Potential of Glutamate-Based Drug Discovery for Next Generation Antidepressants
Abstract
:1. Introduction
2. Clinical and Preclinical Studies of Ketamine
2.1. Clinical Studies of Ketamine
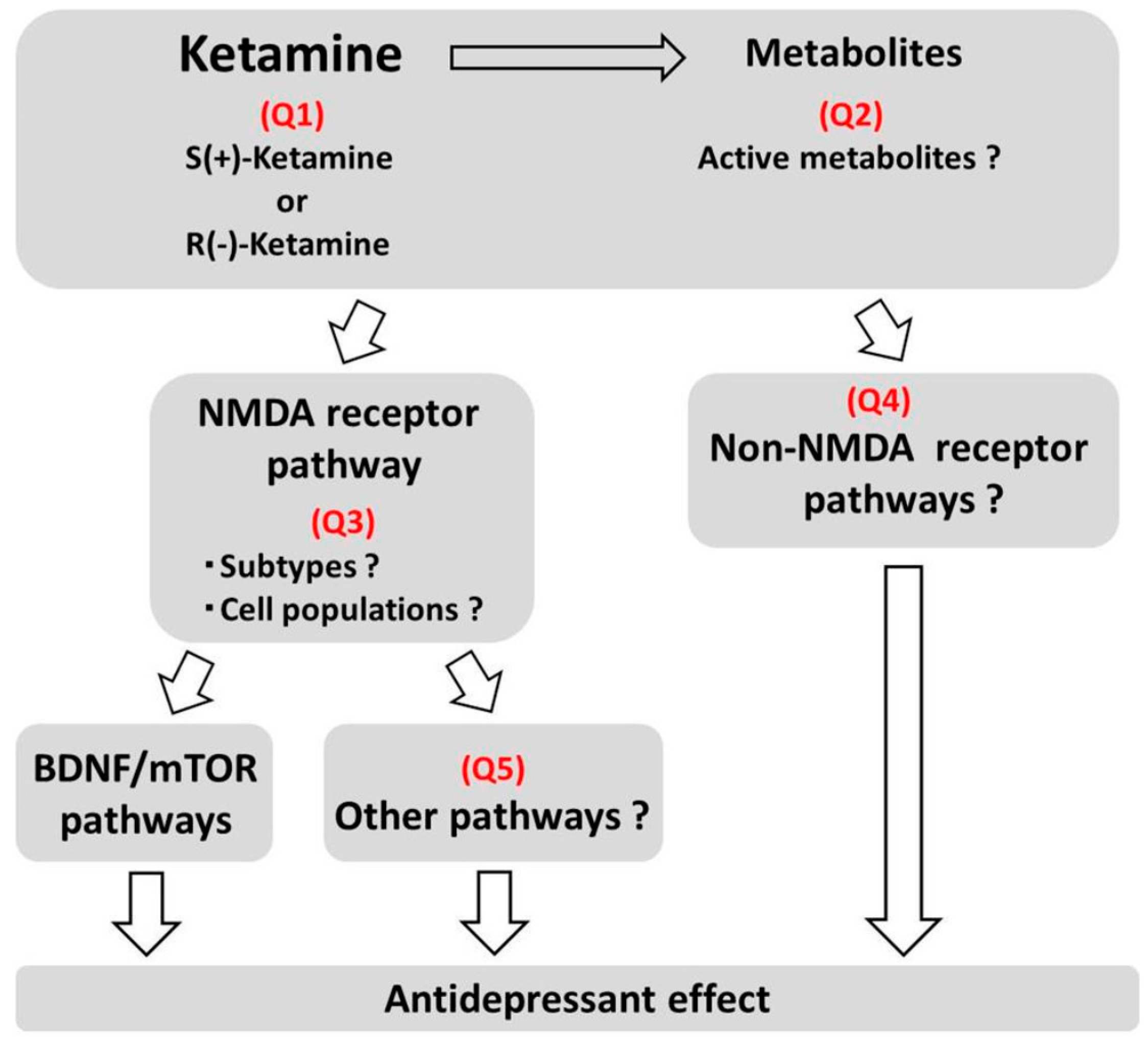
2.2. Mechanisms of Action
3. Possible Alternatives to Ketamine
3.1. Agents Acting on NMDA Receptors
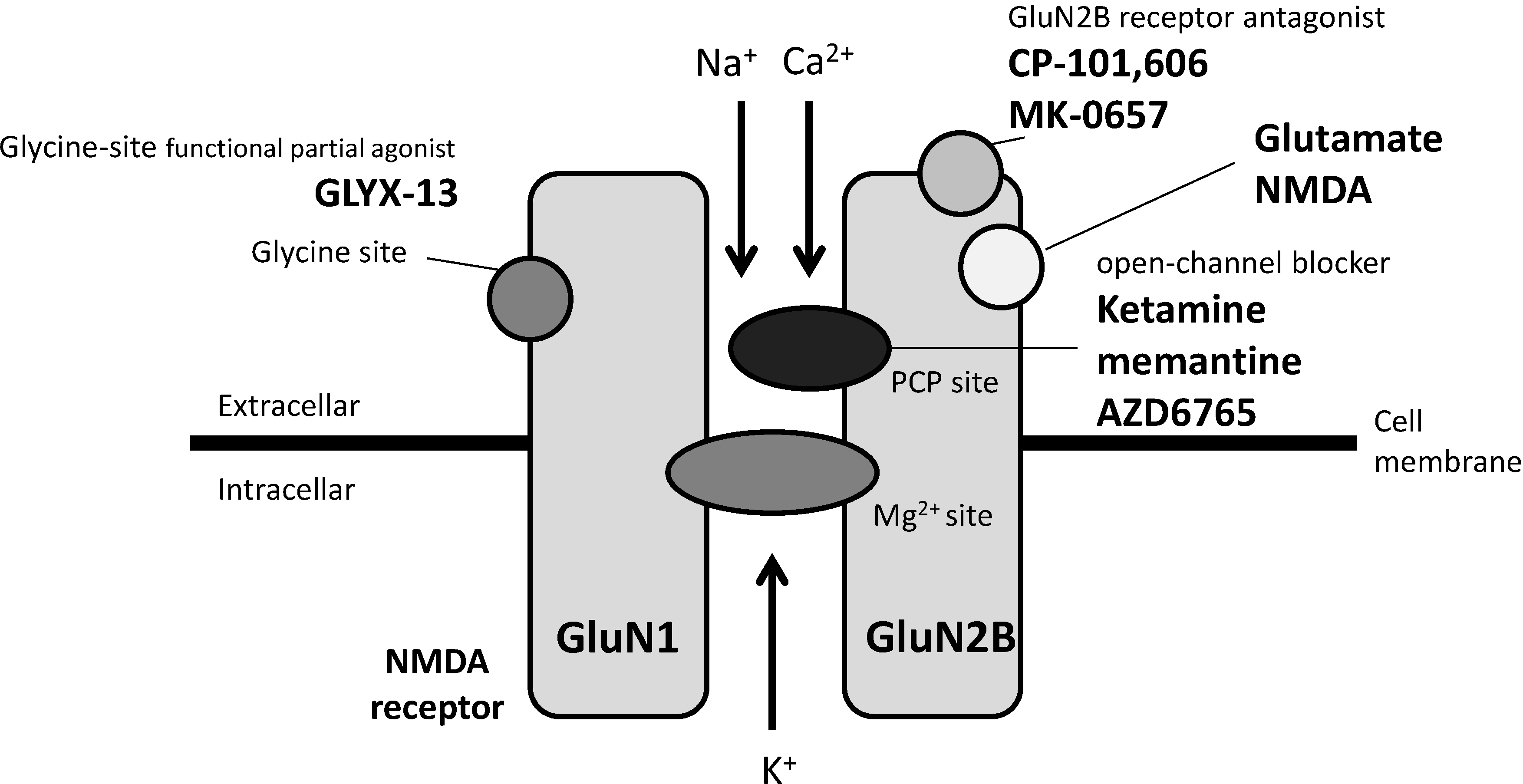
3.2. Agents Acting on mGlu Receptors
4. Conclusion and Future Directions
Conflicts of Interest
References
- Diazgranados, N.; Ibrahim, L.A.; Brutsche, N.E.; Ameli, R.; Henter, I.D.; Luckenbaugh, D.A.; Machado-Vieira, R.; Zarate, C.A., Jr. Rapid resolution of suicidal ideation after a single infusion of an N-methyl-D-aspartate antagonist in patients with treatment-resistant major depressive disorder. J. Clin. Psychiatry 2010, 71, 1605–1611. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, L.; Diazgranados, N.; Luckenbaugh, D.A.; Machado-Vieira, R.; Baumann, J.; Mallinger, A.G.; Zarate, C.A., Jr. Rapid decrease in depressive symptoms with an N-methyl-d-aspartate antagonist in ECT-resistant major depression. Prog. Neuropsychopharmacol. Biol. Psychiatry 2011, 35, 1155–1159. [Google Scholar] [CrossRef] [PubMed]
- Murrough, J.W.; Iosifescu, D.V.; Chang, L.C.; Al Jurdi, R.K.; Green, C.E.; Perez, A.M.; Iqbal, S.; Pillemer, S.; Foulkes, A.; Shah, A.; et al. Antidepressant efficacy of ketamine in treatment-resistant major depression: a two-site randomized controlled trial. Am. J. Psychiatry 2013, 170, 1134–1142. [Google Scholar] [CrossRef] [PubMed]
- Price, R.B.; Nock, M.K.; Charney, D.S.; Mathew, S.J. Effects of intravenous ketamine on explicit and implicit measures of suicidality in treatment-resistant depression. Biol. Psychiatry 2009, 66, 522–526. [Google Scholar] [CrossRef] [PubMed]
- Zarate, C.A., Jr.; Singh, J.B.; Carlson, P.J.; Brutsche, N.E.; Ameli, R.; Luckenbaugh, D.A.; Charney, D.S.; Manji, H.K. A randomized trial of an N-methyl-d-aspartate antagonist in treatment-resistant major depression. Arch. Gen. Psychiatry 2006, 63, 856–864. [Google Scholar] [CrossRef] [PubMed]
- Diazgranados, N.; Ibrahim, L.; Brutsche, N.E.; Newberg, A.; Kronstein, P.; Khalife, S.; Kammerer, W.A.; Quezado, Z.; Luckenbaugh, D.A.; Salvadore, G.; Machado-Vieira, R.; Manji, H.K.; Zarate, C.A., Jr. A randomized add-on trial of an N-methyl-d-aspartate antagonist in treatment-resistant bipolar depression. Arch. Gen. Psychiatry 2010, 67, 793–802. [Google Scholar] [CrossRef] [PubMed]
- Zarate, C.A., Jr.; Brutsche, N.E.; Ibrahim, L.; Franco-Chaves, J.; Diazgranados, N.; Cravchik, A.; Selter, J.; Marquardt, C.A.; Liberty, V.; Luckenbaugh, D.A. Replication of ketamine’s antidepressant efficacy in bipolar depression: a randomized controlled add-on trial. Biol. Psychiatry 2012, 71, 939–946. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, L.; Diaz Granados, N.; Jolkovsky, L.; Brutsche, N.; Luckenbaugh, D.A.; Herring, W.J.; Potter, W.Z.; Zarate, C.A., Jr. A randomized, placebo-controlled, crossover pilot trial of the oral selective NR2B antagonist MK-0657 in patients with treatment-resistant major depressive disorder. J. Clin. Psychopharmacol. 2012, 32, 551–557. [Google Scholar] [CrossRef] [PubMed]
- Preskorn, S.H.; Baker, B.; Kolluri, S.; Menniti, F.S.; Krams, M.; Landen, J.W. An innovative design to establish proof of concept of the antidepressant effects of the NR2B subunit selective N-methyl-d-aspartate antagonist, CP-101,606, in patients with treatment-refractory major depressive disorder. J. Clin. Psychopharmacol. 2008, 28, 631–637. [Google Scholar] [CrossRef] [PubMed]
- Preskorn, S.; Macaluso, M.; Mehra, D.O.; Zammit, G.; Moskal, J.R.; Burch, R.M.; GLYX-13 Clinical Study Group. Randomized proof of concept trial of GLYX-13, an N-methyl-d-aspartate receptor glycine site partial agonist, in major depressive disorder nonresponsive to a previous antidepressant agent. J. Psychiatr. Pract. 2015, 21, 140–149. [Google Scholar] [CrossRef] [PubMed]
- Sanacora, G.; Smith, M.A.; Pathak, S.; Su, H.L.; Boeijinga, P.H.; McCarthy, D.J.; Quirk, M.C. Lanicemine: A low-trapping NMDA channel blocker produces sustained antidepressant efficacy with minimal psychotomimetic adverse effects. Mol. Psychiatry 2014, 19, 978–985. [Google Scholar] [CrossRef] [PubMed]
- Zarate, C.A., Jr.; Mathews, D.; Ibrahim, L.; Chaves, J.F.; Marquardt, C.; Ukoh, I.; Jolkovsky, L.; Brutsche, N.E.; Smith, M.A.; Luckenbaugh, D.A. A randomized trial of a low-trapping nonselective N-methyl-d-aspartate channel blocker in major depression. Biol. Psychiatry 2013, 74, 257–264. [Google Scholar] [CrossRef] [PubMed]
- Berman, R.M.; Cappiello, A.; Anand, A.; Oren, D.A.; Heninger, G.R.; Charney, D.S.; Krystal, J.H. Antidepressant effects of ketamine in depressed patients. Biol. Psychiatry 2000, 47, 351–354. [Google Scholar] [CrossRef]
- aan het Rot, M.; Collins, K.A.; Murrough, J.W.; Perez, A.M.; Reich, D.L.; Charney, D.S.; Mathew, S.J. Safety and efficacy of repeated-dose intravenous ketamine for treatment-resistant depression. Biol. Psychiatry 2010, 67, 139–145. [Google Scholar] [CrossRef] [PubMed]
- Murrough, J.W.; Perez, A.M.; Pillemer, S.; Stern, J.; Parides, M.K.; aan het Rot, M.; Collins, K.A.; Mathew, S.J.; Charney, D.S.; Iosifescu, D.V. Rapid and longer-term antidepressant effects of repeated ketamine infusions in treatment-resistant major depression. Biol. Psychiatry 2013, 74, 250–256. [Google Scholar] [CrossRef] [PubMed]
- Rasmussen, K.G.; Lineberry, T.W.; Galardy, C.W.; Kung, S.; Lapid, M.I.; Palmer, B.A.; Ritter, M.J.; Schak, K.M.; Sola, C.L.; Hanson, A.J.; Frye, M.A. Serial infusions of low-dose ketamine for major depression. J. Psychopharmacol. 2013, 27, 444–450. [Google Scholar] [CrossRef] [PubMed]
- Sinner, B.; Graf, B.M. Ketamine. Handb. Exp. Pharmacol. 2008, 182, 313–333. [Google Scholar] [PubMed]
- Abdallah, C.G.; Sanacora, G.; Duman, R.S.; Krystal, J.H. Ketamine and rapid-acting antidepressants: A window into a new neurobiology for mood disorder therapeutics. Annu. Rev. Med. 2015, 66, 509–523. [Google Scholar] [CrossRef] [PubMed]
- Duman, R.S.; Li, N.; Liu, R.J.; Duric, V.; Aghajanian, G. Signaling pathways underlying the rapid antidepressant actions of ketamine. Neuropharmacology 2012, 62, 35–41. [Google Scholar] [CrossRef] [PubMed]
- Krystal, J.H.; Sanacora, G.; Duman, R.S. Rapid-acting glutamatergic antidepressants: The path to ketamine and beyond. Biol. Psychiatry. 2013, 73, 1133–1141. [Google Scholar] [CrossRef] [PubMed]
- Autry, A.E.; Adachi, M.; Nosyreva, E.; Na, E.S.; Los, M.F.; Cheng, P.F.; Kavalali, E.T.; Monteggia, L.M. NMDA receptor blockade at rest triggers rapid behavioural antidepressant responses. Nature 2011, 475, 91–95. [Google Scholar] [CrossRef] [PubMed]
- Koike, H.; Fukumoto, K.; Iijima, M.; Chaki, S. Role of BDNF/TrkB signaling in antidepressant-like effects of a group II metabotropic glutamate receptor antagonist in animal models of depression. Behav. Brain Res. 2013, 238, 48–52. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.J.; Lee, F.S.; Li, X.Y.; Bambico, F.; Duman, R.S.; Aghajanian, G.K. Brain-derived neurotrophic factor Val66Met allele impairs basal and ketamine-stimulated synaptogenesis in prefrontal cortex. Biol. Psychiatry 2012, 71, 996–1005. [Google Scholar] [CrossRef] [PubMed]
- Laje, G.; Lally, N.; Mathews, D.; Brutsche, N.; Chemerinski, A.; Akula, N.; Kelmendi, B.; Simen, A.; McMahon, F.J.; Sanacora, G.; Zarate, C., Jr. Brain-derived neurotrophic factor Val66Met polymorphism and antidepressant efficacy of ketamine in depressed patients. Biol. Psychiatry 2012, 72, e27–e28. [Google Scholar] [CrossRef] [PubMed]
- Fuchikami, M.; Thomas, A.; Liu, R.; Wohleb, E.S.; Land, B.B.; DiLeone, R.J.; Aghajanian, G.K.; Duman, R.S. Optogenetic stimulation of infralimbic PFC reproduces ketamine's rapid and sustained antidepressant actions. Proc. Natl. Acad. Sci. U.S.A. 2015, 112, 8106–8111. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Lee, B.; Liu, RJ.; Banasr, M.; Dwyer, J.M.; Iwata, M.; Li, X.Y.; Aghajanian, G.; Duman, R.S. mTOR-dependent synapse formation underlies the rapid antidepressant effects of NMDA antagonists. Science 2010, 329, 959–964. [Google Scholar] [CrossRef] [PubMed]
- Maeng, S.; Zarate, C.A., Jr.; Du, J.; Schloesser, R.J.; McCammon, J.; Chen, G.; Manji, H.K. Cellular mechanisms underlying the antidepressant effects of ketamine: role of alpha-amino-3-hydroxy-5-methylisoxazole-4-propionic acid receptors. Biol. Psychiatry 2008, 63, 349–352. [Google Scholar] [CrossRef] [PubMed]
- Pozzi, L.; Dorocic, I.P.; Wang, X.; Carlén, M.; Meletis, K. Mice lacking NMDA receptors in parvalbumin neurons display normal depression-related behavior and response to antidepressant action of NMDAR antagonists. PLoS ONE 2014, 9, e83879. [Google Scholar] [CrossRef] [PubMed]
- Kiselycznyk, C.; Jury, N.J.; Halladay, L.R.; Nakazawa, K.; Mishina, M.; Sprengel, R.; Grant, S.G.; Svenningsson, P.; Holmes, A. NMDA receptor subunits and associated signaling molecules mediating antidepressant-related effects of NMDA-GluN2B antagonism. Behav. Brain Res. 2015, 287, 89–95. [Google Scholar] [CrossRef] [PubMed]
- Miller, O.H.; Yang, L.; Wang, C.C.; Hargroder, E.A.; Zhang, Y.; Delpire, E.; Hall, B.J. GluN2B-containing NMDA receptors regulate depression-like behavior and are critical for the rapid antidepressant actions of ketamine. Elife 2014, 3, e03581. [Google Scholar] [CrossRef] [PubMed]
- Loix, S.; De Kock, M.; Henin, P. The anti-inflammatory effects of ketamine: state of the art. Acta. Anaesthesiol. Belg. 2011, 62, 47–58. [Google Scholar] [PubMed]
- Yang, C.; Shen, J.; Hong, T.; Hu, T.T.; Li, Z.J.; Zhang, H.T.; Zhang, Y.J.; Zhou, Z.Q.; Yang, J.J. Effects of ketamine on lipopolysaccharide-induced depressive-like behavior and the expression of inflammatory cytokines in the rat prefrontal cortex. Mol. Med. Rep. 2013, 8, 887–890. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Li, W.; Zhou, C.; Lu, F.; Gao, T.; Liu, Y.; Cao, J.; Zhang, Y.; Zhang, Y.; Zhou, C. Ketamine inhibits lipopolysaccharide-induced astrocytes activation by suppressing TLR4/NF-ĸB pathway. Cell Physiol. Biochem. 2012, 30, 609–617. [Google Scholar] [CrossRef] [PubMed]
- Ménard, C.; Hodes, G.E.; Russo, S.J. Pathogenesis of depression: Insights from human and rodent studies. Neuroscience 2015. [Google Scholar] [CrossRef] [PubMed]
- Walker, A.K.; Budac, D.P.; Bisulco, S.; Lee, A.W.; Smith, R.A.; Beenders, B.; Kelley, K.W.; Dantzer, R. NMDA receptor blockade by ketamine abrogates lipopolysaccharide-induced depressive-like behavior in C57BL/6J mice. Neuropsychopharmacology 2013, 38, 1609–1616. [Google Scholar] [CrossRef] [PubMed]
- Domino, E.F. Taming the ketamine tiger. 1965. Anesthesiology 2010, 113, 678–684. [Google Scholar] [PubMed]
- Kohrs, R.; Durieux, M.E. Ketamine: Teaching an old drug new tricks. Anesth. Analg. 1998, 87, 1186–1193. [Google Scholar] [PubMed]
- Zhang, J.C.; Li, S.X.; Hashimoto, K. R(−)-ketamine shows greater potency and longer lasting antidepressant effects than S(+)-ketamine. Pharmacol. Biochem. Behav. 2014, 116, 137–141. [Google Scholar] [CrossRef] [PubMed]
- Mion, G.; Villevieille, T. Ketamine pharmacology: an update (pharmacodynamics and molecular aspects, recent findings). CNS Neurosci. Ther. 2013, 19, 370–380. [Google Scholar] [CrossRef] [PubMed]
- Desta, Z.; Moaddel, R.; Ogburn, E.T.; Xu, C.; Ramamoorthy, A.; Venkata, S.L.; Sanghvi, M.; Goldberg, M.E.; Torjman, M.C.; Wainer, I.W. Stereoselective and regiospecific hydroxylation of ketamine and norketamine. Xenobiotica. 2012, 42, 1076–1087. [Google Scholar] [CrossRef] [PubMed]
- Zarate, C.A., Jr.; Brutsche, N.; Laje, G.; Luckenbaugh, D.A.; Venkata, S.L.; Ramamoorthy, A.; Moaddel, R.; Wainer, I.W. Relationship of ketamine’s plasma metabolites with response, diagnosis, and side effects in major depression. Biol. Psychiatry 2012, 72, 331–338. [Google Scholar] [CrossRef] [PubMed]
- Singh, N.S.; Zarate, C.A., Jr.; Moaddel, R.; Bernier, M.; Wainer, I.W. What is hydroxynorketamine and what can it bring to neurotherapeutics? Expert Rev. Neurother. 2014, 14, 1239–1242. [Google Scholar] [CrossRef] [PubMed]
- Paul, R.K.; Singh, N.S.; Khadeer, M.; Moaddel, R.; Sanghvi, M.; Green, C.E.; O'Loughlin, K.; Torjman, M.C.; Bernier, M.; Wainer, I.W. (R,S)-Ketamine metabolites (R,S)-norketamine and (2S,6S)-hydroxynorketamine increase the mammalian target of rapamycin function. Anesthesiology 2014, 121, 149–159. [Google Scholar] [CrossRef] [PubMed]
- Weed, M.R.; Bookbinder, M.; Polino, J.; Keavy, D.; Cardinal, R.N.; Simmermacher-Mayer, J.; Cometa, F.N.; King, D.; Thangathirupathy, S.; Macor, J.E.; Bristow, L.J. Negative allosteric modulators selective for the NR2B subtype of the NMDA receptor impair cognition in multiple domains. Neuropsychopharmacology 2015. [Google Scholar] [CrossRef] [PubMed]
- Mealing, G.A.; Lanthorn, T.H.; Murray, C.L.; Small, D.L.; Morley, P. Differences in degree of trapping of low-affinity uncompetitive N-methyl-d-aspartic acid receptor antagonists with similar kinetics of block. J. Pharmacol. Exp. Ther. 1999, 288, 204–210. [Google Scholar] [PubMed]
- Sanacora, G.; Schatzberg, A.F. Ketamine: Promising path or false prophecy in the development of novel therapeutics for mood disorders? Neuropsychopharmacology 2015, 40, 259–267. [Google Scholar] [CrossRef] [PubMed]
- Burgdorf, J.; Zhang, X.L.; Nicholson, K.L.; Balster, R.L.; Leander, J.D.; Stanton, P.K.; Gross, A.L.; Kroes, R.A.; Moskal, J.R. GLYX-13, a NMDA receptor glycine-site functional partial agonist, induces antidepressant-like effects without ketamine-like side effects. Neuropsychopharmacology 2013, 38, 729–742. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.C.; Wei, I.H.; Huang, C.L.; Chen, K.T.; Tsai, M.H.; Tsai, P.; Tun, R.; Huang, K.H.; Chang, Y.C.; Lane, H.Y.; Tsai, G.E. Inhibition of glycine transporter-I as a novel mechanism for the treatment of depression. Biol. Psychiatry 2013, 74, 734–741. [Google Scholar] [CrossRef] [PubMed]
- Zarate, C.A., Jr.; Singh, J.B.; Quiroz, J.A.; De Jesus, G.; Denicoff, K.K.; Luckenbaugh, D.A.; Manji, H.K.; Charney, D.S. A double-blind, placebo-controlled study of memantine in the treatment of major depression. Am. J. Psychiatry 2006, 163, 153–155. [Google Scholar] [CrossRef] [PubMed]
- Gideons, E.S.; Kavalali, E.T.; Monteggia, L.M. Mechanisms underlying differential effectiveness of memantine and ketamine in rapid antidepressant responses. Proc. Natl. Acad. Sci. U.S.A. 2014, 111, 8649–8654. [Google Scholar] [CrossRef] [PubMed]
- Chiu, C.T.; Scheuing, L.; Liu, G.; Liao, H.M.; Linares, G.R.; Lin, D.; Chuang, D.M. The mood stabilizer lithium potentiates the antidepressant-like effects and ameliorates oxidative stress induced by acute ketamine in a mouse model of stress. Int. J. Neuropsychopharmacol. 2014, 18. pii: pyu102. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.J.; Fuchikami, M.; Dwyer, J.M.; Lepack, A.E.; Duman, R.S.; Aghajanian, G.K. GSK-3 inhibition potentiates the synaptogenic and antidepressant-like effects of subthreshold doses of ketamine. Neuropsychopharmacology 2013, 38, 2268–2277. [Google Scholar] [CrossRef] [PubMed]
- Chaki, S.; Yoshikawa, R.; Hirota, S.; Shimazaki, T.; Maeda, M.; Kawashima, N.; Yoshimizu, T.; Yasuhara, A.; Sakagami, K.; Okuyama, S.; Nakanishi, S.; Nakazato, A. MGS0039: A potent and selective group II metabotropic glutamate receptor antagonist with antidepressant-like activity. Neuropharmacology 2004, 46, 457–467. [Google Scholar] [CrossRef] [PubMed]
- Kawasaki, T.; Ago, Y.; Yano, K.; Araki, R.; Washida, Y.; Onoe, H.; Chaki, S.; Nakazato, A.; Hashimoto, H.; Baba, A.; Takuma, K.; Matsuda, T. Increased binding of cortical and hippocampal group II metabotropic glutamate receptors in isolation-reared mice. Neuropharmacology 2011, 60, 397–404. [Google Scholar] [CrossRef] [PubMed]
- Pałucha-Poniewiera, A.; Wierońska, JM.; Brański, P.; Stachowicz, K.; Chaki, S.; Pilc, A. On the mechanism of the antidepressant-like action of group II mGlu receptor antagonist, MGS0039. Psychopharmacology (Berl.) 2010, 212, 523–535. [Google Scholar] [CrossRef] [PubMed]
- Yoshimizu, T.; Shimazaki, T.; Ito, A.; Chaki, S. An mGluR2/3 antagonist, MGS0039, exerts antidepressant and anxiolytic effects in behavioral models in rats. Psychopharmacology (Berl.) 2006, 186, 587–593. [Google Scholar] [CrossRef] [PubMed]
- Dwyer, J.M.; Lepack, A.E.; Duman, R.S. mGluR2/3 blockade produces rapid and long-lasting reversal of anhedonia caused by chronic stress exposure. J. Mol. Psychiatry 2013, 1, 15. [Google Scholar] [CrossRef] [PubMed]
- Koike, H.; Iijima, M.; Chaki, S. Effects of ketamine and LY341495 on the depressive-like behavior of repeated corticosterone-injected rats. Pharmacol. Biochem. Behav. 2013, 107, 20–23. [Google Scholar] [CrossRef] [PubMed]
- Dwyer, J.M.; Lepack, A.E.; Duman, R.S. mTOR activation is required for the antidepressant effects of mGluR2/3 blockade. Int. J. Neuropsychopharmacol. 2012, 15, 429–434. [Google Scholar] [CrossRef] [PubMed]
- Hascup, E.R.; Hascup, K.N.; Stephens, M.; Pomerleau, F.; Huettl, P.; Gratton, A.; Gerhardt, G.A. Rapid microelectrode measurements and the origin and regulation of extracellular glutamate in rat prefrontal cortex. J. Neurochem. 2010, 115, 1608–1620. [Google Scholar] [CrossRef] [PubMed]
- Koike, H.; Iijima, M.; Chaki, S. Involvement of the mammalian target of rapamycin signaling in the antidepressant-like effect of group II metabotropic glutamate receptor antagonists. Neuropharmacology 2011, 61, 1419–1423. [Google Scholar] [CrossRef] [PubMed]
- Fukumoto, K.; Iijima, M.; Chaki, S. Serotonin-1A receptor stimulation mediates effects of a metabotropic glutamate 2/3 receptor antagonist, 2S-2-amino-2-(1S,2S-2-carboxycycloprop-1-yl)-3-(xanth-9-yl)propanoic acid (LY341495), and an N-methyl-d-aspartate receptor antagonist, ketamine, in the novelty-suppressed feeding test. Psychopharmacology (Berl.) 2014, 231, 2291–2298. [Google Scholar] [PubMed]
- Gigliucci, V.; O'Dowd, G.; Casey, S.; Egan, D.; Gibney, S.; Harkin, A. Ketamine elicits sustained antidepressant-like activity via a serotonin-dependent mechanism. Psychopharmacology (Berl.) 2013, 228, 157–166. [Google Scholar] [CrossRef] [PubMed]
- Gradient, R.A.; Wedel, P.C.; Frisbie, V.M.; Leuchter, A.F.; Targum, S.D.; Truong, C.T.; Hutchinson, J.H. Safety, pharmacokinetic and pharmacodynamics profile of BCI-632, a selective metabotropic glutamate 2/3 receptor antagonist, in healthy human subjects. Abstr. Neurosci. Meeting 2012, 42, 20. [Google Scholar]
- Belozertseva, I.V.; Kos, T.; Popik, P.; Danysz, W.; Bespalov, A.Y. Antidepressant-like effects of mGluR1 and mGluR5 antagonists in the rat forced swim and the mouse tail suspension tests. Eur. Neuropsychopharmacol. 2007, 17, 172–179. [Google Scholar] [CrossRef] [PubMed]
- Hughes, Z.A.; Neal, S.J.; Smith, D.L.; Rizzo, S.S.; Pulicicchio, C.M.; Lotarski, S.; Lu, S.; Dwyer, J.M.; Brennan, J.; Olsen, M.; et al. Negative allosteric modulation of metabotropic glutamate receptor 5 results in broad spectrum activity relevant to treatment resistant depression. Neuropharmacology 2013, 66, 202–214. [Google Scholar] [CrossRef] [PubMed]
- Pałucha, A.; Brański, P.; Szewczyk, B.; Wierońska, J.M.; Kłak, K.; Pilc, A. Potential antidepressant-like effect of MTEP, a potent and highly selective mGluR5 antagonist. Pharmacol. Biochem. Behav. 2005, 81, 901–906. [Google Scholar] [CrossRef] [PubMed]
- Pilc, A.; Kłodzińska, A.; Brański, P.; Nowak, G.; Pałucha, A.; Szewczyk, B.; Tatarczyńska, E.; Chojnacka-Wójcik, E.; Wierońska, J.M. Multiple MPEP administrations evoke anxiolytic- and antidepressant-like effects in rats. Neuropharmacology 2002, 43, 181–187. [Google Scholar] [CrossRef]
- Li, X.; Need, A.B.; Baez, M.; Witkin, J.M. Metabotropic glutamate 5 receptor antagonism is associated with antidepressant-like effects in mice. J. Pharmacol. Exp. Ther. 2006, 319, 254–259. [Google Scholar] [CrossRef] [PubMed]
- Chaki, S.; Ago, Y.; Palucha-Paniewiera, A.; Matrisciano, F.; Pilc, A. mGlu2/3 and mGlu5 receptors: potential targets for novel antidepressants. Neuropharmacology 2013, 66, 40–52. [Google Scholar] [CrossRef] [PubMed]
- Pałucha-Poniewiera, A.; Brański, P.; Wierońska, J.M.; Stachowicz, K.; Sławińska, A.; Pilc, A. The antidepressant-like action of mGlu5 receptor antagonist, MTEP, in the tail suspension test in mice is serotonin dependent. Psychopharmacology (Berl.) 2014, 231, 97–107. [Google Scholar] [CrossRef] [PubMed]
- Fukumoto, K.; Chaki, S. Involvement of serotonergic system in the effect of a metabotropic glutamate 5 receptor antagonist in the novelty-suppressed feeding test. J. Pharmacol. Sci. 2015, 127, 57–61. [Google Scholar] [CrossRef] [PubMed]
- Quiroz, J.A.; Tamburri, P.; Deptula, D.; Banken, L.; Beyer, U.; Fontoura, P.; Santarelli, L. The efficacy and safety of basimglurant as adjunctive therapy in major depression; a randomised, double-blind, placebo-controlled study. In Abstracts of the 27th Congress of the European College of Neuropsychopharmacology, Berlin, 18–21 October 2014. P.2.f.027.
- Podkowa, K.; Rzeźniczek, S.; Marciniak, M.; Acher, F.; Pilc, A.; Pałucha-Poniewiera, A. A novel mGlu4 selective agonist LSP4–2022 increases behavioral despair in mouse models of antidepressant action. Neuropharmacology 2015, 97, 338–345. [Google Scholar] [CrossRef] [PubMed]
- Kalinichev, M.; Le Poul, E.; Boléa, C.; Girard, F.; Campo, B.; Fonsi, M.; Royer-Urios, I.; Browne, S.E.; Uslaner, J.M.; Davis, M.J.; Raber, J.; Duvoisin, R.; Bate, S.T.; Reynolds, I.J.; Poli, S.; Celanire, S. Characterization of the novel positive allosteric modulator of the metabotropic glutamate receptor 4 ADX88178 in rodent models of neuropsychiatric disorders. J. Pharmacol. Exp. Ther. 2014, 350, 495–505. [Google Scholar] [CrossRef] [PubMed]
- Sławińska, A.; Wierońska, J.M.; Stachowicz, K.; Pałucha-Poniewiera, A.; Uberti, M.A.; Bacolod, M.A.; Doller, D.; Pilc, A. Anxiolytic- but not antidepressant-like activity of Lu AF21934, a novel, selective positive allosteric modulator of the mGlu4 receptor. Neuropharmacology 2013, 66, 225–235. [Google Scholar] [CrossRef] [PubMed]
- Wierońska, J.M.; Stachowicz, K.; Pałucha-Poniewiera, A.; Acher, F.; Brański, P.; Pilc, A. Metabotropic glutamate receptor 4 novel agonist LSP1–2111 with anxiolytic, but not antidepressant-like activity, mediated by serotonergic and GABAergic systems. Neuropharmacology 2010, 59, 627–634. [Google Scholar] [CrossRef] [PubMed]
- O'Connor, R.M.; Cryan, J.F. The effects of mGlu₇ receptor modulation in behavioural models sensitive to antidepressant action in two mouse strains. Behav. Pharmacol. 2013, 24, 105–113. [Google Scholar] [CrossRef] [PubMed]
- Pałucha-Poniewiera, A.; Brański, P.; Lenda, T.; Pilc, A. The antidepressant-like action of metabotropic glutamate 7 receptor agonist N,N'-bis(diphenylmethyl)-1,2-ethanediamine (AMN082) is serotonin-dependent. J. Pharmacol. Exp. Ther. 2010, 334, 1066–1074. [Google Scholar] [CrossRef] [PubMed]
- Palucha, A.; Klak, K.; Branski, P.; van der Putten, H.; Flor, P.J.; Pilc, A. Activation of the mGlu7 receptor elicits antidepressant-like effects in mice. Psychopharmacology (Berl.) 2007, 194, 555–562. [Google Scholar] [CrossRef] [PubMed]
- Pałucha-Poniewiera, A.; Pilc, A. A selective mGlu7 receptor antagonist MMPIP reversed antidepressant-like effects of AMN082 in rats. Behav. Brain Res. 2013, 238, 109–112. [Google Scholar] [CrossRef] [PubMed]
- Pałucha-Poniewiera, A.; Szewczyk, B.; Pilc, A. Activation of the mTOR signaling pathway in the antidepressant-like activity of the mGlu5 antagonist MTEP and the mGlu7 agonist AMN082 in the FST in rats. Neuropharmacology 2014, 82, 59–68. [Google Scholar] [CrossRef] [PubMed]
- Cryan, J.F.; Kelly, P.H.; Neijt, H.C.; Sansig, G.; Flor, P.J.; van Der Putten, H. Antidepressant and anxiolytic-like effects in mice lacking the group III metabotropic glutamate receptor mGluR7. Eur. J. Neurosci. 2003, 17, 2409–2417. [Google Scholar] [CrossRef] [PubMed]
- Drevets, W.C.; Zarate, C.A., Jr.; Furey, M.L. Antidepressant effects of the muscarinic cholinergic receptor antagonist scopolamine: A review. Biol. Psychiatry 2013, 73, 1156–1163. [Google Scholar] [CrossRef] [PubMed]
- Voleti, B.; Navarria, A.; Liu, R.J.; Banasr, M.; Li, N.; Terwilliger, R.; Sanacora, G.; Eid, T.; Aghajanian, G.; Duman, R.S. Scopolamine rapidly increases mammalian target of rapamycin complex 1 signaling, synaptogenesis, and antidepressant behavioral responses. Biol. Psychiatry 2013, 74, 742–749. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chaki, S.; Fukumoto, K. Potential of Glutamate-Based Drug Discovery for Next Generation Antidepressants. Pharmaceuticals 2015, 8, 590-606. https://doi.org/10.3390/ph8030590
Chaki S, Fukumoto K. Potential of Glutamate-Based Drug Discovery for Next Generation Antidepressants. Pharmaceuticals. 2015; 8(3):590-606. https://doi.org/10.3390/ph8030590
Chicago/Turabian StyleChaki, Shigeyuki, and Kenichi Fukumoto. 2015. "Potential of Glutamate-Based Drug Discovery for Next Generation Antidepressants" Pharmaceuticals 8, no. 3: 590-606. https://doi.org/10.3390/ph8030590
APA StyleChaki, S., & Fukumoto, K. (2015). Potential of Glutamate-Based Drug Discovery for Next Generation Antidepressants. Pharmaceuticals, 8(3), 590-606. https://doi.org/10.3390/ph8030590




