A Potent (R)-alpha-bis-lipoyl Derivative Containing 8-Hydroxyquinoline Scaffold: Synthesis and Biological Evaluation of Its Neuroprotective Capabilities in SH-SY5Y Human Neuroblastoma Cells
Abstract
:1. Introduction
2. Results and Discussion
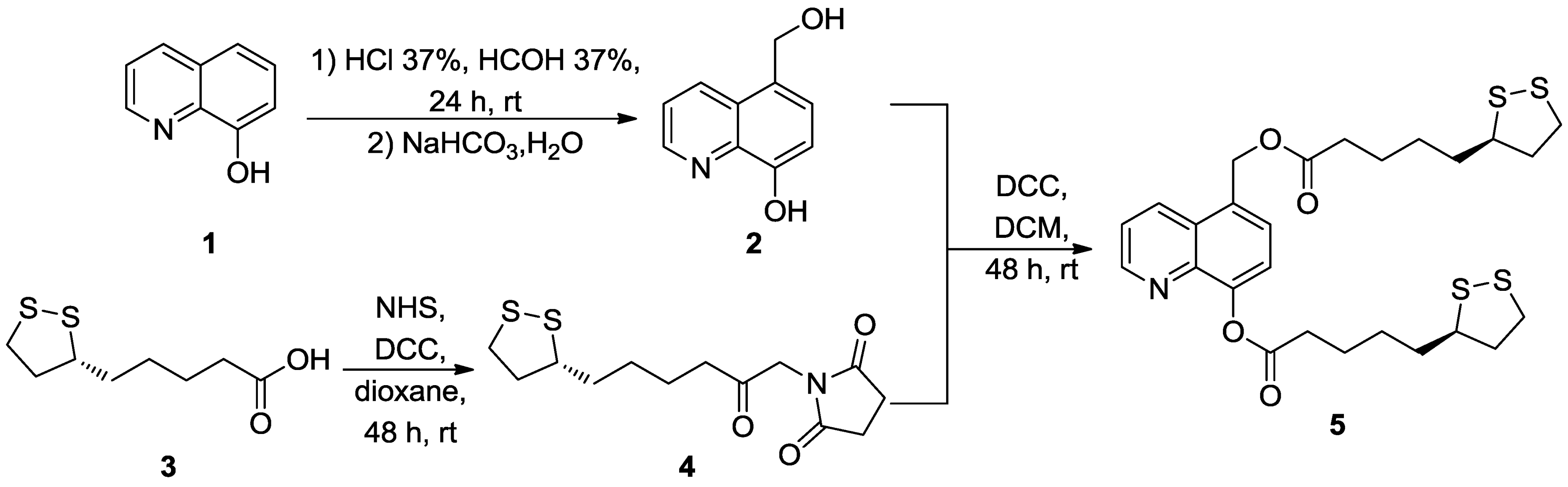


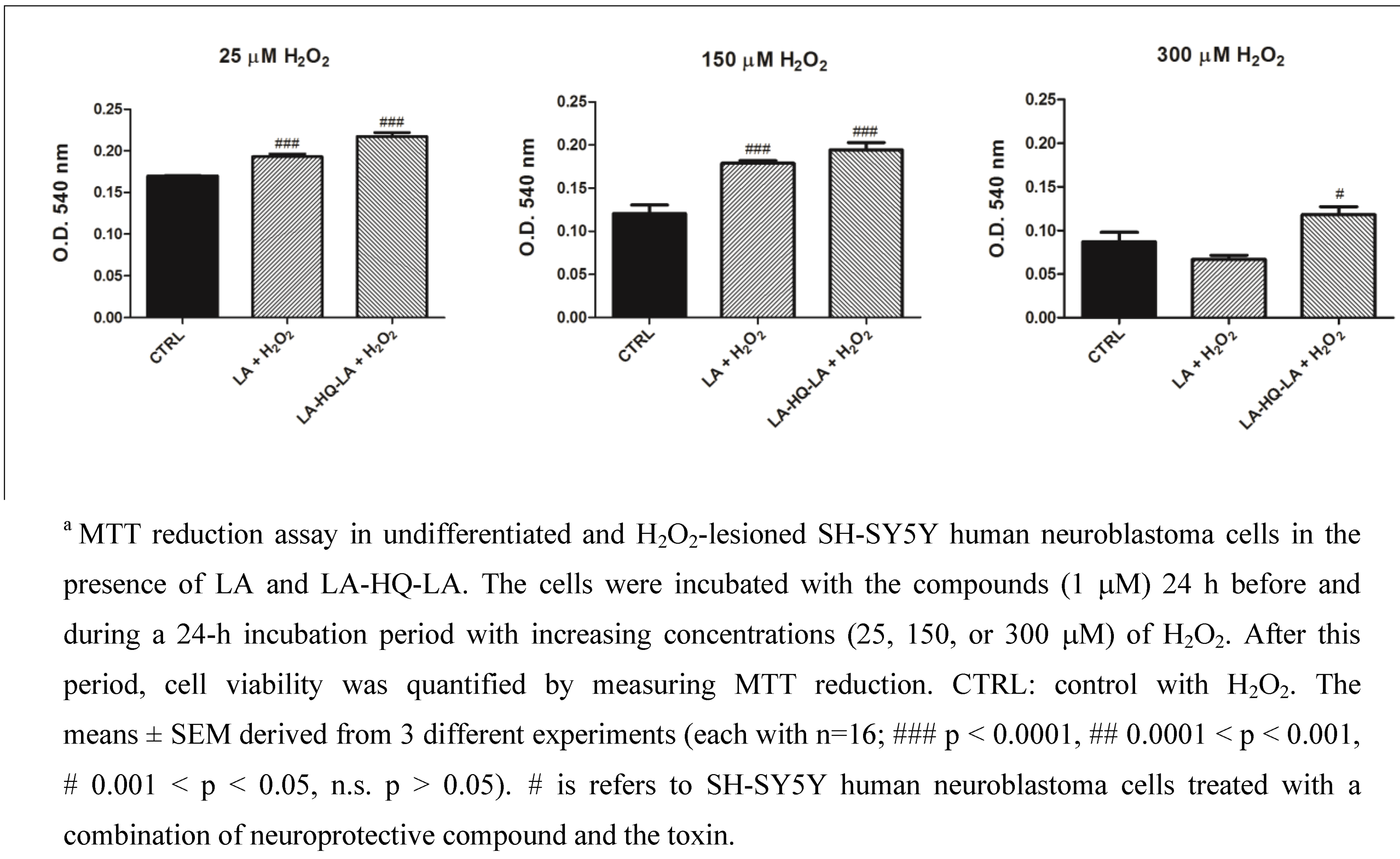
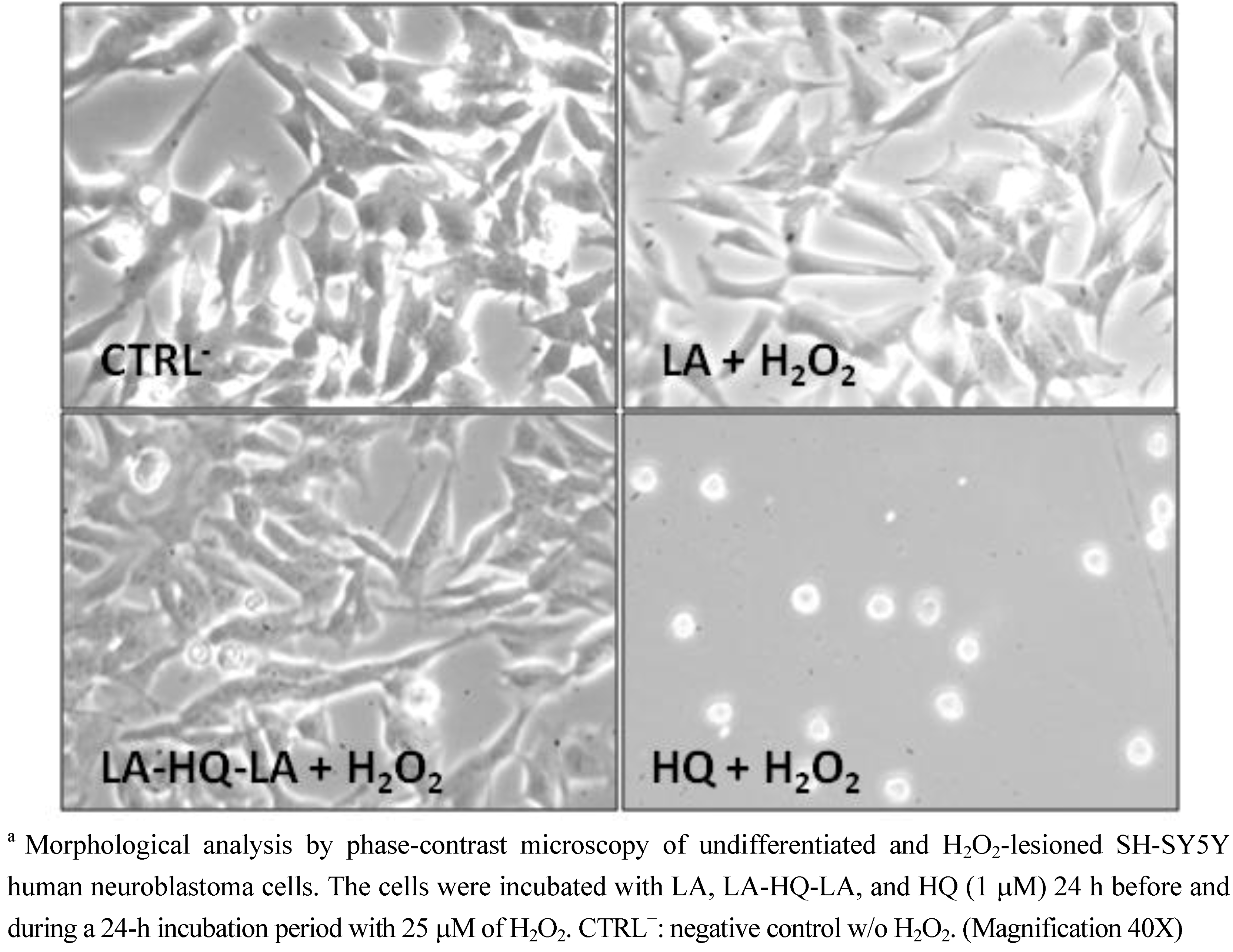
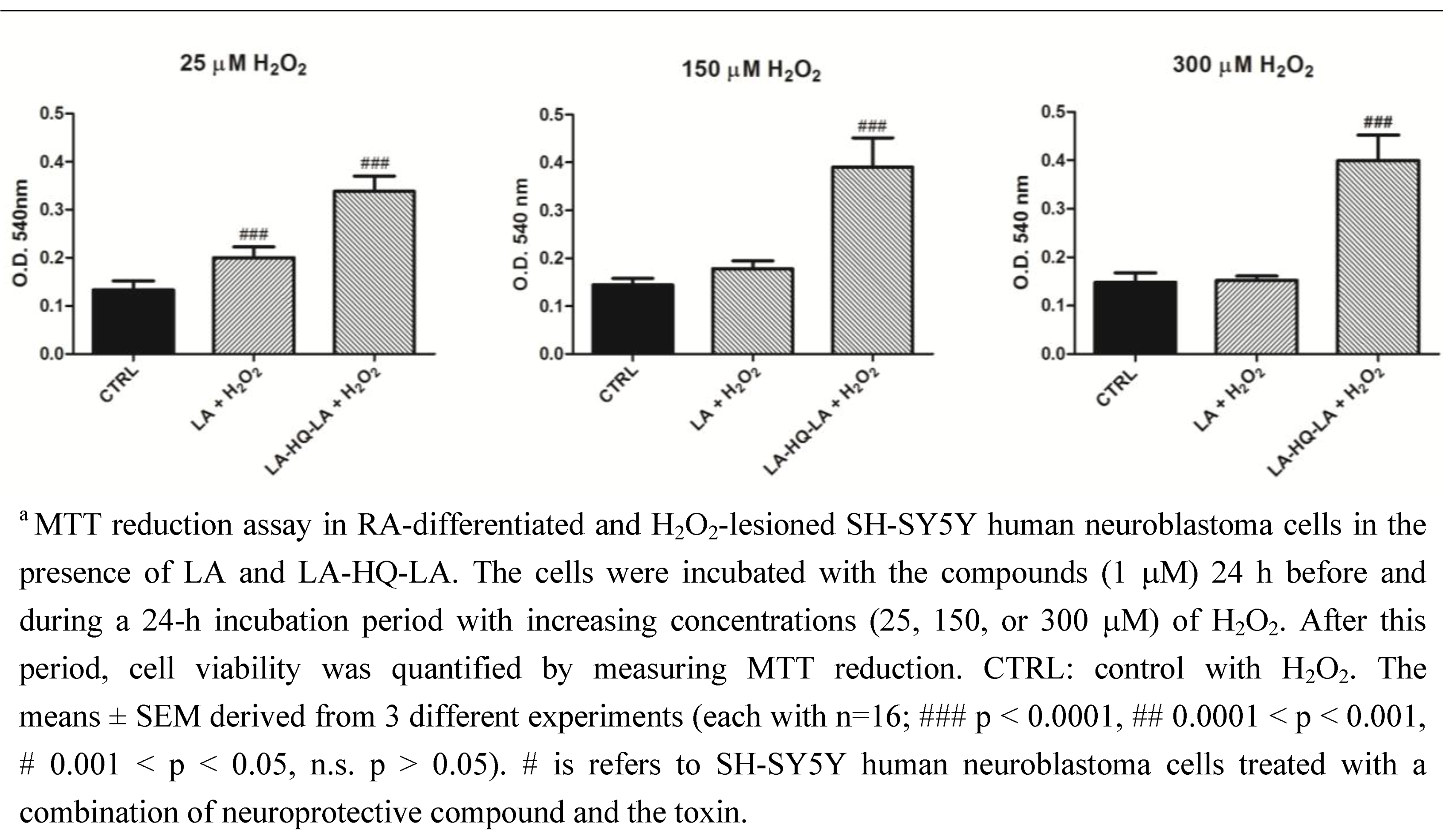
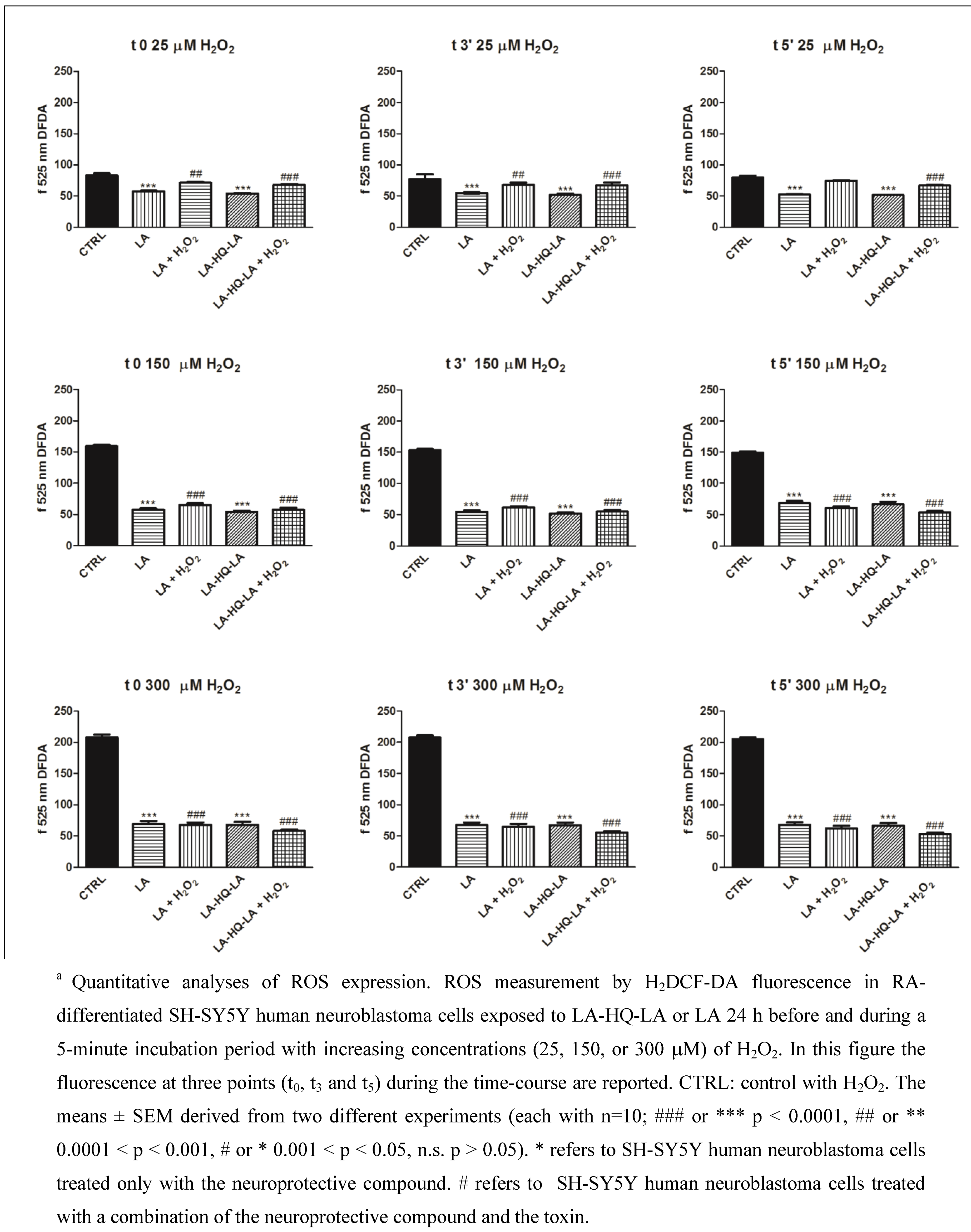
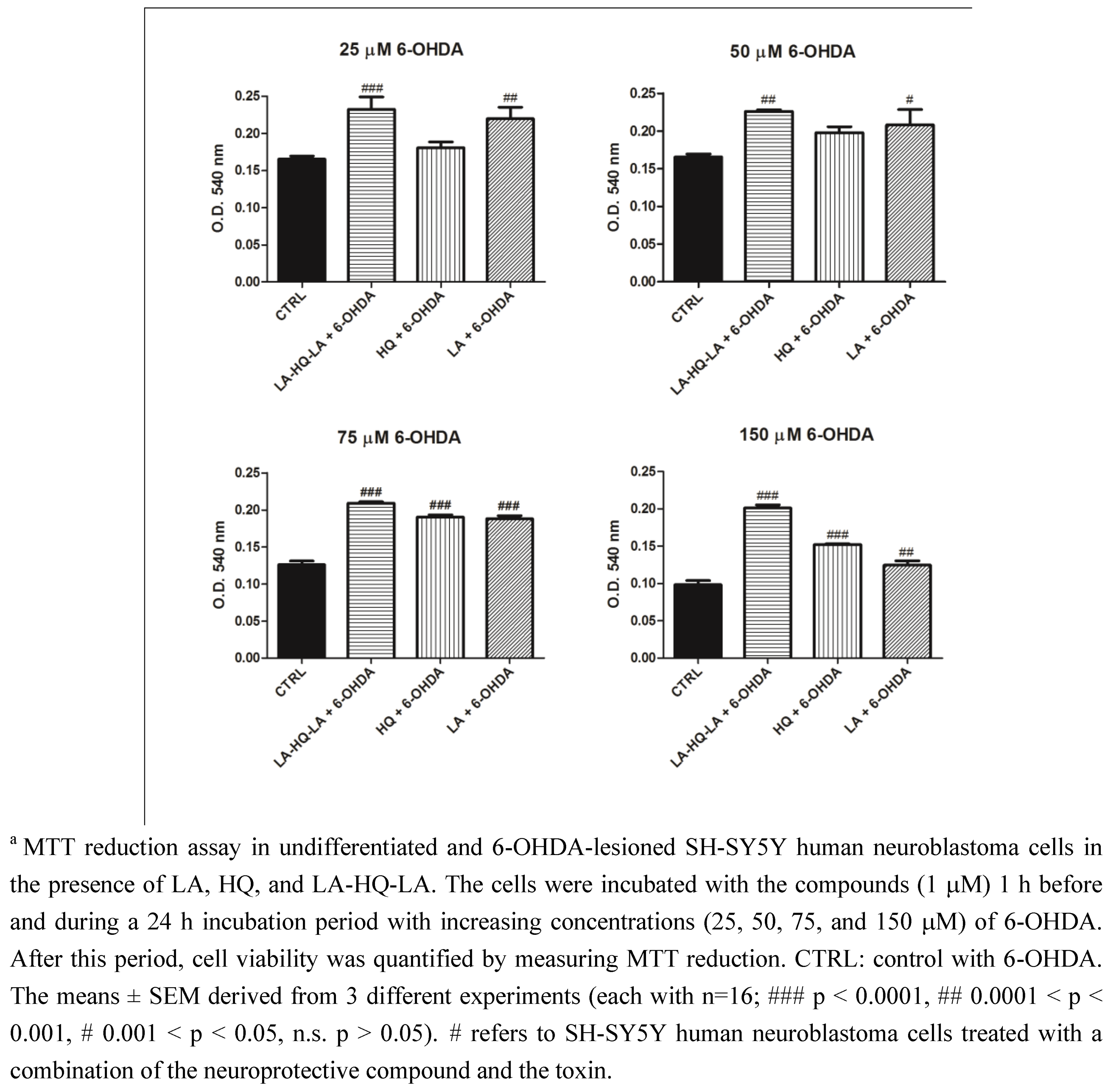
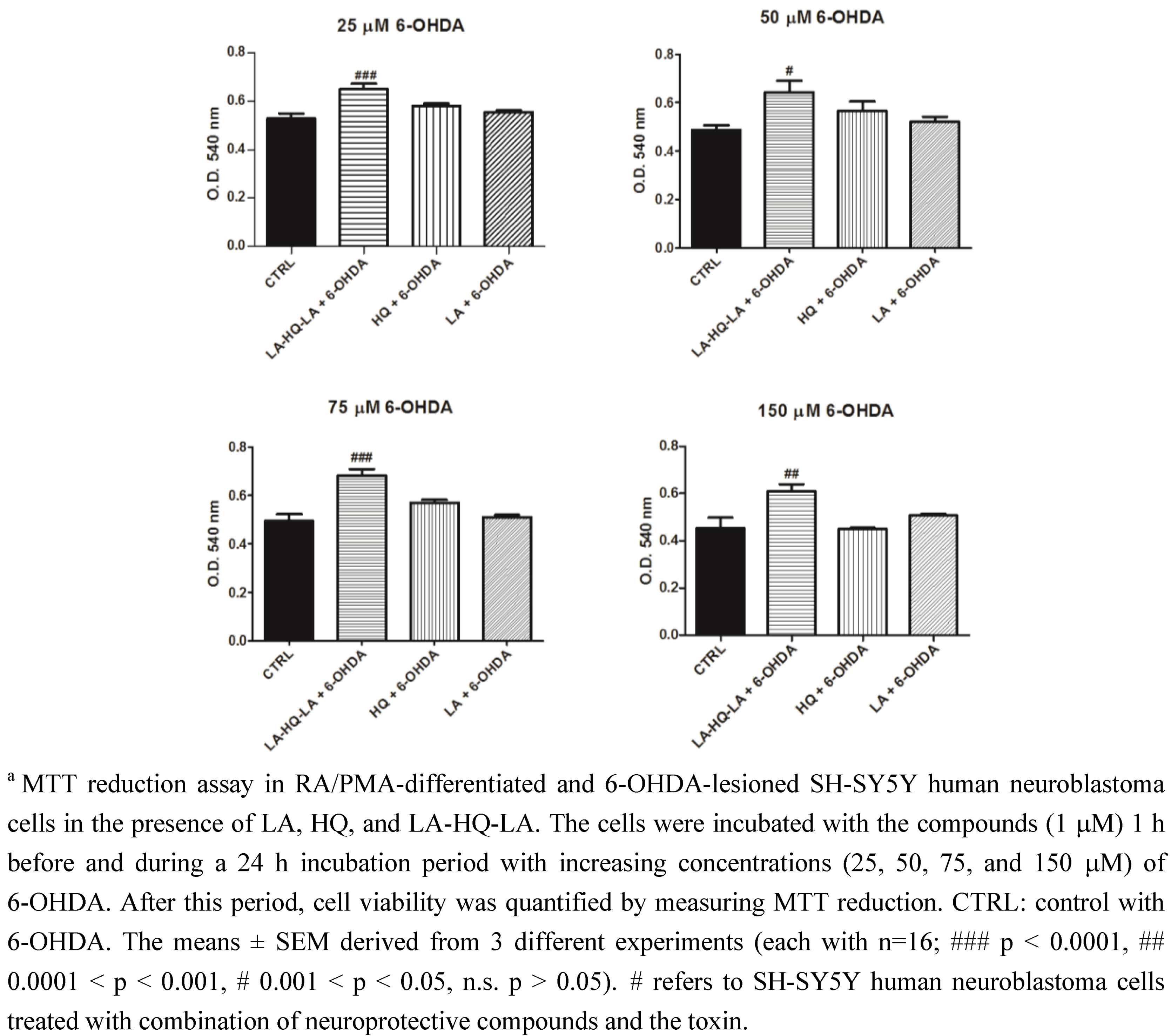
3. Experimental Section
3.1. Chemistry
3.1.1. Synthesis of 5-Hydroxymethyl-8-hydroxyquinoline (2)
3.1.2. Synthesis of 1-(-5-[1,2]dithiolan-3-yl-pentanoyl)-pyrrolidine-2,5-dione (LA-NHS, 4)
3.1.3. Synthesis of LA-HQ-LA (5)
3.2. Neuroprotective Studies
3.2.1. SH-SY5Y Cell Culture
3.2.2. Measurement of Intracellular ROS
4. Conclusions
Acknowledgments
Conflict of Interest
References
- Mark, P.M. Metal-catalyzed disruption of membrane protein and lipid signaling in the pathogenesis of neurodegenerative disorders. Ann. N.Y. Acad. Sci. 2004, 1012, 37–50. [Google Scholar] [CrossRef]
- Jellinger, K.A. General aspects of neurodegeneration. J. Neural Transm. Suppl. 2003, 65, 101–144. [Google Scholar] [CrossRef]
- Lepoivre, M.; Flaman, J.M.; Bobè, P.; Lemaire, G.; Henry, Y. Quenching of the tyrosyl free radical of ribonucleotide reductase by nitric oxide. J. Bio. Chem. 1994, 269, 21891–21897. [Google Scholar]
- Bush, A.I. Metals and neuroscience. Curr. Opin. Chem. Biol. 2000, 4, 184–191. [Google Scholar] [CrossRef]
- Cricthton, R.R.; Dexter, D.T.; Ward, R.J. Metal based neurodegenerative diseases - From molecular mechanism to therapeutic strategies. Coordin. Chem. Rev. 2008, 252, 1189–1199. [Google Scholar] [CrossRef]
- Schulz, J.B.; Lindenau, J.; Seyfried, J.; Dichganz, J. Glutathione, oxidative stress and neurodegeneration. Eur. J. Biochem. 2000, 267, 4904–4911. [Google Scholar] [CrossRef]
- Cuajungco, M.P.; Faget, K.Y.; Huang, X.; Tanzi, R.E.; Bush, A.I. Metal chelation as a potential therapy for Alzheimer’s disease. Ann. N.Y. Acad. Sci. 2000, 920, 292–304. [Google Scholar]
- Sozio, P.; Iannitelli, A.; Cerasa, L.S.; Cacciatore, I.; Cornacchia, C.; Giorgioni, G.; Ricciutelli, M.; Nasuti, C.; Cantalamessa, F.; Di Stefano, A. New L-Dopa codrugs as potential antiparkinson agents. Arch. Pharm. 2008, 341, 412–417. [Google Scholar] [CrossRef]
- Cacciatore, I.; Cornacchia, C.; Pinnen, F.; Mollica, A.; Di Stefano, A. Prodrug approach for increasing cellular glutathione levels. Molecules 2010, 15, 1242–1264. [Google Scholar] [CrossRef]
- Cornacchia, C.; Cacciatore, I.; Baldassarre, L.; Mollica, A.; Feliciani, F.; Pinnen, F. Diketopiperazines as neuroprotective agents. Mini-Rev. Med. Chem. 2012, 12, 2–12. [Google Scholar] [CrossRef]
- Pinnen, F.; Cacciatore, I.; Cornacchia, C.; Mollica, A.; Sozio, P.; Cerasa, L.S.; Iannitelli, A.; Fontana, A.; Nasuti, C.; Di Stefano, A. CNS delivery of L-dopa by a new hybrid glutathione-methionine peptidomimetic prodrug. Amino Acids 2012, 42, 261–269. [Google Scholar] [CrossRef]
- Minelli, A.; Conte, C.; Grottelli, S.; Bellezza, I.; Cacciatore, I.; Bolaños, J. Cyclo(His-Pro) promotes cytoprotection by activating Nrf2-mediated up-regulation of antioxidant defence. J. Cell. Mol. Med. 2009, 13, 1149–1161. [Google Scholar] [CrossRef]
- Minelli, A.; Conte, C.; Prudenzi, E.; Cacciatore, I.; Cornacchia, C.; Taha, E.; Pinnen, F. N-acetyl-L-methionyl-L-Dopa- methyl ester as a dual acting drug that relives L-Dopa-induced oxidative toxicity. Free Radical Bio. Med. 2010, 49, 31–39. [Google Scholar] [CrossRef]
- Minelli, A.; Conte, C.; Cacciatore, I.; Cornacchia, C.; Pinnen, F. Molecular mechanism underlying the cerebral effect of Gly-Pro-Glu tripeptide bound to L-Dopa in a Parkinson's animal model. Amino Acids 2012, 43, 1359–1367. [Google Scholar]
- Cacciatore, I.; Cornacchia, C.; Baldassarre, L.; Fornasari, E.; Mollica, A.; Stefanucci, A.; Pinnen, F. GPE and GPE analogues as promising neuroprotective agents. Mini-Rev. Med. Chem. 2012, 12, 13–23. [Google Scholar] [CrossRef]
- Pinnen, F.; Sozio, P.; Cacciatore, I.; Cornacchia, C.; Mollica, A.; Iannitelli, A.; D’Aurizio, E.; Cataldi, A.; Zara, S.; Nasuti, C.; Di Stefano, A. Ibuprofen and glutathione conjugate as a potential therapeutic agent for treating Alzheimer's disease. Arch. Pharm. 2011, 344, 139–148. [Google Scholar] [CrossRef]
- Bongarzone, S.; Bolognesi, M.L. The concept of privileged structures in rational drug design: Focus on acridine and quinoline scaffolds in neurodegenerative and protozoan diseases. Expert Opin. Drug Dis. 2011, 6, 251–268. [Google Scholar] [CrossRef]
- Frederickson, C.J.; Kasarskis, E.J.; Ringo, D.; Frederickson, R.E. A quinoline fluorescence method for visualizing and assayingthe hystochemically and reactive zinc (bouton zinc) in the brain. J. Neurosci. Methods 1987, 20, 91–103. [Google Scholar]
- Turnquist, T.D.; Sandell, E.B. Stability constants of iron (III)-8-hydroxyquinoline complexes. Anal. Chim. Acta 1968, 42, 239–245. [Google Scholar] [CrossRef]
- Kayyali, R.; Pannala, A.S.; Khodr, H.; Hider, R.C. Comparative radical scavenging ability of bidentate iron (III) chelators. Biochem. Pharmacol. 1998, 55, 1327–1332. [Google Scholar] [CrossRef]
- Ben-Shachar, D.; Kahana, N.; Kampel, V.; Warshawsky, A.; Youdim, M.B. Nroprotection by a novel brain permeable iron chelator, VK-28, against 6-hydroxydopamine lesion in rats. Neuropharmacology 2004, 46, 254–263. [Google Scholar] [CrossRef]
- Dickens, M.G.; Franz, K.J. A Prochelator activated by hydrogen peroxide prevents metal-induced amyloid beta aggregation. Chem. Bio. Chem. 2010, 11, 59–62. [Google Scholar]
- Di Vaira, M.; Bazzicalupi, C.; Orioli, P.; Messori, L.; Bruni, B.; Zatta, P. Clioquinol, a drug for Alzheimer’s disease specifically interfering with brain metal metabolism: structural characterization of its zinc(II) and copper(II) complexes. Inorg. Chem. 2004, 43, 3795–3797. [Google Scholar] [CrossRef]
- Cacciatore, I.; Baldassarre, L.; Fornasari, E.; Mollica, A.; Pinnen, F. Recent advances in the treatment of neurodegenerative diseases based on GSH delivery systems. Oxid. Med. Cell. Longev. 2012, 240146. [Google Scholar]
- Li, L.; Xu, B. Synthesis and characterization of 5-substituted 8-hydroxyquinoline derivatives and their metal complexes. Tetrahedron 2008, 64, 10986–10995. [Google Scholar] [CrossRef]
- Nefkens, G.H. L.; Tesser, G.I. A novel activated ester in peptide syntheses. J. Am. Chem. Soc. 1961, 83, 1263–1263. [Google Scholar]
- Xie, H.; Hu, L.; Li, G. SH-SY5Y human neuroblastoma cells line: in vitro cell model of dopaminergic neurons in Parkinson’s disease. Chin. Med. J. 2010, 123, 1086–1092. [Google Scholar]
- Lombet, A.; Zujovic, V.; Kandouz, M.; Billardon, C.; Carvajal-Gonzalez, S.; Gompel, A.; Rostène, W. Resistance to induced apoptosis in the human neuroblastoma cell line SK-N-SH in relation to neuronal differentiation: Role of Bcl-2 protein family. Eur. J. Biochem. 2001, 268, 1352–1362. [Google Scholar] [CrossRef]
- Ho, R.; Minturn, J.E.; Hishiki, T.; Zhao, H.; Wang, Q.; Cnaan, A.; Maris, J.; Evans, A.E.; Brodeur, G.M. Proliferation of human neuroblastomas mediated by the epidermal growth factor receptor. Cancer Res. 2005, 65, 9868–9875. [Google Scholar] [CrossRef]
- Jantas, D.; Pytel, M.; Mozrzymas, J.W.; Leskiewicz, M.; Regulska, M.; Antkiewicz-Michaluk, L.; Lason, W. The attenuating effect of memantine on staurosporine-, salsolinol- and doxorubicin-induced apoptosis in human neuroblastoma SH-SY5Y cells. Neurochem. Int. 2008, 52, 864–877. [Google Scholar] [CrossRef]
- Middlemas, D.S.; Kihl, B.K.; Moody, N.M. Brain derived neurotrophic factor protects human neuroblastoma cells from DNA damaging agents. J. Neuro-Oncol. 1999, 45, 27–36. [Google Scholar] [CrossRef]
- Tieu, K.; Zuo, D.M.; Yu, P.H. Differential effects of staurosporine and retinoic acid on the vulnerability of the SH-SY5Y neuroblastoma cells: Involvement of Bcl-2 and p53 proteins. J. Neurosci. Res. 1999, 58, 426–435. [Google Scholar]
- LeBel, C.P.; Ischiropoulos, H.; Bondy, S.C. Evaluation of the probe 2′,7′-dichlorofluorescin as an indicator of reactive oxygen species formation and oxidative stress. Chem. Res. Toxicol. 1992, 5, 227–231. [Google Scholar] [CrossRef]
- Lopes, F.M.; Londero, G.F.; de Medeiros, L.M.; da Motta, L.L.; Behr, G.A.; de Oliveira, V.A.; Ibrahim, M.; Moreira, J.C.; de Oliveira Porciúncula, L.; da Rocha, J.B.; Klamt, F. Evaluation of the neurotoxic/neuroprotective role of organoselenides using differentiated human neuroblastoma SH-SY5Y cell line challenged with 6-hydroxydopamine. Neurotox Res. 2012, 22, 138–149. [Google Scholar] [CrossRef]
- Lopes, F.M.; Schröder, R.; da Frota, M.L.; Zanotto-Filho, A.; Müller, C.B.; Pires, A.S.; Meurer, R.T.; Colpo, G.D.; Gelain, D.P.; Kapczinski, F.; Moreira, J.C.; Fernandes Mda, C.; Klamt, F. Comparison between proliferative and neuron-like SH-SY5Y cells as an in vitro model for Parkinson disease studies. Brain Res. 2010, 1337, 85–94. [Google Scholar] [CrossRef]
- Lehmensiek, V.; Tan, E.M.; Liebau, S.; Lenk, T.; Zettlmeisl, H.; Schwarz, J.; Storch, A. Dopamine transporter-mediated cytotoxicity of 6-hydroxydopamine in vitro depends on expression of mutant α-synucleins related to Parkinson's disease. Neurochem. Int. 2006, 48, 329–340. [Google Scholar] [CrossRef]
- Presgraves, S.P.; Ahmed, T.; Borwege, S.; Joyce, J.N. Terminally differentiated SH-SY5Y cells provide a model system for studying neuroprotective effects of dopamine agonists. Neurotox. Res. 2003, 5, 579–598. [Google Scholar] [CrossRef]
- Cacciatore, I.; Baldassarre, L.; Fornasari, E.; Cornacchia, C.; Di Stefano, A.; Sozio, P.; Cerasa, L.S.; Fontana, A.; Fulle, S.; Di Filippo, E.S.; La Rovere, R.M.; Pinnen, F. (R)-alpha-Lipoyl-glycyl-L-prolyl-L-glutamyl dimethyl ester codrug as multifunctional agent with potential neuroprotective activities. Chem. Med. Chem. 2012, 7, 2021–2029. [Google Scholar]
- Menghini, L.; Leporini, L.; Scanu, N.; Pintore, G.; La Rovere, R.; Di Filippo, E.S.; Pietrangelo, T.; Fulle, S. Effect of phytochemicals concentrations on biological activities of cranberry extracts. J. Biol. Reg. Homeos. Ag. 2011, 25, 27–35. [Google Scholar]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Cacciatore, I.; Fornasari, E.; Baldassarre, L.; Cornacchia, C.; Fulle, S.; Di Filippo, E.S.; Pietrangelo, T.; Pinnen, F. A Potent (R)-alpha-bis-lipoyl Derivative Containing 8-Hydroxyquinoline Scaffold: Synthesis and Biological Evaluation of Its Neuroprotective Capabilities in SH-SY5Y Human Neuroblastoma Cells. Pharmaceuticals 2013, 6, 54-69. https://doi.org/10.3390/ph6010054
Cacciatore I, Fornasari E, Baldassarre L, Cornacchia C, Fulle S, Di Filippo ES, Pietrangelo T, Pinnen F. A Potent (R)-alpha-bis-lipoyl Derivative Containing 8-Hydroxyquinoline Scaffold: Synthesis and Biological Evaluation of Its Neuroprotective Capabilities in SH-SY5Y Human Neuroblastoma Cells. Pharmaceuticals. 2013; 6(1):54-69. https://doi.org/10.3390/ph6010054
Chicago/Turabian StyleCacciatore, Ivana, Erika Fornasari, Leonardo Baldassarre, Catia Cornacchia, Stefania Fulle, Ester Sara Di Filippo, Tiziana Pietrangelo, and Francesco Pinnen. 2013. "A Potent (R)-alpha-bis-lipoyl Derivative Containing 8-Hydroxyquinoline Scaffold: Synthesis and Biological Evaluation of Its Neuroprotective Capabilities in SH-SY5Y Human Neuroblastoma Cells" Pharmaceuticals 6, no. 1: 54-69. https://doi.org/10.3390/ph6010054
APA StyleCacciatore, I., Fornasari, E., Baldassarre, L., Cornacchia, C., Fulle, S., Di Filippo, E. S., Pietrangelo, T., & Pinnen, F. (2013). A Potent (R)-alpha-bis-lipoyl Derivative Containing 8-Hydroxyquinoline Scaffold: Synthesis and Biological Evaluation of Its Neuroprotective Capabilities in SH-SY5Y Human Neuroblastoma Cells. Pharmaceuticals, 6(1), 54-69. https://doi.org/10.3390/ph6010054




