Petrographic Record and Conditions of Expansive Hydration of Anhydrite in the Recent Weathering Zone at the Abandoned Dingwall Gypsum Quarry, Nova Scotia, Canada
Abstract
1. Introduction

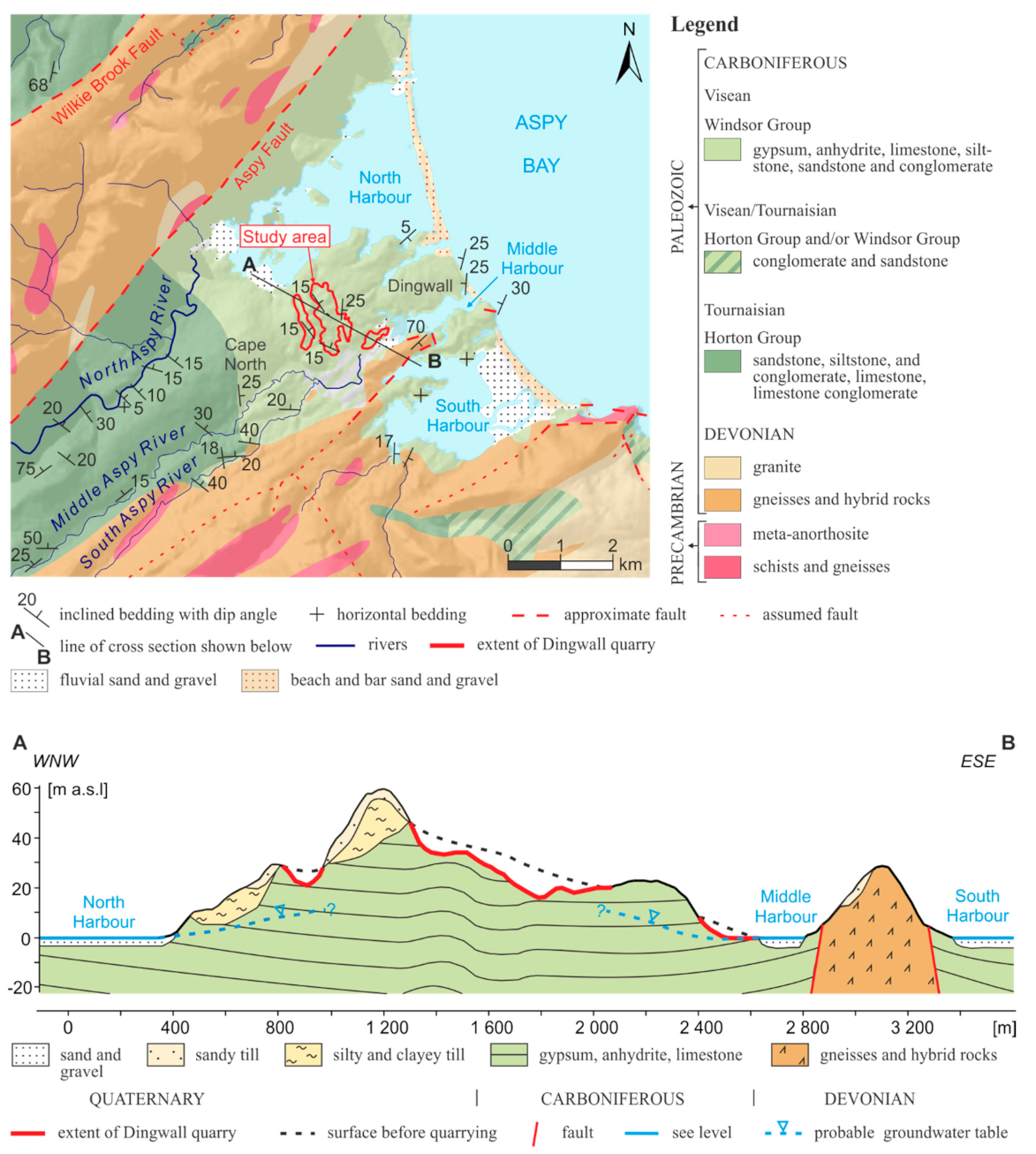
2. Climate and Geology of Studied Area


3. Terminology
4. Materials and Methods
4.1. Field Studies
4.2. Laboratory Studies
5. Weathering Zone and Bedrock
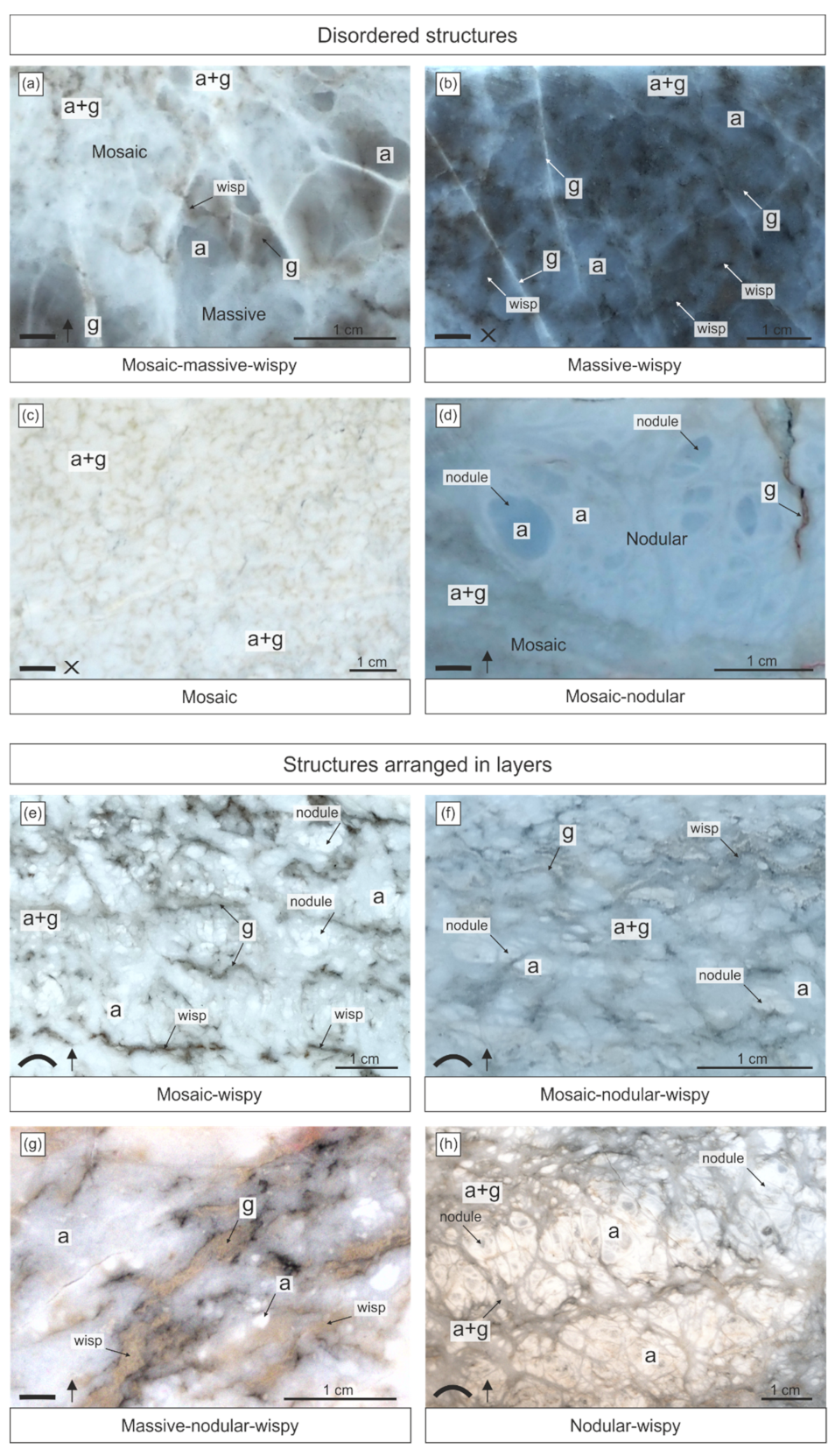
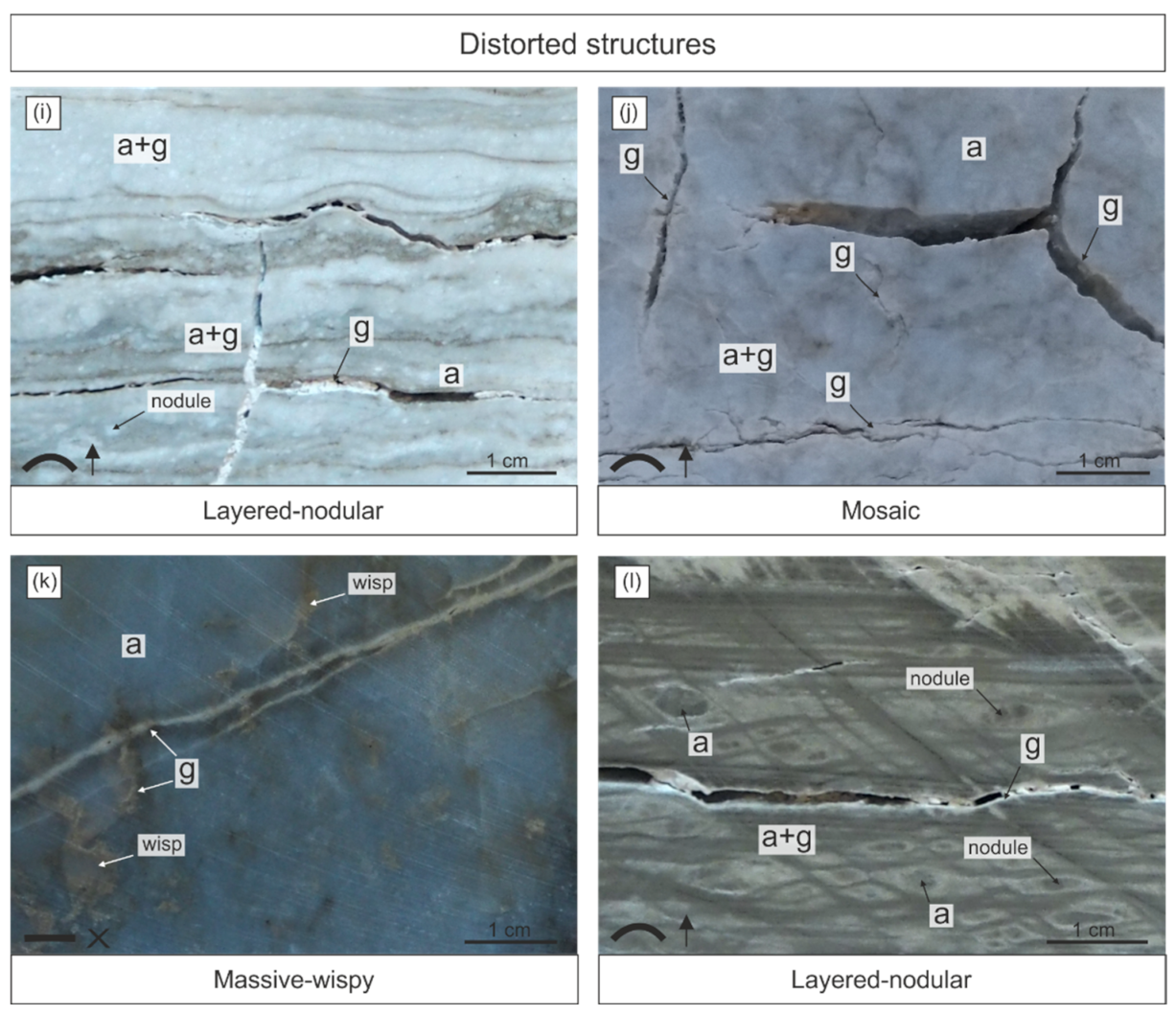
6. Structures
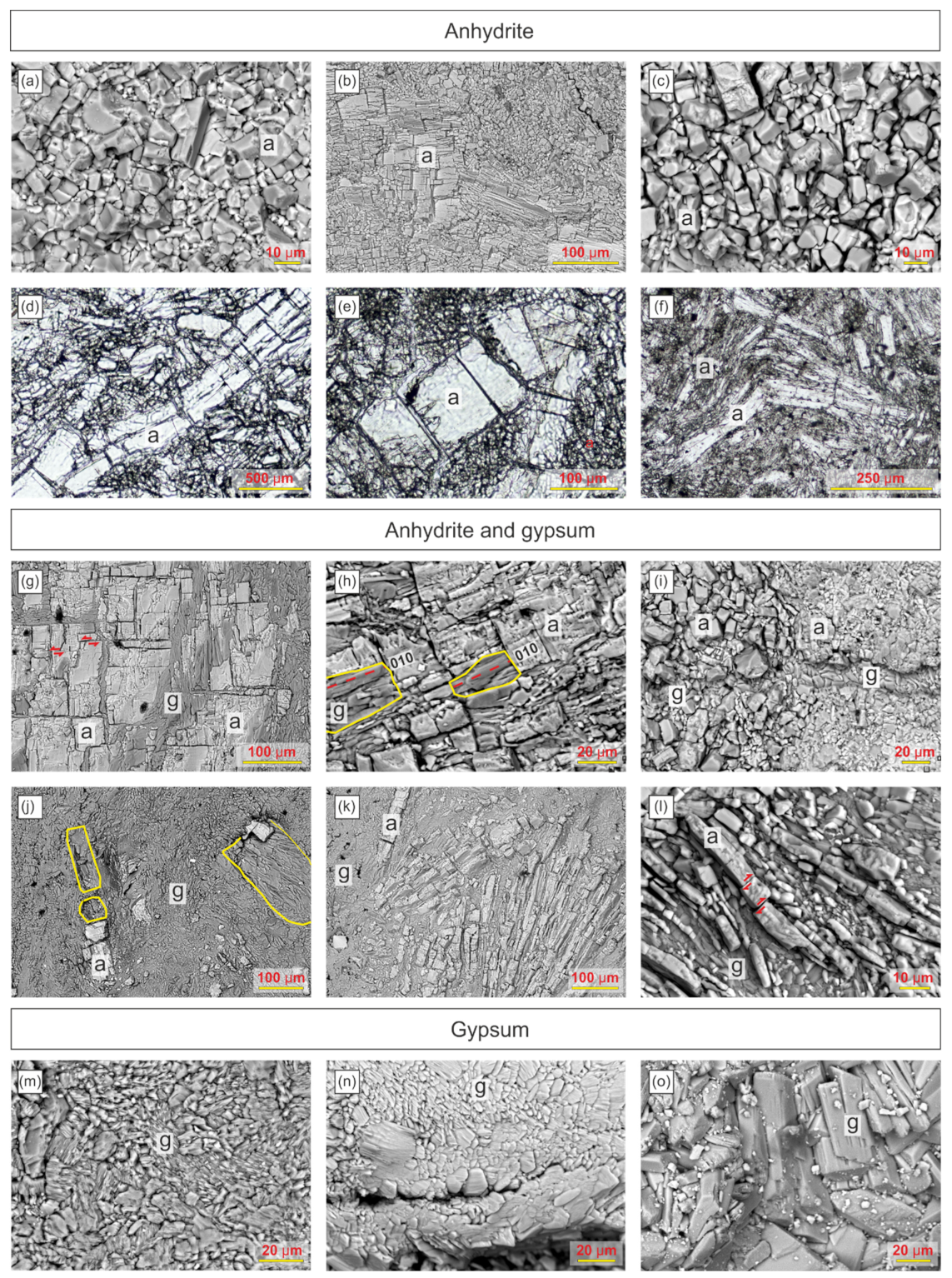
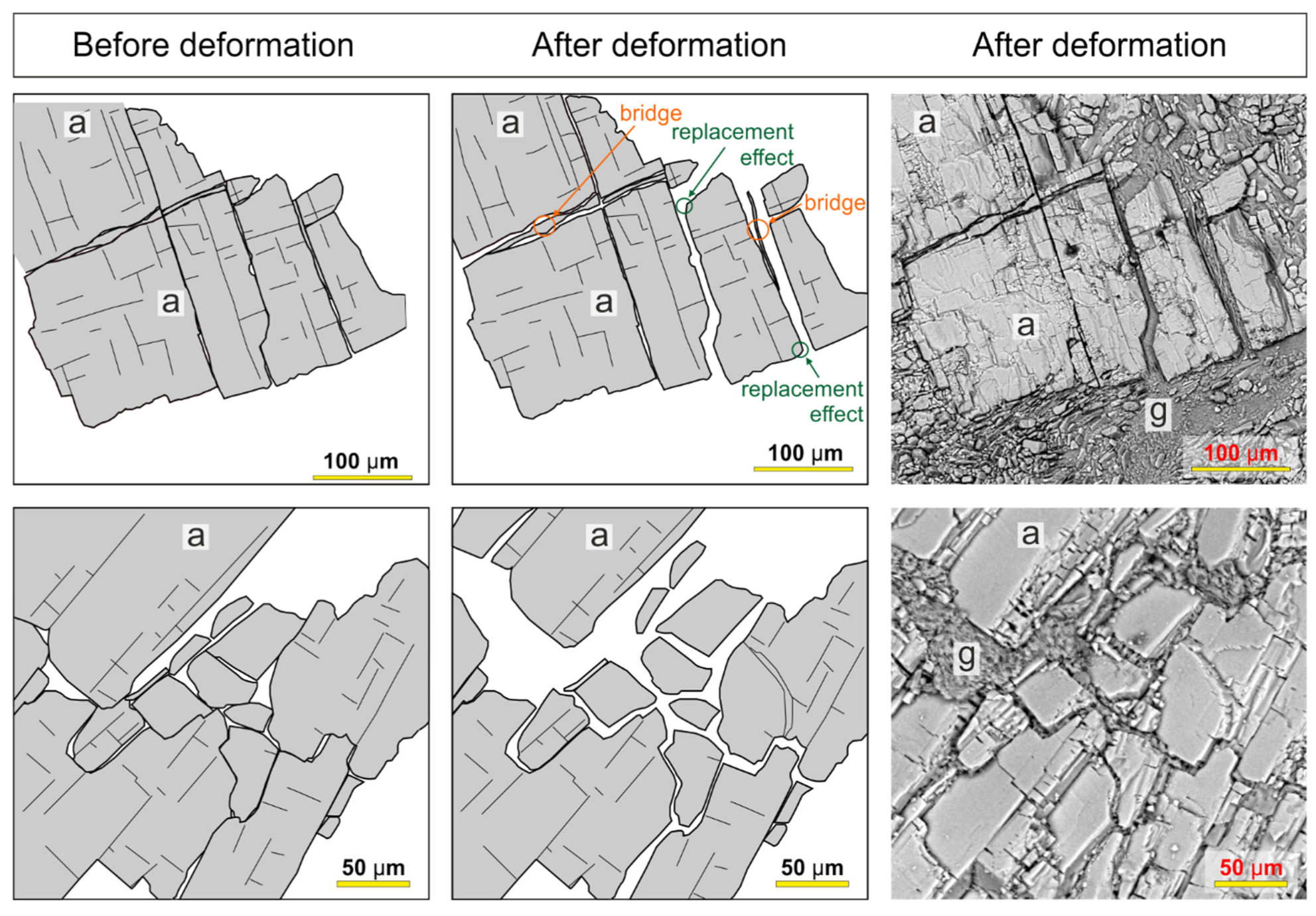
7. Anhydrite and Gypsum Fabrics
7.1. Anhydrite
7.2. Anhydrite and Gypsum
7.3. Gypsum
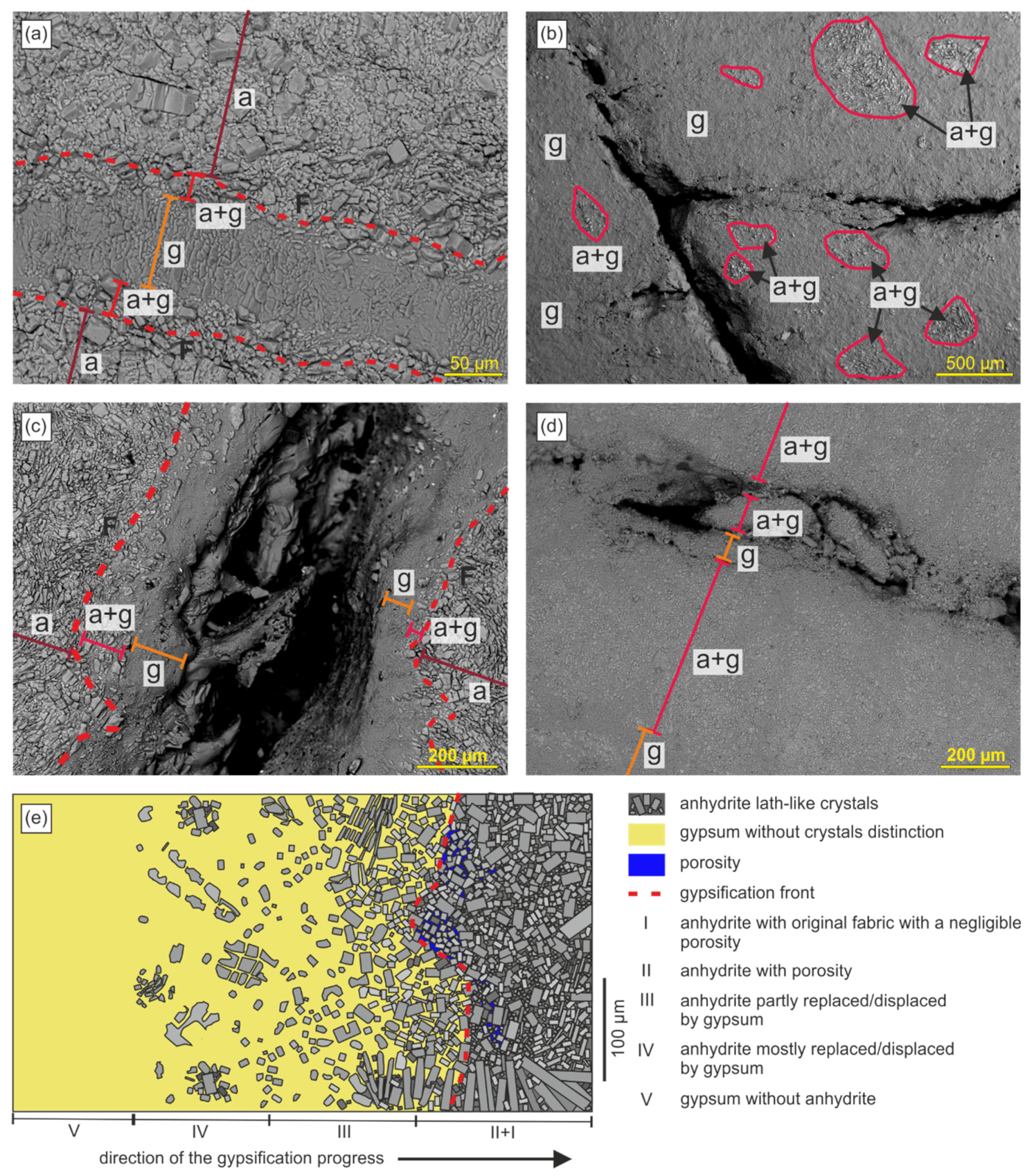
8. Gypsification Zone
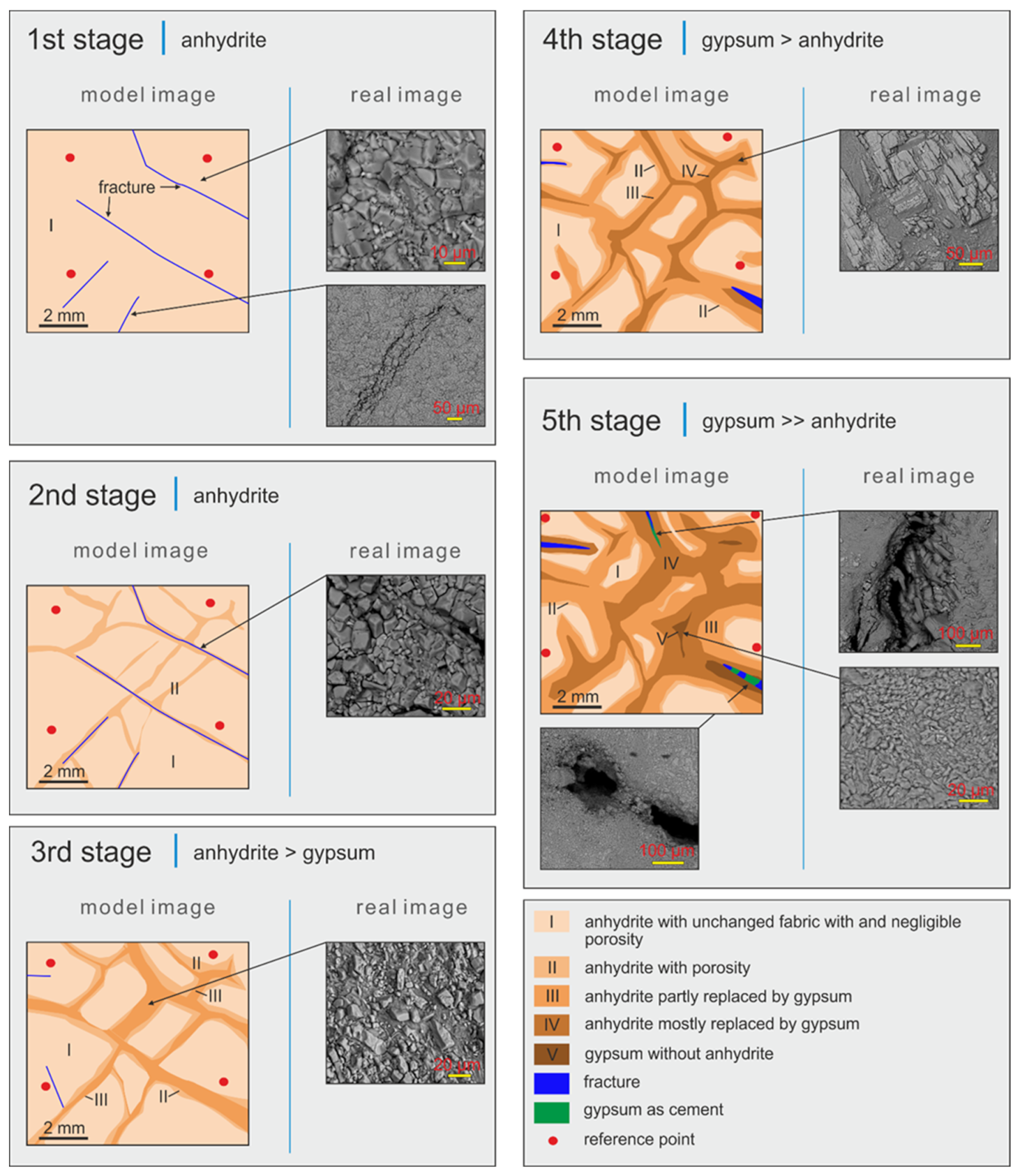
9. Model of Anhydrite Gypsification
10. Conditions of the Expansive Gypsification at Dingwall
10.1. Fracturing and Expansive Hydration
10.2. Advance of Gypsification and Related Processes
11. Summary
- -
- zone of massive to porous anhydrite,
- -
- zone of anhydrite penetrated by gypsum veinlets separating the crystals and their fragments (commonly along cleavage planes),
- -
- zone of gypsum with scattered anhydrite relics, and
- -
- gypsum zone without anhydrite.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sonnenfeld, P. Brines and Evaporites; Academic Press Inc.: Orlando, FL, USA, 1984; pp. 1–613. [Google Scholar]
- Zanbak, C.; Arthur, R.C. Geochemical and engineering aspects of anhydrite/gypsum phase transitions. Bull. Assoc. Eng. Geol. 1986, 23, 419–433. [Google Scholar] [CrossRef]
- Muir, J.L. Anhydrite–gypsum problem of Blaine Formation, Oklahoma. Am. Assoc. Petr. Geol. Bull. 1934, 18, 1297–1312. [Google Scholar] [CrossRef]
- Ham, W.E. Economic geology and petrology of gypsum and anhydrite in Blaine County. Okla. Geol. Surv. Bull. 1962, 89, 100–151. [Google Scholar]
- Holliday, D.W. The petrology of secondary gypsum rocks: A review. J. Sediment. Petrol. 1970, 40, 734–744. [Google Scholar] [CrossRef]
- Kulstad, R.O.; Fairchild, P.; McGregor, D. Gypsum in Kansas. State Geol. Surv. Kans. Bull. 1956, 113, 1–110. [Google Scholar]
- Parfenov, S.I. Specific features in anhydrite gypsification. Lithol. Miner. Resour. Litol. I Polezn. Iskopayemyie 1967, 3, 117–127. (In Russian) [Google Scholar]
- Lugli, S. Timing of post-depositional events in the Burano Formation of the Secchia valley Upper Triassic, Northern Apennines, clues from gypsum-anhydrite transitions and carbonate metasomatism. Sediment. Geol. 2001, 140, 107–122. [Google Scholar] [CrossRef]
- Goldman, M.I. Deformation, metamorphism, and mineralization in gypsum-anhydrite cap rock Sulphur salt dome, Louisiana. Geol. Soc. Am. Mem. 1952, 50, 1–164. [Google Scholar]
- Sharpe, R.D. Effects of karst processes on gypsum mining. Evaporite Karst and Engineering/Environmental Problems in the United States. Okla. Geol. Surv. Circ. 2003, 109, 31–40. [Google Scholar]
- Anderson, R.Y.; Kirkland, D.W. Intrabasin varve correlation. Geol. Soc. Am. Bull. 1966, 77, 241–256. [Google Scholar] [CrossRef]
- Grabau, A.W. Principles of Stratigraphy; A.G. Seiler and Company: New York, NY, USA, 1913; pp. 1–1185. [Google Scholar]
- Grabau, A.W. Geology of the Non-metallic Mineral Deposits Other than Silicates. Principles of Salt Deposition; McGraw-Hill Book Company, Inc.: New York, NY, USA, 1920; Volume 1, pp. 1–435. [Google Scholar]
- Holliday, D.W. Secondary gypsum in Middle Carboniferous rocks of Spitsbergen. Geol. Mag. 1967, 104, 171–177. [Google Scholar] [CrossRef]
- Ortí, F.C. Aproximación al estudio petrográfico de las microestructuras de las rocas de yeso secundario y a su origen. Inst. Investig. Geol. Diput. Prov. Univ. Barc. Rev. 1977, 32, 87–152. [Google Scholar]
- Freyer, D.; Voigt, W. Crystallization and phase stability of CaSO4 and CaSO4–based salts. Monatsh. Chem. 2003, 134, 693–719. [Google Scholar] [CrossRef]
- Amadi, F.O.; Major, R.P.; Baria, L.R. Origin of gypsum in deep carbonate reservoirs: Implications for hydrocarbon exploration and production. Am. Assoc. Petr. Geol. Bull. 2012, 96, 375–390. [Google Scholar] [CrossRef]
- Reimann, M. Geologisch-lagerstättenkundliche und mineralogische Untersuchen zur Vergipsung und Volumenzunahme der Anhydrite verschiedener geologischer Formationen unter natürlichen und labormäßigen Bedingungen. Geol. Jahrb. 1991, 97, 21–125. [Google Scholar]
- Berdugo de Moya, I.R.; Alonso Pérez de Ágreda, E.E.; Romero Morales, E.; Gens Solé, A. Tunnelling and swelling in Triassic sulphate-bearing rocks. Part I–Case studies from Baden-Württemberg. Rev. Épsilon 2009, 12, 13–37. [Google Scholar]
- Butscher, C.; Mutschler, T.; Blum, P. Swelling of clay-sulfate rocks: A review of processes and controls. Rock Mech. Rock Eng. 2016, 49, 1533–1549. [Google Scholar] [CrossRef]
- Jarzyna, A.; Bąbel, M.; Ługowski, D.; Vladi, F.; Yatsyshyn, A.; Olszewska-Nejbert, D.; Nejbert, K.; Bogucki, A. The unique hydration caves and recommended photogrammetric methods for their documentation. Geoheritage 2020, 12, 1–15. [Google Scholar] [CrossRef]
- Ramon, T.A. Expansion Mechanisms in Sulphated Rocks and Soils. Ph.D. Thesis, Universitat Politècnica de Catalunya, Barcelona, Spain, 2014; pp. 1–239. [Google Scholar]
- Alonso, E.E.; Berdugo, I.R.; Ramon, A. Extreme expansive phenomena in anhydritic-gypsiferous claystone: The case of Lilla tunnel. Géotechnique 2013, 63, 584–612. [Google Scholar] [CrossRef]
- Sass, I.; Burbaum, U. Damage to the historic town of Staufen (Germany) caused by geothermal drillings through anhydrite-bearing formations. Acta Carsologica 2010, 39, 233–245. [Google Scholar] [CrossRef]
- Fleuchaus, P.; Blum, P. Damage event analysis of vertical groundsource heat pump systems in Germany. Geotherm. Energy 2017, 5, 1–15. [Google Scholar] [CrossRef]
- Hunt, C.O.; Gale, S.J.; Gilbertson, D.D. The UNESCO Libyan Valley Survey IX: Anhydrite and limestone karst of the Tripolitanian pre-desert. Libyan Stud. 1985, 16, 1–13. [Google Scholar] [CrossRef]
- Holliday, D.W. Early diagenesis in Middle Carboniferous nodular anhydrite of Spitsbergen. Proc. Yorks. Geol. Soc. 1968, 36, 277–283, 292; discussion (with Kent P.E., West I.M., Hemingway J.E., Shearman D.J., Kinsman D.J.J.), 283–292. [Google Scholar] [CrossRef]
- Mossop, G.D.; Shearman, D.J. Origin of secondary gypsum rocks. Trans. Inst. Min. Metall. Sec. B Appl. Earth Sci. 1973, 82, B147–B154. [Google Scholar]
- Reimann, M.; Vladi, F. Zur Entwicklung der sog. Zwergenkirche am Sachsenstein bei Walkenried, Landkreis Osterode am Harz, Niedersachsen und vergleichende Beobachtungen zur rezenten Entstehung von Quellungshöhlen in einem aufgelassenen Gipssteinbruch bei Dingwall, Nova Scotia, Kanada. Mitt. Verb. Dtsch. Höhlen- Karstforscher 2003, 49, 75–77. [Google Scholar]
- Yushkin, N.P. Supergenesis of Carboniferous anhydrites of Novaya Zemlya. Polar Geogr. Geol. 1994, 18, 33–43. [Google Scholar] [CrossRef]
- Bąbel, M.; Olszewska-Nejbert, D.; Ługowski, D.; Nejbert, K.; Jacyszyn, A. Petrogenesis of the zone of present day anhydrite weathering at Pisky near Lviv. In The Weathering of Anhydrite and Gypsum Rocks; Bąbel, M., Olszewska-Nejbert, D., Nejbert, K., Eds.; GIMPO Agencja Wydawniczo-Poligraficzna: Warsaw, Poland, 2020; pp. 145–213, (In Polish with English summary). [Google Scholar] [CrossRef]
- Ługowski, D.; Bąbel, M. Distribution of gypsum content in anhydrite hydration dome from Pisky near Lviv and its development. In The Weathering of Anhydrite and Gypsum Rocks; Bąbel, M., Olszewska-Nejbert, D., Nejbert, K., Eds.; GIMPO Agencja Wydawniczo-Poligraficzna: Warsaw, Poland, 2020; pp. 135–144, (In Polish with English summary). [Google Scholar] [CrossRef]
- Stenson, R.E. The Morphometry and Spatial Distribution of Surface Depressions in Gypsum, with Examples from Nova Scotia, Newfoundland and Manitoba. Ph.D. Thesis, McMaster University, Hamilton, ON, Canada, 1990; pp. 1–134. [Google Scholar]
- Vladi, F.; Bąbel, M. Recent growth and decay of the hydration (swelling) caves in the former gypsum quarry of Dingwall in Cape Breton, Nova Scotia, Canada. In The Weathering of Anhydrite and Gypsum Rocks; Bąbel, M., Olszewska-Nejbert, D., Nejbert, K., Eds.; GIMPO Agencja Wydawniczo-Poligraficzna: Warsaw, Poland, 2020; pp. 223–232. [Google Scholar]
- Vladi, F.; Bąbel, M.; Jarzyna, A. Wachstum und Zerfall rezenter Quellungshöhlen im ehemaligen Gipssteinbruch von Dingwall in Cape Breton, Nova Scotia, Kanada, nebst anmerkungen zum dortigen Sulfatkarst und dem Forschungsstand. Abh. Zur Karst- Und Höhlenkunde 2021, 40, 113–128. [Google Scholar]
- Stenson, R.E.; Ford, D.C. Rillenkarren on gypsum in Nova Scotia. Geogr. Phys. Quatern. 1993, 47, 239–243. [Google Scholar]
- Bath, A.H.; Darling, W.G.; George, I.A.; Milodowski, A.E. 18O/16O and 2H/1H changes during progressive hydration of a Zechstein anhydrite formation. Geochim. Cosmochim. Acta 1987, 51, 3113–3118. [Google Scholar] [CrossRef]
- Reimann, M. Zur Vergipsung der Zechsteinanhydrite Nordwestdeutschlands. Zbl. Geo. Pal. 1991, 4, 1201–1210. [Google Scholar]
- Webb, T.C. Geology and economic development of early Carboniferous marine evaporates, southeastern New Brunswick. New Brunswick Department of Natural Resources. In Lands, Minerals and Petroleum Division; Field Guide No. 6; Lands, Minerals and Petroleum Division, Department of Natural Resources: New Brunswick, NJ, Canada, 2010; pp. 1–71. [Google Scholar]
- Kottek, M.; Grieser, J.; Beck, C.; Rudolf, B.; Rubel, F. World map of the Köppen-Geiger climate classification updated. Meteorol. Z. 2006, 15, 259–263. [Google Scholar] [CrossRef]
- Climate-data.org. Available online: https://en.climate-data.org/north-america/canada/nova-scotia/dalem-lake-98946/ (accessed on 18 September 2021).
- Connor, J.; (Cape North, NS, Canada). Personal communication. 2019. [Google Scholar]
- Gregory, D.J. Gypsum location map and drill hole sections of boreholes through gypsum in Nova Scotia. 1974; 1–37. [Google Scholar]
- Moore, R.G. Lithostratigraphic units in the upper part of the Windsor Group, Minas sub-basin, Nova Scotia. Geol. Assoc. Can. 1967, 4, 1–22. [Google Scholar]
- MacNeil, L.A.; Pufahl, P.K.; James, N.P. Deposition of a saline giant in the Mississippian Windsor group, Nova Scotia, and the nascent late Paleozoic ice age. Sediment. Geol. 2018, 363, 118–135. [Google Scholar] [CrossRef]
- Bell, W.A. Horton-Windsor district, Nova Scotia. In Geological Survey of Canada, Memoir 155; King’s Printer: Ottawa, OT, Canada, 1929; pp. 1–268. [Google Scholar]
- Neale, E.R.W. Geology, Pleasant Bay, Nova Scotia, Map 1119A; Neale, E.R.W., Ed.; Geological Survey of Canada, Department of Mines and Technical Surveys: Ottawa, ON, Canada, 1963.
- Neale, E.R.W. Geology, Dingwall, Nova Scotia, Map 1124A; Neale, E.R.W., Ed.; Geological Survey of Canada, Department of Mines and Technical Surveys: Ottawa, ON, Canada, 1963.
- Adams, G.C. Gypsum and anhydrite resources in Nova Scotia. Econ. Geol. Ser. 1991, 91, 1–293. [Google Scholar]
- Grant, D.R. Late Quaternary movement of Aspy Fault, Nova Scotia. Can. J. Earth Sci. 1990, 27, 984–987. [Google Scholar] [CrossRef]
- Grant, D.R. Quaternary geology. Geol. Surv. Can. 1994, 482, 1–159. [Google Scholar] [CrossRef]
- Moseley, M. Genesis of schlottenkarren on the Avon Peninsula of Nova Scotia (Canada) with implications for the geochronology of evaporite karsts and caves of Atlantic Canada. Int. J. Speleol. 2017, 46, 267–276. [Google Scholar] [CrossRef]
- Beales, F.W.; Oldershaw, A.E. Evaporite-solution brecciation and Devonian carbonate reservoir porosity in Western Canada. Am. Assoc. Petr. Geol. Bull. 1969, 53, 503–512. [Google Scholar] [CrossRef]
- Ługowski, D.; Bąbel, M.; Nejbert, K. Simple method of approximate determination of the quantitative mineralogical composition of gypsum-anhydrite rocks (compared to other analytical methods). In The Weathering of Anhydrite and Gypsum Rocks; Bąbel, M., Olszewska-Nejbert, D., Nejbert, K., Eds.; GIMPO Agencja Wydawniczo-Poligraficzna: Warsaw, Poland, 2020; pp. 125–134, (In Polish with English summary). [Google Scholar] [CrossRef]
- Brodie, K.; Fettes, D.; Harte, B. Structural terms including fault rock terms. In Metamorphic Rocks: A Classification and Glossary of Terms. Recommendations of the International Union of Geological Sciences Subcommission on the Systematics of Metamorphic Rocks; Fettes, D., Desmons, J., Eds.; Cambridge University Press: Cambridge, UK, 2007; pp. 24–31. [Google Scholar]
- Maiklem, W.R.; Bebout, D.G.; Glaister, R.P. Classification of anhydrite—A practical approach. Bull. Can. Petrol. Geol. 1969, 17, 194–233. [Google Scholar] [CrossRef]
- Garrison, R.E.; Schreiber, B.C.; Bernoulli, D.; Fabricius, F.H.; Kidd, R.B.; Mélières, F. Sedimentary petrology and structures of Messinian evaporitic sediments in the Mediterranean Sea, Leg 42A, Deep Sea Drilling Project. In Initial Reports of the Deep Sea Drilling Project; Hsü, K.J., Montadert, L., Eds.; U.S. Government Printing Office: Washington, WA, USA, 1978; Volume 42, pp. 571–611. [Google Scholar] [CrossRef]
- Meyer, F.O. Anhydrite Classification According to Structure; Carbonate Research Consulting: Conifer, CO, USA, 2016; pp. 1–21. Available online: https://linkd.pl/pcru2 (accessed on 8 November 2021).
- Shawkat, M.G. The Sedimentology of the Lower Fars Formation (Miocene) of Northern Iraq. Ph.D. Thesis, University of Newcastle upon Tyne, Newcastle upon Tyne, UK, 1979; pp. 1–164. Available online: https://theses.ncl.ac.uk/dspace/bitstream/10443/597/1/Shawkat79.pdf. (accessed on 8 November 2021).
- Ciarapica, G.; Passeri, L.; Schreiber, B.C. Una proposta di classificazione delle evaporiti solfatiche. Geologica Rom. 1985, 24, 219–232. [Google Scholar]
- Forkner, R.M. An integrated system for macro-scale anhydrite classification. Geol. Q. 2010, 54, 423–430. [Google Scholar]
- Bathurst, R.G.C. Carbonate Sediments and Their Diagenesis, 2nd ed.; Developments in Sedimentology; Elsevier: Amsterdam, The Netherlands, 1975; Volume 12, pp. 1–658. [Google Scholar]
- Friedman, G.M. Terminology of crystallization textures and fabrics in sedimentary rocks. J. Sediment. Petrol. 1965, 35, 643–655. [Google Scholar] [CrossRef]
- Neuendorf, K.K.E.; Mehl, J.P., Jr.; Jackson, J.A. (Eds.) Glossary of Geology, 5th ed.; American Geosciences Institute: Alexandria, VA, USA, 2005; pp. 1–779. [Google Scholar]
- Lindgren, W. The nature of replacement. Econ. Geol. 1912, 7, 521–535. [Google Scholar] [CrossRef]
- Bastin, E.S.; Graton, L.C.; Lindgren, W.; Newhouse, W.H.; Schwartz, G.M.; Short, M.N. Criteria of age relations of minerals, with especial reference to polished sections of ores. Econ. Geol. 1931, 26, 561–610. [Google Scholar] [CrossRef]
- Ramberg, H. The Origin of Metamorphic and Metasomatic rocks: A Treatise on Recrystallization and Replacement in the Earth’s Crust; The University of Chicago Press: Chicago, IL, USA, 1952; pp. 1–317. [Google Scholar]
- Folk, R.L. Some aspects of recrystallization in ancient limestones. Dolomitization and Limestone Diagenesis. In Society of Economic Paleontologists and Mineralogists Special Publication; Pray, L.C., Murray, R.D., Eds.; SEPM Society for Sedimentary Geology: Tulsa, OK, USA, 1965; No. 13; pp. 14–48. [Google Scholar] [CrossRef]
- Ward, W.B.; Reeder, R.J. The use of growth microfabrics and transmission electron microscopy in understanding replacement processes in carbonates. In Carbonate Microfabrics; Rezak, R., Lavoie, D.L., Eds.; Springer: New York, NY, USA, 1993; pp. 253–264. [Google Scholar] [CrossRef]
- Franklin, J.A. Suggest methods for determining water content, porosity, density, absorption and related properties and swelling and slake-durability index properties. Int. J. Rock. Mech. Min. 1979, 16, 141–156. [Google Scholar]
- Anovitz, L.M.; Cole, D.R. Characterization and analysis of porosity and pore structures. Rev. Mineral. Geochem. 2015, 80, 61–164. [Google Scholar] [CrossRef]
- Pye, K.; Krinsley, D.H. Petrographic examination of sedimentary rocks in the SEM using backscattered electron detectors. J. Sediment. Petrol. 1984, 54, 877–888. [Google Scholar] [CrossRef]
- Jordan, P.G.; Düggelin, M.; Mathys, D.; Guggenheim, R. Gypsum-anhydrite differentiation by SEM using the back-scattered electron-signal. J. Sediment. Res. 1991, 61, 616–618. [Google Scholar] [CrossRef]
- Papezik, V.S.; Fong, C.C.K. Howlite and ulexite from the Carboniferous gypsum and anhydrite beds in western Newfoundland. Can. Mineral. 1975, 13, 370–376. [Google Scholar]
- Goodman, N.R. Gypsum and anhydrite in Nova Scotia. In Nova Scotia Department of Mines; Society of Economic Geologists Inc.: Halifax, NS, Canada, 1952; pp. 1–71. [Google Scholar]
- Nova Scotia Well Logs Database. Available online: https://novascotia.ca/natr/meb/download/dp430.asp (accessed on 3 November 2021).
- Baechler, F.; Boehner, R. Karst geology and hydrogeology of Cape Breton Island, Nova Scotia: An overview. Can. J. Earth Sci. 2014, 51, 701–714. [Google Scholar] [CrossRef]
- Wilder, F.A. Gypsum and anhydrite. Am. Min. 1928, 13, 476–480. [Google Scholar]
- Ford, D. Principal features of evaporite karst in Canada. Carbonate Evaporite 1997, 12, 15–23. [Google Scholar] [CrossRef]
- Barnaby, R.J. Quantitative image analysis for geologic core description. J. Sediment. Res. 2017, 87, 460–485. [Google Scholar] [CrossRef]
- Langbein, R. The Zechstein sulphates: The state of art. In The Zechstein Facies in Europe; Lecture Notes in Earth, Sciences; Peryt, T.M., Ed.; Springer: Berlin/Heidelberg, Germany, 1987; Volume 10, pp. 143–188. [Google Scholar]
- Bąbel, M.; Kasprzyk, A. Gypsum ooids from the Middle Miocene (Badenian) evaporites of southern Poland. Acta Geol. Pol. 1990, 40, 215–239. [Google Scholar]
- Mossop, G.D. The evaporites of the Ordovician Baumann Fiord Formation, Ellesmere Island, Arctic Canada. Geol. Surv. Can. Bull. 1979, 298, 1–52. [Google Scholar]
- Fabre, D.; Dayre, M. Proprietes geotechniques de gypses et anhydrites du Trias des Alps de Savoie (France). Bull. Int. Assoc. Eng. Geol. 1982, 25, 91–98. [Google Scholar] [CrossRef]
- Dronkert, H. Diagenesis of Triassic evaporites in northern Swizterland. Eclogae Geol. Helv. 1987, 80, 397–413. [Google Scholar] [CrossRef]
- Schreiber, B.C.; Helman, M.L. Criteria for distinguishing primary evaporite features from deformation features in sulphate evaporites. J. Sediment. Res. 2005, 75, 525–533. [Google Scholar] [CrossRef]
- Holliday, D.W. Field excursion to the Brooks and Bito gypsum quarries, eastern St. Andrew. J. Geol. Soc. Jam. 1970, 11, 36–41. [Google Scholar]
- Holliday, D.W. Origin of Lower Eocene gypsum-anhydrite rocks, southeast St. Andrew, Jamaica. Trans. Inst. Min. Met. 1971, 80, B305–B315. [Google Scholar]
- Ortí, F.; Rosell, L.; Playà, E.; Salvany, J.M. Meganodular anhydritization: A new mechanism of gypsum to anhydrite conversion (Palaeogene–Neogene, Ebro Basin, North-east Spain). Sedimentology 2012, 59, 1257–1277. [Google Scholar] [CrossRef]
- Shearman, D.J.; Fuller, J.G. Anhydrite diagenesis, calcitization, and organic laminites, Winnipegosis Formation, Middle Devonian, Saskatchewan. Bull. Can. Petrol. Geol. 1969, 17, 496–525. [Google Scholar] [CrossRef]
- Zavaritzky, A. Le gypse et l’anhydrite du village Okhlébinino. Bull. Com. Géologique Izvestyia Geol. Kom. 1925, 43, 973–985. (In Russian) [Google Scholar]
- Von Gaertner, H.-R. Petrographie und paläogeographische Stellung der Gipse vom Südrande des Harzes. Jahrb. Preußischen Geol. Landesanst. Berl. 1933, 53, 655–694. [Google Scholar]
- Anrich, H. Zur Frage der Vergipsung in den Sulfatlagern des Mittleren Muschelkalks und Gipskeupers in Südwestdeutschland. Neues Jahrbuch für Geologie und Paläontologie Anhandlungen 1958, 106, 293–338. [Google Scholar]
- Reimann, M. Geologie, Petrographie und Vergipsung der Zechsteinsulfatvorkommen von Stadtoldendorf und Osterode/Harz. Ber. Nat. Ges. Hann. 1987, 129, 57–84. [Google Scholar]
- Hauber, L.; Jordan, P.; Madsen, F.; Nüesch, R.; Vögtli, B.; Flückiger, A.; Ko, L.S.C. Tonminerale und Sulfate als Ursache für druckhaftes Verhalten von Gesteinen. In Ursachen und Wirkungen des Quellvorganges; Forschungsauftrag 55/92 und 52/96 (Nr. 4306.01 und 4306.02) auf Antrag des Bundesamtes für Strassenbau, ASTRA 1996/039; Geologisch-Palaeontologisches Institut der Universität Basel, Abt. Praktische Geologie: Basel, Switzerland, 2005; pp. 1–90. [Google Scholar]
- Webb, T.C. Geology, Development History, and Exploration Alternatives for Gypsum and Anhydrite Resources Near Hillsborough (Part of NTS 21 H/15), Albert County, Southeastern New Brunswick; Open File 2001-6; New Brunswick Department of Natural Resources and Energy, Minerals and Energy Division: Sussex, NB, Canada, 2002; pp. 1–47.
- Ortí, F.C.; Rosell, L.O. Fabricas cristalinas de la anhidrita nodular y laminada. Acta Geol. Hisp. 1981, 16, 235–255. [Google Scholar]
- Brown, L.S. Cap-rock petrography. Am. Assoc. Petr. Geol. Bull. 1931, 15, 509–529. [Google Scholar]
- Carozzi, A.V. Microscopic Sedimentary Petrography; John Wiley Sons., Inc.: New York, NY, USA, 1960; pp. 1–485. [Google Scholar]
- Aleali, M.; Rahimpour-Bonab, H.; Moussavi-Harami, R.; Jahani, D. Environmental and sequence stratigraphic implications of anhydrite textures: A case from the Lower Triassic of the Central Persian Gulf. J. Asian Earth Sci. 2013, 75, 110–125. [Google Scholar] [CrossRef]
- Shearman, D.J. Syndepositional and late diagenetic alteration of primary gypsum to anhydrite. In Proceedings of the Sixth International Symposium on Salt, Toronto, ON, Canada, 24–28 May 1983; Schreiber, B.C., Harner, H.R., Eds.; The Salt Institute: Alexandria, VA, USA, 1985; Volume 1, pp. 41–50. [Google Scholar]
- West, I.M. Evaporite diagenesis in the Lower Purbeck Beds of Dorset. Proceed. Yorkshire Geol. Soc. 1964, 34, 315–330. [Google Scholar] [CrossRef]
- Craig, J.R.; Vaughan, D.J. Ore Microscopy and Ore Petrography, 2nd ed.; John Wiley Sons, Inc.: New York, NY, USA, 1994; pp. 1–434. [Google Scholar]
- Taber, S. The origin of veinlets in the Silurian and Devonian strata of central New York. J. Geol. 1918, 26, 56–73. [Google Scholar] [CrossRef]
- Mossop, G.D. Anhydrite-Carbonate Cycles of the Ordovician Baumann Fiord Formation, Ellesmere Island, Arctic Canada: A Geological History. Ph.D. Thesis, University of London, London, UK, 1973; pp. 1–231. [Google Scholar]
- Marandi, M.; Jahani, D.; Uromeihy, A.; Reihan, M.K. Analysis of structure and textures of anhydrite mineral in Gachsaran Formation in Gotvand area, Iran. Open J. Geol. 2017, 7, 1478–1493. [Google Scholar] [CrossRef]
- James, A.N.; Lupton, A.R.R. Gypsum and anhydrite in foundations of hydraulic structures. Geotechnique 1978, 28, 249–272. [Google Scholar] [CrossRef]
- Gysel, M. Anhydrite dissolution phenomena: Three case histories of anhydrite karst caused by water tunnel operation. Rock Mech. Rock Eng. 2002, 35, 1–21. [Google Scholar] [CrossRef]
- Serafeimidis, K.; Anagnostou, G. The effect of sealing on anhydrite hydration in swelling rocks. In Proceedings of the First International Conference for PhD Students in Civil Engineering, Cluj-Napoca, Romania, 4–7 November 2012; p. 8. [Google Scholar]
- Serafeimidis, K.; Anagnostou, G. On the time-development of sulphate hydration in anhydritic swelling rocks. Rock Mech. Rock Eng. 2013, 46, 619–634. [Google Scholar] [CrossRef]
- Serafeimidis, K. On the Dissolution, Precipitation and Transport Processes in Sulphatic Swelling Rocks. Ph.D. Thesis, Eidgenössische Technische Hochschule Zürich, Zurich, Switzerland, 2014; pp. 1–228. [Google Scholar]
- Wanninger, T.; Pimentel, E.; Anagnostou, G. Experimental investigetions on the self-sealing of anhydritic rock. In Proceedings of the 51st U.S. Rock Mechanics/Geomechanics Symposium, San Francisco, CA, USA, 25–28 June 2017; pp. 1–8. [Google Scholar]
- Wanninger-Huber, T.C. Experimental Investigations for the Modelling of Anhydritic Swelling Claystones. Ph.D. Thesis, Eidgenössische Technische Hochschule Zürich, Zürich, Switzerland, 2019; pp. 1–325. [Google Scholar]
- Shearman, D.J.; Mossop, G.; Dunsmore, H.; Martin, M. Origin of gypsum veins by hydraulic fracture. Inst. Min. Metall. Trans. Sect. B Appl. Earth Sci. 1972, 81, B149–B155. [Google Scholar]
- Hilgers, C.; Urai, J.L. On the arrangement of solid inclusions in fibrous veins and the role of the crack-seal mechanism. J. Struct. Geol. 2005, 27, 481–494. [Google Scholar] [CrossRef]
- Richardson, W.A. The fibrous gypsum of Nottinghamshire. Mineral. Mag. J. Mineral. Soc. 1920, 19, 77–95. [Google Scholar] [CrossRef]
- Fletcher, R.C.; Merino, E. Mineral growth in rocks: Kinetic–rheological models of replacement, vein formation, and syntectonic crystallization. Geochim. Cosmochim. Acta 2001, 65, 3733–3748. [Google Scholar] [CrossRef]
- Bailey, H.B. Hydration factors in gypsum deposits of the Maritime Provinces. In American Institute of Mining and Mettalurgical Engineers; Technical Publication 308; The American Institute of Mining and Metallurgical Engineers: New York, NY, USA, 1930; pp. 1–11. [Google Scholar]
- Boyd, J.M.; Currie, J.B. Fracture porosity in alabaster: An experimental model of rock deformation. Bull. Can. Petrol. Geol. 1969, 17, 117–132. [Google Scholar] [CrossRef]
- Auvray, C.; Homand, F.; Sorgi, C. The aging of gypsum in underground mines. Eng. Geol. 2004, 74, 183–196. [Google Scholar] [CrossRef]
- Putnis, A.; Austrheim, H. Fluid-induced processes: Metasomatism and metamorphism. Geofluids 2010, 10, 254–269. [Google Scholar] [CrossRef]
- Lebedev, A.L.; Avilina, I.A. The kinetics of gypso anhydrites dissolution in water: Experimental studies. Mosc. Univ. Geol. Bull. 2019, 74, 429–432. [Google Scholar] [CrossRef]
- Lutenegger, A.J.; Wollenhaupt, N.C.; Handy, R.L. Laboratory simulation of shale expansion by induced gypsum growth. Can. Geotech. J. 1979, 16, 405–409. [Google Scholar] [CrossRef]
- Shearman, D.J. Displacement of sand grains in sandy gypsum crystals. Geol. Mag. 1981, 118, 303–306. [Google Scholar] [CrossRef]
- Verboom, B.; Grealish, G.; Schoknecht, N.; Omar, S. Influence of gravel on the accumulation of pedogenic gypsum in Kuwait. Arid. Land Res. Manag. 2003, 17, 71–84. [Google Scholar] [CrossRef]
- Hoover, S.E.; Lehmann, D. The expansive effects of concentrated pyritic zones within the Devonian Marcellus Shale Formation of North America. Q. J. Eng. Geol. Hydrogeol. 2009, 42, 157–164. [Google Scholar] [CrossRef]
- Casby-Horton, S.; Herrero, J.; Rolong, N.A. Gypsum soils—Their morphology, classification, function, and landscapes. In Advances in Agronomy; Sparks, D.L., Ed.; Elsevier Academic Press: Amsterdam, The Netherlands, 2015; Volume 130, pp. 231–290. [Google Scholar]
- Bundy, W.M. Petrology of gypsum-anhydrite deposits in southwestern Indiana. J. Sediment. Petrol. 1956, 26, 240–252. [Google Scholar] [CrossRef]
- Flückiger, A.; Nüesch, R.; Madsen, F.T. Anhydritquellung. In Proceedings of the Jahrestagung DGGT, Regensburg, Germany, 13–14 September 1994; Kohler, E.E., Ed.; Berichte der Deutschen Ton- und Tonmineralgruppe. Deutsche Ton- und Tonmineralgruppe DTTG: Dornburg, Germany, 1994; pp. 146–153. [Google Scholar]
- Keulen, N.T.; Den Brok, S.W.J.; Spiers, C.J. Force of crystallisation of gypsum during hydration of anhydrite rock. In Proceedings of the 13th DRT Conference, Deformation Mechanisms, Rheology, and Tectonics, Noordwijkerhout, The Netherlands, 2–4 April 2001; p. 1. [Google Scholar]
- Ramon, A.; Olivella, S.; Alonso, E.E. Swelling of a gypsiferous claystone and its modelling. In Proceedings of the 17th International Conference on Soil Mechanics and Geotechnical Engineering, Alexandria, Egypt, 5–9 October 2009; Hamza, M., Shahien, M., El-Mossallam, Y., Eds.; IOS Press: Amsterdam, The Netherlands, 2009; pp. 730–733. [Google Scholar] [CrossRef]
- Ramon, A.; Alonso, E.E. Heave of a railway bridge: Modelling gypsum crystal growth. Géotechnique 2013, 63, 720–732. [Google Scholar] [CrossRef]
- Serafeimidis, K.; Anagnostou, G. On the crystallisation pressure of gypsum. Environ. Earth. Sci. 2014, 72, 4985–4994. [Google Scholar] [CrossRef]
- Khaimov-Malkov, V.Y. Experimental measurement of crystallization pressure. In Growth of Crystals; Shubnikov, A.V., Sheftal, N.N., Eds.; Consultants Bureau, Inc.: New York, NY, USA; Chapman Hall, Ltd.: London, UK, 1959; Volume 2, pp. 14–19. [Google Scholar]
- Scherer, G.W. Factors affecting crystallization pressure. In Internal Sulfate Attack and Delayed Ettringite Formation, Proceedings of the International RILEM TC 186-ISA Workshop on Internal Sulfate Attack and Delayed Ettringite Formation, Villars, Switzerland, 4–6 September 2002; Scrivener, K., Skalny, J., Scrivener, K., Skalny, J., Eds.; RILEM Publications: Bagneux, France, 2004; pp. 139–154. ISBN 2-912143-44-46. [Google Scholar]
- Flatt, R.J.; Steiger, M.; Scherer, G.W. A commented translation of the paper by C.W. Correns and W. Steinborn on crystallization pressure. Environ. Geol. 2007, 52, 187–203. [Google Scholar] [CrossRef]
- Sekine, K.; Okamoto, A.; Hayashi, K. In situ observation of the crystallization pressure induced by halite crystal growth in a microfluidic channel. Am. Mineral. 2011, 96, 1012–1019. [Google Scholar] [CrossRef]
- Steiger, M.; Charola, A.E.; Sterflinger, S. Weathering and deterioration. In Stone in Architecture, Properties, Durability, 5th ed.; Siegesmund, S., Snethlage, S., Eds.; Springer: Heidelberg, Germany, 2014; pp. 225–316. [Google Scholar] [CrossRef]
- Meldrum, F.C.; O’Shaughnessy, C. Crystallization in confinement. Adv. Mater. 2020, 32, 1–64. [Google Scholar] [CrossRef]
- Oguchi, C.T.; Yu, S. A review of theoretical salt weathering studies for stone heritage. Prog. Earth Planet. Sci. 2021, 8, 1–23. [Google Scholar] [CrossRef]
- Bard, J.P. Microtextures of Igneous and Metamorphic Rocks; Reidel, D., Ed.; Publishing Company: Dordrecht, The Netherlands, 1986; pp. 1–264. [Google Scholar]
- Nahon, D.; Merino, E. Pseudomorphic replacement in tropical weathering: Evidence, geochemical consequences, and kinetic-rheological origin. Am. J. Sci. 1997, 297, 393–417. [Google Scholar] [CrossRef]
- Fletcher, R.C.; Merino, E. Mineral growth in rocks: Interacting stress and kinetics in vein growth, replacement, and water-rock interaction. In Water-Rock Interaction, Proceedings of the Tenth International Symposium on Water-Rock Interaction, WRI-10, Villasimius, Italy, 10–15 July 2001; Cidu, R., Ed.; A.A. Balkema: Lisse, The Netherlands, 2001; pp. 161–164. [Google Scholar]
- Chen, Q.; You, L.; Kang, Y.; Dou, L.; Sheng, J.J. Gypsum-crystallization-induced fracturing during shale−fluid reactions and application for shale stimulation. Energy Fuels 2018, 32, 10367–10381. [Google Scholar] [CrossRef]
- Bell, F.G. A survey of the engineering properties of some anhydrite and gypsum from the north and midlands of England. Eng. Geol. 1994, 38, 1–23. [Google Scholar] [CrossRef]
- Zhang, B.; Wang, H.; Wang, L.; Xu, N. Stability analysis of a group of underground anhydrite caverns used for crude oil storage considering rock tensile properties. Bull. Eng. Geol. Environ. 2019, 78, 6249–6265. [Google Scholar] [CrossRef]
- Bilgin, N. The cuttability of evaporites. Bull. Int. Assoc. Eng. Geol. 1982, 25, 85–90. [Google Scholar] [CrossRef]
- Papadopoulos, Z.; Kolaiti, E.; Mourtzas, N. The efect of crystal size on geotechnical properties of Neogene gypsum in Crete. Q. J. Eng. Geol. 1994, 27, 267–273. [Google Scholar] [CrossRef]
- Serafeimidis, K.; Anagnostou, G.; Vrakas, A. Scale effects in relation to swelling pressure in anhydritic claystones. Geomechnics from Micro to Macro. In Proceedings of the TC105 ISSMGE International Symposium on Geomechanics from Micro to Macro, Cambridge, UK, 1–3 September 2014; Soga, K., Kumar, K., Biscontin, G., Kuo, M., Eds.; Taylor Francis Group: London, UK, 2015; Volume 2, pp. 795–800. [Google Scholar]
- Imam, R.; Mahmoudi, A.; Farsghoshooni, A. Experimental study of gypsum and anhydrite dissolution due to surface flow. In Proceedings of the GeoEdmonton 2018—71st Canadian Geotechnical Conference and the 13th Joint CGS/IAH-CNC Groundwater Conference, Paper 259. Edmonton, AL, Canada, 23–26 September 2018; p. 10. [Google Scholar]
- Kaufmann, G.; Romanov, D. Modelling speleogenesis in soluble rocks: A case study from the Permian Zechstein sequences exposed along the southern Harz Mountains and the Kyffhäuser Hills, German. Acta Carsologica 2019, 48, 173–197. [Google Scholar] [CrossRef]
- Rauh, F.; Thuro, K. Investigations on the swelling behavior of pure anhydrites. In Proceedings of the 1st Canada–U.S. Rock Mechanics Symposium, Vancouver, WA, Canada, 27–31 May 2007. [Google Scholar] [CrossRef]
- McWhae, J.R.H. The Carboniferous breccias of Billefjorden, Vestspitsbergen. Geol. Mag. 1953, 95, 287–298. [Google Scholar] [CrossRef]
- Bell, F.G. Engineering Properties of Soils and Rocks, 3rd ed.; Butterworth-Heinemann Ltd.: Oxford, UK, 1992; pp. 1–345. [Google Scholar]
- Pina, C.M. Nanoscale dissolution and growth on anhydrite cleavage faces. Geochim. Cosmochim. Acta 2009, 73, 7034–7044. [Google Scholar] [CrossRef][Green Version]
- Anagnostou, G.; Pimentel, E.; Serafeimidis, K. Swelling of sulphatic claystones–some fundamental questions and their practical relevance. Geomech. Tunn. 2010, 3, 567–572. [Google Scholar] [CrossRef]
- Serafeimidis, K.; Anagnostou, G. The solubilities and thermodynamic equilibrium of anhydrite and gypsum. Rock Mech. Rock Eng. 2015, 48, 15–31. [Google Scholar] [CrossRef]
- Kontrec, J.; Kralj, D.; Brečević, L. Transformation of anhydrous calcium sulphate into calcium sulphate dihydrate in aqueous solutions. J. Cryst. Growth 2002, 240, 203–211. [Google Scholar] [CrossRef]
- Serafeimidis, K.; Anagnostou, G. Simultaneous anhydrite dissolution and gypsum precipitation in a closed swelling rock system. In Proceedings of the 46th US Rock Mechanics/Geomechanics Symposium, Symposium Proceedings, Chicago, IL, USA, 24–27 June 2012; Bobet, A., Ewy, R., Gadde, M., Labuz, J., Pyrak-Nolte, L., Westman, E., Eds.; American Rock Mechanics Association (ARMA): Alexandria, VA, USA, 2012; Volume 3, pp. 2249–2259. [Google Scholar]
- Van Driessche, A.E.S.; Stawski, T.M.; Benning, L.B.; Kellermeier, M. Calcium sulfate precipitation throughout its phase diagram. In New Perspectives on Mineral Nucleation and Growth from Solution Precursors to Solid Materials; Van Driessche, A.E.S., Kellermeier, M., Benning, L.G., Gebauer, D., Eds.; Springer International Publishing: Cham, Switzerland, 2017; pp. 227–256. [Google Scholar] [CrossRef]
- Allen, D.G. The origin of sheet fractures in the Galore Creek copper deposits, British Columbia. Can. J. Earth Sci. 1971, 8, 704–711. [Google Scholar] [CrossRef]
- Otálora, F.; Garcia-Ruiz, J.M. Nucleation and growth of the Naica giant gypsum crystals. Chem. Soc. Rev. 2014, 43, 2013–2026. [Google Scholar] [CrossRef]
- Alonso, J.; Moya, M.; Navarro, V.; Asensio, L.; Aguado, J.A. Temperature effect on gypsum-bearing soil and supported building foundations: The case of the Central Storage Facility of Villar de Cañas, Spain. Eng. Geol. 2021, 284, 1–13. [Google Scholar] [CrossRef]
- Berdugo, I.; Romero, E.; Saaltink, M.; Albis, M. On the behaviour of the Ca–SO4–H2O system. Rev. Acad. Colomb. Cienc. 2008, 32, 545–557. [Google Scholar]
- Dutton, S.P.; Kreitler, C.W.; Bracken, B.R. Formation and diagenesis of salt-dome cap rock, Texas gulf coast. In Depositional and Diagenetic Spectra of Evaporites; Handford, C.R., Loucks, R.G., Davies, G.T., Eds.; Society of Economic Paleontologists and Mineralogists, Core Workshop: Calgary, AB, Canada, 1982; pp. 100–129. [Google Scholar] [CrossRef]
- Thordarson, W. Hydrogeology of anhydrite. In Anhydrite Deposits of the United States and Characteristics of Anhydrite Important for Storage of Radioactive Wastes; Dean, W.E., Johnson, K.S., Eds.; U.S. Geological Survey Bulletin; U.S. Government Printing Office: Washington, WA, USA, 1989; Volume 1794, pp. 95–105. [Google Scholar]
- Boidin, E.; Homand, F.; Thomas, F.; Yvon, J. Anhydrite–gypsum transition in the argillites of flooded salt workings in eastern France. Environ. Geol. 2009, 58, 531–542. [Google Scholar] [CrossRef]
- Plümper, O.; Røyne, A.; Magrasó, A.; Jamtveit, B. The interface-scale mechanism of reaction-induced fracturing during serpentinization. Geology 2012, 40, 1103–1106. [Google Scholar] [CrossRef]
- Okamoto, A.; Shimizu, H. Contrasting fracture patterns induced by volume-increasing and -decreasing reactions: Implications for the progress of metamorphic reactions. Earth Planet. Sci. Lett. 2015, 417, 9–18. [Google Scholar] [CrossRef]
- Renard, F. Reaction-induced fracturing: When chemistry breaks rocks. J. Geophys. Res.-Sol. Ea. 2021, 126, e2020JB021451. [Google Scholar] [CrossRef]
- Iyer, K.; Jamtveit, B.; Mathiesen, J.; Malthe-Sørenssen, A.; Feder, J. Reaction-assisted hierarchical fracturing during serpentinization. Earth Planet. Sci. Lett. 2008, 267, 503–516. [Google Scholar] [CrossRef]
- Jamtveit, B.; Putnis, C.V.; Malthe-Sørenssen, A. Reaction induced fracturing during replacement processes. Contrib. Mineral. Petrol. 2009, 157, 127–133. [Google Scholar] [CrossRef]
- Ulven, O.I.; Jamtveit, B.; Malthe-Sørenssen, A. Reaction-driven fracturing of porous rock. J. Geophys. Res. Solid Earth 2014, 119, 7473–7486. [Google Scholar] [CrossRef]
- Røyne, A.; Jamtveit, B. Pore-scale controls on reaction-driven fracturing. Pore-scale geochemical processes. Rev. Mineral. Geochem. 2015, 80, 25–44. [Google Scholar] [CrossRef]
- Schenk, C.J.; Richardson, R.W. Recognition of interstitial anhydrite dissolution: A cause of secondary porosity, San Andres limestone, New Mexico, and Upper Minnelusa Formation, Wyoming. Am. Assoc. Petr. Geol. Bull. 1985, 69, 1064–1076. [Google Scholar] [CrossRef]
- Shindo, H.; Seo, A.; Itasaka, M.; Odaki, T.; Tanaka, K. Stability of surface atomic structures of ionic crystals studied by atomic force microscopy observation of various faces of CaSO4 crystal in solutions. J. Vac. Sci. Technol. 1996, 14, 1365–1368. [Google Scholar] [CrossRef]
- Hall, K. Mechanical weathering in cold regions. In Treatise on Geomorphology; Pope, G.A., Ed.; Academic Press: San Diego, CA, USA, 2013; Volume 4, pp. 258–276. [Google Scholar] [CrossRef]
- Kaiser, P.K.; Amann, F.; Steiner, W. How highly stressed brittle rock failure impacts tunnel design. Rock Mechanics in Civil and Environmental Engineering. In Proceedings of the European Rock Mechanics Symposium (EUROCK) 2010, Lausanne, Switzerland, 15–18 June 2010; Zhao, J., Labiouse, V., Dudt, J.-P., Mathier, J.-F., Eds.; CRC Press/Balkema: London, UK, 2010; pp. 27–38. [Google Scholar]
- Amann, F.; Kaiser, P.K.; Steiner, W. Triggering swelling potential of anhydrite clay rocks by brittle failure processes. Rock Mechanics in Civil and Environmental Engineering. In Proceedings of the European Rock Mechanics Symposium (EUROCK) 2010, Lausanne, Switzerland, 15–18 June 2010; Zhao, J., Labiouse, V., Dudt, J.-P., Mathier, J.-F., Eds.; CRC Press/Balkema: London, UK, 2010; pp. 339–342. [Google Scholar]
- Steiner, W.; Kaiser, P.K.; Spaun, G. Role of brittle fracture in swelling behaviour: Evidence from tunnelling case histories. Geomech. Tunn. 2011, 4, 141–156. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jarzyna, A.; Bąbel, M.; Ługowski, D.; Vladi, F. Petrographic Record and Conditions of Expansive Hydration of Anhydrite in the Recent Weathering Zone at the Abandoned Dingwall Gypsum Quarry, Nova Scotia, Canada. Minerals 2022, 12, 58. https://doi.org/10.3390/min12010058
Jarzyna A, Bąbel M, Ługowski D, Vladi F. Petrographic Record and Conditions of Expansive Hydration of Anhydrite in the Recent Weathering Zone at the Abandoned Dingwall Gypsum Quarry, Nova Scotia, Canada. Minerals. 2022; 12(1):58. https://doi.org/10.3390/min12010058
Chicago/Turabian StyleJarzyna, Adrian, Maciej Bąbel, Damian Ługowski, and Firouz Vladi. 2022. "Petrographic Record and Conditions of Expansive Hydration of Anhydrite in the Recent Weathering Zone at the Abandoned Dingwall Gypsum Quarry, Nova Scotia, Canada" Minerals 12, no. 1: 58. https://doi.org/10.3390/min12010058
APA StyleJarzyna, A., Bąbel, M., Ługowski, D., & Vladi, F. (2022). Petrographic Record and Conditions of Expansive Hydration of Anhydrite in the Recent Weathering Zone at the Abandoned Dingwall Gypsum Quarry, Nova Scotia, Canada. Minerals, 12(1), 58. https://doi.org/10.3390/min12010058





