Differential Activation of TRP Channels in the Adult Rat Spinal Substantia Gelatinosa by Stereoisomers of Plant-Derived Chemicals
Abstract
:1. TRP Channels Involved in Nociceptive Transmission through Dorsal Root Ganglion Neurons
2. Spinal Substantia Gelatinosa Involved in Regulating Nociceptive Transmission
3. TRP Channels in Nociception
4. Actions of Plant-Derived Stereoisomers on Spontaneous Excitatory Transmission in Substantia Gelatinosa Neurons
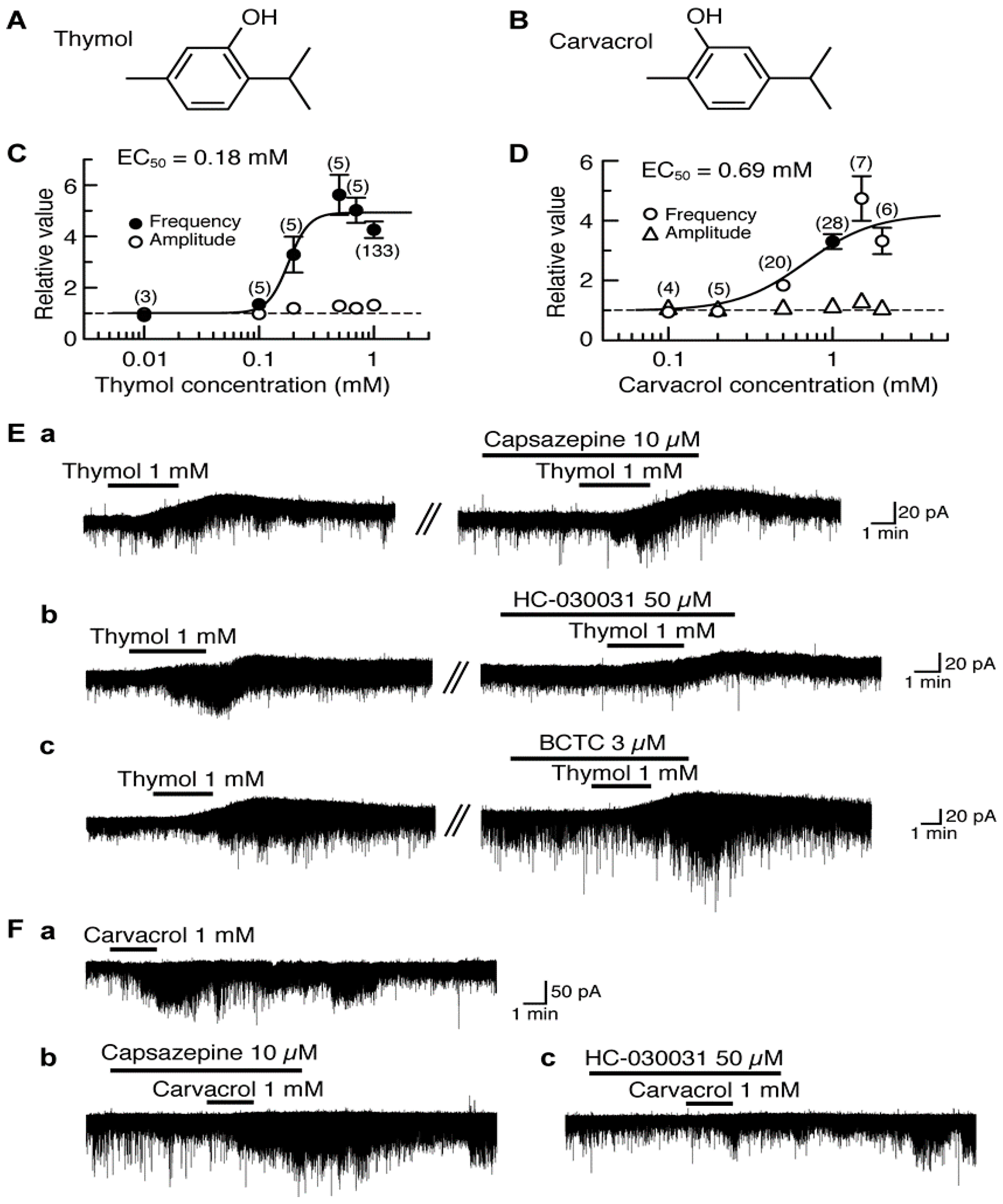
4.1. Actions of Thymol and Carvacrol
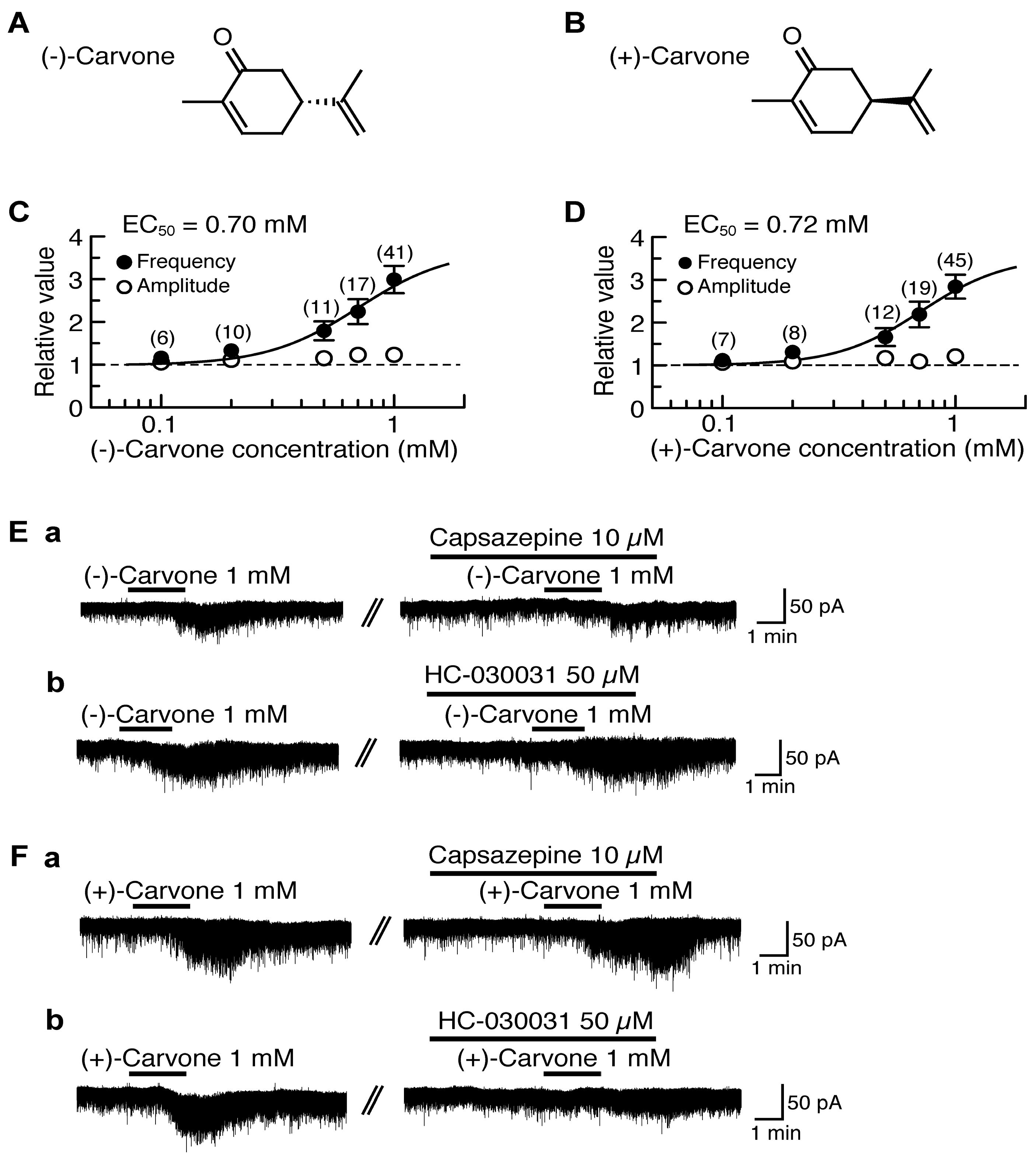
4.2. Actions of (−)-Carvone and (+)-Carvone
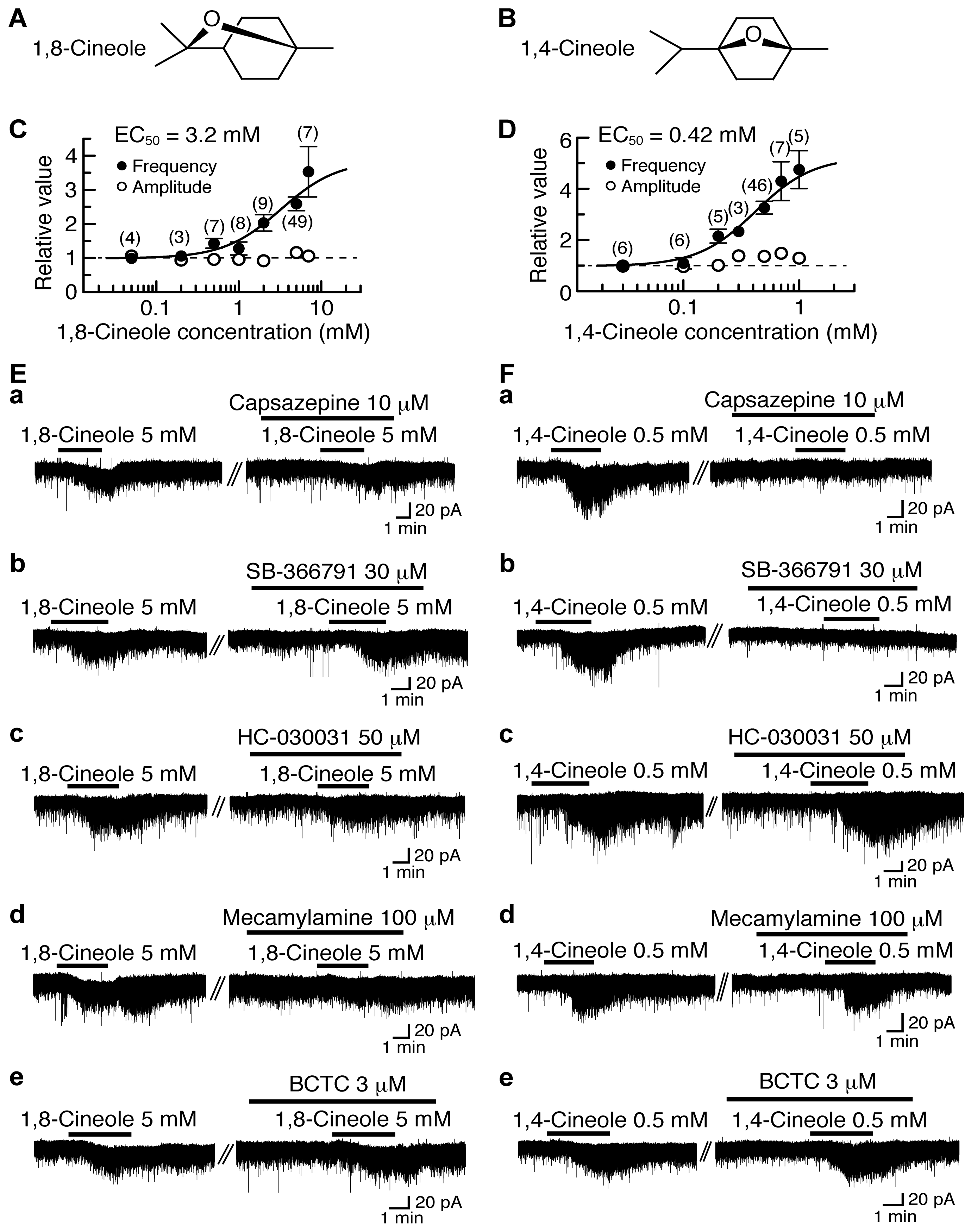
4.3. Actions of 1,8-Cineole and 1,4-Cineole
5. Activation by Plant-Derived Stereoisomers of TRP Channels in the Substantia Gelatinosa in a Different Manner
6. Conclusions
Acknowledgments
Conflicts of Interest
References
- Patapoutian, A.; Tate, S.; Woolf, C.J. Transient receptor potential channels: Targeting pain at the source. Nat. Rev. Drug Discov. 2009, 8, 55–68. [Google Scholar] [CrossRef] [PubMed]
- Story, G.M.; Peier, A.M.; Reeve, A.J.; Eid, S.R.; Mosbacher, J.; Hricik, T.R.; Earley, T.J.; Hergarden, A.C.; Andersson, D.A.; Hwang, S.W.; et al. ANKTM1, a TRP-like channel expressed in nociceptive neurons, is activated by cold temperatures. Cell 2003, 112, 819–829. [Google Scholar] [CrossRef]
- Kobayashi, K.; Fukuoka, T.; Obata, K.; Yamanaka, H.; Dai, Y.; Tokunaga, A.; Noguchi, K. Distinct expression of TRPM8, TRPA1, and TRPV1 mRNAs in rat primary afferent neurons with Aδ/C-fibers and colocalization with Trk receptors. J. Comp. Neurol. 2005, 493, 596–606. [Google Scholar] [CrossRef] [PubMed]
- Tominaga, M. TRP channels and nociception. In Cellular and Molecular Mechanisms for the Modulation of Nociceptive Transmission in the Peripheral and Central Nervous Systems; Kumamoto, E., Ed.; Research Signpost: Kelara, India, 2007; pp. 23–40. [Google Scholar]
- Julius, D. TRP channels and pain. Annu. Rev. Cell Dev. Biol. 2013, 29, 355–384. [Google Scholar] [CrossRef] [PubMed]
- Caterina, M.J.; Schumacher, M.A.; Tominaga, M.; Rosen, T.A.; Levine, J.D.; Julius, D. The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature 1997, 389, 816–824. [Google Scholar] [PubMed]
- Caterina, M.J.; Julius, D. The vanilloid receptor: a molecular gateway to the pain pathway. Annu. Rev. Neurosci. 2001, 24, 487–517. [Google Scholar] [CrossRef] [PubMed]
- Bandell, M.; Story, G.M.; Hwang, S.W.; Viswanath, V.; Eid, S.R.; Petrus, M.J.; Earley, T.J.; Patapoutian, A. Noxious cold ion channel TRPA1 is activated by pungent compounds and bradykinin. Neuron 2004, 41, 849–857. [Google Scholar] [CrossRef]
- Jordt, S.E.; Bautista, D.M.; Chuang, H.H.; McKemy, D.D.; Zygmunt, P.M.; Högestätt, E.D.; Meng, I.D.; Julius, D. Mustard oils and cannabinoids excite sensory nerve fibres through the TRP channel ANKTM1. Nature 2004, 427, 260–265. [Google Scholar] [CrossRef] [PubMed]
- Nilius, B.; Voets, T. TRP channels: a TR(I)P through a world of multifunctional cation channels. Pflügers Arch. 2005, 451, 1–10. [Google Scholar] [CrossRef] [PubMed]
- McKemy, D.D.; Neuhausser, W.M.; Julius, D. Identification of a cold receptor reveals a general role for TRP channels in thermosensation. Nature 2002, 416, 52–58. [Google Scholar] [CrossRef] [PubMed]
- Peier, A.M.; Moqrich, A.; Hergarden, A.C.; Reeve, A.J.; Andersson, D.A.; Story, G.M.; Earley, T.J.; Dragoni, I.; McIntyre, P.; Bevan, S.; et al. A TRP channel that senses cold stimuli and menthol. Cell 2002, 108, 705–715. [Google Scholar] [CrossRef]
- Kumazawa, T.; Perl, E.R. Excitation of marginal and substantia gelatinosa neurons in the primate spinal cord: indications of their place in dorsal horn functional organization. J. Comp. Neurol. 1978, 177, 417–434. [Google Scholar] [CrossRef] [PubMed]
- Sugiura, Y.; Lee, C.L.; Perl, E.R. Central projections of identified, unmyelinated (C) afferent fibers innervating mammalian skin. Science 1986, 234, 358–361. [Google Scholar] [CrossRef] [PubMed]
- Yang, K; Kumamoto, E.; Furue, H.; Yoshimura, M. Capsaicin facilitates excitatory but not inhibitory synaptic transmission in substantia gelatinosa of the rat spinal cord. Neurosci. Lett. 1998, 255, 135–138. [Google Scholar] [CrossRef]
- Morisset, V.; Urbán, L. Cannabinoid-induced presynaptic inhibition of glutamatergic EPSCs in substantia gelatinosa neurons of the rat spinal cord. J. Neurophysiol. 2001, 86, 40–48. [Google Scholar] [PubMed]
- Baccei, M.L.; Bardoni, R.; Fitzgerald, M. Development of nociceptive synaptic inputs to the neonatal rat dorsal horn: glutamate release by capsaicin and menthol. J. Physiol. 2003, 549, 231–242. [Google Scholar] [CrossRef] [PubMed]
- Jiang, C.-Y.; Fujita, T.; Yue, H.-Y.; Piao, L.-H.; Liu, T.; Nakatsuka, T.; Kumamoto, E. Effect of resiniferatoxin on glutamatergic spontaneous excitatory synaptic transmission in substantia gelatinosa neurons of the adult rat spinal cord. Neuroscience 2009, 164, 1833–1844. [Google Scholar] [CrossRef] [PubMed]
- Kosugi, M.; Nakatsuka, T.; Fujita, T.; Kuroda, Y.; Kumamoto, E. Activation of TRPA1 channel facilitates excitatory synaptic transmission in substantia gelatinosa neurons of the adult rat spinal cord. J. Neurosci. 2007, 27, 4443–4451. [Google Scholar] [CrossRef] [PubMed]
- Uta, D.; Furue, H.; Pickering, A.E.; Rashid, M.H.; Mizuguchi-Takase, H.; Katafuchi, T.; Imoto, K.; Yoshimura, M. TRPA1-expressing primary afferents synapse with a morphologically identified subclass of substantia gelatinosa neurons in the adult rat spinal cord. Eur. J. Neurosci. 2010, 31, 1960–1973. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, S.C.; Furue, H.; Koga, K.; Jiang, N.; Nohmi, M.; Shimazaki, Y.; Katoh-Fukui, Y.; Yokoyama, M.; Yoshimura, M.; Takeichi, M. Cadherin-8 is required for the first relay synapses to receive functional inputs from primary sensory afferents for cold sensation. J. Neurosci. 2007, 27, 3466–3476. [Google Scholar] [CrossRef] [PubMed]
- Wrigley, P.J.; Jeong, H.-J.; Vaughan, C.W. Primary afferents with TRPM8 and TRPA1 profiles target distinct subpopulations of rat superficial dorsal horn neurones. Br. J. Pharmacol. 2009, 157, 371–380. [Google Scholar] [CrossRef] [PubMed]
- Kumamoto, E.; Fujita, T.; Jiang, C.-Y. TRP channels involved in spontaneous l-glutamate release enhancement in the adult rat spinal substantia gelatinosa. Cells 2014, 3, 331–362. [Google Scholar] [CrossRef] [PubMed]
- Melzack, R.; Wall, P.D. Pain mechanisms: a new theory. Science 1965, 150, 971–979. [Google Scholar] [CrossRef] [PubMed]
- Willis, W.D., Jr.; Coggeshall, R.E. Sensory Mechanisms of the Spinal Cord, 2nd ed.; Plenum: New York, NY, USA, 1991. [Google Scholar]
- Todd, A.J. Neuronal circuitry for pain processing in the dorsal horn. Nat. Rev. Neurosci. 2010, 11, 823–836. [Google Scholar] [CrossRef] [PubMed]
- Jiang, C.-Y.; Wang, C.; Xu, N.-X.; Fujita, T.; Murata, Y.; Kumamoto, E. 1,8- and 1,4-cineole enhance spontaneous excitatory transmission by activating different types of transient receptor potential channels in the rat spinal substantia gelatinosa. J. Neurochem. 2016, 136, 764–777. [Google Scholar] [CrossRef] [PubMed]
- Yoshimura, M.; Nishi, S. Blind patch-clamp recordings from substantia gelatinosa neurons in adult rat spinal cord slices: pharmacological properties of synaptic currents. Neuroscience 1993, 53, 519–526. [Google Scholar] [CrossRef]
- Nakatsuka, T.; Park, J.-S.; Kumamoto, E.; Tamaki, T.; Yoshimura, M. Plastic changes in sensory inputs to rat substantia gelatinosa neurons following peripheral inflammation. Pain 1999, 82, 39–47. [Google Scholar] [CrossRef]
- Ito, A.; Kumamoto, E.; Takeda, M.; Takeda, M.; Shibata, K.; Sagai, H.; Yoshimura, M. Mechanisms for ovariectomy-induced hyperalgesia and its relief by calcitonin: participation of 5-HT1A-like receptor on C-afferent terminals in substantia gelatinosa of the rat spinal cord. J. Neurosci. 2000, 20, 6302–6308. [Google Scholar] [PubMed]
- Fürst, S. Transmitters involved in antinociception in the spinal cord. Brain Res. Bull. 1999, 48, 129–141. [Google Scholar] [CrossRef]
- Yoshimura, M.; North, R.A. Substantia gelatinosa neurones hyperpolarized in vitro by enkephalin. Nature 1983, 305, 529–530. [Google Scholar] [CrossRef] [PubMed]
- Fujita, T.; Nakatsuka, T.; Kumamoto, E. Opioid receptor activation in spinal dorsal horn. In Cellular and Molecular Mechanisms for the Modulation of Nociceptive Transmission in the Peripheral and Central Nervous Systems; Kumamoto, E., Ed.; Research Signpost: Kelara, India, 2007; pp. 87–111. [Google Scholar]
- Lai, C.C.; Wu, S.Y.; Dun, S.L.; Dun, N.J. Nociceptin-like immunoreactivity in the rat dorsal horn and inhibition of substantia gelatinosa neurons. Neuroscience 1997, 81, 887–891. [Google Scholar] [CrossRef]
- Luo, C.; Kumamoto, E.; Furue, H.; Yoshimura, M. Nociceptin-induced outward current in substantia gelatinosa neurones of the adult rat spinal cord. Neuroscience 2001, 108, 323–330. [Google Scholar] [CrossRef]
- Kangrga, I.; Jiang, M.; Randić, M. Actions of (−)-baclofen on rat dorsal horn neurons. Brain Res. 1991, 562, 265–275. [Google Scholar] [CrossRef]
- Yang, K.; Kumamoto, E.; Furue, H.; Li, Y.-Q.; Yoshimura, M. Capsaicin induces a slow inward current which is not mediated by substance P in substantia gelatinosa neurons of the rat spinal cord. Neuropharmacology 2000, 39, 2185–2194. [Google Scholar] [CrossRef]
- Koga, A.; Fujita, T.; Totoki, T.; Kumamoto, E. Tramadol produces outward currents by activating μ-opioid receptors in adult rat substantia gelatinosa neurones. Br. J. Pharmacol. 2005, 145, 602–607. [Google Scholar] [CrossRef] [PubMed]
- Sonohata, M.; Furue, H.; Katafuchi, T.; Yasaka, T.; Doi, A.; Kumamoto, E.; Yoshimura, M. Actions of noradrenaline on substantia gelatinosa neurones in the rat spinal cord revealed by in vivo patch recording. J. Physiol. 2004, 555, 515–526. [Google Scholar] [CrossRef] [PubMed]
- Abe, K.; Kato, G.; Katafuchi, T.; Tamae, A.; Furue, H.; Yoshimura, M. Responses to 5-HT in morphologically identified neurons in the rat substantia gelatinosa in vitro. Neuroscience 2009, 159, 316–324. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Perl, E.R. Adenosine inhibition of synaptic transmission in the substantia gelatinosa. J. Neurophysiol. 1994, 72, 1611–1621. [Google Scholar] [PubMed]
- Liu, T.; Fujita, T.; Kawasaki, Y.; Kumamoto, E. Regulation by equilibrative nucleoside transporter of adenosine outward currents in adult rat spinal dorsal horn neurons. Brain Res. Bull. 2004, 64, 75–83. [Google Scholar] [CrossRef] [PubMed]
- Kumamoto, E.; Fujita, T. Role of adenosine in regulating nociceptive transmission in the spinal dorsal horn. In Recent Research Developments in Physiology; Pandalai, S.G., Ed.; Research Signpost: Kerala, India, 2005; Volume 3, pp. 39–57. [Google Scholar]
- Jiang, N.; Furue, H.; Katafuchi, T.; Yoshimura, M. Somatostatin directly inhibits substantia gelatinosa neurons in adult rat spinal dorsal horn in vitro. Neurosci. Res. 2003, 47, 97–107. [Google Scholar] [CrossRef]
- Nakatsuka, T.; Fujita, T.; Inoue, K.; Kumamoto, E. Activation of GIRK channels in substantia gelatinosa neurones of the adult rat spinal cord: a possible involvement of somatostatin. J. Physiol. 2008, 586, 2511–2522. [Google Scholar] [CrossRef] [PubMed]
- Tamae, A.; Nakatsuka, T.; Koga, K.; Kato, G.; Furue, H.; Katafuchi, T.; Yoshimura, M. Direct inhibition of substantia gelatinosa neurones in the rat spinal cord by activation of dopamine D2-like receptors. J. Physiol. 2005, 568, 243–253. [Google Scholar] [CrossRef] [PubMed]
- Taniguchi, W.; Nakatsuka, T.; Miyazaki, N.; Yamada, H.; Takeda, D.; Fujita, T.; Kumamoto, E.; Yoshida, M. In vivo patch-clamp analysis of dopaminergic antinociceptive actions on substantia gelatinosa neurons in the spinal cord. Pain 2011, 152, 95–105. [Google Scholar] [CrossRef] [PubMed]
- Yue, H.-Y.; Fujita, T.; Kumamoto, E. Biphasic modulation by galanin of excitatory synaptic transmission in substantia gelatinosa neurons of adult rat spinal cord slices. J. Neurophysiol. 2011, 105, 2337–2349. [Google Scholar] [CrossRef] [PubMed]
- Kohno, T.; Kumamoto, E.; Higashi, H.; Shimoji, K.; Yoshimura, M. Actions of opioids on excitatory and inhibitory transmission in substantia gelatinosa of adult rat spinal cord. J. Physiol. 1999, 518, 803–813. [Google Scholar] [CrossRef] [PubMed]
- Liebel, J.T.; Swandulla, D.; Zeilhofer, H.U. Modulation of excitatory synaptic transmission by nociceptin in superficial dorsal horn neurones of the neonatal rat spinal cord. Br. J. Pharmacol. 1997, 121, 425–432. [Google Scholar] [CrossRef] [PubMed]
- Luo, C.; Kumamoto, E.; Furue, H.; Chen, J.; Yoshimura, M. Nociceptin inhibits excitatory but not inhibitory transmission to substantia gelatinosa neurones of adult rat spinal cord. Neuroscience 2002, 109, 349–358. [Google Scholar] [CrossRef]
- Ataka, T.; Kumamoto, E.; Shimoji, K.; Yoshimura, M. Baclofen inhibits more effectively C-afferent than Aδ-afferent glutamatergic transmission in substantia gelatinosa neurons of adult rat spinal cord slices. Pain 2000, 86, 273–282. [Google Scholar] [CrossRef]
- Iyadomi, M.; Iyadomi, I.; Kumamoto, E.; Tomokuni, K.; Yoshimura, M. Presynaptic inhibition by baclofen of miniature EPSCs and IPSCs in substantia gelatinosa neurons of the adult rat spinal dorsal horn. Pain 2000, 85, 385–393. [Google Scholar] [CrossRef]
- Luo, C.; Kumamoto, E.; Furue, H.; Chen, J.; Yoshimura, M. Anandamide inhibits excitatory transmission to rat substantia gelatinosa neurones in a manner different from that of capsaicin. Neurosci. Lett. 2002, 321, 17–20. [Google Scholar] [CrossRef]
- Kawasaki, Y.; Kumamoto, E.; Furue, H.; Yoshimura, M. α2 Adrenoceptor-mediated presynaptic inhibition of primary afferent glutamatergic transmission in rat substantia gelatinosa neurons. Anesthesiology 2003, 98, 682–689. [Google Scholar] [CrossRef] [PubMed]
- Lao, L.-J.; Kumamoto, E.; Luo, C.; Furue, H.; Yoshimura, M. Adenosine inhibits excitatory transmission to substantia gelatinosa neurons of the adult rat spinal cord through the activation of presynaptic A1 adenosine receptor. Pain 2001, 94, 315–324. [Google Scholar] [CrossRef]
- Lao, L.-J.; Kawasaki, Y.; Yang, K.; Fujita, T.; Kumamoto, E. Modulation by adenosine of Aδ and C primary-afferent glutamatergic transmission in adult rat substantia gelatinosa neurons. Neuroscience 2004, 125, 221–231. [Google Scholar] [CrossRef] [PubMed]
- Alier, K.A.; Chen, Y.; Sollenberg, U.E.; Langel, Ü.; Smith, P.A. Selective stimulation of GalR1 and GalR2 in rat substantia gelatinosa reveals a cellular basis for the anti- and pro-nociceptive actions of galanin. Pain 2008, 137, 138–146. [Google Scholar] [CrossRef] [PubMed]
- Hudson, L.J.; Bevan, S.; Wotherspoon, G.; Gentry, C.; Fox, A.; Winter, J. VR1 protein expression increases in undamaged DRG neurons after partial nerve injury. Eur. J. Neurosci. 2001, 13, 2105–2114. [Google Scholar] [CrossRef] [PubMed]
- Walker, K.M.; Urbán, L.; Medhurst, S.J.; Patel, S.; Panesar, M.; Fox, A.J.; McIntyre, P. The VR1 antagonist capsazepine reverses mechanical hyperalgesia in models of inflammatory and neuropathic pain. J. Pharmacol. Exp. Ther. 2003, 304, 56–62. [Google Scholar] [CrossRef] [PubMed]
- Gavva, N.R.; Tamir, R.; Qu, Y.; Klionsky, L.; Zhang, T.J.; Immke, D.; Wang, J.; Zhu, D.; Vanderah, T.W.; Porreca, F.; et al. AMG 9810 [(E)-3-(4-t-butylphenyl)-N-(2,3-dihydrobenzo[b][1,4] dioxin-6-yl)acrylamide], a novel vanilloid receptor 1 (TRPV1) antagonist with antihyperalgesic properties. J. Pharmacol. Exp. Ther. 2005, 313, 474–484. [Google Scholar] [CrossRef] [PubMed]
- Culshaw, A.J.; Bevan, S.; Christiansen, M.; Copp, P.; Davis, A.; Davis, C.; Dyson, A.; Dziadulewicz, E.K.; Edwards, L.; Eggelte, H.; et al. Identification and biological characterization of 6-aryl-7-isopropylquinazolinones as novel TRPV1 antagonists that are effective in models of chronic pain. J. Med. Chem. 2006, 49, 471–474. [Google Scholar] [CrossRef] [PubMed]
- Davis, J.B.; Gray, J.; Gunthorpe, M.J.; Hatcher, J.P.; Davey, P.T.; Overend, P.; Harries, M.H.; Latcham, J.; Clapham, C.; Atkinson, K.; et al. Vanilloid receptor-1 is essential for inflammatory thermal hyperalgesia. Nature 2000, 405, 183–187. [Google Scholar] [CrossRef] [PubMed]
- Bron, R.; Klesse, L.J.; Shah, K.; Parada, L.F.; Winter, J. Activation of Ras is necessary and sufficient for upregulation of vanilloid receptor type 1 in sensory neurons by neurotrophic factors. Mol. Cell. Neurosci. 2003, 22, 118–132. [Google Scholar] [CrossRef]
- Yiangou, Y.; Facer, P.; Dyer, N.H.C.; Chan, C.L.H.; Knowles, C.; Williams, N.S.; Anand, P. Vanilloid receptor 1 immunoreactivity in inflamed human bowel. Lancet 2001, 357, 1338–1339. [Google Scholar] [CrossRef]
- Chan, C.L.H.; Facer, P.; Davis, J.B.; Smith, G.D.; Egerton, J.; Bountra, C.; Williams, N.S.; Anand, P. Sensory fibres expressing capsaicin receptor TRPV1 in patients with rectal hypersensitivity and faecal urgency. Lancet 2003, 361, 385–391. [Google Scholar] [CrossRef]
- Lappin, S.C.; Randall, A.D.; Gunthorpe, M.J.; Morisset, V. TRPV1 antagonist, SB-366791, inhibits glutamatergic synaptic transmission in rat spinal dorsal horn following peripheral inflammation. Eur. J. Pharmacol. 2006, 540, 73–81. [Google Scholar] [CrossRef] [PubMed]
- Szallasi, A.; Blumberg, P.M. Resiniferatoxin, a phorbol-related diterpene, acts as an ultrapotent analog of capsaicin, the irritant constituent in red pepper. Neuroscience 1989, 30, 515–520. [Google Scholar] [CrossRef]
- Brown, D.C.; Iadarola, M.J.; Perkowski, S.Z.; Erin, H.; Shofer, F.; Laszlo, K.J.; Olah, Z.; Mannes, A.J. Physiologic and antinociceptive effects of intrathecal resiniferatoxin in a canine bone cancer model. Anesthesiology 2005, 103, 1052–1059. [Google Scholar] [CrossRef] [PubMed]
- Ghilardi, J.R.; Röhrich, H.; Lindsay, T.H.; Sevcik, M.A.; Schwei, M.J.; Kubota, K.; Halvorson, K.G.; Poblete, J.; Chaplan, S.R.; Dubin, A.E.; et al. Selective blockade of the capsaicin receptor TRPV1 attenuates bone cancer pain. J. Neurosci. 2005, 25, 3126–3131. [Google Scholar] [CrossRef] [PubMed]
- Prevarskaya, N.; Zhang, L.; Barritt, G. TRP channels in cancer. Biochim. Biophys. Acta 2007, 1772, 937–946. [Google Scholar] [CrossRef] [PubMed]
- Watabiki, T.; Kiso, T.; Tsukamoto, M.; Aoki, T.; Matsuoka, N. Intrathecal administration of AS1928370, a transient receptor potential vanilloid 1 antagonist, attenuates mechanical allodynia in a mouse model of neuropathic pain. Biol. Pharm. Bull. 2011, 34, 1105–1108. [Google Scholar] [CrossRef] [PubMed]
- Namer, B.; Seifert, F.; Handwerker, H.O.; Maihöfner, C. TRPA1 and TRPM8 activation in humans: effects of cinnamaldehyde and menthol. NeuroReport 2005, 16, 955–959. [Google Scholar] [CrossRef] [PubMed]
- Obata, K.; Katsura, H.; Mizushima, T.; Yamanaka, H.; Kobayashi, K.; Dai, Y.; Fukuoka, T.; Tokunaga, A.; Tominaga, M.; Noguchi, K. TRPA1 induced in sensory neurons contributes to cold hyperalgesia after inflammation and nerve injury. J. Clin. Investig. 2005, 115, 2393–2401. [Google Scholar] [CrossRef] [PubMed]
- da Costa, D.S.M.; Meotti, F.C.; Andrade, E.L.; Leal, P.C.; Motta, E.M.; Calixto, J.B. The involvement of the transient receptor potential A1 (TRPA1) in the maintenance of mechanical and cold hyperalgesia in persistent inflammation. Pain 2010, 148, 431–437. [Google Scholar] [CrossRef] [PubMed]
- Proudfoot, C.J.; Garry, E.M.; Cottrell, D.F.; Rosie, R.; Anderson, H.; Robertson, D.C.; Fleetwood-Walker, S.M.; Mitchell, R. Analgesia mediated by the TRPM8 cold receptor in chronic neuropathic pain. Curr. Biol. 2006, 16, 1591–1605. [Google Scholar] [CrossRef] [PubMed]
- Xing, H.; Chen, M.; Ling, J.; Tan, W.; Gu, J.G. TRPM8 mechanism of cold allodynia after chronic nerve injury. J. Neurosci. 2007, 27, 13680–13690. [Google Scholar] [CrossRef] [PubMed]
- Kono, T.; Satomi, M.; Suno, M.; Kimura, N.; Yamazaki, H.; Furukawa, H.; Matsubara, K. Oxaliplatin-induced neurotoxicity involves TRPM8 in the mechanism of acute hypersensitivity to cold sensation. Brain Behav. 2012, 2, 68–73. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Fan, L.; Balakrishna, S.; Sui, A.; Morris, J.B.; Jordt, S.-E. TRPM8 is the principal mediator of menthol-induced analgesia of acute and inflammatory pain. Pain 2013, 154, 2169–2177. [Google Scholar] [CrossRef] [PubMed]
- Edwards, J.G. TRPV1 in the central nervous system: synaptic plasticity, function, and pharmacological implications. Prog. Drug Res. 2014, 68, 77–104. [Google Scholar] [PubMed]
- Zygmunt, P.M.; Högestätt, E.D. TRPA1. Handb. Exp. Pharmacol. 2014, 222, 583–630. [Google Scholar] [PubMed]
- Soudijn, W.; van Wijngaarden, I.; IJzerman, A.P. Stereoselectivity of drug-receptor interactions. IDrugs 2003, 6, 43–56. [Google Scholar]
- Valenzuela, C.; Moreno, C.; de la Cruz, A.; Macías, Á.; Prieto, Á.; González, T. Stereoselective interactions between local anesthetics and ion channels. Chirality 2012, 24, 944–950. [Google Scholar] [CrossRef] [PubMed]
- Karashima, Y.; Damann, N.; Prenen, J.; Talavera, K.; Segal, A.; Voets, T.; Nilius, B. Bimodal action of menthol on the transient receptor potential channel TRPA1. J. Neurosci. 2007, 27, 9874–9884. [Google Scholar] [CrossRef] [PubMed]
- Yang, K.; Li, Y.-Q.; Kumamoto, E.; Furue, H.; Yoshimura, M. Voltage-clamp recordings of postsynaptic currents in substantia gelatinosa neurons in vitro and its applications to assess synaptic transmission. Brain Res. Protoc. 2001, 7, 235–240. [Google Scholar] [CrossRef]
- Baser, K.H.C. Biological and pharmacological activities of carvacrol and carvacrol bearing essential oils. Curr. Pharm. Des. 2008, 14, 3106–3119. [Google Scholar] [CrossRef] [PubMed]
- Angeles-López, G.; Pérez-Vásquez, A.; Hernández-Luis, F.; Déciga-Campos, M.; Bye, R.; Linares, E.; Mata, R. Antinociceptive effect of extracts and compounds from Hofmeisteria schaffneri. J. Ethnopharmacol. 2010, 131, 425–432. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.-H.; Wang, C.; Fujita, T.; Jiang, C.-Y.; Kumamoto, E. Action of thymol on spontaneous excitatory transmission in adult rat spinal substantia gelatinosa neurons. Neurosci. Lett. 2015, 606, 94–99. [Google Scholar] [CrossRef] [PubMed]
- Luo, Q.-T.; Fujita, T.; Jiang, C.-Y.; Kumamoto, E. Carvacrol presynaptically enhances spontaneous excitatory transmission and produces outward current in adult rat spinal substantia gelatinosa neurons. Brain Res. 2014, 1592, 44–54. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Delling, M.; Jun, J.C.; Clapham, D.E. Oregano, thyme and clove-derived flavors and skin sensitizers activate specific TRP channels. Nat. Neurosci. 2006, 9, 628–635. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.P.; Buber, M.T.; Yang, Q.; Cerne, R.; Cortés, R.Y.; Sprous, D.G.; Bryant, R.W. Thymol and related alkyl phenols activate the hTRPA1 channel. Br. J. Pharmacol. 2008, 153, 1739–1749. [Google Scholar] [CrossRef] [PubMed]
- Ortar, G.; Morera, L.; Moriello, A.S.; Morera, E.; Nalli, M.; Di Marzo, V.; De Petrocellis, L. Modulation of thermo-transient receptor potential (thermo-TRP) channels by thymol-based compounds. Bioorg. Med. Chem. Lett. 2012, 22, 3535–3539. [Google Scholar] [CrossRef] [PubMed]
- Vogt-Eisele, A.K.; Weber, K.; Sherkheli, M.A.; Vielhaber, G.; Panten, J.; Gisselmann, G.; Hatt, H. Monoterpenoid agonists of TRPV3. Br. J. Pharmacol. 2007, 151, 530–540. [Google Scholar] [CrossRef] [PubMed]
- de la Roche, J.; Eberhardt, M.J.; Klinger, A.B.; Stanslowsky, N.; Wegner, F.; Koppert, W.; Reeh, P.W.; Lampert, A.; Fischer, M.J.M.; Leffler, A. The molecular basis for species-specific activation of human TRPA1 protein by protons involves poorly conserved residues within transmembrane domains 5 and 6. J. Biol. Chem. 2013, 288, 20280–20292. [Google Scholar] [CrossRef] [PubMed]
- McNamara, C.R.; Mandel-Brehm, J.; Bautista, D.M.; Siemens, J.; Deranian, K.L.; Zhao, M.; Hayward, N.J.; Chong, J.A.; Julius, D.; Moran, M.M.; et al. TRPA1 mediates formalin-induced pain. Proc. Natl. Acad. Sci. USA 2007, 104, 13525–13530. [Google Scholar] [CrossRef] [PubMed]
- Urban, L.; Dray, A. Capsazepine, a novel capsaicin antagonist, selectively antagonises the effects of capsaicin in the mouse spinal cord in vitro. Neurosci. Lett. 1991, 134, 9–11. [Google Scholar] [CrossRef]
- Madrid, R.; Donovan-Rodríguez, T.; Meseguer, V.; Acosta, M.C.; Belmonte, C.; Viana, F. Contribution of TRPM8 channels to cold transduction in primary sensory neurons and peripheral nerve terminals. J. Neurosci. 2006, 26, 12512–12525. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, J.C.R.; Silveira, A.L.; de Souza, H.D.N.; Nery, A.A.; Prado, V.F.; Prado, M.A.M.; Ulrich, H.; Araújo, D.A.M. The monoterpene (−)-carvone: a novel agonist of TRPV1 channels. Cytometry Part A 2013, 83, 212–219. [Google Scholar] [CrossRef] [PubMed]
- Buchbauer, G.; Jäger, W.; Gruber, A.; Dietrich, H. R-(+)- and S-(−)-carvone: influence of chirality on locomotion activity in mice. Flavour Fragr. J. 2005, 20, 686–689. [Google Scholar] [CrossRef]
- de Sousa, D.P.; de Farias Nóbrega, F.F.; de Almeida, R.N. Influence of the chirality of (R)-(−)- and (S)-(+)-carvone in the central nervous system: a comparative study. Chirality 2007, 19, 264–268. [Google Scholar] [CrossRef] [PubMed]
- Kang, Q.; Jiang, C.-Y.; Fujita, T.; Kumamoto, E. Spontaneous l-glutamate release enhancement in rat substantia gelatinosa neurons by (−)-carvone and (+)-carvone which activate different types of TRP channel. Biochem. Biophys. Res. Commun. 2015, 459, 498–503. [Google Scholar] [CrossRef] [PubMed]
- Liapi, C.; Anifantis, G.; Chinou, I.; Kourounakis, A.P.; Theodosopoulos, S.; Galanopoulou, P. Antinociceptive properties of 1,8-cineole and β-pinene, from the essential oil of Eucalyptus camaldulensis leaves, in rodents. Planta Med. 2007, 73, 1247–1254. [Google Scholar] [CrossRef] [PubMed]
- Romagni, J.G.; Allen, S.N.; Dayan, F.E. Allelopathic effects of volatile cineoles on two weedy plant species. J. Chem. Ecol. 2000, 26, 303–313. [Google Scholar] [CrossRef]
- Behrendt, H.-J.; Germann, T.; Gillen, C.; Hatt, H.; Jostock, R. Characterization of the mouse cold-menthol receptor TRPM8 and vanilloid receptor type-1 VR1 using a fluorometric imaging plate reader (FLIPR) assay. Br. J. Pharmacol. 2004, 141, 737–745. [Google Scholar] [CrossRef] [PubMed]
- Takaishi, M.; Fujita, F.; Uchida, K.; Yamamoto, S.; Sawada Shimizu, M.; Hatai Uotsu, C.; Shimizu, M.; Tominaga, M. 1,8-Cineole, a TRPM8 agonist, is a novel natural antagonist of human TRPA1. Mol. Pain 2012, 8, 86. [Google Scholar] [CrossRef] [PubMed]
- Talavera, K.; Gees, M.; Karashima, Y.; Meseguer, V.M.; Vanoirbeek, J.A.J.; Damann, N.; Everaerts, W.; Benoit, M.; Janssens, A.; Vennekens, R.; et al. Nicotine activates the chemosensory cation channel TRPA1. Nat. Neurosci. 2009, 12, 1293–1299. [Google Scholar] [CrossRef] [PubMed]
- Zygmunt, P.M.; Petersson, J.; Andersson, D.A.; Chuang, H.-h.; Sørgård, M.; Di Marzo, V.; Julius, D.; Högestätt, E.D. Vanilloid receptors on sensory nerves mediate the vasodilator action of anandamide. Nature 1999, 400, 452–457. [Google Scholar] [PubMed]
- Hwang, S.W.; Cho, H.; Kwak, J.; Lee, S.-Y.; Kang, C.-J.; Jung, J.; Cho, S.; Min, K.H.; Suh, Y.-G.; Kim, D.; et al. Direct activation of capsaicin receptors by products of lipoxygenases: endogenous capsaicin-like substances. Proc. Natl. Acad. Sci. USA 2000, 97, 6155–6160. [Google Scholar] [CrossRef] [PubMed]
- Starowicz, K.; Niga, S.; Di Marzo, V. Biochemistry and pharmacology of endovanilloids. Pharmacol. Ther. 2007, 114, 13–33. [Google Scholar] [CrossRef] [PubMed]
- Yue, H.-Y.; Fujita, T.; Kawasaki, Y.; Kumamoto, E. AM404 enhances the spontaneous release of l-glutamate in a manner sensitive to capsazepine in adult rat substantia gelatinosa neurones. Brain Res. 2004, 1018, 283–287. [Google Scholar] [CrossRef] [PubMed]
- Niforatos, W.; Zhang, X.-F.; Lake, M.R.; Walter, K.A.; Neelands, T.; Holzman, T.F.; Scott, V.E.; Faltynek, C.R.; Moreland, R.B.; Chen, J. Activation of TRPA1 channels by the fatty acid amide hydrolase inhibitor 3′-carbamoylbiphenyl-3-yl cyclohexylcarbamate (URB597). Mol. Pharmacol. 2007, 71, 1209–1216. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Orengo, L.; Dhaka, A.; Heuermann, R.J.; Young, T.J.; Montana, M.C.; Cavanaugh, E.J.; Kim, D.; Story, G.M. Cutaneous nociception evoked by 15-delta PGJ2 via activation of ion channel TRPA1. Mol. Pain 2008, 4, 30. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Kohno, T.; Amaya, F.; Brenner, G.J.; Ito, N.; Allchorne, A.; Ji, R.-R.; Woolf, C.J. Bradykinin produces pain hypersensitivity by potentiating spinal cord glutamatergic synaptic transmission. J. Neurosci. 2005, 25, 7986–7992. [Google Scholar] [CrossRef] [PubMed]
- Asuthkar, S.; Elustondo, P.A.; Demirkhanyan, L.; Sun, X.; Baskaran, P.; Velpula, K.K.; Thyagarajan, B.; Pavlov, E.V.; Zakharian, E. The TRPM8 protein is a testosterone receptor: I. Biochemical evidence for direct TRPM8-testosterone interactions. J. Biol. Chem. 2015, 290, 2659–2669. [Google Scholar] [CrossRef] [PubMed]
- Asuthkar, S.; Demirkhanyan, L.; Sun, X.; Elustondo, P.A.; Krishnan, V.; Baskaran, P.; Velpula, K.K.; Thyagarajan, B.; Pavlov, E.V.; Zakharian, E. The TRPM8 protein is a testosterone receptor: II. Functional evidence for an ionotropic effect of testosterone on TRPM8. J. Biol. Chem. 2015, 290, 2670–2688. [Google Scholar] [CrossRef] [PubMed]
- Leffler, A.; Fischer, M.J.; Rehner, D.; Kienel, S.; Kistner, K.; Sauer, S.K.; Gavva, N.R.; Reeh, P.W.; Nau, C. The vanilloid receptor TRPV1 is activated and sensitized by local anesthetics in rodent sensory neurons. J. Clin. Invest. 2008, 118, 763–776. [Google Scholar] [CrossRef] [PubMed]
- Leffler, A.; Lattrell, A.; Kronewald, S.; Niedermirtl, F.; Nau, C. Activation of TRPA1 by membrane permeable local anesthetics. Mol. Pain 2011, 7, 62. [Google Scholar] [CrossRef] [PubMed]
- Piao, L.-H.; Fujita, T.; Jiang, C.-Y.; Liu, T.; Yue, H.-Y.; Nakatsuka, T.; Kumamoto, E. TRPA1 activation by lidocaine in nerve terminals results in glutamate release increase. Biochem. Biophys. Res. Commun. 2009, 379, 980–984. [Google Scholar] [CrossRef] [PubMed]
- Szallasi, A. Piperine: researchers discover new flavor in an ancient spice. Trends Pharmacol. Sci. 2005, 26, 437–439. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Fujita, T.; Jiang, C.-Y.; Piao, L.-H.; Yue, H.-Y.; Mizuta, K.; Kumamoto, E. TRPV1 agonist piperine but not olvanil enhances glutamatergic spontaneous excitatory transmission in rat spinal substantia gelatinosa neurons. Biochem. Biophys. Res. Commun. 2011, 410, 841–845. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Simon, S.A. Similarities and differences in the currents activated by capsaicin, piperine, and zingerone in rat trigeminal ganglion cells. J. Neurophysiol. 1996, 76, 1858–1869. [Google Scholar] [PubMed]
- Liu, L.; Lo, Y.-C.; Chen, I.-J.; Simon, S.A. The responses of rat trigeminal ganglion neurons to capsaicin and two nonpungent vanilloid receptor agonists, olvanil and glyceryl nonamide. J. Neurosci. 1997, 17, 4101–4111. [Google Scholar] [PubMed]
- Yang, B.H.; Piao, Z.G.; Kim, Y.-B.; Lee, C.-H.; Lee, J.K.; Park, K.; Kim, J.S.; Oh, S.B. Activation of vanilloid receptor 1 (VR1) by eugenol. J. Dent. Res. 2003, 82, 781–785. [Google Scholar] [CrossRef] [PubMed]
- Inoue, M.; Fujita, T.; Goto, M.; Kumamoto, E. Presynaptic enhancement by eugenol of spontaneous excitatory transmission in rat spinal substantia gelatinosa neurons is mediated by transient receptor potential A1 channels. Neuroscience 2012, 210, 403–415. [Google Scholar] [CrossRef] [PubMed]
- Yue, H.-Y.; Jiang, C.-Y.; Fujita, T.; Kumamoto, E. Zingerone enhances glutamatergic spontaneous excitatory transmission by activating TRPA1 but not TRPV1 channels in the adult rat substantia gelatinosa. J. Neurophysiol. 2013, 110, 658–671. [Google Scholar] [CrossRef] [PubMed]
- Park, C.-K.; Xu, Z.-Z.; Berta, T.; Han, Q.; Chen, G.; Liu, X.-J.; Ji, R.-R. Extracellular microRNAs activate nociceptor neurons to elicit pain via TLR7 and TRPA1. Neuron 2014, 82, 47–54. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Fujita, T.; Jiang, C.-Y.; Kumamoto, E. Enhancement by citral of glutamatergic spontaneous excitatory transmission in adult rat substantia gelatinosa neurons. NeuroReport 2016, 27, 166–171. [Google Scholar] [CrossRef] [PubMed]
- Andersson, D.A.; Gentry, C.; Alenmyr, L.; Killander, D.; Lewis, S.E.; Andersson, A.; Bucher, B.; Galzi, J.-L.; Sterner, O.; Bevan, S.; et al. TRPA1 mediates spinal antinociception induced by acetaminophen and the cannabinoid Δ9-tetrahydrocannabiorcol. Nat. Commun. 2011, 2, 551. [Google Scholar] [CrossRef] [PubMed]
- Furue, H.; Narikawa, K.; Kumamoto, E.; Yoshimura, M. Responsiveness of rat substantia gelatinosa neurones to mechanical but not thermal stimuli revealed by in vivo patch-clamp recording. J. Physiol. 1999, 521, 529–535. [Google Scholar]
- Yamanaka, M.; Taniguchi, W.; Nishio, N.; Hashizume, H.; Yamada, H.; Yoshida, M.; Nakatsuka, T. In vivo patch-clamp analysis of the antinociceptive actions of TRPA1 activation in the spinal dorsal horn. Mol. Pain 2015, 11, 20. [Google Scholar] [CrossRef] [PubMed]
- Todd, A.J.; Watt, C.; Spike, R.C.; Sieghart, W. Colocalization of GABA, glycine and their receptors at synapses in the rat spinal cord. J. Neurosci. 1996, 16, 974–982. [Google Scholar] [PubMed]
- Coggeshall, R.E.; Carlton, S.M. Receptor localization in the mammalian dorsal horn and primary afferent neurons. Brain Res. Rev. 1997, 24, 28–66. [Google Scholar] [CrossRef]
- Moore, K.A.; Kohno, T.; Karchewski, L.A.; Scholz, J.; Baba, H.; Woolf, C.J. Partial peripheral nerve injury promotes a selective loss of GABAergic inhibition in the superficial dorsal horn of the spinal cord. J. Neurosci. 2002, 22, 6724–6731. [Google Scholar] [PubMed]
- Kohno, T. A role of spinal inhibition in neuropathic pain. In Cellular and Molecular Mechanisms for the Modulation of Nociceptive Transmission in the Peripheral and Central Nervous Systems; Kumamoto, E., Ed.; Research Signpost: Kelara, India, 2007; pp. 131–145. [Google Scholar]
- Coull, J.A.M.; Boudreau, D.; Bachand, K.; Prescott, S.A.; Nault, F.; Sik, A.; de Koninck, P.; de Koninck, Y. Trans-synaptic shift in anion gradient in spinal lamina I neurons as a mechanism of neuropathic pain. Nature 2003, 424, 938–942. [Google Scholar] [CrossRef] [PubMed]
- Müller, F.; Heinke, B.; Sandkühler, J. Reduction of glycine receptor-mediated miniature inhibitory postsynaptic currents in rat spinal lamina I neurons after peripheral inflammation. Neuroscience 2003, 122, 799–805. [Google Scholar] [CrossRef] [PubMed]
- Tanabe, M.; Takasu, K.; Yamaguchi, S.; Kodama, D.; Ono, H. Glycine transporter inhibitors as a potential therapeutic strategy for chronic pain with memory impairment. Anesthesiology 2008, 108, 929–937. [Google Scholar] [CrossRef] [PubMed]
- Sandkühler, J. Models and mechanisms of hyperalgesia and allodynia. Physiol. Rev. 2009, 89, 707–758. [Google Scholar] [CrossRef] [PubMed]
- Zeilhofer, H.U.; Wildner, H.; Yévenes, G.E. Fast synaptic inhibition in spinal sensory processing and pain control. Physiol. Rev. 2012, 92, 193–235. [Google Scholar] [CrossRef] [PubMed]
- Baba, H.; Kohno, T.; Okamoto, M.; Goldstein, P.A.; Shimoji, K.; Yoshimura, M. Muscarinic facilitation of GABA release in substantia gelatinosa of the rat spinal dorsal horn. J. Physiol. 1998, 508, 83–93. [Google Scholar] [CrossRef] [PubMed]
- Baba, H.; Goldstein, P.A.; Okamoto, M.; Kohno, T.; Ataka, T.; Yoshimura, M.; Shimoji, K. Norepinephrine facilitates inhibitory transmission in substantia gelatinosa of adult rat spinal cord (part 2): effects on somatodendritic sites of GABAergic neurons. Anesthesiology 2000, 92, 485–492. [Google Scholar] [CrossRef] [PubMed]
- Baba, H.; Shimoji, K.; Yoshimura, M. Norepinephrine facilitates inhibitory transmission in substantia gelatinosa of adult rat spinal cord (part 1): effects on axon terminals of GABAergic and glycinergic neurons. Anesthesiology 2000, 92, 473–484. [Google Scholar] [CrossRef] [PubMed]
- Takeda, D.; Nakatsuka, T.; Papke, R.; Gu, J.G. Modulation of inhibitory synaptic activity by a non-α4β2, non-α7 subtype of nicotinic receptors in the substantia gelatinosa of adult rat spinal cord. Pain 2003, 101, 13–23. [Google Scholar] [CrossRef]
- Fukushima, T.; Ohtsubo, T.; Tsuda, M.; Yanagawa, Y.; Hori, Y. Facilitatory actions of serotonin type 3 receptors on GABAergic inhibitory synaptic transmission in the spinal superficial dorsal horn. J. Neurophysiol. 2009, 102, 1459–1471. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Fujita, T.; Kumamoto, E. Acetylcholine and norepinephrine mediate GABAergic but not glycinergic transmission enhancement by melittin in adult rat substantia gelatinosa neurons. J. Neurophysiol. 2011, 106, 233–246. [Google Scholar] [CrossRef] [PubMed]
- Bíró, T.; Tóth, B.I.; Marincsák, R.; Dobrosi, N.; Géczy, T.; Paus, R. TRP channels as novel players in the pathogenesis and therapy of itch. Biochim. Biophys. Acta 2007, 1772, 1004–1021. [Google Scholar] [CrossRef] [PubMed]
- Sikand, P.; Shimada, S.G.; Green, B.G.; LaMotte, R.H. Similar itch and nociceptive sensations evoked by punctate cutaneous application of capsaicin, histamine and cowhage. Pain 2009, 144, 66–75. [Google Scholar] [CrossRef] [PubMed]
- Akiyama, T.; Carstens, E. Neural processing of itch. Neuroscience 2013, 250, 697–714. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Ji, R.-R. New insights into the mechanisms of itch: are pain and itch controlled by distinct mechanisms? Pflügers Arch. 2013, 465, 1671–1685. [Google Scholar] [CrossRef] [PubMed]
- Fujita, T.; Liu, T.; Nakatsuka, T.; Kumamoto, E. Proteinase-activated receptor-1 activation presynaptically enhances spontaneous glutamatergic excitatory transmission in adult rat substantia gelatinosa neurons. J. Neurophysiol. 2009, 102, 312–319. [Google Scholar] [CrossRef] [PubMed]
- Jordt, S.-E.; Julius, D. Molecular basis for species-specific sensitivity to "hot" chili peppers. Cell 2002, 108, 421–430. [Google Scholar] [CrossRef]
- Gavva, N.R.; Klionsky, L.; Qu, Y.; Shi, L.; Tamir, R.; Edenson, S.; Zhang, T.J.; Viswanadhan, V.N.; Toth, A.; Pearce, L.V.; et al. Molecular determinants of vanilloid sensitivity in TRPV1. J. Biol. Chem. 2004, 279, 20283–20295. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Zhang, X.-F.; Kort, M.E.; Huth, J.R.; Sun, C.; Miesbauer, L.J.; Cassar, S.C.; Neelands, T.; Scott, V.E.; Moreland, R.B.; et al. Molecular determinants of species-specific activation or blockade of TRPA1 channels. J. Neurosci. 2008, 28, 5063–5071. [Google Scholar] [CrossRef] [PubMed]
- Nagatomo, K.; Kubo, Y. Caffeine activates mouse TRPA1 channels but suppresses human TRPA1 channels. Proc. Natl. Acad. Sci. USA 2008, 105, 17373–17378. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Joshi, S.K.; DiDomenico, S.; Perner, R.J.; Mikusa, J.P.; Gauvin, D.M.; Segreti, J.A.; Han, P.; Zhang, X.-F.; Niforatos, W.; et al. Selective blockade of TRPA1 channel attenuates pathological pain without altering noxious cold sensation or body temperature regulation. Pain 2011, 152, 1165–1172. [Google Scholar] [CrossRef] [PubMed]
- Banzawa, N.; Saito, S.; Imagawa, T.; Kashio, M.; Takahashi, K.; Tominaga, M.; Ohta, T. Molecular basis determining inhibition/activation of nociceptive receptor TRPA1 protein: a single amino acid dictates species-specific actions of the most potent mammalian TRPA1 antagonist. J. Biol. Chem. 2014, 289, 31927–31939. [Google Scholar] [CrossRef] [PubMed]
| Plant-Derived Chemicals | TRPV1 (mM) | TRPA1 (mM) | References |
|---|---|---|---|
| Resiniferatoxin | 2.1 × 10−4 | − | [18] |
| Piperine | 0.052 | − | [120] |
| Eugenol | − | 3.8 | [124] |
| Zingerone | − | 1.3 | [125] |
| (−)-Carvone | 0.70 | − | [101] |
| (+)-Carvone | − | 0.72 | [101] |
| Carvacrol | − | 0.69 | [89] |
| Thymol | − | 0.18 | [88] |
| 1,8-Cineole | − | 3.2 | [27] |
| 1,4-Cineole | 0.42 | − | [27] |
| Cital | − | 0.58 | [127] |
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kumamoto, E.; Fujita, T. Differential Activation of TRP Channels in the Adult Rat Spinal Substantia Gelatinosa by Stereoisomers of Plant-Derived Chemicals. Pharmaceuticals 2016, 9, 46. https://doi.org/10.3390/ph9030046
Kumamoto E, Fujita T. Differential Activation of TRP Channels in the Adult Rat Spinal Substantia Gelatinosa by Stereoisomers of Plant-Derived Chemicals. Pharmaceuticals. 2016; 9(3):46. https://doi.org/10.3390/ph9030046
Chicago/Turabian StyleKumamoto, Eiichi, and Tsugumi Fujita. 2016. "Differential Activation of TRP Channels in the Adult Rat Spinal Substantia Gelatinosa by Stereoisomers of Plant-Derived Chemicals" Pharmaceuticals 9, no. 3: 46. https://doi.org/10.3390/ph9030046
APA StyleKumamoto, E., & Fujita, T. (2016). Differential Activation of TRP Channels in the Adult Rat Spinal Substantia Gelatinosa by Stereoisomers of Plant-Derived Chemicals. Pharmaceuticals, 9(3), 46. https://doi.org/10.3390/ph9030046





