Abstract
Menopause is associated with health concerns including vasomotor symptoms, vulvar/vaginal atrophy (VVA), and osteoporosis. Estrogen therapy or combined estrogen-progestin therapy (EPT) are primary treatment options for menopausal symptom relief and osteoporosis prevention. Because EPT has been associated with some safety/tolerability concerns relating to undesirable effects of estrogen and progestin, alternative options are needed. The tissue selective estrogen complex (TSEC) is a novel class of agents pairing a selective estrogen receptor modulator (SERM) with 1 or more estrogens. The TSEC combines the established efficacy of estrogens on menopausal symptoms and bone with the protective effects of a SERM on the reproductive tract. The pairing of bazedoxifene (BZA) with conjugated estrogens (CE) has been evaluated in a series of phase 3 clinical trials. BZA 20 mg/CE 0.45 mg and BZA 20 mg/CE 0.625 mg have shown efficacy in reducing the frequency and severity of hot flushes, relieving VVA symptoms, and maintaining bone mass while protecting the endometrium and breast. These BZA/CE doses have been associated with a favorable safety/tolerability profile, with higher rates of cumulative amenorrhea and lower incidences of breast pain than those reported for EPT. Thus, BZA/CE may be a promising alternative to conventional EPT for treating non-hysterectomized, postmenopausal women.
1. Introduction
The menopausal transition is associated with a decline in ovarian production of endogenous estrogens [1,2], leading to a state of estrogen deficiency that can result in some bothersome symptoms and the loss of bone mass. Vasomotor symptoms (VMS) are experienced by 60% to 90% of women and can interfere with sleep and daily activities [3]. Vulvar/vaginal atrophy (VVA) can result in uncomfortable symptoms such as vaginal dryness [4]; it has been reported that the percentage of women with vaginal dryness increases from 25% in the first year postmenopause to 47% in the third year postmenopause [5]. Moreover, the loss of bone mass associated with postmenopausal estrogen deficiency can increase the risk of osteoporosis and bone fractures [6,7]. Overall, menopausal symptoms can adversely affect the quality of life for women and impose a significant economic burden in the form of decreased productivity and increased health care costs [4,8]. Hormone therapy (HT) is the only treatment option for both menopausal symptoms and osteoporosis prevention; other options are available for either treatment of menopausal symptoms or osteoporosis, but each of these options carries a unique risk/benefit profile and thus may not be appropriate for all women. The objective of this article is to review HT and other therapies for the treatment of menopausal symptoms and prevention of osteoporosis, and to describe the tissue selective estrogen complex (TSEC), a promising new class of therapy for postmenopausal women.
2. Hormone Therapy
2.1. Types of HT
HT, in the form of estrogen therapy (ET; for hysterectomized women) or combined estrogen-progestin therapy (EPT; for non-hysterectomized women), is the established treatment for postmenopausal women with moderate-to-severe VMS and VVA. ET and EPT are also approved for the prevention of postmenopausal osteoporosis [9,10]. Estrogens have broad and varied pharmacologic effects in different tissues, some of which are desired and others undesired. Unopposed estrogens are associated with an increased risk of endometrial carcinoma [11,12,13,14], and so progestins are added in EPT for women with an intact uterus in order to protect the endometrium [9,15]. Progestins also have varied pharmacologic effects, which are complex, both desirable and undesirable, and may also depend on estrogen. There are multiple formulations available for both ET and EPT; in the United States, oral conjugated estrogens (CE) is the most commonly prescribed ET and medroxyprogesterone acetate (MPA) is the most commonly used progestin for EPT [16].
2.2. Efficacy of HT on Menopausal Symptoms
HT is primarily indicated for the treatment of moderate-to-severe VMS [9], and the efficacy of HT in relieving VMS has been established in numerous clinical trials in postmenopausal women [16,17,18,19,20,21,22]. A systematic review of randomized, double-blind, and placebo (PBO)-controlled studies of oral HT (including both ET and EPT) showed a 75% reduction in the frequency of hot flushes (95% confidence interval [CI], 64.3–82.3) for HT compared with PBO [17]. HT was also associated with a significant reduction in the severity of hot flushes (odds ratio [OR], 0.13; 95% CI, 0.07–0.23) compared with PBO [17].
HT has been shown to be effective in improving VVA symptoms in postmenopausal women, with ET and EPT both showing improvements in measures of VVA, including vaginal maturation index and vaginal dryness, compared with PBO [16,21,23]. A meta-analysis reported that local application of estrogens is as effective as systemic estrogens for the treatment of VVA symptoms [23]; local estrogens are recommended for women who are seeking treatment for VVA symptoms only [9,24]. As noted by the North American Menopause Society, hepatic metabolism that occurs with oral administration may result in the requirement for higher doses compared with vaginal estrogen delivery to achieve local concentrations of estrogens high enough to provide symptomatic relief and reversal of atrophic changes [24]. For example, local estrogen delivery using a low-dose (0.3 mg) CE cream had little to no impact on serum estrogen levels compared with oral administration at the same dose [25], but significantly improved participant-reported most bothersome symptom of VVA compared with PBO and significantly decreased dyspareunia compared with PBO at 12 weeks [26].
2.3. Effects of HT on Bone
HT has demonstrated efficacy in preventing bone loss and is approved for the prevention of postmenopausal osteoporosis [9,10]. In the Women’s Health Initiative (WHI) trial, ET with conjugated equine estrogens (CEE) (p < 0.0001) [27] and EPT with CEE/MPA (p < 0.001) [28] significantly improved total hip bone mineral density (BMD) compared with PBO over 6 and 3 years, respectively. These findings are consistent with those from the previous Women’s Health, Osteoporosis, Progestin, Estrogen (HOPE) trial showing a significant improvement in spine and hip BMD over 2 years for women who received CEE or CEE/MPA compared with those who received PBO (p < 0.001) [29]. More importantly, both CEE and CEE/MPA also showed significant reductions in total fracture risk in the WHI trial (hazard ratio [HR] of 0.71 and 95% CI of 0.64–0.80 for CEE; HR of 0.76 and 95% CI of 0.69–0.83 for CEE/MPA) compared with PBO [27,28]. Similarly, meta-analyses of randomized trials have also shown reduced risk of fractures and improvement in BMD for HT [30,31]. In a meta-analysis of 22 trials in which women received at least 12 months of HT, there was a 27% reduction in nonvertebral fracture risk favoring HT (relative risk [RR], 0.73; 95% CI, 0.56–0.94; p = 0.02) [30]. Interestingly, nonvertebral fracture risk reduction was greater in women randomized to HT with mean age <60 years (RR, 0.67; 95% CI, 0.46–0.98; p = 0.03) than in those with mean age ≥60 years (RR, 0.88; 95% CI, 0.71–1.08; p = 0.22). In another meta-analysis that included 57 studies of postmenopausal women randomized to HT or control (PBO or calcium/vitamin D) for at least 1 year, there was a nonsignificant trend toward a reduced incidence of vertebral (RR, 0.66; 95% CI, 0.41–1.07; 5 trials) and nonvertebral fractures (RR, 0.87; 95% CI, 0.71–1.08; 6 trials) with HT and consistent, favorable effects on BMD [31]. Taken together, these data suggest that HT may be appropriate for a younger population that may require long-term osteoporosis prevention [9].
2.4. Effects of HT on Sleep and Quality of Life
HT has been reported to improve sleep parameters in several studies. In the WHI trial, both CEE and CEE/MPA provided small but statistically significant improvements in sleep disturbance compared with PBO at 1 year (p < 0.001) [32,33]. These results are consistent with findings from previous studies showing improvements in sleep parameters for ET and EPT [34,35]. The relief of VMS with ET was shown to be the most important predictive factor for a positive treatment effect on sleep parameters [34]. In contrast, studies examining the effects of HT on measures of quality of life have shown mixed results. One study showed a significant improvement in the Menopause-Specific Quality of Life (MENQOL) summary score for EPT (estradiol/norgestimate) compared with PBO (p < 0.001) [36] and the Heart and Estrogen/Progestin Replacement Study (HERS) trial showed improved mental health and depressive symptoms for EPT (CEE/MPA) compared with PBO (p = 0.04 and p = 0.01, respectively) for women experiencing hot flushes at baseline, but not for those without hot flushes at baseline [37]. Other studies, including the WHI trial, have shown no clinically meaningful improvements in measures of quality of life for ET (estradiol) and EPT (CEE/MPA) [32,33,38].
2.5. Safety and Tolerability of HT
Findings from the WHI trial have raised some safety concerns with HT and, in particular, with CEE/MPA [39].
2.5.1. Estrogen Therapy
CEE alone showed no overall increase in the incidence of coronary heart disease (CHD; HR, 0.91; 95% CI, 0.75–1.12) in the WHI trial, although a trend toward increased risk of peripheral arterial events (categorized as carotid artery disease, abdominal aortic aneurysm, or lower extremity arterial disease) was observed (HR, 1.32; 95% CI, 0.99–1.77) [40,41]. Risk of CHD associated with CEE therapy was somewhat increased in women aged 70 to 79 years (HR, 1.13; 95% CI, 0.82–1.54) but there was reduced risk of CHD in women aged 50 to 59 years (HR, 0.63; 95% CI, 0.36–1.09) (Table 1) [42]. In an ancillary substudy of women aged 50 to 59 years participating in the WHI trial, calcified-plaque burden in the coronary arteries, as measured by change in coronary artery calcium scores, was significantly lower with CEE vs. PBO (p = 0.02) [43].
An increased risk of stroke and venous thromboembolic events (VTEs) was observed with CEE during the WHI trial [41], and these risks were generally greater in older women compared with younger women [44,45]. The overall HR for stroke was 1.37 (95% CI, 1.09–1.73; p = 0.008) [44]; however, CEE alone was associated with a decreased risk of stroke in a subgroup of younger women aged 50 to 59 years [42]. For VTEs, there was a 32% increased risk with CEE overall (HR, 1.32; 95% CI, 0.99–1.75); risk was lower in women aged 50 to 59 years at baseline (HR, 1.37; 95% CI, 0.70–2.68) than in women aged 70 to 79 years at baseline (HR, 3.77; 95% CI, 2.07–6.89) (Table 1) [45].
HT, particularly EPT, has also been associated with some breast-related safety concerns, which are discussed below. No increase in the risk of invasive breast cancer was initially observed for CEE compared with PBO in the WHI trial (HR, 0.80; 95% CI, 0.62–1.04) [46]. At a mean follow-up of 10.7 years, CEE showed a significantly decreased risk of breast cancer compared with PBO (HR, 0.77; 95% CI, 0.62–0.95; p = 0.02) and no increased or decreased risk of total mortality (HR, 1.02; 95% CI, 0.91–1.15) [47].

Table 1.
Summary of key cardiovascular outcomes with hormone therapy analyzed by age group.
| HR (95% CI) | Age group at randomization | ||
|---|---|---|---|
| 50 to 59 years | 60 to 69 years | 70 to 79 years | |
| CHD [42] | |||
| CEE alone | 0.63 (0.36–1.09) | 0.94 (0.71–1.24) | 1.13 (0.82–1.54) |
| CEE/MPA | 1.29 (0.79–2.12) | 1.03 (0.74–1.43) | 1.48 (1.04–2.11) |
| Stroke [42] | |||
| CEE alone | 0.89 (0.47–1.69) | 1.62 (1.15–2.27) | 1.21 (0.84–1.75) |
| CEE/MPA | 1.41 (0.75–2.65) | 1.37 (0.95–1.97) | 1.21 (0.82–1.78) |
| VTE [45,48] | |||
| CEE alone | 1.37 (0.70–2.68) | 2.82 (1.59–5.01) | 3.77 (2.07–6.89) |
| CEE/MPA | 2.27 (1.19–4.33) | 4.28 (2.38–7.72) | 7.46 (4.32–14.38) |
HR, hazard ratio; CI, confidence interval; CHD, coronary heart disease; CEE, conjugated equine estrogens; MPA, medroxyprogesterone acetate; VTE, venous thromboembolism.
2.5.2. Estrogen-Progestin Therapy
In contrast to CEE alone, CEE/MPA was associated with a 29% increase in the incidence of CHD (HR, 1.29; 95% CI, 1.02–1.63; p < 0.05) in the WHI trial [39]. Further analyses have shown that the risk of CHD correlates with the timing of CEE/MPA initiation, with increased risk of CHD observed for women who were ≥20 years past menopause (HR, 1.66; 95% CI, 1.14–2.41) while younger women <10 years past the onset of menopause at HT initiation showed a risk of CHD comparable to PBO (HR, 0.88; 95% CI, 0.54–1.43); this trend toward decreased risk with decreasing time since menopause was statistically significant (p = 0.05) [42]. When analyzed by age at initiation of CEE/MPA, CHD risk remained consistent across age groups (Table 1) [42].
Similar to CEE alone, CEE/MPA was also associated with an increased risk of stroke and VTEs during the WHI trial [39], with risks generally greater in older vs. younger women [48,49]. The overall HR for stroke was 1.31 (95% CI, 1.02–1.68) for CEE/MPA [49], with an increased risk in the subgroup of younger women aged 50 to 59 years (Table 1) [42]. When stroke risk data from the CEE and CEE/MPA studies were combined, there was no significant increase in the risk of stroke in this subgroup (HR, 1.13; 95% CI, 0.73–1.76). For VTEs, greater risk was observed with CEE/MPA, which showed a 2-fold increase (HR, 2.06; 95% CI, 1.57–2.70), compared with the 32% increase with CEE described above [45,48]. The HR for VTEs in women aged 50 to 59 years at baseline was lower (2.27 [95% CI, 1.19–4.33]) compared with that for women aged 70 to 79 years at baseline (7.46 [95% CI, 4.32–14.38] for EPT) (Table 1) [48]. For women aged 50 to 59 years at randomization, the absolute excess risk of VTEs with either CEE or CEE/MPA is considered rare [9].
As mentioned above, EPT has been associated with some breast-related safety concerns. HT has been shown to increase mammographic breast density in postmenopausal women, with EPT having a greater effect on breast density compared with ET [50,51,52,53]. High mammographic breast density has been shown to be a risk factor for breast cancer [54], as confirmed in a recent systemic meta-analysis [55]; however, this relationship is not well understood. High breast density may also decrease the sensitivity of mammograms for detecting breast abnormalities [56]. In the WHI trial, CEE/MPA was associated with a 26% increase in the incidence of invasive breast cancer (HR, 1.26; 95% CI, 1.00–1.59) [39,57] and with increased breast cancer mortality (HR, 1.96; 95% CI, 1.00–4.04) [58] compared with PBO. The increase in absolute risk of invasive breast cancer for CEE/MPA compared with PBO was 8 cases per 10,000 women-years, which is considered rare [9].
EPT has also been associated with some tolerability issues including increased incidences of breast pain and irregular vaginal bleeding [35,59,60]. In the WHI trial, women receiving CEE/MPA who were asymptomatic at baseline were significantly more likely to develop breast tenderness than women receiving PBO (p < 0.001) [60]; additional analyses have also linked new-onset breast tenderness after initiating CEE/MPA (but not CEE) to an increased risk of breast cancer (HR, 1.33; 95% CI, 1.02–1.72; p = 0.03) [61]. Vaginal bleeding was reported by 51% of women on CEE/MPA and only 5% of women on PBO at 6 months during the WHI trial [60]. Likewise, in the Heart and Estrogen/Progestin Replacement Study, women receiving CEE/MPA were more likely to experience bleeding and breast symptoms than those receiving PBO [35]. In the Postmenopausal Estrogen/Progestin Interventions Trial, breast tenderness was associated with EPT but not ET [18]. In a retrospective study to determine compliance with HT among 821 women attending a menopause clinic, the most common reason women discontinued therapy was irregular vaginal bleeding (23%) [59].
The North American Menopause Society currently recommends that HT be used at the lowest effective dose to treat postmenopausal women for whom the benefits of treatment outweigh the risks [9]. Overall, the benefit-to-risk ratio for HT, particularly ET, is favorable for younger women initiating therapy close to the onset of menopause than for older women who are many years postmenopause [9]. In general, when a specific population is carefully chosen, ET is not associated with an increased risk of many of these safety concerns. Assessment of the benefits and risks of HT for each individual woman is therefore important for determining whether HT may be appropriate.
Review of the clinical data above suggests that many of the concerns associated with HT appear to be related to the progestin component, as CHD risk, increased breast density, breast cancer risk, breast pain, and irregular vaginal bleeding are more often associated with EPT (particularly CEE/MPA) than with ET (i.e., CEE alone). These effects are broadly consistent with the known pharmacologic and physiologic effects of progestins. For example, in normal menstrual cycles in pre-menopausal women, progesterone acts to protect the endometrium from the proliferative effect of estrogens, but also promotes menstrual bleeding [62]. Moreover, there are differential effects with different types of progestins. In a French study that compared the association between different HTs and breast cancer risk, there was no association between route of estrogen administration and risk; however, risk of breast cancer with estrogen-progesterone compared with women who had never used HT (RR, 1.00; 95% CI, 0.83–1.22) was similar to the risk with estrogen alone (RR, 1.29; 95% CI, 1.02–1.65) and significantly lower than the risk with estrogen-other progestogens, including MPA (RR, 1.69; 95% CI, 1.50–1.91; p for homogeneity <0.001) [63]. Therefore, alternatives to progestin are needed that will protect the endometrium while avoiding other progestin-associated effects and preserving the desired effects of estrogens in postmenopausal women.
3. Other Therapies for Menopausal Symptoms or Postmenopausal Osteoporosis
For women who cannot or do not wish to take HT, other options include therapies that are targeted specifically to either menopausal symptoms or the treatment and/or prevention of osteoporosis. Some nonhormonal therapies have been prescribed off-label for the treatment of VMS. Agents such as selective serotonin reuptake inhibitors, serotonin norepinephrine reuptake inhibitors, clonidine, and gabapentin have shown some efficacy in reducing VMS, but are generally not as effective as HT [64]. Nonprescription remedies such as soy-based isoflavones have also been reported to provide some relief of menopausal symptoms, although clinical trials have shown mixed efficacy results [65,66]. For the treatment and/or prevention of postmenopausal osteoporosis, pharmacologic options include bisphosphonates, salmon calcitonin, parathyroid hormone, strontium ranelate (outside of the U.S.), denosumab, and selective estrogen receptor modulators (SERMs) [10,67]. Some of these therapies are associated with adverse effects with long-term treatment, are indicated for a restricted period of treatment, or are recommended only for women who are ≥5 years past the onset of menopause [10], thus limiting the treatment options for younger women at risk for osteoporosis who expect to be on therapy for many years. There is therefore an ongoing need for new treatment options for postmenopausal women seeking a comprehensive therapy for menopausal symptoms and prevention/treatment of osteoporosis that has a favorable long-term safety and tolerability profile.
3.1. The Tissue Selective Estrogen Complex
A novel menopausal therapy in clinical development is the tissue selective estrogen complex (TSEC), which partners a SERM with one or more estrogens [68]. SERMs can have either agonist or antagonist effects on the estrogen receptor (ER) depending on tissue type [69]. The goal of a SERM/CE pairing is to blend the positive effects of estrogens on menopausal symptoms and bone with the protective effects of a SERM on the endometrium and breast in women with a uterus [70]. The pairing of a SERM with CE may be an alternative to the use of progestins in EPT for treating non-hysterectomized, postmenopausal women.
Preclinical Evidence for Potential SERM/CE Combinations
The key issues a potential TSEC should address include endometrial and breast protection without counteracting the positive effects of estrogens on the central nervous system, skeleton, and vagina. Because each SERM and CE pairing will exhibit a different tissue selectivity profile, some pairings may not adequately achieve these goals. Results from preclinical studies evaluating various SERM/CE combinations further support this concept.
Preclinical studies assessing different SERM/CE combinations have shown promising results for the pairing of bazedoxifene (BZA) with CE, while data do not support the combination of CE with other SERMs. In mature/reproductively competent, ovariectomized (OVX) rats, coadministration of BZA with CE prevented CE-induced increases in uterine wet weight and preserved BMD [70,71]. Studies in OVX, sexually immature mice showed that BZA was more effective than raloxifene (RLX) and lasofoxifene (LAS) in inhibiting CE-induced increases in uterine wet weight [72]. In this model, BZA also demonstrated less agonist activity in the mammary gland and was a more effective antagonist of CE-induced breast stimulation than RLX and LAS [72]. Moreover, the addition of BZA did not inhibit the efficacy of CE in reducing tail skin temperature in a model of VMS [70].
Taken together, these preclinical data suggest that BZA/CE is a promising TSEC pairing, while the combination of RLX or LAS with CE may not be suitable, as these pairings cause unacceptable uterine stimulation. Published clinical studies of RLX combined with oral or transdermal estrogens further reinforce these preclinical findings and show endometrial stimulation with this SERM/estrogen combination [73,74,75]. In 2 clinical trials evaluating the combination of RLX and an oral estrogen (the first with 17β-estradiol [73] and the second with esterified CE) [75], both showed an increase in endometrial thickness from baseline after 52 weeks and 3 months of therapy, respectively. Another study reported a reduction in endometrial thickness with RLX plus PBO (−0.9 mm) compared with an increase in endometrial thickness with RLX plus low-dose transdermal estradiol after 8 weeks of therapy (0.8 mm; p = 0.021) [74]. In contrast, studies of other estrogen formulations (e.g., 17β-estradiol percutaneous or vaginal ring) administered in combination with RLX did not show signs of endometrial stimulation [76,77]. Based on these findings, BZA/CE was selected for further development and is the first TSEC in clinical development.
3.2. Clinical Studies of BZA/CE
The efficacy and safety of BZA/CE have been evaluated in non-hysterectomized, postmenopausal women in a series of randomized, double-blind, PBO- and active-controlled, phase 3 trials called the Selective estrogens, Menopause, And Response to Therapy (SMART) trials (Table 2). The SMART-1 trial (N = 3,397) was a 2-year study that evaluated the efficacy and safety of BZA/CE compared with RLX and PBO in women aged 40 to 75 years [78,79,80,81]. The primary endpoint was the incidence of endometrial hyperplasia. BZA 20 mg was shown in this study to be the lowest effective dose for protecting the endometrium from CE stimulation [81]. Based on these findings, BZA 20 mg/CE 0.45 and 0.625 mg were selected for evaluation in subsequent SMART trials. The 12-week SMART-2 trial (N = 318) evaluated BZA 20 mg/CE 0.45 and 0.625 mg compared with PBO in women aged 40 to 65 years who had ≥7 moderate-to-severe hot flushes daily at baseline [82,83]. The primary endpoint was the change from baseline in frequency and severity of hot flushes. The 12-week SMART-3 trial (N = 652) evaluated BZA 20 mg/CE 0.45 and 0.625 mg compared with BZA 20 mg and PBO in women aged 40 to 65 years with ≥1 moderate-to-severe VVA symptom [84,85]. The primary endpoint was the change from baseline in measures of VVA. Generally speaking, exclusion criteria for the SMART trials were based on the prescribing information for HT; therefore, criteria were similar for both the WHI and SMART trials. More specifically, relative to the WHI study population, women in the SMART trials were generally younger (WHI included women ages 50–79 years) and exclusion criteria were more restrictive in terms of cardiovascular risk factors, excluding women with a history or presence of thromboembolic disease, cerebrovascular event, or myocardial infarction/ischemic heart disease. In both the WHI and SMART trials, women with breast cancer were excluded from participation.

Table 2.
Summary of the SMART trial study designs.
| SMART-1 [78,79,80,81,86,87,88] | SMART-2 [82,83] | SMART-3 [84,85] |
|---|---|---|
| Enrolled non-hysterectomized postmenopausal women | ||
| Aged 40–75 years with acceptable endometrial biopsy results at screening (N = 3,397) | Aged 40–65 years with acceptable endometrial biopsy results and ≥7 moderate-to-severe hot flushes/d at screening (N = 318) | Aged 40–65 years with acceptable endometrial biopsy results and ≥1 moderate-to-severe VVA symptom at screening (N = 652) |
| Substudies | ||
| N/A | N/A |
| Study duration | ||
| 2 years | 12 weeks | 12 weeks |
|
|
|
| Primary endpoints | ||
|
|
|
| Secondary endpoints | ||
|
|
|
SMART, Selective estrogens, Menopause, And Response to Therapy; VVA, vulvar/vaginal atrophy; BMD, bone mineral density; BZA, bazedoxifene; CE, conjugated estrogens; RLX, raloxifene; PBO, placebo; BTM, bone turnover markers; AE, adverse events; MOS, Medical Outcomes Study; QOL, quality of life; MENQOL, Menopause-Specific Quality of Life; MS-TSQ, Menopause Symptoms-Treatment Satisfaction Questionnaire; ASEX, Arizona Sexual Experiences.
3.2.1. Efficacy of BZA/CE on VMS
The effect of BZA/CE on VMS was evaluated in the SMART-1 trial in a subset of women with ≥7 moderate-to-severe hot flushes daily at baseline (n = 216) and in the SMART-2 trial (n = 310) [80,82]. In both studies, BZA 20 mg/CE 0.45 and 0.625 mg showed significantly greater decreases from baseline in the mean daily number and severity of hot flushes compared with PBO at 12 weeks (p < 0.05 for all; Figure 1 and Table 3). The efficacy of BZA/CE on VMS was sustained over 2 years of treatment in the SMART-1 trial [87]. In the SMART-2 trial, the efficacy of BZA/CE in reducing the frequency and severity of hot flushes compared with PBO was observed as early as Week 3; both BZA/CE doses also showed significantly higher percentages of subjects with at least a 50% or 75% decrease in the number of moderate-to-severe hot flushes at Weeks 4 and 12 compared with PBO (p < 0.001). Moreover, in a subanalysis of the SMART-2 study, the number of hot flush symptom-free days was significantly increased with both BZA/CE doses vs. PBO (p < 0.0001) over 12 weeks of therapy [89].
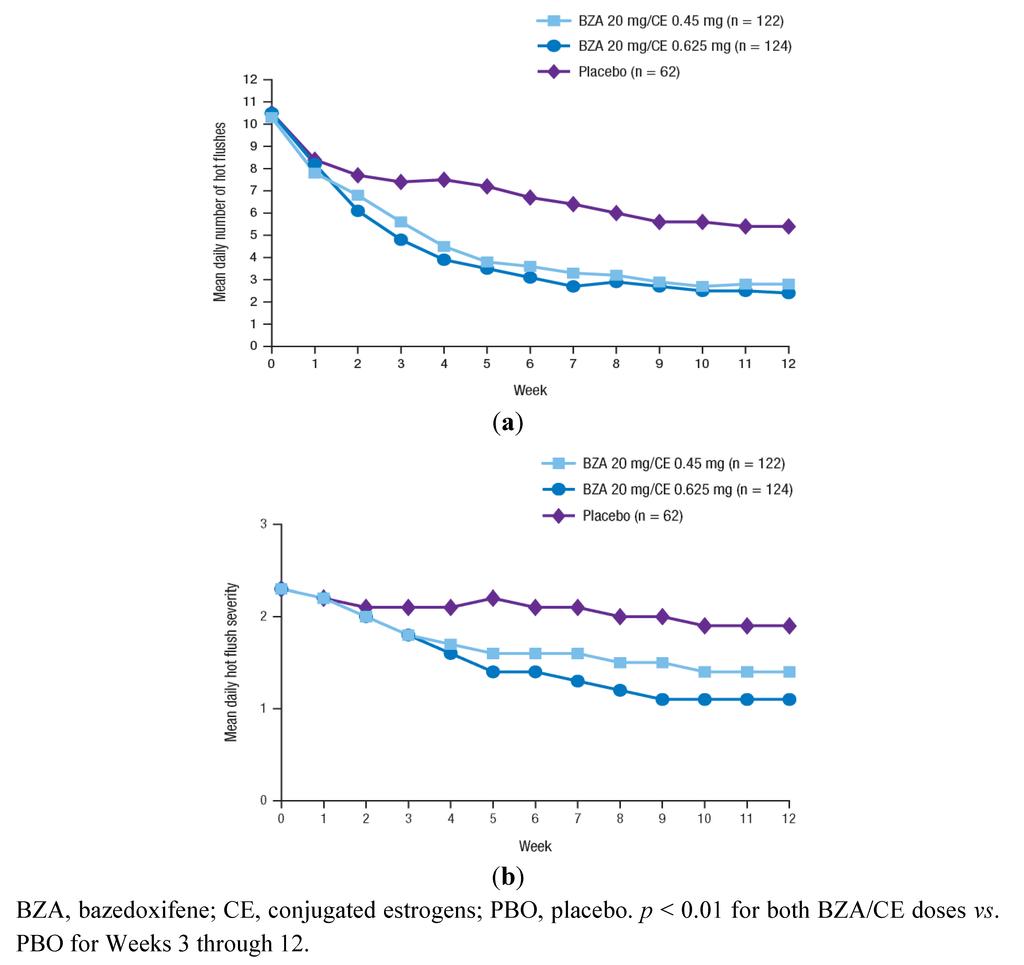
Figure 1.
The mean daily number (a) and severity (b) of moderate-to-severe hot flushes over 12 weeks in the SMART-2 trial. Reprinted from Pinkerton et al. [82] with permission from Wolters Kluwer Health.
Figure 1.
The mean daily number (a) and severity (b) of moderate-to-severe hot flushes over 12 weeks in the SMART-2 trial. Reprinted from Pinkerton et al. [82] with permission from Wolters Kluwer Health.
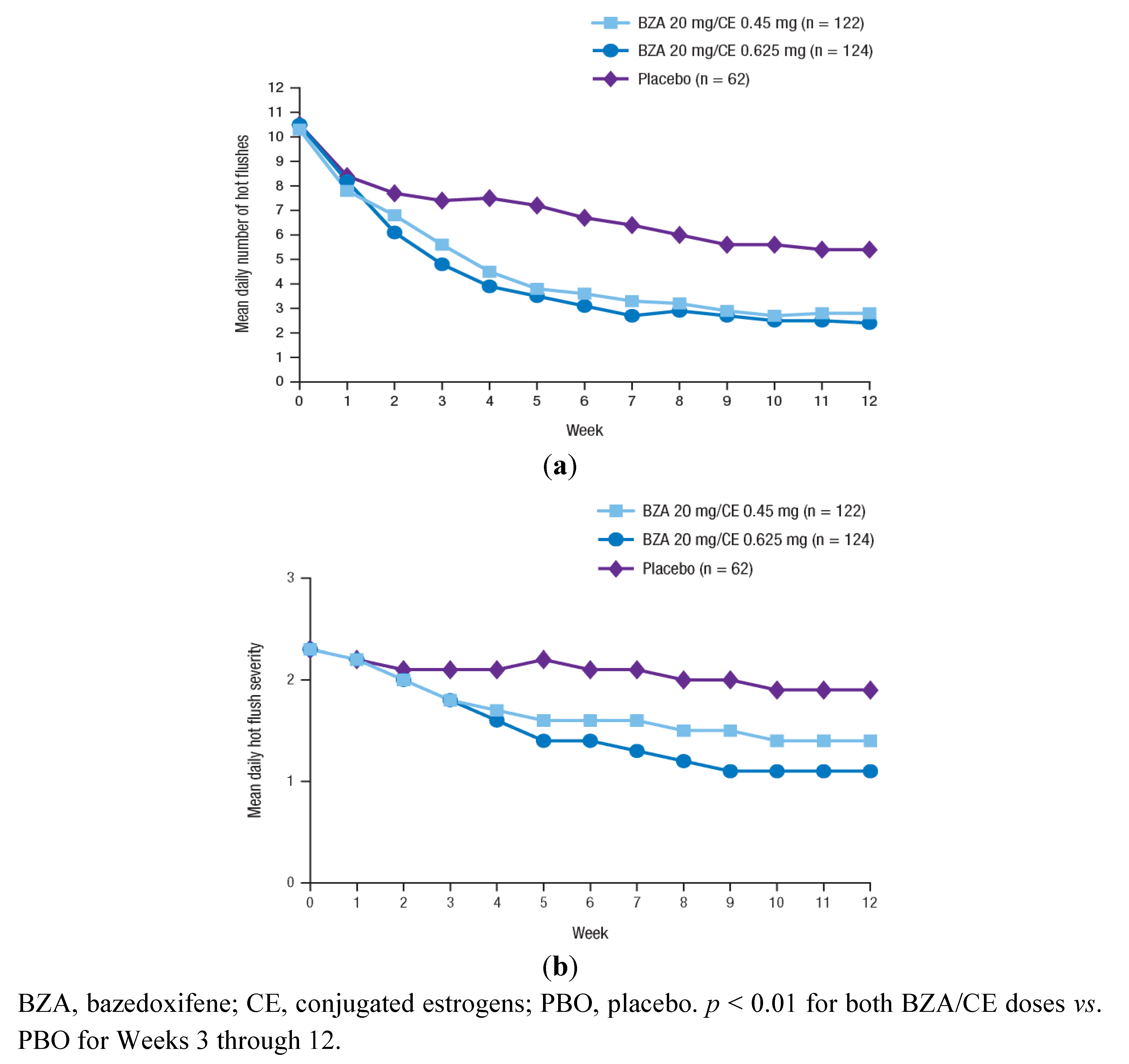

Table 3.
Summary of key efficacy findings in the SMART trials.
| SMART-1 | SMART-2 | SMART-3 |
|---|---|---|
| Efficacy on vasomotor symptoms | ||
|
| N/A |
| Efficacy on vulvar/vaginal atrophy | ||
| N/A |
|
| Effects on bone | ||
| N/A | N/A |
| Effects on sleep and quality of life | ||
| N/A |
|
|
| Satisfaction with treatment | ||
| N/A |
|
|
SMART, Selective estrogens, Menopause, And Response to Therapy; BZA, bazedoxifene; CE, conjugated estrogens; PBO, placebo; N/A, not applicable; VVA, vulvar/vaginal atrophy; BMD, bone mineral density; BTM, bone turnover markers; MENQOL, Menopause-Specific Quality of Life.
Quality of life and patient satisfaction tools were also used to assess the efficacy of BZA/CE on VMS in the SMART-2 study. Significant improvements were reported with BZA 20 mg/CE 0.45 and 0.625 mg at 12 weeks compared with PBO in the vasomotor function domain of the MENQOL questionnaire and significantly greater satisfaction in the ability to control hot flushes during the day and night according to the Menopause Symptoms Treatment Satisfaction Questionnaire (MS-TSQ) [83]. A range of components of the Medical Outcomes Study (MOS) sleep scale also showed significant improvements with BZA/CE vs. PBO, including significant decreases in sleep disturbance (effect size [95% CI] vs. PBO: −0.65 [−0.98 to −0.31] for BZA 20 mg/CE 0.45 mg, −0.75 [−1.08 to −0.41] for BZA 20 mg/CE 0.625 mg) and significant increases in sleep adequacy (effect size [95% CI] vs. PBO: 0.55 [0.22 to 0.88] for BZA 20 mg/CE 0.45 mg, 0.59 [0.25 to 0.92] for BZA 20 mg/CE 0.625 mg) [83]. In summary, BZA/CE was associated with a clinically meaningful reduction in VMS, comparable to that observed with HT. Moreover, this efficacy was also correlated with improvements in sleep and menopause-related quality of life.
3.2.2. Efficacy of BZA/CE on VVA
The efficacy of BZA/CE on measures of VVA was assessed in the SMART-1 trial in a subset of women with ≤5% superficial cells at screening who had a baseline and ≥1 on-therapy measurement (n = 1,867) [80]. In the SMART-3 trial, VVA parameters were evaluated in subjects who had a baseline and ≥1 on-therapy value for the parameter being assessed (n = 617, n = 637, and n = 634, respectively, for assessments of vaginal epithelial maturation, vaginal pH, and the most bothersome VVA symptom) [85]. Significantly greater increases from baseline in the mean proportion of superficial cells compared with PBO were observed for BZA 20 mg/CE 0.625 mg at 24 months in the SMART-1 trial and for BZA 20 mg/CE 0.45 and 0.625 mg at 12 weeks in the SMART-3 trial (p < 0.01 vs. PBO for all; Table 3). Both BZA/CE doses also showed significantly greater improvements from baseline in the mean proportions of intermediate and parabasal cells compared with PBO at 24 months and 12 weeks, respectively, in the SMART-1 and SMART-3 studies (p < 0.01 vs. PBO for all; Table 3). In the SMART-1 trial, the incidence of dyspareunia was significantly lower for subjects who received BZA 20 mg/CE 0.45 and 0.625 mg during Weeks 9 to 12 compared with those who received PBO (p < 0.05). In the SMART-2 trial, both BZA/CE doses showed a significantly greater improvement from baseline in vaginal dryness at Week 12 vs. PBO (p < 0.05). BZA 20 mg/CE 0.625 mg also showed a significantly greater decrease from baseline vs. PBO in vaginal pH (p < 0.001) and in the subjects’ most bothersome VVA symptom (p < 0.05) at Week 12. Taken together, these data support the ability of BZA/CE to improve vaginal health for non-hysterectomized postmenopausal women.
3.2.3. Effects of BZA/CE on Bone
The SMART-1 trial included 2 osteoporosis substudies [79]. Substudy I (n = 1,454) enrolled women who had a BMD T-score between −1 and −2.5 at screening and ≥1 additional risk factor for osteoporosis, and whose last menstrual period (LMP) was >5 years prior to screening. Substudy II (n = 861) enrolled women with ≥1 risk factor for osteoporosis whose LMP was between 1 and 5 years prior to screening. In both substudies, all BZA/CE doses showed significantly greater increases from baseline in lumbar spine (p < 0.001) and total hip (p < 0.01) BMD at 12 and 24 months compared with PBO, which showed decreases from baseline (Table 3). The BZA/CE groups had significantly higher percentages of responders for lumbar spine BMD (defined as subjects who had no change or an increase from baseline in lumbar spine BMD at Months 12 and 24) compared with PBO in both substudies (p < 0.001). Substudy II also evaluated BTM, with all BZA/CE doses showing a significantly greater reduction from baseline in serum levels of osteocalcin and C-telopeptide (p < 0.001) compared with PBO at all time points assessed. These data for BZA/CE are supported by the established efficacy of each agent alone in osteoporosis. Single-agent BZA 20 and 40 mg/day significantly reduced the risk of new vertebral fractures compared with PBO in a large 3-year, phase 3 study; in a post hoc subgroup analysis of women at higher risk for fracture, BZA 20 mg was associated with a 50% reduction in nonvertebral fracture risk compared with PBO (p = 0.02) and of 44% compared with RLX 60 mg (p = 0.05) [90]. Likewise, as mentioned previously, ET showed significant reductions in total fracture risk during the WHI trial compared with PBO [27]. Taken together, these results suggest that BZA/CE may provide benefits in terms of fracture risk reduction in the postmenopausal osteoporosis population.
3.2.4. Effects of BZA/CE on Sleep, Quality of Life, and Satisfaction with Treatment
BZA/CE was associated with improvement in quality of life and sleep. The effects of BZA/CE on sleep parameters were evaluated in the SMART-2 trial using the MOS sleep scale in randomized subjects who received ≥1 dose of study medication and had a baseline and ≥1 on-therapy measurement [83]. At Week 12, BZA 20 mg/CE 0.45 and 0.625 mg showed significant improvements in various sleep parameters including time to fall asleep, sleep disturbance, sleep adequacy, and sleep problems indexes I and II compared with PBO (p < 0.001; Table 3). BZA 20 mg/CE 0.625 mg also showed a significant improvement in sleep quantity vs. PBO (p = 0.01). Based on regression analysis, improvements in sleep parameters were significantly associated with reductions in the frequency of moderate-to-severe hot flushes.
Quality of life was assessed using the MENQOL questionnaire in the SMART-2 and SMART-3 trials in randomized subjects who received ≥1 dose of study medication and had a baseline and ≥1 on-therapy measurement [83,84]. In both trials, BZA 20 mg/CE 0.45 and 0.625 mg showed clinically significant improvements in vasomotor function and total MENQOL scores at 12 weeks compared with PBO (p ≤ 0.001; Table 3). Significant improvement in sexual function score was observed for BZA 20 mg/CE 0.625 mg in the SMART-2 trial and for both BZA/CE doses in the SMART-3 trial compared with PBO (p < 0.01 for all). BZA 20 mg/CE 0.625 mg also showed significant improvements vs. PBO in physical function score in both trials, and in psychosocial score in the SMART-2 trial (p < 0.0 for all). In the SMART-3 trial, sexual function was also evaluated using the Arizona Sexual Experiences (ASEX) scale. At Week 12, both BZA/CE doses showed significant improvement in ease of lubrication compared with PBO (p < 0.05).
In the SMART-2 and SMART-3 trials, satisfaction with treatment was evaluated using the MS-TSQ in subjects who received ≥1 dose of study medication and had ≥1 on-therapy measurement [83,84]. In both trials, significantly higher proportions of subjects who received BZA/CE reported overall satisfaction with treatment at Week 12 compared with those who received PBO (SMART-2 trial, 73.5% to 78.2% for BZA/CE vs. 44.4% for PBO; SMART-3 trial, 62.6% to 69.4% for BZA/CE vs. 47.4% for PBO; p < 0.05 for all; Table 3). BZA/CE-treated subjects reported significantly greater satisfaction than those who received PBO with the ability to control hot flushes during the day and night, the effect on quality of sleep, and the effect on mood or emotions (p < 0.05 for all).
3.2.5. Safety and Tolerability of BZA/CE
BZA/CE treatment has been shown in the SMART trials to be generally safe and well tolerated in postmenopausal women with a uterus. Across the SMART-1, SMART-2, and SMART-3 trials, no significant differences were observed between the BZA/CE and PBO groups in the overall incidences of adverse events (AEs) or study discontinuations due to AEs (Table 4) [80,82,85,91].
3.2.5.1. Cardiovascular
The incidences of cardiovascular AEs and VTEs were similar among the BZA/CE and PBO groups [80,82,85]. In the SMART-1 trial, cardiovascular AEs (including myocardial infarction, coronary artery disease, and coronary artery insufficiency) occurred in <1% and at a comparable incidence across treatment groups, and the relative risk of VTE for BZA/CE vs. placebo was 0.48 (95% CI, 0.05–4.66) [80].
3.2.5.2. Endometrium
In the SMART-1 trial, BZA 20 mg/CE 0.45 and 0.625 mg showed low rates (<1%) of endometrial hyperplasia over 2 years of treatment, similar to those observed for PBO (Table 4) [81]. There were no significant differences between these BZA/CE doses and PBO in the mean change from baseline in endometrial thickness at 2 years. The incidence of endometrial polyps was generally similar with the BZA/CE groups compared with PBO (1.3%–1.6%) at all time points, with the exception of BZA 10 mg/CE 0.625 mg (6.25%) and BZA 20 mg/CE 0.45 mg (5.67%) at Month 24. Reports of ovarian cysts were distributed similarly among treatment groups. There were no cases of endometrial hyperplasia reported in the SMART-2 trial and the change from baseline in mean endometrial thickness at 12 weeks was similar among the BZA/CE and PBO groups [82]. The SMART-3 trial showed no increase in the incidence of endometrial disorders or ovarian cysts for BZA 20 mg/CE 0.45 and 0.625 mg compared with PBO [85].
3.2.5.3. Breast
Based on the favorable breast safety observed with the individual components of BZA/CE [46,47,92,93,94,95], in theory, one would speculate that the combination would also exhibit a favorable breast safety profile. Indeed, breast safety findings for BZA/CE are consistent with these expectations. An ancillary study of the SMART-1 trial demonstrated that BZA 20 mg/CE 0.45 and 0.625 mg treatment did not increase mammographic breast density over two years of treatment compared with PBO (Table 4) [88]. Breast cancer risk in the SMART-1 trial was low and similar to that observed for PBO [96]. Consistent with this observation, a pooled analysis of data for BZA 20 mg/CE 0.45 mg and PBO from the SMART-1, SMART-2, and SMART-3 trials showed no differences between these groups in the incidences of breast-related AEs [91]. There were also no differences between the BZA/CE and PBO groups in the incidence of breast pain in the SMART-1, SMART-2, and SMART-3 trials [80,82,85].

Table 4.
Summary of key safety findings in the SMART trials.
| SMART-1 | SMART-2 | SMART-3 |
|---|---|---|
| Overall safety | ||
|
|
|
| Endometrial safety | ||
|
|
|
| Tolerability | ||
|
|
|
SMART, Selective estrogens, Menopause, And Response to Therapy; AE, adverse event; BZA, bazedoxifene; CE, conjugated estrogens; PBO, placebo; VTE, venous thromboembolic event.
3.2.5.4. Uterine Bleeding
BZA 20 mg/CE 0.45 and 0.625 mg were associated with high rates of cumulative amenorrhea over 2 years of treatment, similar to that observed with PBO [78,86]. A pooled analysis of data for BZA 20 mg/CE 0.45 mg and PBO from the SMART-1, SMART-2, and SMART-3 trials showed similar incidences of bleeding-related AEs between those groups [91].
4. TSEC Summary: Menopause Symptom Relief without a Progestin
Taken together, results from the phase 3 SMART clinical trial program indicate that BZA/CE has comparable efficacy to HT in terms of its beneficial effects on VMS, VVA, bone, sleep, and quality of life with an improved safety and tolerability profile, particularly in terms of a more favorable breast and bleeding profile. As mentioned earlier, data suggest that many of the tolerability concerns associated with HT appear to be related to the progestin component, particularly irregular bleeding [97,98] and breast pain/tenderness [16,60,99]. The favorable tolerability profile of BZA/CE relative to EPT (particularly CE/MPA) may be linked in part to the contrasting pharmacology of BZA vs. progestins. For example, irregular vaginal bleeding is a known treatment effect of EPT [97,98]; progestins cause changes in the endometrium associated with bleeding (i.e., increased blood supply and angiogenesis). While these effects are considered a normal part of the menstrual cycle for premenopausal women, irregular bleeding and spotting are troublesome for the postmenopausal population and are a common reason for discontinuation of EPT [59,100]. The goal of a TSEC such as BZA/CE is to achieve an optimal blend of tissue-selective activities without the need for a progestin in women with a uterus, thus minimizing some of the tolerability concerns associated with this component. BZA in combination with estrogens, such as CE, acts as an estrogen receptor antagonist in the endometrium and on breast cancer cells [101,102]. These antagonist effects in the endometrium and breast may contribute at least in part to the favorable bleeding and breast profile observed with BZA/CE relative to that observed with EPT.
5. Conclusions
HT is the conventional and established therapy option for postmenopausal women, with EPT recommended for women with an intact uterus. Both ET and EPT have demonstrated efficacy in relieving VMS and symptoms associated with VVA, as well as in preventing osteoporosis. However, EPT, particularly CEE/MPA, may be associated with some safety and tolerability concerns. The appropriateness of HT should therefore be evaluated based on the benefits and risks for each individual woman. For non-hysterectomized women, there is a need for alternatives to EPT for treating menopausal symptoms and preventing osteoporosis while ensuring reproductive safety.
The TSEC provides a novel approach to treating menopausal symptoms and preventing osteoporosis while maintaining endometrial and breast safety through the pairing of a SERM with 1 or more estrogens. BZA 20 mg/CE 0.45 and 0.625 mg have been shown in the SMART trials to be effective in relieving VMS and VVA symptoms and preserving bone mass while protecting the endometrium and breast. In addition, these BZA/CE doses were associated with a favorable overall safety and tolerability profile over two years of treatment, with no increases in the incidences of VTEs and cardiovascular events compared with PBO. Consistent with findings from the SMART-1, SMART-2, and SMART-3 trials, the recently completed SMART-5 trial also demonstrated the positive effects of BZA 20 mg/CE 0.45 and 0.625 mg on bone, sleep parameters, and QOL [103,104], as well as its overall safety and tolerability in postmenopausal women [105,106]. Based on these findings, BZA/CE may be a promising alternative to conventional EPT for non-hysterectomized, postmenopausal women who are seeking a safe and comprehensive therapy for the relief of menopausal symptoms and the prevention of postmenopausal osteoporosis.
Conflict of Interest
Barry S. Komm and Sebastian Mirkin are employees of Pfizer Inc.
Acknowledgements
Medical writing support for this manuscript was provided by Joy Loh of MedErgy and was funded by Pfizer Inc. The authors retained full editorial control over the content of the article.
References
- Burger, H.G.; Dudley, E.C.; Robertson, D.M.; Dennerstein, L. Hormonal changes in the menopause transition. Recent Prog. Horm. Res. 2002, 57, 257–275. [Google Scholar] [CrossRef]
- Burger, H.G. The endocrinology of the menopause. Maturitas 1996, 23, 129–136. [Google Scholar] [CrossRef]
- Levine, J.P. Treating menopausal symptoms with a tissue-selective estrogen complex. Gend. Med. 2011, 8, 57–68. [Google Scholar] [CrossRef]
- Lewis, V. Undertreatment of menopausal symptoms and novel options for comprehensive management. Curr. Med. Res. Opin. 2009, 25, 2689–2698. [Google Scholar]
- Dennerstein, L.; Dudley, E.C.; Hopper, J.L.; Guthrie, J.R.; Burger, H.G. A prospective population-based study of menopausal symptoms. Obstet. Gynecol. 2000, 96, 351–358. [Google Scholar]
- Riggs, B.L.; Khosla, S.; Melton, L.J., III. A unitary model for involutional osteoporosis: Estrogen deficiency causes both type I and type II osteoporosis in postmenopausal women and contributes to bone loss in aging men. J. Bone Miner. Res. 1998, 13, 763–773. [Google Scholar]
- National Osteoporosis Foundation. Fast facts on osteoporosis. Available online: http://www.nof.org/node/40/ (accessed on 17 November 2011).
- Utian, W.H. Psychosocial and socioeconomic burden of vasomotor symptoms in menopause: A comprehensive review. Health. Qual. Life Outcomes 2005, 3, 47. [Google Scholar] [CrossRef]
- North American Menopause Society. The 2012 hormone therapy position statement of The North American Menopause Society. Menopause 2012, 19, 257–271.
- North American Menopause Society. Management of osteoporosis in postmenopausal women: 2010 Position statement of The North American Menopause Society. Menopause 2010, 17, 25–54.
- Grady, D.; Gebretsadik, T.; Kerlikowske, K.; Ernster, V.; Petitti, D. Hormone replacement therapy and endometrial cancer risk: A meta-analysis. Obstet. Gynecol. 1995, 85, 304–313. [Google Scholar]
- Smith, D.C.; Prentice, R.; Thompson, D.J.; Herrmann, W.L. Association of exogenous estrogen and endometrial carcinoma. N. Engl. J. Med. 1975, 293, 1164–1167. [Google Scholar] [CrossRef]
- Weiderpass, E.; Adami, H.O.; Baron, J.A.; Magnusson, C.; Bergstrom, R.; Lindgren, A.; Correia, N.; Persson, I. Risk of endometrial cancer following estrogen replacement with and without progestins. J. Natl. Cancer Inst. 1999, 91, 1131–1137. [Google Scholar]
- Ziel, H.K.; Finkle, W.D. Increased risk of endometrial carcinoma among users of conjugated estrogens. N. Engl. J. Med. 1975, 293, 1167–1170. [Google Scholar]
- North American Menopause Society. Role of progestogen in hormone therapy for postmenopausal women: Position statement of The North American Menopause Society. Menopause 2003, 10, 113–132.
- Utian, W.H.; Shoupe, D.; Bachmann, G.; Pinkerton, J.V.; Pickar, J.H. Relief of vasomotor symptoms and vaginal atrophy with lower doses of conjugated equine estrogens and medroxyprogesterone acetate. Fertil. Steril. 2001, 75, 1065–1079. [Google Scholar]
- MacLennan, A.H.; Broadbent, J.L.; Lester, S.; Moore, V. Oral oestrogen and combined oestrogen/progestogen therapy versus placebo for hot flushes. Cochrane Database Syst. Rev. 2004, CD002978. [Google Scholar]
- Greendale, G.A.; Reboussin, B.A.; Hogan, P.; Barnabei, V.M.; Shumaker, S.; Johnson, S.; Barrett-Connor, E. Symptom relief and side effects of postmenopausal hormones: Results from the Postmenopausal Estrogen/Progestin Interventions Trial. Obstet. Gynecol. 1998, 92, 982–988. [Google Scholar]
- Pornel, B.; Spielmann, D. A study of the control of climacteric symptoms in postmenopausal women following sequential regimens of 1 mg 17b-estradiol and trimegestone compared with a regimen containing 1 mg estradiol valerate and norethisterone over a 2-year period. Gynecol. Endocrinol. 2005, 21, 74–81. [Google Scholar] [CrossRef]
- Gambacciani, M.; Spielmann, D.; Genazzani, A.R. Efficacy on climacteric symptoms of a continuous combined regimen of 1 mg 17b-estradiol and trimegestone versus two regimens combining 1 or 2 mg 17b-estradiol and norethisterone acetate. Gynecol. Endocrinol. 2005, 21, 65–73. [Google Scholar] [CrossRef]
- Schurmann, R.; Holler, T.; Benda, N. Estradiol and drospirenone for climacteric symptoms in postmenopausal women: A double-blind, randomized, placebo-controlled study of the safety and efficacy of three dose regimens. Climacteric 2004, 7, 189–196. [Google Scholar]
- Rowan, J.P.; Simon, J.A.; Speroff, L.; Ellman, H. Effects of low-dose norethindrone acetate plus ethinyl estradiol (0.5 mg/2.5 mg) in women with postmenopausal symptoms: Updated analysis of three randomized, controlled trials. Clin. Ther. 2006, 28, 921–932. [Google Scholar]
- Cardozo, L.; Bachmann, G.; McClish, D.; Fonda, D.; Birgerson, L. Meta-analysis of estrogen therapy in the management of urogenital atrophy in postmenopausal women: Second report of the Hormones and Urogenital Therapy Committee. Obstet. Gynecol. 1998, 92, 722–727. [Google Scholar] [CrossRef]
- North American Menopause Society. The role of local vaginal estrogen for treatment of vaginal atrophy in postmenopausal women: 2007 Position statement of The North American Menopause Society. Menopause 2007, 14, 357–369.
- Dorr, M.B.; Nelson, A.L.; Mayer, P.R.; Ranganath, R.P.; Norris, P.M.; Helzner, E.C.; Preston, R.A. Plasma estrogen concentrations after oral and vaginal estrogen administration in women with atrophic vaginitis. Fertil. Steril. 2010, 94, 2365–2368. [Google Scholar]
- Bachmann, G.; Bouchard, C.; Hoppe, D.; Ranganath, R.; Altomare, C.; Vieweg, A.; Graepel, J.; Helzner, E. Efficacy and safety of low-dose regimens of conjugated estrogens cream administered vaginally. Menopause 2009, 16, 719–727. [Google Scholar]
- Jackson, R.D.; Wactawski-Wende, J.; LaCroix, A.Z.; Pettinger, M.; Yood, R.A.; Watts, N.B.; Robbins, J.A.; Lewis, C.E.; Beresford, S.A.; Ko, M.G.; et al. Effects of conjugated equine estrogen on risk of fractures and BMD in postmenopausal women with hysterectomy: Results from the women’s health initiative randomized trial. J. Bone Miner. Res. 2006, 21, 817–828. [Google Scholar]
- Cauley, J.A.; Robbins, J.; Chen, Z.; Cummings, S.R.; Jackson, R.D.; LaCroix, A.Z.; LeBoff, M.; Lewis, C.E.; McGowan, J.; Neuner, J.; et al. Effects of estrogen plus progestin on risk of fracture and bone mineral density: The Women’s Health Initiative randomized trial. JAMA 2003, 290, 1729–1738. [Google Scholar]
- Lindsay, R.; Gallagher, J.C.; Kleerekoper, M.; Pickar, J.H. Effect of lower doses of conjugated equine estrogens with and without medroxyprogesterone acetate on bone in early postmenopausal women. JAMA 2002, 287, 2668–2676. [Google Scholar] [CrossRef]
- Torgerson, D.J.; Bell-Syer, S.E. Hormone replacement therapy and prevention of nonvertebral fractures: A meta-analysis of randomized trials. JAMA 2001, 285, 2891–2897. [Google Scholar]
- Wells, G.; Tugwell, P.; Shea, B.; Guyatt, G.; Peterson, J.; Zytaruk, N.; Robinson, V.; Henry, D.; O’Connell, D.; Cranney, A. Meta-analyses of therapies for postmenopausal osteoporosis. V. Meta-analysis of the efficacy of hormone replacement therapy in treating and preventing osteoporosis in postmenopausal women. Endocr. Rev. 2002, 23, 529–539. [Google Scholar]
- Hays, J.; Ockene, J.K.; Brunner, R.L.; Kotchen, J.M.; Manson, J.E.; Patterson, R.E.; Aragaki, A.K.; Shumaker, S.A.; Brzyski, R.G.; LaCroix, A.Z.; et al. Effects of estrogen plus progestin on health-related quality of life. N. Engl. J. Med. 2003, 348, 1839–1854. [Google Scholar]
- Brunner, R.L.; Gass, M.; Aragaki, A.; Hays, J.; Granek, I.; Woods, N.; Mason, E.; Brzyski, R.; Ockene, J.; Assaf, A.; et al. Effects of conjugated equine estrogen on health-related quality of life in postmenopausal women with hysterectomy: Results from the Women’s Health Initiative Randomized Clinical Trial. Arch. Intern. Med. 2005, 165, 1976–1986. [Google Scholar]
- Polo-Kantola, P.; Erkkola, R.; Helenius, H.; Irjala, K.; Polo, O. When does estrogen replacement therapy improve sleep quality? Am. J. Obstet. Gynecol. 1998, 178, 1002–1009. [Google Scholar] [CrossRef]
- Barnabei, V.M.; Grady, D.; Stovall, D.W.; Cauley, J.A.; Lin, F.; Stuenkel, C.A.; Stefanick, M.L.; Pickar, J.H. Menopausal symptoms in older women and the effects of treatment with hormone therapy. Obstet. Gynecol. 2002, 100, 1209–1218. [Google Scholar]
- Gelfand, M.M.; Moreau, M.; Ayotte, N.J.; Hilditch, J.R.; Wong, B.A.; Lau, C.Y. Clinical assessment and quality of life of postmenopausal women treated with a new intermittent progestogen combination hormone replacement therapy: A placebo-controlled study. Menopause 2003, 10, 29–36. [Google Scholar]
- Hlatky, M.A.; Boothroyd, D.; Vittinghoff, E.; Sharp, P.; Whooley, M.A. Quality-of-life and depressive symptoms in postmenopausal women after receiving hormone therapy: Results from the Heart and Estrogen/Progestin Replacement Study (HERS) trial. JAMA 2002, 287, 591–597. [Google Scholar]
- Haines, C.J.; Yim, S.F.; Chung, T.K.; Lam, C.W.; Lau, E.W.; Ng, M.H.; Chin, R.; Lee, D.T. A prospective, randomized, placebo-controlled study of the dose effect of oral oestradiol on menopausal symptoms, psychological well being, and quality of life in postmenopausal Chinese women. Maturitas 2003, 44, 207–214. [Google Scholar] [CrossRef]
- Rossouw, J.E.; Anderson, G.L.; Prentice, R.L.; LaCroix, A.Z.; Kooperberg, C.; Stefanick, M.L.; Jackson, R.D.; Beresford, S.A.; Howard, B.V.; Johnson, K.C.; et al. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: Principal results from the Women’s Health Initiative randomized controlled trial. JAMA 2002, 288, 321–333. [Google Scholar]
- Hsia, J.; Criqui, M.H.; Herrington, D.M.; Manson, J.E.; Wu, L.; Heckbert, S.R.; Allison, M.; McDermott, M.M.; Robinson, J.; Masaki, K. Conjugated equine estrogens and peripheral arterial disease risk: The Women’s Health Initiative. Am. Heart J. 2006, 152, 170–176. [Google Scholar]
- Anderson, G.L.; Limacher, M.; Assaf, A.R.; Bassford, T.; Beresford, S.A.; Black, H.; Bonds, D.; Brunner, R.; Brzyski, R.; Caan, B.; et al. Effects of conjugated equine estrogen in postmenopausal women with hysterectomy: The Women’s Health Initiative randomized controlled trial. JAMA 2004, 291, 1701–1712. [Google Scholar] [CrossRef]
- Rossouw, J.E.; Prentice, R.L.; Manson, J.E.; Wu, L.; Barad, D.; Barnabei, V.M.; Ko, M.; LaCroix, A.Z.; Margolis, K.L.; Stefanick, M.L. Postmenopausal hormone therapy and risk of cardiovascular disease by age and years since menopause. JAMA 2007, 297, 1465–1477. [Google Scholar]
- Manson, J.E.; Allison, M.A.; Rossouw, J.E.; Carr, J.J.; Langer, R.D.; Hsia, J.; Kuller, L.H.; Cochrane, B.B.; Hunt, J.R.; Ludlam, S.E.; et al. Estrogen therapy and coronary-artery calcification. N. Engl. J. Med. 2007, 356, 2591–2602. [Google Scholar]
- Hendrix, S.L.; Wassertheil-Smoller, S.; Johnson, K.C.; Howard, B.V.; Kooperberg, C.; Rossouw, J.E.; Trevisan, M.; Aragaki, A.; Baird, A.E.; Bray, P.F.; et al. Effects of conjugated equine estrogen on stroke in the Women’s Health Initiative. Circulation 2006, 113, 2425–2434. [Google Scholar]
- Curb, J.D.; Prentice, R.L.; Bray, P.F.; Langer, R.D.; van Horn, L.; Barnabei, V.M.; Bloch, M.J.; Cyr, M.G.; Gass, M.; Lepine, L.; et al. Venous thrombosis and conjugated equine estrogen in women without a uterus. Arch. Intern. Med. 2006, 166, 772–780. [Google Scholar]
- Stefanick, M.L.; Anderson, G.L.; Margolis, K.L.; Hendrix, S.L.; Rodabough, R.J.; Paskett, E.D.; Lane, D.S.; Hubbell, F.A.; Assaf, A.R.; Sarto, G.E.; et al. Effects of conjugated equine estrogens on breast cancer and mammography screening in postmenopausal women with hysterectomy. JAMA 2006, 295, 1647–1657. [Google Scholar] [CrossRef]
- LaCroix, A.Z.; Chlebowski, R.T.; Manson, J.E.; Aragaki, A.K.; Johnson, K.C.; Martin, L.; Margolis, K.L.; Stefanick, M.L.; Brzyski, R.; Curb, J.D.; et al. Health outcomes after stopping conjugated equine estrogens among postmenopausal women with prior hysterectomy: A randomized controlled trial. JAMA 2011, 305, 1305–1314. [Google Scholar]
- Cushman, M.; Kuller, L.H.; Prentice, R.; Rodabough, R.J.; Psaty, B.M.; Stafford, R.S.; Sidney, S.; Rosendaal, F.R. Estrogen plus progestin and risk of venous thrombosis. JAMA 2004, 292, 1573–1580. [Google Scholar]
- Wassertheil-Smoller, S.; Hendrix, S.L.; Limacher, M.; Heiss, G.; Kooperberg, C.; Baird, A.; Kotchen, T.; Curb, J.D.; Black, H.; Rossouw, J.E.; et al. Effect of estrogen plus progestin on stroke in postmenopausal women: The Women’s Health Initiative: A randomized trial. JAMA 2003, 289, 2673–2684. [Google Scholar] [CrossRef]
- Topal, N.B.; Ayhan, S.; Topal, U.; Bilgin, T. Effects of hormone replacement therapy regimens on mammographic breast density: The role of progestins. J. Obstet. Gynaecol. Res. 2006, 32, 305–308. [Google Scholar] [CrossRef]
- Kaewrudee, S.; Anuwutnavin, S.; Kanpittaya, J.; Soontrapa, S.; Sakondhavat, C. Effect of estrogen-progestin and estrogen on mammographic density. J. Reprod. Med. 2007, 52, 513–520. [Google Scholar]
- Santen, R. Menopausal hormone therapies: Their effect on mammographic density and breast cancer risk. Gynecol. Endocrinol. 2005, 21, 12–16. [Google Scholar] [CrossRef]
- Chen, F.P.; Cheung, Y.C.; Soong, Y.K. Factors that influence changes in mammographic density with postmenopausal hormone therapy. Taiwan J. Obstet. Gynecol. 2010, 49, 413–418. [Google Scholar]
- Boyd, N.F.; Rommens, J.M.; Vogt, K.; Lee, V.; Hopper, J.L.; Yaffe, M.J.; Paterson, A.D. Mammographic breast density as an intermediate phenotype for breast cancer. Lancet Oncol. 2005, 6, 798–808. [Google Scholar]
- McCormack, V.A.; dos Santos Silva, I. Breast density and parenchymal patterns as markers of breast cancer risk: A meta-analysis. Cancer Epidemiol. Biomarkers Prev. 2006, 15, 1159–1169. [Google Scholar] [CrossRef]
- Pinsky, R.W.; Helvie, M.A. Mammographic breast density: Effect on imaging and breast cancer risk. J. Natl. Compr. Canc. Netw. 2010, 8, 1157–1164. [Google Scholar]
- Anderson, G.L.; Chlebowski, R.T.; Rossouw, J.E.; Rodabough, R.J.; McTiernan, A.; Margolis, K.L.; Aggerwal, A.; David, C.J.; Hendrix, S.L.; Allan, H.F.; et al. Prior hormone therapy and breast cancer risk in the Women’s Health Initiative randomized trial of estrogen plus progestin. Maturitas 2006, 55, 103–115. [Google Scholar] [CrossRef]
- Chlebowski, R.T.; Anderson, G.L.; Gass, M.; Lane, D.S.; Aragaki, A.K.; Kuller, L.H.; Manson, J.E.; Stefanick, M.L.; Ockene, J.; Sarto, G.E.; et al. Estrogen plus progestin and breast cancer incidence and mortality in postmenopausal women. JAMA 2010, 304, 1684–1692. [Google Scholar] [CrossRef]
- Manonai, J.; Theppisai, U.; Suchartwatnachai, C.; Jetsawangsri, T.; Chittacharoen, A. Compliance with hormone replacement therapy in Thai women. Maturitas 2003, 44, 201–205. [Google Scholar] [CrossRef]
- Barnabei, V.M.; Cochrane, B.B.; Aragaki, A.K.; Nygaard, I.; Williams, R.S.; McGovern, P.G.; Young, R.L.; Wells, E.C.; O’Sullivan, M.J.; Chen, B.; et al. Menopausal symptoms and treatment-related effects of estrogen and progestin in the Women’s Health Initiative. Obstet. Gynecol. 2005, 105, 1063–1073. [Google Scholar]
- Crandall, C.J.; Aragaki, A.K.; Cauley, J.A.; McTiernan, A.; Manson, J.E.; Anderson, G.; Chlebowski, R.T. Breast tenderness and breast cancer risk in the estrogen plus progestin and estrogen-alone women’s health initiative clinical trials. Breast Cancer Res. Treat. 2012, 132, 275–285. [Google Scholar] [CrossRef]
- Textbook of Medical Physiology; Guyton, A.; Hall, J. (Eds.) W.B. Saunders Company: Philadelphia, PA, USA, 1996; pp. 1017–1032.
- Fournier, A.; Berrino, F.; Clavel-Chapelon, F. Unequal risks for breast cancer associated with different hormone replacement therapies: Results from the E3N cohort study. Breast Cancer Res. Treat. 2008, 107, 103–111. [Google Scholar]
- Nelson, H.D.; Vesco, K.K.; Haney, E.; Fu, R.; Nedrow, A.; Miller, J.; Nicolaidis, C.; Walker, M.; Humphrey, L. Nonhormonal therapies for menopausal hot flashes: Systematic review and meta-analysis. JAMA 2006, 295, 2057–2071. [Google Scholar]
- North American Menopause Society. The role of soy isoflavones in menopausal health: Report of The North American Menopause Society/Wulf H. Utian Translational Science Symposium in Chicago, IL (October 2010). Menopause 2011, 18, 732–753.
- Howes, L.G.; Howes, J.B.; Knight, D.C. Isoflavone therapy for menopausal flushes: A systematic review and meta-analysis. Maturitas 2006, 55, 203–211. [Google Scholar] [CrossRef]
- Ettinger, B.; Black, D.M.; Mitlak, B.H.; Knickerbocker, R.K.; Nickelsen, T.; Genant, H.K.; Christiansen, C.; Delmas, P.D.; Zanchetta, J.R.; Stakkestad, J.; et al. Reduction of vertebral fracture risk in postmenopausal women with osteoporosis treated with raloxifene: Results from a 3-year randomized clinical trial. JAMA 1999, 282, 637–645. [Google Scholar] [CrossRef]
- Komm, B.S. A new approach to menopausal therapy: The tissue selective estrogen complex. Reprod. Sci. 2008, 15, 984–992. [Google Scholar] [CrossRef]
- McDonnell, D.P.; Connor, C.E.; Wijayaratne, A.; Chang, C.Y.; Norris, J.D. Definition of the molecular and cellular mechanisms underlying the tissue-selective agonist/antagonist activities of selective estrogen receptor modulators. Recent Prog. Horm. Res. 2002, 57, 295–316. [Google Scholar] [CrossRef]
- Kharode, Y.; Bodine, P.V.; Miller, C.P.; Lyttle, C.R.; Komm, B.S. The pairing of a selective estrogen receptor modulator, bazedoxifene, with conjugated estrogens as a new paradigm for the treatment of menopausal symptoms and osteoporosis prevention. Endocrinology 2008, 149, 6084–6091. [Google Scholar] [CrossRef]
- Komm, B.S.; Vlasseros, F.; Samadfam, R.; Chouinard, L.; Smith, S.Y. Skeletal effects of bazedoxifene paired with conjugated estrogens in ovariectomized rats. Bone 2011, 49, 376–386. [Google Scholar]
- Peano, B.J.; Crabtree, J.S.; Komm, B.S.; Winneker, R.C.; Harris, H.A. Effects of various selective estrogen receptor modulators with or without conjugated estrogens on mouse mammary gland. Endocrinology 2009, 150, 1897–1903. [Google Scholar]
- Stovall, D.W.; Utian, W.H.; Gass, M.L.; Qu, Y.; Muram, D.; Wong, M.; Plouffe, L., Jr. The effects of combined raloxifene and oral estrogen on vasomotor symptoms and endometrial safety. Menopause 2007, 14, 510–517. [Google Scholar]
- Davis, S.R.; O’Neill, S.M.; Eden, J.; Baber, R.; Ekangaki, A.; Stocks, J.M.; Thiebaud, D. Transition from estrogen therapy to raloxifene in postmenopausal women: Effects on treatment satisfaction and the endometrium-a pilot study. Menopause 2004, 11, 167–175. [Google Scholar] [CrossRef]
- Carranza-Lira, S.; Gooch, A.L.; Saldivar, N.; Osterwalder, M.S. Climacteric symptom control after the addition of low-dose esterified conjugated estrogens to raloxifene standard doses. Int. J. Fertil. Womens Med. 2007, 52, 93–96. [Google Scholar]
- Pinkerton, J.V.; Shifren, J.L.; La, V.J.; Rosen, A.; Roesinger, M.; Siddhanti, S. Influence of raloxifene on the efficacy of an estradiol-releasing ring for treating vaginal atrophy in postmenopausal women. Menopause 2003, 10, 45–52. [Google Scholar]
- Valiati, B.; Capp, E.; Edelweiss, M.I.; de Freitas, F.M.; Wender, M.C. Effect of raloxifene and low-dose percutaneous 17beta-estradiol on menopause symptoms and endometrium—A randomized controlled trial. Maturitas 2009, 62, 81–84. [Google Scholar] [CrossRef]
- Archer, D.F.; Lewis, V.; Carr, B.R.; Olivier, S.; Pickar, J.H. Bazedoxifene/conjugated estrogens (BZA/CE): Incidence of uterine bleeding in postmenopausal women. Fertil. Steril. 2009, 92, 1039–1044. [Google Scholar]
- Lindsay, R.; Gallagher, J.C.; Kagan, R.; Pickar, J.H.; Constantine, G. Efficacy of tissue-selective estrogen complex of bazedoxifene/conjugated estrogens for osteoporosis prevention in at-risk postmenopausal women. Fertil. Steril. 2009, 92, 1045–1052. [Google Scholar]
- Lobo, R.A.; Pinkerton, J.V.; Gass, M.L.; Dorin, M.H.; Ronkin, S.; Pickar, J.H.; Constantine, G. Evaluation of bazedoxifene/conjugated estrogens for the treatment of menopausal symptoms and effects on metabolic parameters and overall safety profile. Fertil. Steril. 2009, 92, 1025–1038. [Google Scholar]
- Pickar, J.H.; Yeh, I.-T.; Bachmann, G.; Speroff, L. Endometrial effects of a tissue selective estrogen complex containing bazedoxifene/conjugated estrogens as a menopausal therapy. Fertil. Steril. 2009, 92, 1018–1024. [Google Scholar]
- Pinkerton, J.V.; Utian, W.H.; Constantine, G.D.; Olivier, S.; Pickar, J.H. Relief of vasomotor symptoms with the tissue-selective estrogen complex containing bazedoxifene/conjugated estrogens: A randomized, controlled trial. Menopause 2009, 16, 1116–1124. [Google Scholar]
- Utian, W.; Yu, H.; Bobula, J.; Mirkin, S.; Olivier, S.; Pickar, J.H. Bazedoxifene/conjugated estrogens and quality of life in postmenopausal women. Maturitas 2009, 63, 329–335. [Google Scholar]
- Bachmann, G.; Bobula, J.; Mirkin, S. Effects of bazedoxifene/conjugated estrogens on quality of life in postmenopausal women with symptoms of vulvar/vaginal atrophy. Climacteric 2010, 13, 132–140. [Google Scholar] [CrossRef]
- Kagan, R.; Williams, R.S.; Pan, K.; Mirkin, S.; Pickar, J.H. A randomized, placebo- and active-controlled trial of bazedoxifene/conjugated estrogens for treatment of moderate to severe vulvar/vaginal atrophy in postmenopausal women. Menopause 2010, 17, 281–289. [Google Scholar]
- Fenton, A.; Chines, A.; Mirkin, S. Endometrial safety and bleeding profile of bazedoxifene paired with conjugated estrogens: Results from 2 years of therapy. In Presentation at the 4th Triennial Scientific Meeting of the Asia Pacific Menopause Federation, Sydney, Australia, 26–29 September 2010.
- Fenton, A.; Chines, A.; Mirkin, S. Effect of bazedoxifene paired with conjugated estrogens on vasomotor symptoms over 2 years of therapy. In Presented at the 4th Triennial Scientific Meeting of the Asia Pacific Menopause Federation, Sydney, Australia, 26–29 September 2010.
- Harvey, J.A.; Pinkerton, J.V.; Baracat, E.C.; Shi, H.; Mirkin, S.; Chines, A.A. Evaluation of changes in mammographic breast density associated with bazedoxifene/conjugated estrogens in postmenopausal women. Endocr. Rev. 2011, 32, P1–P79, (Abstract). [Google Scholar]
- Yu, H.; Racketa, J.; Mirkin, S.; Chines, A. Hot flush symptom-free days with bazedoxifene/conjugated estrogens in a randomized, controlled trial of postmenopausal women. Menopause 2010, 17, 1238, Abstract P-56. [Google Scholar]
- Silverman, S.L.; Christiansen, C.; Genant, H.K.; Vukicevic, S.; Zanchetta, J.R.; de Villiers, T.J.; Constantine, G.D.; Chines, A.A. Efficacy of bazedoxifene in reducing new vertebral fracture risk in postmenopausal women with osteoporosis: Results from a 3-year, randomized, placebo- and active-controlled clinical trial. J. Bone Miner. Res. 2008, 23, 1923–1934. [Google Scholar] [CrossRef]
- Haines, C.J.; Pan, K.; Mirkin, S.; Chines, A.A. Safety and tolerability of bazedoxifene and conjugated estrogens: Pooled analysis from the Selective estrogens, Menopause And Response to Therapy (SMART)-1, SMART-2, and SMART-3 trials. In Poster presented at the 4th Triennial Scientific Meeting of the Asia Pacific Menopause Federation, Sydney, Australia, 26–29 September 2010.
- Christiansen, C.; Chesnut, C.H., III; Adachi, J.D.; Brown, J.P.; Fernandes, C.E.; Kung, A.W.; Palacios, S.; Levine, A.B.; Chines, A.A.; Constantine, G.D. Safety of bazedoxifene in a randomized, double-blind, placebo- and active-controlled phase 3 study of postmenopausal women with osteoporosis. BMC Musculoskelet. Disord. 2010, 11, 130. [Google Scholar]
- Pinkerton, J.V.; Archer, D.F.; Utian, W.H.; Menegoci, J.C.; Levine, A.B.; Chines, A.A.; Constantine, G.D. Bazedoxifene effects on the reproductive tract in postmenopausal women at risk for osteoporosis. Menopause 2009, 16, 1102–1108. [Google Scholar] [CrossRef]
- Archer, D.F.; Pinkerton, J.V.; Utian, W.H.; Menegoci, J.C.; de Villiers, T.J.; Yuen, C.K.; Levine, A.B.; Chines, A.A.; Constantine, G.D. Bazedoxifene, a selective estrogen receptor modulator: Effects on the endometrium, ovaries, and breast from a randomized controlled trial in osteoporotic postmenopausal women. Menopause 2009, 16, 1109–1115. [Google Scholar]
- Harvey, J.A.; Holm, M.K.; Ranganath, R.; Guse, P.A.; Trott, E.A.; Helzner, E. The effects of bazedoxifene on mammographic breast density in postmenopausal women with osteoporosis. Menopause 2009, 16, 1193–1196. [Google Scholar] [CrossRef]
- Pinkerton, J.A.; Constantine, G.D.; Komm, B.S.; Yu, H.; Pickar, J.H. Breast effects of bazedoxifene/conjugated estrogens in a randomized, controlled trial of postmenopausal women. Menopause 2008, 15, 1221, Abstract P-46. [Google Scholar]
- Archer, D.F.; Dorin, M.; Lewis, V.; Schneider, D.L.; Pickar, J.H. Effects of lower doses of conjugated equine estrogens and medroxyprogesterone acetate on endometrial bleeding. Fertil. Steril. 2001, 75, 1080–1087. [Google Scholar]
- Archer, D.F.; Pickar, J.H.; Bottiglioni, F. Bleeding patterns in postmenopausal women taking continuous combined or sequential regimens of conjugated estrogens with medroxyprogesterone acetate. Menopause Study Group. Obstet. Gynecol. 1994, 83, 686–692. [Google Scholar]
- Crandall, C.J.; Karlamangla, A.; Huang, M.H.; Ursin, G.; Guan, M.; Greendale, G.A. Association of new-onset breast discomfort with an increase in mammographic density during hormone therapy. Arch. Intern. Med. 2006, 166, 1578–1584. [Google Scholar] [CrossRef]
- Wattanakumtornkul, S.; Chichareon, S.; Geater, A.; Suwan, K. Compliance with hormone replacement therapy at Songklanagarind Hospital. J. Obstet. Gynaecol. Res. 2003, 29, 380–387. [Google Scholar] [CrossRef]
- Komm, B.S.; Lyttle, C.R. Developing a SERM: Stringent preclinical selection criteria leading to an acceptable candidate (WAY-140424) for clinical evaluation. Ann. NY Acad. Sci. 2001, 949, 317–326. [Google Scholar]
- Komm, B.S.; Kharode, Y.P.; Bodine, P.V.; Harris, H.A.; Miller, C.P.; Lyttle, C.R. Bazedoxifene acetate: A selective estrogen receptor modulator with improved selectivity. Endocrinology 2005, 146, 3999–4008. [Google Scholar]
- Gallagher, J.C.; Lindsay, R.; Pan, K.; Mirkin, S.; Chines, A. Effects of bazedoxifene/conjugated estrogens on bone mineral density and bone turnover markers in postmenopausal women: A double-blind, randomized, placebo- and active-controlled phase 3 study. J. Bone Miner. Res. 2011, 26, S46, Abstract 1133. [Google Scholar]
- Pinkerton, J.V.; Pan, K.; Abraham, L.; Racketa, J.; Chines, A.A.; Mirkin, S. Effects of bazedoxifene/conjugated estrogens on sleep parameters and health-related quality of life in postmenopausal women. Menopause 2011, 18, 1346, Abstract S-13. [Google Scholar]
- Archer, D.F.; Lobo, R.A.; Pan, K.; Chines, A.A.; Mirkin, S. Safety and tolerability of bazedoxifene/conjugated estrogens in postmenopausal women: Findings from a 1-year, randomized, placebo- and active-controlled, phase 3 trial. Menopause 2011, 18, 1355–1356, Abstract P-25. [Google Scholar]
- Harvey, J.A.; Pinkerton, J.V.; Pan, K.; Thompson, J.R.; Ryan, K.A.; Mirkin, S.; Chines, A.A. The effects of bazedoxifene/conjugated estrogens on breast density in postmenopausal women. Menopause 2011, 18, 1342, Abstract S-1. [Google Scholar]
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
