ezzat, S.M.; Salem, M.A.; Merghany, R.M.; El Mahdy, N.M.; Klllç, C.S.; Sytar, O.; et al. Therapeutic Potential of Quercetin: New Insights and Perspectives for Human Health. ACS Omega 2020, 5, 11849–11872. [Google Scholar] [CrossRef]
- Yim, S.; Malhotra, A.; Veves, A. Antioxidants and CVD in diabetes: Where do we stand now? Curr. Diab. Rep. 2007, 7, 8–13. [Google Scholar] [CrossRef][Green Version]
- Ragavan, G.; Muralidaran, Y.; Sridharan, B.; Nachiappa Ganesh, R.; Viswanathan, P. Evaluation of garlic oil in nano-emulsified form: Optimization and its efficacy in high-fat diet induced dyslipidemia in Wistar rats. Food Chem. Toxicol. 2017, 105, 203–213. [Google Scholar] [CrossRef]
- Sangeetha, M.K.; Eazhisai Vallabi, D.; Sali, V.K.; Thanka, J.; Vasanthi, H.R. Sub-acute toxicity profile of a modified resveratrol supplement. Food Chem. Toxicol. 2013, 59, 492–500. [Google Scholar] [CrossRef]
- Chen, P.; Chen, J.; Zheng, Q.; Chen, W.; Wang, Y.; Xu, X. Pioglitazone, extract of compound Danshen dripping pill, and quercetin ameliorate diabetic nephropathy in diabetic rats. J. Endocrinol. Investig. 2013, 36, 422–427. [Google Scholar] [CrossRef]

| Run | Independent Variables | Response Values | ||||
|---|---|---|---|---|---|---|
| Smix * (%) | % Amplitude | Sonication Time (min) | Droplet Size (nm) | PDI | EE (%) | |
| 1 | 7 | 30 | 7.5 | 155.0±12.83 | 0.44 ± 0.07 | 78.32 ± 5.59 |
| 2 | 7 | 25 | 5.0 | 157.0 ± 15.30 | 0.43 ± 0.04 | 73.28 ± 7.56 |
| 3 | 7 | 30 | 2.5 | 143.5 ± 16.43 | 0.08 ± 0.02 | 77.40 ± 4.88 |
| 4 | 9 | 20 | 5.0 | 120.9 ± 10.00 | 0.17 ± 0.05 | 87.84 ± 2.91 |
| 5 | 7 | 20 | 7.5 | 147.8 ± 15.77 | 0.10 ± 0.04 | 71.84 ± 2.74 |
| 6 | 7 | 20 | 2.5 | 161.6 ± 27.03 | 0.28 ± 0.05 | 7.000 ± 3.61 |
| 7 | 7 | 25 | 5.0 | 154.5 ± 15.93 | 0.50 ± 0.08 | 77.52 ± 5.61 |
| 8 | 9 | 30 | 5.0 | 129.3 ± 14.03 | 0.06 ± 0.04 | 87.52 ± 2.72 |
| 9 | 7 | 25 | 5.0 | 153.2 ± 20.05 | 0.43 ± 0.07 | 75.92 ± 5.59 |
| 10 | 9 | 25 | 7.5 | 139.8 ± 21.02 | 0.05 ± 0.02 | 87.52 ± 3.97 |
| 11 | 5 | 25 | 7.5 | 175.5 ± 19.33 | 0.59 ± 0.03 | 66.16 ± 5.91 |
| 12 | 5 | 25 | 2.5 | 199.5 ± 27.68 | 0.31 ± 0.06 | 60.16 ± 4.49 |
| 13 | 7 | 25 | 5.0 | 163.4 ± 2012 | 0.33 ± 0.05 | 76.64 ± 3.78 |
| 14 | 5 | 30 | 5.0 | 156.1 ± 15.53 | 0.05 ± 0.01 | 68.40 ± 5.59 |
| 15 | 7 | 25 | 5.0 | 156.9 ± 19.40 | 0.40 ± 0.04 | 74.72 ± 1.67 |
| 16 | 9 | 25 | 2.5 | 124.4 ± 12.64 | 0.15 ± 0.04 | 91.08 ± 2.95 |
| 17 | 5 | 20 | 5.0 | 189.7 ± 19.39 | 0.36 ± 0.04 | 63.84 ± 4.61 |
| Source | Response 1 Droplet Size (nm) | Response 2 PDI * | Response 3 % EE | |||
|---|---|---|---|---|---|---|
| ANOVA ANALYSIS | ||||||
| F-Value | p-Value | F-Value | p-Value | F-Value | p-Value | |
| Model | 43.55 | <0.0001 | 3.52 | 0.0555 | 67.53 | <0.0001 |
| A-Smix | 307.91 | <0.0001 | 7.58 | 0.0284 | 194.99 | <0.0001 |
| B-% Amplitude | 9.42 | <0.0181 | 0.8011 | 0.4005 | 7.03 | 0.0199 |
| C-Sonication time | 0.8587 | <0.3849 | 1.23 | 0.3036 | 0.5793 | 0.4602 |
| AB | 25.50 | 0.0015 | 0.7472 | 0.4160 | - | - |
| AC | 22.44 | 0.0021 | 2.91 | 0.1316 | - | - |
| BC | 9.25 | 0.0188 | 5.65 | 0.0491 | - | - |
| A2 | 0.0019 | 0.9668 | 3.69 | 0.0963 | - | - |
| B2 | 15.24 | 0.0059 | 7.58 | 0.0284 | - | - |
| C2 | 2.03 | 0.1973 | 0.4818 | 0.5100 | - | - |
| Lack of fit | 1.28 | 0.3937 | 6.01 | 0.0580 | 2.61 | 0.1842 |
| Fit Statistics | ||||||
| R2 | 0.9825 | 0.8191 | 0.9397 | |||
| Adjusted R2 | 0.9599 | 0.5865 | 0.9258 | |||
| Adequate Precision | 24.9500 | 5.8970 | 24.2220 | |||
| Regression Coefficient Values | ||||||
| Intercept | 157.00 | 0.4182 | 75.77 | |||
| A-Smix | −25.80 | −0.1104 | 11.92 | |||
| B-% Amplitude | −4.51 | −0.0359 | 2.27 | |||
| C-Sonication time | −1.36 | 0.0445 | 0.6500 | |||
| AB | 10.50 | 0.0490 | - | |||
| AC | 9.85 | −0.0968 | - | |||
| BC | 6.32 | 0.1348 | - | |||
| A2 | −0.0875 | −0.1061 | - | |||
| B2 | −7.91 | −0.1521 | - | |||
| C2 | 2.89 | 0.0384 | - | |||
| Response 1: Droplet size = +157.00 − 25.80 · A − 4.51 · B − 1.36 · C + 10.50 · A · B + 9.85 · A · C + 6.32 · B · C − 0.0875 · A2 − 7.91 · B2 + 2.89 · C2 |
| Response 2: PDI = +0.4182 − 0.1104 · A − 0.0359 · B + 0.0445 · C + 0.0490 · A · B − 0.0968 · A · C + 0.1348 · B · C − 0.1061 · A2 − 0.1521 · B2 − 0.0384 · C2 |
| Response 3: %EE = +75.77 + 11.92 · A + 2.27 · B + 0.6500 · C |
| BBD-Optimized Condition | ||
|---|---|---|
| Factors | Independent Variables | Actual Levels |
| A | Smix ratio | 9 |
| B | % Amplitude | 25 |
| C | Sonication time (mins) | 2.5 |
| Responses | Predicted values | Experimental values |
| Droplet size (nm) | 125.51 | 127.51 ± 7.71 |
| PDI * | 0.210 | 0.178 ± 0.02 |
| EE (%) * | 87.04 | 85.26 ± 4.69 |
| Pharmacokinetic Parameter | Que-PD | Que-NE |
|---|---|---|
| T max (h) | 2 ± 0.0 | 4 ± 0.0 |
| Cmax (ng/mL) | 1634.28 ± 70.18 | 5962.74 ± 238.54 ** |
| C48 (ng/mL) | 160.01 ± 38.93 | 798.62 ± 71.52 ** |
| T1/2 (h) | 21.79 ± 6.78 | 37.96 ± 7.74 * |
| AUC0–48 (ng/h/mL) | 18,748.47 ± 930.16 | 83,667.94 ± 1610.30 ** |
| AUC0–∞ (ng/h/mL) | 24,079.09 ± 3556.69 | 128,205.28 ± 14677.35 ** |
| Ke (h−1) | 0.03 ± 0.00 | 0.01 ± 0.00 * |
| Vd (mL) | 16,096 ± 2988.32 | 5279.78 ± 606.20 * |
| Cl (mL/h) | 535.75 ± 97.77 | 98.02 ± 8.43 * |
| MRT (h) | 28.78 ± 8.44 | 46.13 ± 9.91 * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mahadev, M.; Nandini, H.S.; Ramu, R.; Gowda, D.V.; Almarhoon, Z.M.; Al-Ghorbani, M.; Mabkhot, Y.N. Fabrication and Evaluation of Quercetin Nanoemulsion: A Delivery System with Improved Bioavailability and Therapeutic Efficacy in Diabetes Mellitus. Pharmaceuticals 2022, 15, 70. https://doi.org/10.3390/ph15010070
Mahadev M, Nandini HS, Ramu R, Gowda DV, Almarhoon ZM, Al-Ghorbani M, Mabkhot YN. Fabrication and Evaluation of Quercetin Nanoemulsion: A Delivery System with Improved Bioavailability and Therapeutic Efficacy in Diabetes Mellitus. Pharmaceuticals. 2022; 15(1):70. https://doi.org/10.3390/ph15010070
Chicago/Turabian StyleMahadev, Manohar, Hittanahalli S. Nandini, Ramith Ramu, Devegowda V. Gowda, Zainab M. Almarhoon, Mohammed Al-Ghorbani, and Yahia N. Mabkhot. 2022. "Fabrication and Evaluation of Quercetin Nanoemulsion: A Delivery System with Improved Bioavailability and Therapeutic Efficacy in Diabetes Mellitus" Pharmaceuticals 15, no. 1: 70. https://doi.org/10.3390/ph15010070
APA StyleMahadev, M., Nandini, H. S., Ramu, R., Gowda, D. V., Almarhoon, Z. M., Al-Ghorbani, M., & Mabkhot, Y. N. (2022). Fabrication and Evaluation of Quercetin Nanoemulsion: A Delivery System with Improved Bioavailability and Therapeutic Efficacy in Diabetes Mellitus. Pharmaceuticals, 15(1), 70. https://doi.org/10.3390/ph15010070






