Dynamic Cross Talk between S1P and CXCL12 Regulates Hematopoietic Stem Cells Migration, Development and Bone Remodeling
Abstract
:1. Introduction
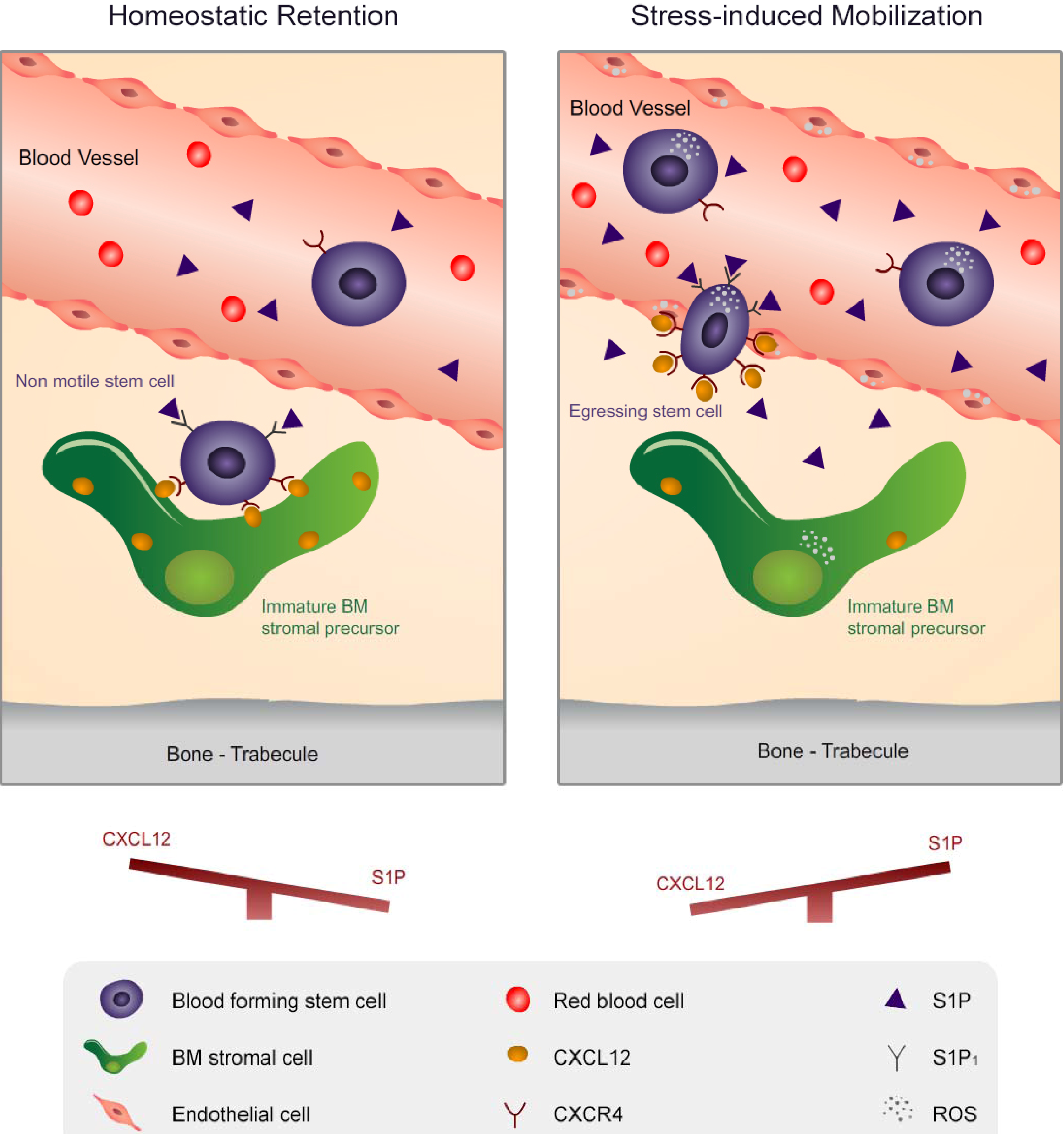
2. Migration of Hematopoietic Stem Cells and Mature Leukocytes is Dynamically Regulated by the Levels of CXCL12 in the BM and S1P in the Blood
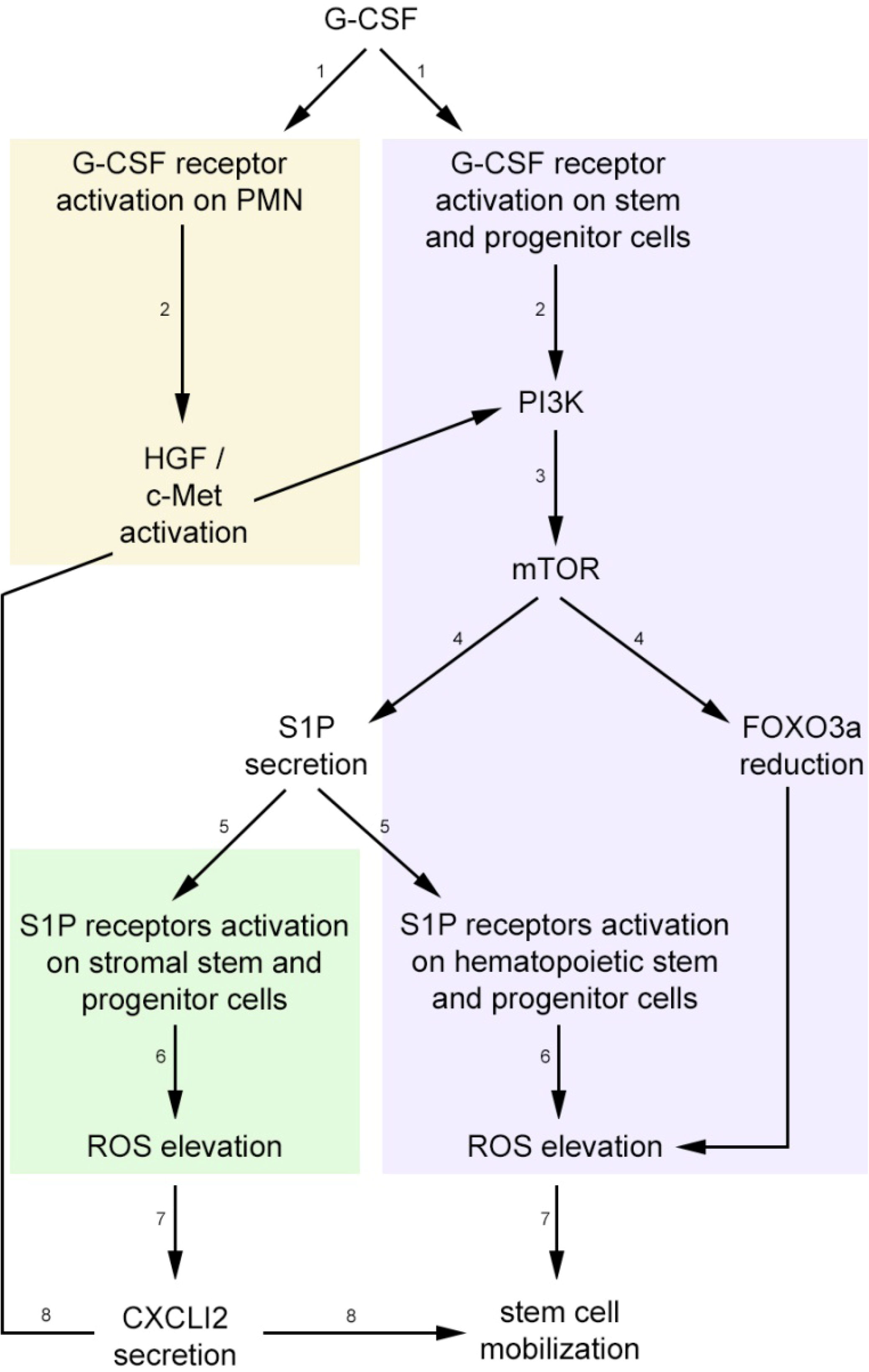
3. Stress-Induced Stem and Progenitor Cell Mobilization is Orchestrated by Dynamic CXCL12 and S1P Regulation via ROS Signaling
4. S1P and CXCL12 Regulate Bone Turnover and the Dynamic BM Microenvironment, Indirectly Facilitating Stem Cell Development and Migration
5. Conclusions, Clinical Aspects and Future Directions
Acknowledgments
Conflicts of Interest
References
- Ponomaryov, T.; Peled, A.; Petit, I.; Taichman, R.S.; Habler, L.; Sandbank, J.; Arenzana-Seisdedos, F.; Magerus, A.; Caruz, A.; Fujii, N.; et al. Induction of the chemokine stromal-derived factor-1 following DNA damage improves human stem cell function. J. Clin. Invest. 2000, 106, 1331–1339. [Google Scholar] [CrossRef]
- Petit, I.; Szyper-Kravitz, M.; Nagler, A.; Lahav, M.; Peled, A.; Habler, L.; Ponomaryov, T.; Taichman, R.S.; Arenzana-Seisdedos, F.; Fujii, N.; et al. G-csf induces stem cell mobilization by decreasing bone marrow sdf-1 and up-regulating cxcr4. Nat. Immunol. 2002, 3, 687–694. [Google Scholar] [CrossRef]
- Lapidot, T.; Petit, I. Current understanding of stem cell mobilization: The roles of chemokines, proteolytic enzymes, adhesion molecules, cytokines, and stromal cells. Exp. Hematol. 2002, 30, 973–981. [Google Scholar] [CrossRef]
- Lapidot, T.; Kollet, O. The brain-bone-blood triad: Traffic lights for stem-cell homing and mobilization. Hematol. Am. Soc. Hematol. Educ. Program 2010, 2010, 1–6. [Google Scholar] [CrossRef]
- Levesque, J.P.; Winkler, I.G.; Rasko, J.E. Nichotherapy for stem cells: There goes the neighborhood. Bioessays 2013, 35, 183–190. [Google Scholar] [CrossRef]
- Laird, D.J.; von Andrian, U.H.; Wagers, A.J. Stem cell trafficking in tissue development, growth, and disease. Cell 2008, 132, 612–630. [Google Scholar] [CrossRef]
- Mazo, I.B.; Massberg, S.; von Andrian, U.H. Hematopoietic stem and progenitor cell trafficking. Trends Immunol. 2011, 32, 493–503. [Google Scholar] [CrossRef]
- Greenbaum, A.M.; Link, D.C. Mechanisms of g-csf-mediated hematopoietic stem and progenitor mobilization. Leukemia 2010, 25, 211–217. [Google Scholar]
- Papayannopoulou, T.; Scadden, D.T. Stem-cell ecology and stem cells in motion. Blood 2008, 111, 3923–3930. [Google Scholar] [CrossRef]
- Hoggatt, J.; Pelus, L.M. Many mechanisms mediating mobilization: An alliterative review. Curr. Opin. Hematol. 2011, 18, 231–238. [Google Scholar] [CrossRef]
- Broxmeyer, H.E.; Orschell, C.M.; Clapp, D.W.; Hangoc, G.; Cooper, S.; Plett, P.A.; Liles, W.C.; Li, X.; Graham-Evans, B.; Campbell, T.B.; et al. Rapid mobilization of murine and human hematopoietic stem and progenitor cells with amd3100, a cxcr4 antagonist. J. Exp. Med. 2005, 201, 1307–1318. [Google Scholar] [CrossRef]
- Pusic, I.; DiPersio, J.F. Update on clinical experience with amd3100, an sdf-1/cxcl12-cxcr4 inhibitor, in mobilization of hematopoietic stem and progenitor cells. Curr. Opin. Hematol. 2010, 17, 319–326. [Google Scholar] [CrossRef]
- Frenette, P.S.; Weiss, L. Sulfated glycans induce rapid hematopoietic progenitor cell mobilization: Evidence for selectin-dependent and independent mechanisms. Blood 2000, 96, 2460–2468. [Google Scholar]
- Sweeney, E.A.; Lortat-Jacob, H.; Priestley, G.V.; Nakamoto, B.; Papayannopoulou, T. Sulfated polysaccharides increase plasma levels of sdf-1 in monkeys and mice: Involvement in mobilization of stem/progenitor cells. Blood 2002, 99, 44–51. [Google Scholar] [CrossRef]
- Suda, T.; Arai, F.; Shimmura, S. Regulation of stem cells in the niche. Cornea 2005, 24, S12–S17. [Google Scholar] [CrossRef]
- Mendez-Ferrer, S.; Lucas, D.; Battista, M.; Frenette, P.S. Haematopoietic stem cell release is regulated by circadian oscillations. Nature 2008, 452, 442–447. [Google Scholar] [CrossRef]
- Spiegel, A.; Kalinkovich, A.; Shivtiel, S.; Kollet, O.; Lapidot, T. Stem cell regulation via dynamic interactions of the nervous and immune systems with the microenvironment. Cell Stem Cell 2008, 3, 484–492. [Google Scholar] [CrossRef]
- Kollet, O.; Dar, A.; Shivtiel, S.; Kalinkovich, A.; Lapid, K.; Sztainberg, Y.; Tesio, M.; Samstein, R.M.; Goichberg, P.; Spiegel, A.; et al. Osteoclasts degrade endosteal components and promote mobilization of hematopoietic progenitor cells. Nat. Med. 2006, 12, 657–664. [Google Scholar] [CrossRef]
- Broxmeyer, H.E.; Kohli, L.; Kim, C.H.; Lee, Y.; Mantel, C.; Cooper, S.; Hangoc, G.; Shaheen, M.; Li, X.; Clapp, D.W. Stromal cell-derived factor-1/cxcl12 directly enhances survival/antiapoptosis of myeloid progenitor cells through cxcr4 and g(alpha)i proteins and enhances engraftment of competitive, repopulating stem cells. J. Leukoc. Biol. 2003, 73, 630–638. [Google Scholar] [CrossRef]
- Broxmeyer, H.E. Chemokines in hematopoiesis. Curr. Opin. Hematol. 2008, 15, 49–58. [Google Scholar] [CrossRef]
- Peled, A.; Kollet, O.; Ponomaryov, T.; Petit, I.; Franitza, S.; Grabovsky, V.; Slav, M.M.; Nagler, A.; Lider, O.; Alon, R.; et al. The chemokine sdf-1 activates the integrins lfa-1, vla-4, and vla-5 on immature human cd34(+) cells: Role in transendothelial/stromal migration and engraftment of nod/scid mice. Blood 2000, 95, 3289–3296. [Google Scholar]
- Avigdor, A.; Goichberg, P.; Shivtiel, S.; Dar, A.; Peled, A.; Samira, S.; Kollet, O.; Hershkoviz, R.; Alon, R.; Hardan, I.; et al. Cd44 and hyaluronic acid cooperate with sdf-1 in the trafficking of human cd34+ stem/progenitor cells to bone marrow. Blood 2004, 103, 2981–2989. [Google Scholar] [CrossRef]
- Sugiyama, T.; Kohara, H.; Noda, M.; Nagasawa, T. Maintenance of the hematopoietic stem cell pool by cxcl12-cxcr4 chemokine signaling in bone marrow stromal cell niches. Immunity 2006, 25, 977–988. [Google Scholar] [CrossRef]
- Alvarez, S.E.; Milstien, S.; Spiegel, S. Autocrine and paracrine roles of sphingosine-1-phosphate. Trends Endocrinol. Metab. 2007, 18, 300–307. [Google Scholar] [CrossRef]
- Pebay, A.; Bonder, C.S.; Pitson, S.M. Stem cell regulation by lysophospholipids. Prostaglandins Other Lipid Mediat. 2007, 84, 83–97. [Google Scholar] [CrossRef]
- Venkataraman, K.; Lee, Y.M.; Michaud, J.; Thangada, S.; Ai, Y.; Bonkovsky, H.L.; Parikh, N.S.; Habrukowich, C.; Hla, T. Vascular endothelium as a contributor of plasma sphingosine 1-phosphate. Circ. Res. 2008, 102, 669–676. [Google Scholar] [CrossRef]
- Bendall, L.J.; Basnett, J. Role of sphingosine 1-phosphate in trafficking and mobilization of hematopoietic stem cells. Curr. Opin. Hematol. 2013, 20, 281–288. [Google Scholar] [CrossRef]
- Schwab, S.R.; Pereira, J.P.; Matloubian, M.; Xu, Y.; Huang, Y.; Cyster, J.G. Lymphocyte sequestration through s1p lyase inhibition and disruption of s1p gradients. Science 2005, 309, 1735–1739. [Google Scholar] [CrossRef]
- Ito, K.; Anada, Y.; Tani, M.; Ikeda, M.; Sano, T.; Kihara, A.; Igarashi, Y. Lack of sphingosine 1-phosphate-degrading enzymes in erythrocytes. Biochem. Biophys. Res. Commun. 2007, 357, 212–217. [Google Scholar] [CrossRef]
- Liu, J.; Hsu, A.; Lee, J.F.; Cramer, D.E.; Lee, M.J. To stay or to leave: Stem cells and progenitor cells navigating the s1p gradient. World J. Biol. Chem. 2011, 2, 1–13. [Google Scholar] [CrossRef]
- Pappu, R.; Schwab, S.R.; Cornelissen, I.; Pereira, J.P.; Regard, J.B.; Xu, Y.; Camerer, E.; Zheng, Y.W.; Huang, Y.; Cyster, J.G.; et al. Promotion of lymphocyte egress into blood and lymph by distinct sources of sphingosine-1-phosphate. Science 2007, 316, 295–298. [Google Scholar] [CrossRef]
- Hisano, Y.; Kobayashi, N.; Yamaguchi, A.; Nishi, T. Mouse spns2 functions as a sphingosine-1-phosphate transporter in vascular endothelial cells. PLoS One 2012, 7, e38941. [Google Scholar]
- Fukuhara, S.; Simmons, S.; Kawamura, S.; Inoue, A.; Orba, Y.; Tokudome, T.; Sunden, Y.; Arai, Y.; Moriwaki, K.; Ishida, J.; et al. The sphingosine-1-phosphate transporter spns2 expressed on endothelial cells regulates lymphocyte trafficking in mice. J. Clin. Invest. 2012, 122, 1416–1426. [Google Scholar] [CrossRef]
- Venkataraman, K.; Thangada, S.; Michaud, J.; Oo, M.L.; Ai, Y.; Lee, Y.M.; Wu, M.; Parikh, N.S.; Khan, F.; Proia, R.L.; et al. Extracellular export of sphingosine kinase-1a contributes to the vascular s1p gradient. Biochem. J. 2006, 397, 461–471. [Google Scholar] [CrossRef]
- Ratajczak, M.Z.; Kim, C.; Janowska-Wieczorek, A.; Ratajczak, J. The expanding family of bone marrow homing factors for hematopoietic stem cells: Stromal derived factor 1 is not the only player in the game. Sci. World J. 2012, 2012, 758512. [Google Scholar]
- Goetzl, E.J.; Wang, W.; McGiffert, C.; Liao, J.J.; Huang, M.C. Sphingosine 1-phosphate as an intracellular messenger and extracellular mediator in immunity. Acta Paediatr. Suppl. 2007, 96, 49–52. [Google Scholar]
- Kimura, T.; Boehmler, A.M.; Seitz, G.; Kuci, S.; Wiesner, T.; Brinkmann, V.; Kanz, L.; Mohle, R. The sphingosine 1-phosphate receptor agonist fty720 supports cxcr4-dependent migration and bone marrow homing of human cd34+ progenitor cells. Blood 2004, 103, 4478–4486. [Google Scholar] [CrossRef]
- Golan, K.; Vagima, Y.; Ludin, A.; Itkin, T.; Cohen-Gur, S.; Kalinkovich, A.; Kollet, O.; Kim, C.; Schajnovitz, A.; Ovadya, Y.; et al. S1p promotes murine progenitor cell egress and mobilization via s1p1-mediated ros signaling and sdf-1 release. Blood 2012, 119, 2478–2488. [Google Scholar] [CrossRef]
- Hla, T. Immunology. Dietary factors and immunological consequences. Science 2005, 309, 1682–1683. [Google Scholar]
- Nagahashi, M.; Kim, E.Y.; Yamada, A.; Ramachandran, S.; Allegood, J.C.; Hait, N.C.; Maceyka, M.; Milstien, S.; Takabe, K.; Spiegel, S. Spns2, a transporter of phosphorylated sphingoid bases, regulates their blood and lymph levels, and the lymphatic network. FASEB J. 2013, 27, 1001–1011. [Google Scholar] [CrossRef]
- Sobue, S.; Hagiwara, K.; Banno, Y.; Tamiya-Koizumi, K.; Suzuki, M.; Takagi, A.; Kojima, T.; Asano, H.; Nozawa, Y.; Murate, T. Transcription factor specificity protein 1 (sp1) is the main regulator of nerve growth factor-induced sphingosine kinase 1 gene expression of the rat pheochromocytoma cell line, pc12. J. Neurochem. 2005, 95, 940–949. [Google Scholar] [CrossRef]
- Nervi, B.; Link, D.C.; DiPersio, J.F. Cytokines and hematopoietic stem cell mobilization. J. Cell. Biochem. 2006, 99, 690–705. [Google Scholar] [CrossRef]
- Lapid, K.; Vagima, Y.; Kollet, O.; Lapidot, T. Egress and mobilization of hematopoietic stem and progenitor cells. Available online: http://www.stembook.org/node/558 (accessed on 18 August 2013).
- Kollet, O.; Dar, A.; Lapidot, T. The multiple roles of osteoclasts in host defense: Bone remodeling and hematopoietic stem cell mobilization. Annu. Rev. Immunol. 2007, 25, 51–69. [Google Scholar] [CrossRef]
- Spiegel, A.; Shivtiel, S.; Kalinkovich, A.; Ludin, A.; Netzer, N.; Goichberg, P.; Azaria, Y.; Resnick, I.; Hardan, I.; Ben-Hur, H.; et al. Catecholaminergic neurotransmitters regulate migration and repopulation of immature human cd34+ cells through wnt signaling. Nat. Immunol. 2007, 8, 1123–1131. [Google Scholar] [CrossRef]
- Wright, D.E.; Bowman, E.P.; Wagers, A.J.; Butcher, E.C.; Weissman, I.L. Hematopoietic stem cells are uniquely selective in their migratory response to chemokines. J. Exp. Med. 2002, 195, 1145–1154. [Google Scholar] [CrossRef]
- Peled, A.; Petit, I.; Kollet, O.; Magid, M.; Ponomaryov, T.; Byk, T.; Nagler, A.; Ben-Hur, H.; Many, A.; Shultz, L.; et al. Dependence of human stem cell engraftment and repopulation of nod/scid mice on cxcr4. Science 1999, 283, 845–848. [Google Scholar] [CrossRef]
- Nagasawa, T.; Hirota, S.; Tachibana, K.; Takakura, N.; Nishikawa, S.; Kitamura, Y.; Yoshida, N.; Kikutani, H.; Kishimoto, T. Defects of b-cell lymphopoiesis and bone-marrow myelopoiesis in mice lacking the cxc chemokine pbsf/sdf-1. Nature 1996, 382, 635–638. [Google Scholar] [CrossRef]
- Tzeng, Y.S.; Li, H.; Kang, Y.L.; Chen, W.C.; Cheng, W.C.; Lai, D.M. Loss of cxcl12/sdf-1 in adult mice decreases the quiescent state of hematopoietic stem/progenitor cells and alters the pattern of hematopoietic regeneration after myelosuppression. Blood 2011, 117, 429–439. [Google Scholar] [CrossRef]
- Ara, T.; Tokoyoda, K.; Sugiyama, T.; Egawa, T.; Kawabata, K.; Nagasawa, T. Long-term hematopoietic stem cells require stromal cell-derived factor-1 for colonizing bone marrow during ontogeny. Immunity 2003, 19, 257–267. [Google Scholar] [CrossRef]
- Rueda, P.; Richart, A.; Recalde, A.; Gasse, P.; Vilar, J.; Guerin, C.; Lortat-Jacob, H.; Vieira, P.; Baleux, F.; Chretien, F.; et al. Homeostatic and tissue reparation defaults in mice carrying selective genetic invalidation of cxcl12/proteoglycan interactions. Circulation 2012, 126, 1882–1895. [Google Scholar] [CrossRef]
- Nagasawa, T.; Kikutani, H.; Kishimoto, T. Molecular cloning and structure of a pre-b-cell growth-stimulating factor. Proc. Natl. Acad. Sci. USA 1994, 91, 2305–2309. [Google Scholar] [CrossRef]
- Nie, Y.; Han, Y.C.; Zou, Y.R. Cxcr4 is required for the quiescence of primitive hematopoietic cells. J. Exp. Med. 2008, 205, 777–783. [Google Scholar] [CrossRef]
- Dar, A.; Goichberg, P.; Shinder, V.; Kalinkovich, A.; Kollet, O.; Netzer, N.; Margalit, R.; Zsak, M.; Nagler, A.; Hardan, I.; et al. Chemokine receptor cxcr4-dependent internalization and resecretion of functional chemokine sdf-1 by bone marrow endothelial and stromal cells. Nat. Immunol. 2005, 6, 1038–1046. [Google Scholar] [CrossRef]
- Kortesidis, A.; Zannettino, A.; Isenmann, S.; Shi, S.; Lapidot, T.; Gronthos, S. Stromal-derived factor-1 promotes the growth, survival, and development of human bone marrow stromal stem cells. Blood 2005, 105, 3793–3801. [Google Scholar] [CrossRef]
- Schajnovitz, A.; Itkin, T.; D'Uva, G.; Kalinkovich, A.; Golan, K.; Ludin, A.; Cohen, D.; Shulman, Z.; Avigdor, A.; Nagler, A.; et al. Cxcl12 secretion by bone marrow stromal cells is dependent on cell contact and mediated by connexin-43 and connexin-45 gap junctions. Nat. Immunol. 2011, 12, 391–398. [Google Scholar] [CrossRef]
- Naumann, U.; Cameroni, E.; Pruenster, M.; Mahabaleshwar, H.; Raz, E.; Zerwes, H.G.; Rot, A.; Thelen, M. Cxcr7 functions as a scavenger for cxcl12 and cxcl11. PLoS One 2010, 5, e9175. [Google Scholar] [CrossRef]
- Brinkmann, V.; Cyster, J.G.; Hla, T. Fty720: Sphingosine 1-phosphate receptor-1 in the control of lymphocyte egress and endothelial barrier function. Am. J. Transplant. 2004, 4, 1019–1025. [Google Scholar] [CrossRef]
- Dev, K.K.; Mullershausen, F.; Mattes, H.; Kuhn, R.R.; Bilbe, G.; Hoyer, D.; Mir, A. Brain sphingosine-1-phosphate receptors: Implication for fty720 in the treatment of multiple sclerosis. Pharm. Ther. 2008, 117, 77–93. [Google Scholar] [CrossRef]
- Xin, C.; Ren, S.; Eberhardt, W.; Pfeilschifter, J.; Huwiler, A. The immunomodulator fty720 and its phosphorylated derivative activate the smad signalling cascade and upregulate connective tissue growth factor and collagen type iv expression in renal mesangial cells. Br. J Pharmacol. 2006, 147, 164–174. [Google Scholar] [CrossRef]
- Ratajczak, M.Z.; Shin, D.M.; Ratajczak, J.; Kucia, M.; Bartke, A. A novel insight into aging: Are there pluripotent very small embryonic-like stem cells (vsels) in adult tissues overtime depleted in an igf-1-dependent manner? Aging 2010, 2, 875–883. [Google Scholar]
- Schwab, S.R.; Cyster, J.G. Finding a way out: Lymphocyte egress from lymphoid organs. Nat. Immunol. 2007, 8, 1295–1301. [Google Scholar] [CrossRef]
- Pereira, J.P.; Cyster, J.G.; Xu, Y. A role for s1p and s1p1 in immature-b cell egress from mouse bone marrow. PloS One 2010, 5, e9277. [Google Scholar] [CrossRef]
- Seitz, G.; Boehmler, A.M.; Kanz, L.; Mohle, R. The role of sphingosine 1-phosphate receptors in the trafficking of hematopoietic progenitor cells. Ann. N. Y. Acad. Sci. 2005, 1044, 84–89. [Google Scholar]
- Ratajczak, M.Z.; Lee, H.; Wysoczynski, M.; Wan, W.; Marlicz, W.; Laughlin, M.J.; Kucia, M.; Janowska-Wieczorek, A.; Ratajczak, J. Novel insight into stem cell mobilization-plasma sphingosine-1-phosphate is a major chemoattractant that directs the egress of hematopoietic stem progenitor cells from the bone marrow and its level in peripheral blood increases during mobilization due to activation of complement cascade/membrane attack complex. Leukemia 2010, 24, 976–985. [Google Scholar] [CrossRef]
- Juarez, J.G.; Harun, N.; Thien, M.; Welschinger, R.; Baraz, R.; Pena, A.D.; Pitson, S.M.; Rettig, M.; DiPersio, J.F.; Bradstock, K.F.; et al. Sphingosine-1-phosphate facilitates trafficking of hematopoietic stem cells and their mobilization by cxcr4 antagonists in mice. Blood 2012, 119, 707–716. [Google Scholar] [CrossRef]
- Massberg, S.; Schaerli, P.; Knezevic-Maramica, I.; Kollnberger, M.; Tubo, N.; Moseman, E.A.; Huff, I.V.; Junt, T.; Wagers, A.J.; Mazo, I.B.; et al. Immunosurveillance by hematopoietic progenitor cells trafficking through blood, lymph, and peripheral tissues. Cell 2007, 131, 994–1008. [Google Scholar] [CrossRef]
- Massberg, S.; von Andrian, U.H. Novel trafficking routes for hematopoietic stem and progenitor cells. Ann. N. Y. Acad. Sci. 2009, 1176, 87–93. [Google Scholar]
- Sarraj, B.; Massberg, S.; Li, Y.; Kasorn, A.; Subramanian, K.; Loison, F.; Silberstein, L.E.; von Andrian, U.; Luo, H.R. Myeloid-specific deletion of tumor suppressor pten augments neutrophil transendothelial migration during inflammation. J. Immunol. 2009, 182, 7190–7200. [Google Scholar] [CrossRef]
- Whetton, A.D.; Lu, Y.; Pierce, A.; Carney, L.; Spooncer, E. Lysophospholipids synergistically promote primitive hematopoietic cell chemotaxis via a mechanism involving vav 1. Blood 2003, 102, 2798–2802. [Google Scholar] [CrossRef]
- Sanchez-Aguilera, A.; Lee, Y.J.; Lo Celso, C.; Ferraro, F.; Brumme, K.; Mondal, S.; Kim, C.; Dorrance, A.; Luo, H.R.; Scadden, D.T.; et al. Guanine nucleotide exchange factor vav1 regulates perivascular homing and bone marrow retention of hematopoietic stem and progenitor cells. Proc. Natl. Acad. Sci. USA 2011, 108, 9607–9612. [Google Scholar] [CrossRef]
- Ryser, M.F.; Ugarte, F.; Lehmann, R.; Bornhauser, M.; Brenner, S. S1p(1) overexpression stimulates s1p-dependent chemotaxis of human cd34+ hematopoietic progenitor cells but strongly inhibits sdf-1/cxcr4-dependent migration and in vivo homing. Mol. Immunol. 2008, 46, 166–171. [Google Scholar] [CrossRef]
- Lapid, K.; Itkin, T.; D'Uva, G.; Ovadya, Y.; Ludin, A.; Caglio, G.; Kalinkovich, A.; Golan, K.; Porat, Z.; Zollo, M.; et al. Gsk3beta regulates physiological migration of stem/progenitor cells via cytoskeletal rearrangement. J. Clin. Invest. 2013, 123, 1705–1717. [Google Scholar] [CrossRef]
- Kollet, O.; Vagima, Y.; D'Uva, G.; Golan, K.; Canaani, J.; Itkin, T.; Gur-Cohen, S.; Kalinkovich, A.; Caglio, G.; Medaglia, C.; et al. Physiologic corticosterone oscillations regulate murine hematopoietic stem/progenitor cell proliferation and cxcl12 expression by bone marrow stromal progenitors. Leukemia 2013. [CrossRef]
- Katayama, Y.; Battista, M.; Kao, W.M.; Hidalgo, A.; Peired, A.J.; Thomas, S.A.; Frenette, P.S. Signals from the sympathetic nervous system regulate hematopoietic stem cell egress from bone marrow. Cell 2006, 124, 407–421. [Google Scholar] [CrossRef]
- Kalinkovich, A.; Spiegel, A.; Shivtiel, S.; Kollet, O.; Jordaney, N.; Piacibello, W.; Lapidot, T. Blood-forming stem cells are nervous: Direct and indirect regulation of immature human cd34+ cells by the nervous system. Brain Behav. Immun. 2009, 23, 1059–1065. [Google Scholar] [CrossRef]
- Scheiermann, C.; Kunisaki, Y.; Frenette, P.S. Circadian control of the immune system. Nat. Rev. Immunol. 2013, 13, 190–198. [Google Scholar] [CrossRef]
- Scadden, D.T. Circadian rhythms: Stem cells traffic in time. Nature 2008, 452, 416–417. [Google Scholar] [CrossRef]
- Dar, A.; Schajnovitz, A.; Lapid, K.; Kalinkovich, A.; Itkin, T.; Ludin, A.; Kao, W.M.; Battista, M.; Tesio, M.; Kollet, O.; et al. Rapid mobilization of hematopoietic progenitors by amd3100 and catecholamines is mediated by cxcr4-dependent sdf-1 release from bone marrow stromal cells. Leukemia 2011, 25, 1286–1296. [Google Scholar] [CrossRef]
- Levesque, J.P.; Hendy, J.; Winkler, I.G.; Takamatsu, Y.; Simmons, P.J. Granulocyte colony-stimulating factor induces the release in the bone marrow of proteases that cleave c-kit receptor (cd117) from the surface of hematopoietic progenitor cells. Exp. Hematol. 2003, 31, 109–117. [Google Scholar] [CrossRef]
- Semerad, C.L.; Christopher, M.J.; Liu, F.; Short, B.; Simmons, P.J.; Winkler, I.; Levesque, J.P.; Chappel, J.; Ross, F.P.; Link, D.C. G-csf potently inhibits osteoblast activity and cxcl12 mrna expression in the bone marrow. Blood 2005, 106, 3020–3027. [Google Scholar] [CrossRef]
- Levesque, J.P.; Winkler, I.G.; Hendy, J.; Williams, B.; Helwani, F.; Barbier, V.; Nowlan, B.; Nilsson, S.K. Hematopoietic progenitor cell mobilization results in hypoxia with increased hypoxia-inducible transcription factor-1 alpha and vascular endothelial growth factor a in bone marrow. Stem Cells 2007, 25, 1954–1965. [Google Scholar] [CrossRef]
- Tesio, M.; Golan, K.; Corso, S.; Giordano, S.; Schajnovitz, A.; Vagima, Y.; Shivtiel, S.; Kalinkovich, A.; Caione, L.; Gammaitoni, L.; et al. Enhanced c-met activity promotes g-csf-induced mobilization of hematopoietic progenitor cells via ros signaling. Blood 2011, 117, 419–428. [Google Scholar] [CrossRef]
- Naka, K.; Muraguchi, T.; Hoshii, T.; Hirao, A. Regulation of reactive oxygen species and genomic stability in hematopoietic stem cells. Antioxid. Redox. Signal. 2008, 10, 1883–1894. [Google Scholar] [CrossRef]
- Jang, Y.Y.; Sharkis, S.J. A low level of reactive oxygen species selects for primitive hematopoietic stem cells that may reside in the low-oxygenic niche. Blood 2007, 110, 3056–3063. [Google Scholar] [CrossRef]
- Ito, K.; Hirao, A.; Arai, F.; Takubo, K.; Matsuoka, S.; Miyamoto, K.; Ohmura, M.; Naka, K.; Hosokawa, K.; Ikeda, Y.; et al. Reactive oxygen species act through p38 mapk to limit the lifespan of hematopoietic stem cells. Nat. Med. 2006, 12, 446–451. [Google Scholar] [CrossRef]
- Juntilla, M.M.; Patil, V.D.; Calamito, M.; Joshi, R.P.; Birnbaum, M.J.; Koretzky, G.A. Akt1 and akt2 maintain hematopoietic stem cell function by regulating reactive oxygen species. Blood 2010, 115, 4030–4038. [Google Scholar] [CrossRef]
- Lee, H.M.; Wysoczynski, M.; Liu, R.; Shin, D.M.; Kucia, M.; Botto, M.; Ratajczak, J.; Ratajczak, M.Z. Mobilization studies in complement-deficient mice reveal that optimal amd3100 mobilization of hematopoietic stem cells depends on complement cascade activation by amd3100-stimulated granulocytes. Leukemia 2010, 24, 573–582. [Google Scholar] [CrossRef]
- Tepper, O.M.; Carr, J.; Allen, R.J., Jr.; Chang, C.C.; Lin, C.D.; Tanaka, R.; Gupta, S.M.; Levine, J.P.; Saadeh, P.B.; Warren, S.M. Decreased circulating progenitor cell number and failed mechanisms of stromal cell-derived factor-1alpha mediated bone marrow mobilization impair diabetic tissue repair. Diabetes 2010, 59, 1974–1983. [Google Scholar] [CrossRef]
- McQuibban, G.A.; Butler, G.S.; Gong, J.H.; Bendall, L.; Power, C.; Clark-Lewis, I.; Overall, C.M. Matrix metalloproteinase activity inactivates the cxc chemokine stromal cell-derived factor-1. J. Biol. Chem. 2001, 276, 43503–43508. [Google Scholar]
- Valenzuela-Fernandez, A.; Planchenault, T.; Baleux, F.; Staropoli, I.; Le-Barillec, K.; Leduc, D.; Delaunay, T.; Lazarini, F.; Virelizier, J.L.; Chignard, M.; et al. Leukocyte elastase negatively regulates stromal cell-derived factor-1 (sdf-1)/cxcr4 binding and functions by amino-terminal processing of sdf-1 and cxcr4. J. Biol. Chem. 2002, 277, 15677–15689. [Google Scholar] [CrossRef]
- Levesque, J.P.; Hendy, J.; Takamatsu, Y.; Simmons, P.J.; Bendall, L.J. Disruption of the cxcr4/cxcl12 chemotactic interaction during hematopoietic stem cell mobilization induced by gcsf or cyclophosphamide. J. Clin. Invest. 2003, 111, 187–196. [Google Scholar]
- Hsieh, H.L.; Wu, C.B.; Sun, C.C.; Liao, C.H.; Lau, Y.T.; Yang, C.M. Sphingosine-1-phosphate induces cox-2 expression via pi3k/akt and p42/p44 mapk pathways in rat vascular smooth muscle cells. J. Cell. Physiol. 2006, 207, 757–766. [Google Scholar] [CrossRef]
- Nakahara, T.; Iwase, A.; Nakamura, T.; Kondo, M.; Bayasula; Kobayashi, H.; Takikawa, S.; Manabe, S.; Goto, M.; Kotani, T.; et al. Sphingosine-1-phosphate inhibits h2o2-induced granulosa cell apoptosis via the pi3k/akt signaling pathway. Fertil. Steril. 2012, 98, 1001–1008.e1. [Google Scholar] [CrossRef]
- Vagima, Y.; Avigdor, A.; Goichberg, P.; Shivtiel, S.; Tesio, M.; Kalinkovich, A.; Golan, K.; Dar, A.; Kollet, O.; Petit, I.; et al. Mt1-mmp and reck are involved in human cd34+ progenitor cell retention, egress, and mobilization. J. Clin. Invest. 2009, 119, 492–503. [Google Scholar] [CrossRef]
- Hanel, P.; Andreani, P.; Graler, M.H. Erythrocytes store and release sphingosine 1-phosphate in blood. FASEB J. 2007, 21, 1202–1209. [Google Scholar] [CrossRef]
- Obinata, H.; Hla, T. Sphingosine 1-phosphate in coagulation and inflammation. Semin. Immunopathol. 2012, 34, 73–91. [Google Scholar] [CrossRef]
- Ratajczak, J.; Reca, R.; Kucia, M.; Majka, M.; Allendorf, D.J.; Baran, J.T.; Janowska-Wieczorek, A.; Wetsel, R.A.; Ross, G.D.; Ratajczak, M.Z. Mobilization studies in mice deficient in either c3 or c3a receptor (c3ar) reveal a novel role for complement in retention of hematopoietic stem/progenitor cells in bone marrow. Blood 2004, 103, 2071–2078. [Google Scholar] [CrossRef]
- Lee, H.M.; Wu, W.; Wysoczynski, M.; Liu, R.; Zuba-Surma, E.K.; Kucia, M.; Ratajczak, J.; Ratajczak, M.Z. Impaired mobilization of hematopoietic stem/progenitor cells in c5-deficient mice supports the pivotal involvement of innate immunity in this process and reveals novel promobilization effects of granulocytes. Leukemia 2009, 23, 2052–2062. [Google Scholar] [CrossRef]
- Amara, U.; Flierl, M.A.; Rittirsch, D.; Klos, A.; Chen, H.; Acker, B.; Bruckner, U.B.; Nilsson, B.; Gebhard, F.; Lambris, J.D.; et al. Molecular intercommunication between the complement and coagulation systems. J. Immunol. 2010, 185, 5628–5636. [Google Scholar] [CrossRef]
- Spiel, A.O.; Siller-Matula, J.; Firbas, C.; Leitner, J.M.; Russmueller, G.; Jilma, B. Single dose granulocyte colony-stimulating factor markedly enhances shear-dependent platelet function in humans. Platelets 2010, 21, 464–469. [Google Scholar] [CrossRef]
- Zhang, L.; Orban, M.; Lorenz, M.; Barocke, V.; Braun, D.; Urtz, N.; Schulz, C.; von Bruhl, M.L.; Tirniceriu, A.; Gaertner, F.; et al. A novel role of sphingosine 1-phosphate receptor s1pr1 in mouse thrombopoiesis. J. Exp. Med. 2012, 209, 2165–2181. [Google Scholar] [CrossRef]
- Hla, T.; Galvani, S.; Rafii, S.; Nachman, R. S1p and the birth of platelets. J. Exp. Med. 2012, 209, 2137–2140. [Google Scholar] [CrossRef]
- Christopher, M.J.; Liu, F.; Hilton, M.J.; Long, F.; Link, D.C. Suppression of cxcl12 production by bone marrow osteoblasts is a common and critical pathway for cytokine-induced mobilization. Blood 2009, 114, 1331–1339. [Google Scholar] [CrossRef]
- Winkler, I.G.; Pettit, A.R.; Raggatt, L.J.; Jacobsen, R.N.; Forristal, C.E.; Barbier, V.; Nowlan, B.; Cisterne, A.; Bendall, L.J.; Sims, N.A.; et al. Hematopoietic stem cell mobilizing agents g-csf, cyclophosphamide or amd3100 have distinct mechanisms of action on bone marrow hsc niches and bone formation. Leukemia 2012, 26, 1594–1601. [Google Scholar] [CrossRef]
- Li, S.; Zhai, Q.; Zou, D.; Meng, H.; Xie, Z.; Li, C.; Wang, Y.; Qi, J.; Cheng, T.; Qiu, L. A pivotal role of bone remodeling in granulocyte colony stimulating factor induced hematopoietic stem/progenitor cells mobilization. J. Cell. Physiol. 2013, 228, 1002–1009. [Google Scholar] [CrossRef]
- Takamatsu, Y.; Simmons, P.J.; Moore, R.J.; Morris, H.A.; To, L.B.; Levesque, J.P. Osteoclast-mediated bone resorption is stimulated during short-term administration of granulocyte colony-stimulating factor but is not responsible for hematopoietic progenitor cell mobilization. Blood 1998, 92, 3465–3473. [Google Scholar]
- Lapidot, T.; Dar, A.; Kollet, O. How do stem cells find their way home? Blood 2005, 106, 1901–1910. [Google Scholar] [CrossRef]
- Boyce, B.F. Advances in osteoclast biology reveal potential new drug targets and new roles for osteoclasts. J. Bone Miner. Res. 2013, 28, 711–722. [Google Scholar] [CrossRef]
- Novack, D.V.; Teitelbaum, S.L. The osteoclast: Friend or foe? Annu. Rev. Pathol. 2008, 3, 457–484. [Google Scholar] [CrossRef]
- Kollet, O.; Canaani, J.; Kalinkovich, A.; Lapidot, T. Regulatory cross talks of bone cells, hematopoietic stem cells and the nervous system maintain hematopoiesis. Inflam. Allergy Drug Targets 2012, 11, 170–180. [Google Scholar] [CrossRef]
- Fulzele, K.; Krause, D.S.; Panaroni, C.; Saini, V.; Barry, K.J.; Liu, X.; Lotinun, S.; Baron, R.; Bonewald, L.; Feng, J.Q.; et al. Myelopoiesis is regulated by osteocytes through gsalpha-dependent signaling. Blood 2013, 121, 930–939. [Google Scholar] [CrossRef]
- Honma, M.; Ikebuchi, Y.; Kariya, Y.; Hayashi, M.; Hayashi, N.; Aoki, S.; Suzuki, H. Rankl subcellular trafficking and regulatory mechanisms in osteocytes. J. Bone Miner. Res. 2013, 28, 1936–1949. [Google Scholar] [CrossRef]
- Calvi, L.M.; Adams, G.B.; Weibrecht, K.W.; Weber, J.M.; Olson, D.P.; Knight, M.C.; Martin, R.P.; Schipani, E.; Divieti, P.; Bringhurst, F.R.; et al. Osteoblastic cells regulate the haematopoietic stem cell niche. Nature 2003, 425, 841–846. [Google Scholar] [CrossRef]
- Zhang, J.; Niu, C.; Ye, L.; Huang, H.; He, X.; Tong, W.G.; Ross, J.; Haug, J.; Johnson, T.; Feng, J.Q.; et al. Identification of the haematopoietic stem cell niche and control of the niche size. Nature 2003, 425, 836–841. [Google Scholar] [CrossRef]
- Visnjic, D.; Kalajzic, Z.; Rowe, D.W.; Katavic, V.; Lorenzo, J.; Aguila, H.L. Hematopoiesis is severely altered in mice with an induced osteoblast deficiency. Blood 2004, 103, 3258–3264. [Google Scholar] [CrossRef]
- Itkin, T.; Ludin, A.; Gradus, B.; Gur-Cohen, S.; Kalinkovich, A.; Schajnovitz, A.; Ovadya, Y.; Kollet, O.; Canaani, J.; Shezen, E.; et al. Fgf-2 expands murine hematopoietic stem and progenitor cells via proliferation of stromal cells, c-kit activation, and cxcl12 down-regulation. Blood 2012, 120, 1843–1855. [Google Scholar] [CrossRef]
- Shivtiel, S.; Kollet, O.; Lapid, K.; Schajnovitz, A.; Goichberg, P.; Kalinkovich, A.; Shezen, E.; Tesio, M.; Netzer, N.; Petit, I.; et al. Cd45 regulates retention, motility, and numbers of hematopoietic progenitors, and affects osteoclast remodeling of metaphyseal trabecules. J. Exp. Med. 2008, 205, 2381–2395. [Google Scholar] [CrossRef]
- Aicher, A.; Kollet, O.; Heeschen, C.; Liebner, S.; Urbich, C.; Ihling, C.; Orlandi, A.; Lapidot, T.; Zeiher, A.M.; Dimmeler, S. The wnt antagonist dickkopf-1 mobilizes vasculogenic progenitor cells via activation of the bone marrow endosteal stem cell niche. Circ. Res. 2008, 103, 796–803. [Google Scholar] [CrossRef]
- Fleming, H.E.; Janzen, V.; Lo Celso, C.; Guo, J.; Leahy, K.M.; Kronenberg, H.M.; Scadden, D.T. Wnt signaling in the niche enforces hematopoietic stem cell quiescence and is necessary to preserve self-renewal in vivo. Cell Stem Cell 2008, 2, 274–283. [Google Scholar] [CrossRef]
- Ryu, J.; Kim, H.J.; Chang, E.J.; Huang, H.; Banno, Y.; Kim, H.H. Sphingosine 1-phosphate as a regulator of osteoclast differentiation and osteoclast-osteoblast coupling. EMBO J. 2006, 25, 5840–5851. [Google Scholar] [CrossRef]
- Grey, A.; Xu, X.; Hill, B.; Watson, M.; Callon, K.; Reid, I.R.; Cornish, J. Osteoblastic cells express phospholipid receptors and phosphatases and proliferate in response to sphingosine-1-phosphate. Calcif. Tissue Int. 2004, 74, 542–550. [Google Scholar] [CrossRef]
- Ishii, M.; Kikuta, J. Sphingosine-1-phosphate signaling controlling osteoclasts and bone homeostasis. Biochim. Biophys. Acta 2013, 1831, 223–227. [Google Scholar]
- Grey, A.; Chen, Q.; Callon, K.; Xu, X.; Reid, I.R.; Cornish, J. The phospholipids sphingosine-1-phosphate and lysophosphatidic acid prevent apoptosis in osteoblastic cells via a signaling pathway involving g(i) proteins and phosphatidylinositol-3 kinase. Endocrinology 2002, 143, 4755–4763. [Google Scholar] [CrossRef]
- Pederson, L.; Ruan, M.; Westendorf, J.J.; Khosla, S.; Oursler, M.J. Regulation of bone formation by osteoclasts involves wnt/bmp signaling and the chemokine sphingosine-1-phosphate. Proc. Natl. Acad. Sci. USA 2008, 105, 20764–20769. [Google Scholar] [CrossRef]
- Lotinun, S.; Kiviranta, R.; Matsubara, T.; Alzate, J.A.; Neff, L.; Luth, A.; Koskivirta, I.; Kleuser, B.; Vacher, J.; Vuorio, E.; et al. Osteoclast-specific cathepsin k deletion stimulates s1p-dependent bone formation. J. Clin. Invest. 2013, 123, 666–681. [Google Scholar]
- Sato, C.; Iwasaki, T.; Kitano, S.; Tsunemi, S.; Sano, H. Sphingosine 1-phosphate receptor activation enhances bmp-2-induced osteoblast differentiation. Biochem. Biophys. Res. Commun. 2012, 423, 200–205. [Google Scholar] [CrossRef]
- Yu, X.; Huang, Y.; Collin-Osdoby, P.; Osdoby, P. Stromal cell-derived factor-1 (sdf-1) recruits osteoclast precursors by inducing chemotaxis, matrix metalloproteinase-9 (mmp-9) activity, and collagen transmigration. J. Bone Miner. Res. 2003, 18, 1404–1418. [Google Scholar] [CrossRef]
- Wright, L.M.; Maloney, W.; Yu, X.; Kindle, L.; Collin-Osdoby, P.; Osdoby, P. Stromal cell-derived factor-1 binding to its chemokine receptor cxcr4 on precursor cells promotes the chemotactic recruitment, development and survival of human osteoclasts. Bone 2005, 36, 840–853. [Google Scholar] [CrossRef]
- Diamond, P.; Labrinidis, A.; Martin, S.K.; Farrugia, A.N.; Gronthos, S.; To, L.B.; Fujii, N.; O’Loughlin, P.D.; Evdokiou, A.; Zannettino, A.C. Targeted disruption of the cxcl12/cxcr4 axis inhibits osteolysis in a murine model of myeloma-associated bone loss. J. Bone Miner. Res. 2009, 24, 1150–1161. [Google Scholar] [CrossRef]
- Mendez-Ferrer, S.; Michurina, T.V.; Ferraro, F.; Mazloom, A.R.; Macarthur, B.D.; Lira, S.A.; Scadden, D.T.; Ma’ayan, A.; Enikolopov, G.N.; Frenette, P.S. Mesenchymal and haematopoietic stem cells form a unique bone marrow niche. Nature 2010, 466, 829–834. [Google Scholar] [CrossRef]
- Li, C.; Yang, G.; Ruan, J. Sphingosine kinase-1/sphingosine-1-phosphate receptor type 1 signalling axis is induced by transforming growth factor-beta1 and stimulates cell migration in raw264.7 macrophages. Biochem. Biophys. Res. Commun. 2012, 426, 415–420. [Google Scholar] [CrossRef]
- Ishii, M.; Kikuta, J.; Shimazu, Y.; Meier-Schellersheim, M.; Germain, R.N. Chemorepulsion by blood s1p regulates osteoclast precursor mobilization and bone remodeling in vivo. J. Exp. Med. 2010, 207, 2793–2798. [Google Scholar] [CrossRef]
- Quint, P.; Ruan, M.; Pederson, L.; Kassem, M.; Westendorf, J.J.; Khosla, S.; Oursler, M.J. Sphingosine 1-phosphate (s1p) receptors 1 and 2 coordinately induce mesenchymal cell migration through s1p activation of complementary kinase pathways. J. Biol. Chem. 2013, 288, 5398–5406. [Google Scholar]
- Ishii, M.; Egen, J.G.; Klauschen, F.; Meier-Schellersheim, M.; Saeki, Y.; Vacher, J.; Proia, R.L.; Germain, R.N. Sphingosine-1-phosphate mobilizes osteoclast precursors and regulates bone homeostasis. Nature 2009, 458, 524–528. [Google Scholar] [CrossRef]
- Wu, X.; Pang, L.; Lei, W.; Lu, W.; Li, J.; Li, Z.; Frassica, F.J.; Chen, X.; Wan, M.; Cao, X. Inhibition of sca-1-positive skeletal stem cell recruitment by alendronate blunts the anabolic effects of parathyroid hormone on bone remodeling. Cell Stem Cell 2011, 7, 571–580. [Google Scholar]
- Roelofsen, T.; Akkers, R.; Beumer, W.; Apotheker, M.; Steeghs, I.; van de Ven, J.; Gelderblom, C.; Garritsen, A.; Dechering, K. Sphingosine-1-phosphate acts as a developmental stage specific inhibitor of platelet-derived growth factor-induced chemotaxis of osteoblasts. J. Cell. Biochem. 2008, 105, 1128–1138. [Google Scholar] [CrossRef]
- Mollard, R.C.; Weiler, H.A. Bone resorption varies as a function of time of day and quantity of dietary long chain polyunsaturated fatty acids. J. Nutr. Biochem. 2008, 19, 482–488. [Google Scholar] [CrossRef]
- Ostrowska, Z.; Kos-Kudla, B.; Marek, B.; Kajdaniuk, D.; Wolkowska-Pokrywa, K. Circadian concentrations of free testosterone, selected markers of bone metabolism, osteoprotegerin and its ligand srankl in obese postmenopausal women. Postepy. Hig. Med. Dosw. 2011, 65, 658–667. [Google Scholar] [CrossRef]
- Iimura, T.; Nakane, A.; Sugiyama, M.; Sato, H.; Makino, Y.; Watanabe, T.; Takagi, Y.; Numano, R.; Yamaguchi, A. A fluorescence spotlight on the clockwork development and metabolism of bone. J. Bone Miner. Metab. 2012, 30, 254–269. [Google Scholar] [CrossRef]
- Cyster, J.G.; Schwab, S.R. Sphingosine-1-phosphate and lymphocyte egress from lymphoid organs. Annu. Rev. Immunol. 2012, 30, 69–94. [Google Scholar] [CrossRef]
- Cohen, J.A.; Chun, J. Mechanisms of fingolimod’s efficacy and adverse effects in multiple sclerosis. Annals of Neurology 2011, 69, 759–777. [Google Scholar] [CrossRef]
- Kappos, L.; Radue, E.W.; O'Connor, P.; Polman, C.; Hohlfeld, R.; Calabresi, P.; Selmaj, K.; Agoropoulou, C.; Leyk, M.; Zhang-Auberson, L.; et al. A placebo-controlled trial of oral fingolimod in relapsing multiple sclerosis. N Engl. J. Med. 2010, 362, 387–401. [Google Scholar] [CrossRef]
- Hu, P.F.; Chen, Y.; Cai, P.F.; Jiang, L.F.; Wu, L.D. Sphingosine-1-phosphate: A potential therapeutic target for rheumatoid arthritis. Mol. Biol. Rep. 2011, 38, 4225–4230. [Google Scholar] [CrossRef]
- Pyne, N.J.; Tonelli, F.; Lim, K.G.; Long, J.S.; Edwards, J.; Pyne, S. Sphingosine 1-phosphate signalling in cancer. Biochem. Soc. Trans. 2012, 40, 94–100. [Google Scholar] [CrossRef]
- Loh, K.C.; Baldwin, D.; Saba, J.D. Sphingolipid signaling and hematopoietic malignancies: To the rheostat and beyond. Anticancer Agents Med. Chem. 2011, 11, 782–793. [Google Scholar] [CrossRef]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Golan, K.; Kollet, O.; Lapidot, T. Dynamic Cross Talk between S1P and CXCL12 Regulates Hematopoietic Stem Cells Migration, Development and Bone Remodeling. Pharmaceuticals 2013, 6, 1145-1169. https://doi.org/10.3390/ph6091145
Golan K, Kollet O, Lapidot T. Dynamic Cross Talk between S1P and CXCL12 Regulates Hematopoietic Stem Cells Migration, Development and Bone Remodeling. Pharmaceuticals. 2013; 6(9):1145-1169. https://doi.org/10.3390/ph6091145
Chicago/Turabian StyleGolan, Karin, Orit Kollet, and Tsvee Lapidot. 2013. "Dynamic Cross Talk between S1P and CXCL12 Regulates Hematopoietic Stem Cells Migration, Development and Bone Remodeling" Pharmaceuticals 6, no. 9: 1145-1169. https://doi.org/10.3390/ph6091145
APA StyleGolan, K., Kollet, O., & Lapidot, T. (2013). Dynamic Cross Talk between S1P and CXCL12 Regulates Hematopoietic Stem Cells Migration, Development and Bone Remodeling. Pharmaceuticals, 6(9), 1145-1169. https://doi.org/10.3390/ph6091145



