The Antistaphylococcal Activity of Citropin 1.1 and Temporin A against Planktonic Cells and Biofilms Formed by Isolates from Patients with Atopic Dermatitis: An Assessment of Their Potential to Induce Microbial Resistance Compared to Conventional Antimicrobials
Abstract
:1. Introduction
2. Results and Discussion
2.1. Results
2.1.1. SA Isolates
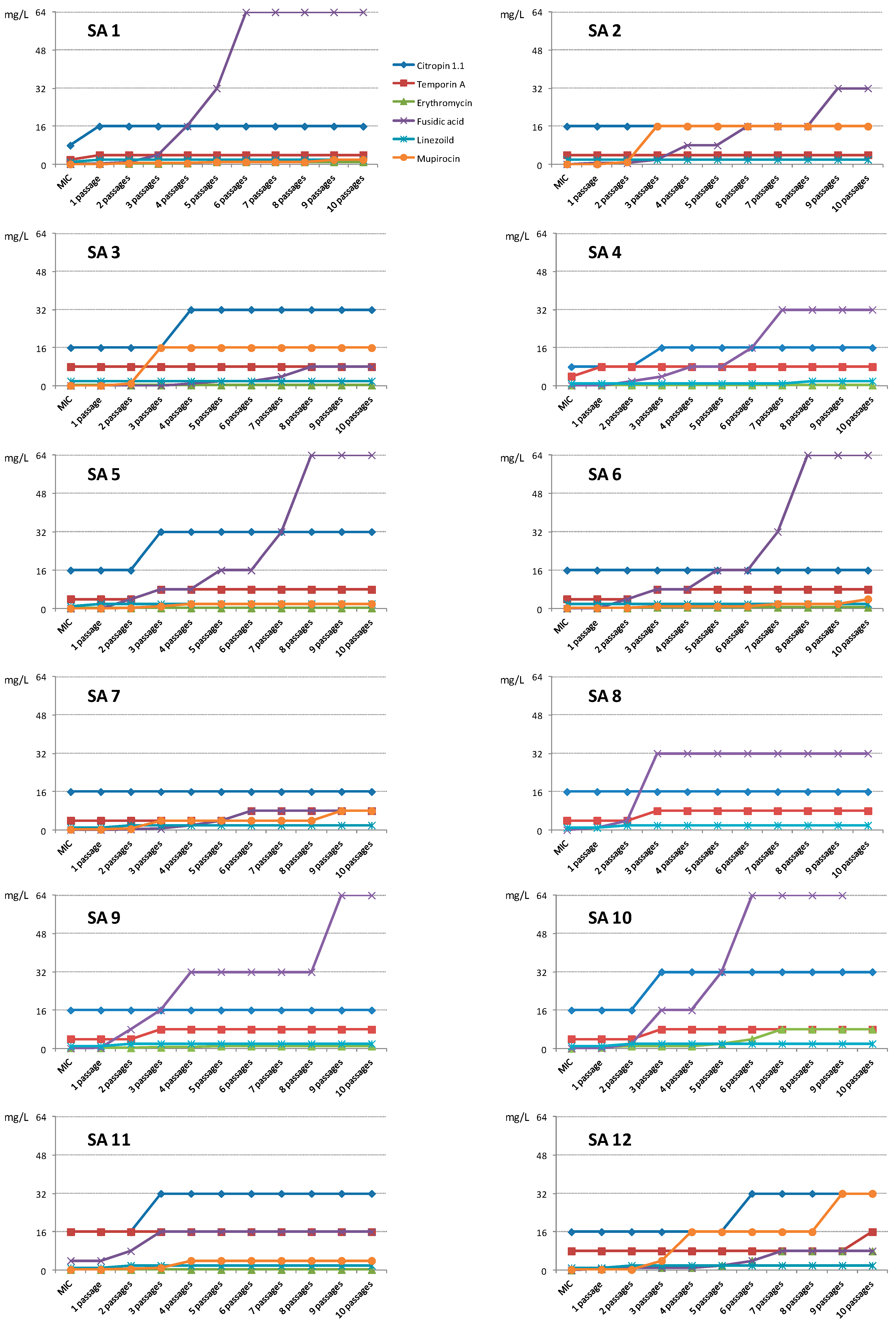
2.1.2. Activity against Planktonic Cells
2.1.3. Activity against Staphylococcal Biofilm
2.2. Discussion
3. Experimental Section
3.1. Peptides
3.2. Bacterial Isolates
Superantigen Detection
3.3. Toxin Gene Detection Using the PCR Technique
3.4. Identification of S. Aureus agr Groups I, II, III, IV
3.5. Antimicrobial Assay
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Percival, S.L.; Emanuel, C.; Cutting, K.F.; Williams, D.W. Microbiology of the skin and the role of biofilms in infection. Int. Wound J. 2012, 9, 14–32. [Google Scholar] [CrossRef] [PubMed]
- So-Young, N.; Joo-Young, R.; Jeung-Min, K.; Migma, D.T.; Jong-Rok, L. Analysis of Colonization and Genotyping of the Exotoxins of Staphylococcus aureus in Patients with Atopic dermatitis. Ann. Dermatol. 2012, 24, 413–419. [Google Scholar]
- Pastuszka, M.; Matych, M.; Kaszuba, A.; Poznańska-Kurowska, K. Microorganisms in the etiopathogenesis of atopic dermatitis. Postep. Derm. Alergol. 2012, 3, 215–221. [Google Scholar]
- Williams, H.C. Clinical practice. Atopic dermatitis. N. Engl. J. Med. 2005, 352, 2314–2324. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.K.; Thammavongsa, V.; Schneewind, O.; Missiakas, D. Recurrent infections and immune evasion strategies of Staphylococcus aureus. Curr. Opin. Microbiol. 2012, 15, 92–99. [Google Scholar] [CrossRef] [PubMed]
- Kurlenda, J.; Grinholc, M. Alternative therapies in Staphylococcus aureus diseases. Act. Pol. Bichim. 2012, 59, 215–221. [Google Scholar]
- Wertheim, H.F.; Melles, D.C.; Vos, M.C.; van Leeuwen, W.; van Belkum, A.; Verbrugh, H.A.; Nouwen, J.L. The role of nasal carriage in Staphylococcus aureus infections. Lancet Infect. Dis. 2005, 5, 751–762. [Google Scholar] [CrossRef]
- Antunes, L.C.M.; Ferreira, R.B.R.; Buckner, M.M.C.; Finlay, B.R. Quorum sensing in bacterial virulence. Microbiology 2010, 156, 2271–2282. [Google Scholar] [CrossRef] [PubMed]
- Boles, B.R.; Horswill, A.R. Staphylococcal biofilm disassembly. Trends Microbiol. 2011, 19, 449–455. [Google Scholar] [CrossRef] [PubMed]
- Speziale, P.; Visai, L.; Rindi, S.; Pietrocola, G.; Provenza, G.; Provenzano, M. Prevention and treatment of staphylococcal biofilms. Curr. Med. Chem. 2008, 15, 3185–3195. [Google Scholar] [CrossRef] [PubMed]
- Mahgoub, D.M.; El-Eishi, N.H.; Rashed, L.A. A quantitative study of the antimicrobial peptide granulysin and its possible role in atopic dermatitis. J. Egypt. Women Dermatol. 2010, 7, 177–122. [Google Scholar]
- Lee, H.; Kim, S.M.; Kim, J.M.; Oh, B.M.; Kim, J.Y.; Jung, H.J.; Lim, H.J.; Kim, B.S.; Lee, W.J.; Lee, S.; et al. Potential immunoinflammatory role of staphylococcal enterotoxin A in atopic dermatitis: Immunohistopathological analysis and in vitro assay. Ann. Dermatol. 2013, 25, 173–180. [Google Scholar] [CrossRef] [PubMed]
- Ong, P.Y.; Ohtake, T.; Brandt, C.; Strickland, I.; Boguniewicz, M.; Ganz, T.; Gallo, R.L.; Leung, D.Y. Endogenous antimicrobial peptides and skin infections in atopic dermatitis. N. Engl. J. Med. 2002, 347, 1151–1160. [Google Scholar] [CrossRef] [PubMed]
- Nakatsuji, T.; Gallo, R.L. Antimicrobial peptides: Old molecules with new ideas. J. Investig. Dermatol. 2012, 132, 887–895. [Google Scholar] [CrossRef] [PubMed]
- Harder, J.; Schroeder, J.M.; Glaeser, R. The skin surface as antimicrobial barrier: Present concepts and future outlooks. Exp. Dermatol. 2013, 22, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Sikorska, E.; Greber, K.; Motowidło-Rodziewicz, S.; Szultka, Ł.; Łukasiak, J.; Kamysz, W. Synthesis and antimicrobial activity of truncated fragments and analogs of citropin 1.1: The solution structure of the SDS micelle-bound citropin-like peptides. J. Struct. Biol. 2009, 168, 250–258. [Google Scholar] [CrossRef] [PubMed]
- Wade, D.; Silberring, J.; Soliymani, R.; Heikkinen, S.; Kilpeläinen, I.; Lankinen, H.; Kuusela, P. Antimicrobial activities of temporin A analogs. FEBS Lett. 2000, 479, 6–9. [Google Scholar] [CrossRef]
- Ou, L.S.; Leing, D.Y. Advances in atopic dermatitis. Chang Gung Med. J. 2005, 28, 1–8. [Google Scholar] [PubMed]
- Sung, H.C.; Jung, H.D.; Park, K.D.; Lee, W.J.; Lee, S.J.; Kim, D.W. A quantitative culture study of Staphylococcus aureus in adolescent and adult patients with atopic dermatitis using the contact-plate sampling technique. Korean J. Dermatol. 2007, 45, 673–679. [Google Scholar]
- Lebon, A.; Labout, J.A.; Verbrugh, H.A.; Jaddoe, V.W.; Hofman, A.; van Wamel, W.J.; van Belkum, A.; Moll, H.A. Role of Staphylococcus aureus nasal colonization in atopic dermatitis in infants: The Generation R Study. Arch. Pediatr. Adolesc. Med. 2009, 163, 745–749. [Google Scholar] [CrossRef] [PubMed]
- Manders, S.M. Toxin-mediated streptococcal and staphylococcal disease. J. Am. Acad. Dermatol. 1998, 39, 383–398. [Google Scholar] [CrossRef]
- Balaban, N.; Rasooly, A. Staphylococcal enterotoxins. Int. J. Food Microbiol. 2000, 61, 1–10. [Google Scholar] [CrossRef]
- Mirani, Z.A.; Aziz, M.; Khan, M.N.; Lal, I.; Hassan ul, N.; Khan, S.I. Biofilm formation and dispersal of Staphylococcus aureus under the influence of oxacillin. Microb Pathog. 2013, 61–61, 66–72. [Google Scholar] [CrossRef] [PubMed]
- Sakoulas, G.; Eliopoulos, G.M.; Moellering, R.C., Jr.; Novick, R.P.; Venkataraman, L.; Wennersten, C.; DeGirolami, P.C.; Schwaber, M.J.; Gold, H.S. Staphylococcus aureus accesory gene regulator (agr) group II: Is there a relationship to the development of intermediate-level glycopeptides resistance? J. Invest. Dermatol. 2003, 187, 929–938. [Google Scholar]
- Baranska-Rybak, W.; Cirioni, O.; Dawgul, M.; Sokolowska-Wojdylo, M.; Naumiuk, L.; Szczerkowska-Dobosz, A.; Nowicki, R.; Roszkiewicz, J.; Kamysz, W. Activity of antimicrobial peptides and conventional antibiotics against superantigen positive Staphylococcus aureus isolated from the patients with neoplastic and inflammatory erythrodermia. Chemother. Res. Pract. 2011, 2011, 270932. [Google Scholar] [PubMed]
- Gherardi, G.; De Florio, L.; Lorino, J.; Fico, L.; Dicuonzo, G. Macrolide resistance genotypes and phenotypesamong erythromycin-resistant clinical isolates of Staphylococcus aureus and coagulase-negative staphylococci. FEMS Immunol. Med. Microbiol. 2009, 55, 62–67. [Google Scholar] [CrossRef] [PubMed]
- Gadepalli, R.; Dhawan, B.; Mohanty, S.; Kapil, A.; Das, B.K.; Chaudhry, R.; Samantaray, J.C. Mupirocin resistance in Staphylococcus aureus in an Indian hospital. Diagn. Microbiol. Infect. Dis. 2007, 58, 125–127. [Google Scholar] [CrossRef] [PubMed]
- Jones, J.C.; Rogers, T.J.; Brookmeyer, P.; Dunne, W.M., Jr.; Storch, G.A.; Coopersmith, C.M.; Fraser, V.J.; Warren, D.K. Mupirocin resistance in patients colonized with methicillin-resistant Staphylococcus aureus in a surgical intensive care unit. Clin. Infect. Dis. 2007, 45, 541–547. [Google Scholar] [CrossRef] [PubMed]
- Wertheim, H.F.L.; Verveer, J.; Boelens, H.A.M.; van Belkum, A.; Verbrugh, H.A.; Vos, M.C. Effect of Mupirocin Treatment on Nasal, Pharyngeal, and Perineal Carriage of Staphylococcus aureus in Healthy Adults. Antimicrob. Agents. Chemother. 2005, 49, 1465–1467. [Google Scholar] [CrossRef] [PubMed]
- Abbasi-Montazeri, E.; Khosravi, A.D.; Feizabadi, M.M.; Goodarzi, H.; Khoramrooz, S.S.; Mirzaii, M.; Kalantar, E.; Darban-Sarokhalil, D. The prevalence of methicillin resistant Staphylococcus aureus (MRSA) isolates with high-level mupirocin resistance from patients and personnel in a burn center. Burns 2013, 39, 650–654. [Google Scholar] [CrossRef] [PubMed]
- Park, S.-H.; Kim, S.-Y.; Lee, J.-H.; Park, C.; Lee, D.-G. Community-genotype strains of methicillin-resistant Staphylococcus aureus with high-level mupirocin resistance in a neonatal intensive care unit. Early Hum. Dev. 2013, 89, 661–665. [Google Scholar] [CrossRef] [PubMed]
- Schöfer, H.; Simonsen, L. Fusidic acid in dermatology: An updated review. Eur. J. Dermatol. 2010, 20, 6–15. [Google Scholar] [PubMed]
- Heng, Y.K.; Tan, K.T.; Sen, P.; Chow, A.; Leo, Y.S.; Lye, D.C.; Chan, R.K. Staphylococcus aureus and topical fusidic acid use: Results of a clinical audit on antimicrobial resistance. Int. J. Dermatol. 2013, 52, 876–881. [Google Scholar] [CrossRef] [PubMed]
- Ager, S.; Gould, K. Clinical update on linezolid in the treatment of Gram-positive bacterial infections. Inf. Drug Resist. 2012, 5, 87–102. [Google Scholar]
- Schauber, J.; Gallo, R.L. Antimicrobial peptides and skin immune defense system. J. Allergy Clin. Immunol. 2009, 124, 13–18. [Google Scholar] [CrossRef] [PubMed]
- Cirioni, O. Lipopeptide Laur-CKK-NH 2 dimer preserves daptomycin susceptibility and enhances its activity against Enterococcus faecalis. J. Antimicrob. Chemother. 2011, 66, 859–862. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Parente, J.; Harris, S.M.; Woods, D.E.; Hancock, R.E.W.; Falla, T.J. Antimicrobial peptide therapeutics for cystic fibrosis. Antimicrob. Agents Chemother. 2005, 49, 2921–2927. [Google Scholar] [CrossRef] [PubMed]
- Silva, N.C.; Sarmento, B.; Pintado, M. The importance of antimicrobial peptides and their potential for therapeutic use in ophthalmology. Int. J. Antimicrob. Agents 2013, 41, 5–10. [Google Scholar] [CrossRef] [PubMed]
- Huang, Q.; Yu, H.J.; Liu, G.D.; Huang, X.K.; Zhang, L.Y.; Zhou, Y.G.; Chen, J.Y.; Lin, F.; Wang, Y.; Fei, J. Comparison of the effects of human β-defensin 3, vancomycin, and clindamycin on Staphylococcus aureus biofilm formation. Orthopedic 2012, 35, 53–60. [Google Scholar]
- Sigurdardotti, T.; Andersson, P.; Davoudi, M.; Malmsten, M.; Schmidtchen, A. In silico identification and biological evaluation of antimicrobial peptides based on human cathelicidin LL-37. Antimicrob. Agents. Chemother. 2006, 50, 2983–2989. [Google Scholar] [CrossRef] [PubMed]
- Hell, E.; Giske, C.G.; Nelson, A.; Römling, U.; Marchini, G. Human cathelicidin peptide LL37 inhibits both attachment capability and biofilm formation of Staphylococcus epidermidis. Lett. Appl. Microbiol. 2010, 50, 211–215. [Google Scholar] [CrossRef] [PubMed]
- Giacometti, A.; Cirioni, O.; Kamysz, W.; Silvestri, C.; Licci, A.; D’Amato, G.; Nadolski, P.; Riva, A.; Lukasiak, J.; Scalise, G. In vitro activity and killing effect of uperin 3.6 against gram-positive cocci isolated from immunocompromised patients. Antimicrob. Agents. Chemother. 2005, 49, 3933–3936. [Google Scholar] [CrossRef] [PubMed]
- Giacometti, A.; Cirioni, O.; Riva, A.; Kamysz, W.; Silvestri, C.; Nadolski, P.; della Vittoria, A.; Łukasiak, J.; Scalise, G. In vitro activity of aurein 1.2 alone and in combination with antibiotics against gram-positive nosocomial cocci. Antimicrob. Agents. Chemother. 2007, 51, 1494–1496. [Google Scholar] [CrossRef] [PubMed]
- Cirioni, O.; Giacometti, A.; Ghiselli, R.; Kamysz, W.; Orlando, F.; Mocchegiani, F.; Silvestri, C.; Licci, A.; Chiodi, L.; Lukasiak, J.; et al. Citropin 1.1-treated central venous catheters improve the efficacy of hydrophobic antibiotics in the treatment of experimental staphylococcal catheter-related infection. Peptides 2006, 27, 1210–1216. [Google Scholar] [CrossRef] [PubMed]
- Simonetti, O.; Cirioni, O.; Goteri, G.; Ghiselli, R.; Kamysz, W.; Kamysz, E.; Silvestri, C.; Orlando, F.; Barucca, C.; Scalise, A.; et al. Temporin A is effective in MRSA-infected wounds through bactericidal activity and acceleration of wound repair in a murine model. Peptides 2008, 29, 520–528. [Google Scholar] [CrossRef] [PubMed]
- Potera, C. Biofilm dispersing agent rejuvenates older antibiotics. Environ. Health Persp. 2010, 118, 288. [Google Scholar] [CrossRef]
- Dawgul, M.; Baranska-Rybak, W.; Kamysz, E.; Karafova, A.; Nowicki, R.; Kamysz, W. Activity of short lipopeptides and conventional antimicrobials against planktonic cells and biofilms formed by clinical strains of Staphylococcus aureus. Future Med. Chem. 2012, 4, 1541–1551. [Google Scholar] [CrossRef] [PubMed]
- Barańska-Rybak, W.; Pikuła, M.; Dawgul, M.; Kamysz, W.; Trzonkowski, P.; Roszkiewicz, J. Safety profile of antimicrobial peptides: Camel, citropin, protegrin, temporin A and lipopeptide on HaCaT keratinocytes. Acta Pol. Pharm. Drug Res. 2013, 70, 795–801. [Google Scholar]
- Fields, G.B.; Noble, R.L. Solid phase peptide synthesis utilizing 9-fluorenylmethoxycarbonyl amino acids. Int. J. Pept. Protein Res. 1990, 35, 161–214. [Google Scholar] [CrossRef] [PubMed]
- Barski, P.; Piechowicz, L.; Galiński, J.; Kur, J. Rapid assay for detection of methicillin-resistant Staphylococcus aureus using multiplex PCR. Mol. Cell. Probes. 1996, 10, 471–475. [Google Scholar] [CrossRef] [PubMed]
- Mehrotra, M.; Wang, G.; Johnson, W.M. Multiplex PCR for detection of genes for Staphylococcus aureus enterotoxins, exfoliative toxins, toxic shock syndrome toxin 1, and methicillin resistance. J. Clin. Microbiol. 2000, 38, 1032–1035. [Google Scholar] [PubMed]

| Strain No. | Toxins | Agr | ||||
|---|---|---|---|---|---|---|
| SEA | SEB | SEC | SED | TSS | ||
| 1 | SEA | - | - | - | - | III |
| 2 | SEA | - | - | - | - | III |
| 3 | - | - | - | - | - | I |
| 4 | SEA | - | - | - | - | III |
| 5 | - | - | - | - | - | I |
| 6 | - | - | SEC | SED | - | II |
| 7 | SEA | - | - | - | - | III |
| 8 | SEA | - | - | - | - | III |
| 9 | - | - | - | SED | - | I |
| 10 | - | - | - | - | - | I |
| 11 | - | - | SEC | - | - | I |
| 12 | - | - | - | SED | - | I |
| Compound/SA strain | SA | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Citropin 1.1 | MIC | 8 | 16 | 16 | 8 | 16 | 16 | 16 | 16 | 16 | 16 | 16 | 16 |
| MIC10 | 16 | 16 | 32 | 16 | 32 | 16 | 16 | 16 | 16 | 32 | 32 | 32 | |
| MBEC | 32 | 64 | 64 | 64 | 32 | 64 | 128 | 64 | 128 | 32 | 32 | 32 | |
| Temporin A | MIC | 2 | 4 | 8 | 4 | 4 | 4 | 4 | 4 | 4 | 4 | 16 | 8 |
| MIC10 | 4 | 4 | 8 | 8 | 8 | 8 | 8 | 8 | 8 | 8 | 16 | 16 | |
| MBEC | 32 | 32 | 64 | 64 | 32 | 16 | 16 | 32 | 64 | 32 | 32 | 256 | |
| Erythromycin | MIC | 0.25 | >512 | 0.25 | 0.25 | 0.25 | 0.25 | >512 | >512 | 0.25 | 0.125 | 0.25 | 0.25 |
| MIC10 | 1 | >512 | 0.25 | 0.25 | 0.25 | 0.5 | >512 | >512 | 1 | 8 | 0.25 | 8 | |
| MBEC | >512 | >512 | 512 | >512 | 512 | >512 | >512 | >512 | >512 | >512 | 512 | 512 | |
| Fusidic acid | MIC | 0.125 | 0.125 | 0.0625 | 0.125 | 0.0625 | 0.0625 | 0.125 | 0.0625 | 0.0625 | 0.125 | 4 | 0.0625 |
| MIC10 | 64 | 32 | 8 | 32 | 64 | 64 | 8 | 32 | 64 | 128 | 16 | 8 | |
| MBEC | >512 | >512 | >512 | >512 | 128 | 512 | >512 | 256 | 32 | 16 | 32 | 16 | |
| Linezolid | MIC | 1 | 2 | 2 | 1 | 1 | 2 | 1 | 1 | 1 | 1 | 1 | 1 |
| MIC10 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | |
| MBEC | >512 | 512 | 256 | >512 | 32 | >512 | >512 | 256 | 128 | 64 | 64 | 32 | |
| Mupirocin | MIC | 0.125 | 0.125 | 0.125 | >512 | 0.125 | 0.25 | 0.25 | >512 | >512 | >512 | 0.25 | 0.25 |
| MIC10 | 2 | 16 | 16 | >512 | 2 | 4 | 8 | >512 | >512 | >512 | 4 | 32 | |
| MBEC | >512 | 256 | 128 | >512 | >512 | 512 | >512 | >512 | >512 | >512 | 128 | 512 |
| Compound | Amino Acid Sequence |
|---|---|
| Citropin 1.1 | GLFDVIKKVASVIGGL-NH2 |
| Temporin A | FLPLIGRVLSGIL-NH2 |
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dawgul, M.; Baranska-Rybak, W.; Piechowicz, L.; Bauer, M.; Neubauer, D.; Nowicki, R.; Kamysz, W. The Antistaphylococcal Activity of Citropin 1.1 and Temporin A against Planktonic Cells and Biofilms Formed by Isolates from Patients with Atopic Dermatitis: An Assessment of Their Potential to Induce Microbial Resistance Compared to Conventional Antimicrobials. Pharmaceuticals 2016, 9, 30. https://doi.org/10.3390/ph9020030
Dawgul M, Baranska-Rybak W, Piechowicz L, Bauer M, Neubauer D, Nowicki R, Kamysz W. The Antistaphylococcal Activity of Citropin 1.1 and Temporin A against Planktonic Cells and Biofilms Formed by Isolates from Patients with Atopic Dermatitis: An Assessment of Their Potential to Induce Microbial Resistance Compared to Conventional Antimicrobials. Pharmaceuticals. 2016; 9(2):30. https://doi.org/10.3390/ph9020030
Chicago/Turabian StyleDawgul, Malgorzata, Wioletta Baranska-Rybak, Lidia Piechowicz, Marta Bauer, Damian Neubauer, Roman Nowicki, and Wojciech Kamysz. 2016. "The Antistaphylococcal Activity of Citropin 1.1 and Temporin A against Planktonic Cells and Biofilms Formed by Isolates from Patients with Atopic Dermatitis: An Assessment of Their Potential to Induce Microbial Resistance Compared to Conventional Antimicrobials" Pharmaceuticals 9, no. 2: 30. https://doi.org/10.3390/ph9020030
APA StyleDawgul, M., Baranska-Rybak, W., Piechowicz, L., Bauer, M., Neubauer, D., Nowicki, R., & Kamysz, W. (2016). The Antistaphylococcal Activity of Citropin 1.1 and Temporin A against Planktonic Cells and Biofilms Formed by Isolates from Patients with Atopic Dermatitis: An Assessment of Their Potential to Induce Microbial Resistance Compared to Conventional Antimicrobials. Pharmaceuticals, 9(2), 30. https://doi.org/10.3390/ph9020030







