Evaluation of the Combined Effects of Stilbenoid from Shorea gibbosa and Vancomycin against Methicillin-Resistant Staphylococcus aureus (MRSA)
Abstract
:1. Introduction
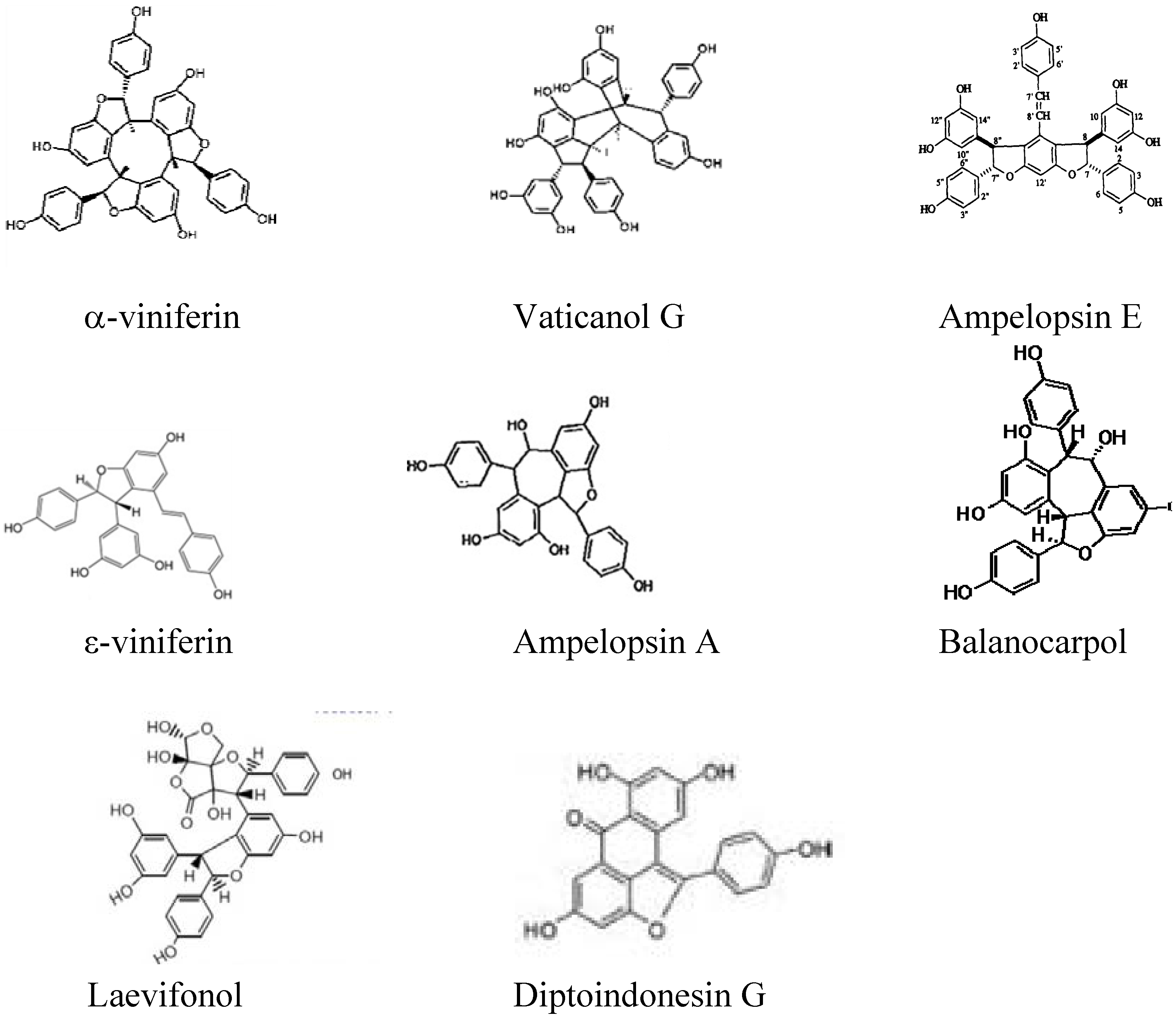
2. Results and Discussion
2.1. MIC of Stilbenoids and Vancomycin
| Concentration (μg/mL) | ATCC 33591 | HUKM strain | ||||
|---|---|---|---|---|---|---|
| ε-Viniferin | α-Viniferin | Johorenol A | ε-viniferin | α-viniferin | Johorenol A | |
| 400 | - | - | - | - | - | - |
| 200 | + | - | - | + | - | - |
| 100 | + | - | - | + | - | + |
| 50 | + | + | + | + | + | + |
| 25 | + | + | + | + | + | + |
| 12.5 | + | + | + | + | + | + |
| 6.25 | + | + | + | + | + | + |
| 3.13 | + | + | + | + | + | + |
| 1.56 | + | + | + | + | + | + |
| 0.78 | + | + | + | + | + | + |
| Concentration (μg/mL) | Vancomycin | |
|---|---|---|
| ATCC 33591 | HUKM strain | |
| 250 | - | - |
| 125 | - | - |
| 62.5 | - | - |
| 31.25 | - | - |
| 15.6 | - | - |
| 7.8 | - | - |
| 3.9 | - | - |
| 2 | - | - |
| 1 | + | + |
| 0.5 | + | + |
2.2. MBCs of Stilbenoids
| MIC (μg/mL) | ATCC 33591 | MRSA HUKM | ||||
|---|---|---|---|---|---|---|
| ε-Vineferin | α-Vineferin | Johorenol A | ε-Vineferin | α-Vineferin | Johorenol A | |
| 400 | + | + | + | + | + | + |
| 200 | ND | + | + | ND | + | + |
| 100 | ND | + | + | ND | + | ND |
| 50 | ND | ND | ND | ND | ND | ND |
| 25 | ND | ND | ND | ND | ND | ND |
2.3. FIC of Stilbenoids and Vancomycin
| Strains | Agent | MIC (μg/mL) | FIC (μg/mL) | Outcome | ||
|---|---|---|---|---|---|---|
| Alone | Combination | FIC | FICI− | |||
| ATCC 33591 | ε-viniferin | 400 | 200 | 0.5 | 0.5625 | Additive |
| Vancomycin | 1.5 | 0.0938 | 0.0625 | |||
| α-viniferin | 100 | 50 | 0.5 | 0.5625 | Additive | |
| Vancomycin | 2 | 0.125 | 0.0625 | |||
| Johorenol A | 100 | 6.25 | 0.0625 | 0.3125 | Synergistic | |
| Vancomycin | 2 | 0.5 | 0.25 | |||
| MRSA HUKM | ε-viniferin | 400 | 200 | 0.5 | 0.5625 | Additive |
| Vancomycin | 1.5 | 0.0938 | 0.0625 | |||
| α-viniferin | 100 | 100 | 1.0 | 1.0625 | Additive | |
| Vancomycin | 2 | 0.125 | 0.0625 | |||
| Johorenol A | 200 | 100 | 0.5 | 0.5625 | Additive | |
| Vancomycin | 2 | 0.125 | 0.0625 | |||
3. Experimental
3.1. Bacterial Strains
3.2. Antimicrobial Agents
3.3. Bacterial Suspensions
3.4. MIC Determination
3.5. MBC Determination
3.6. FIC Determination


4. Conclusions
Acknowledgments
Conflict of Interest
References
- Bauman, R.W. Pathogenic Gram-Positive Cocci and Bacilli. In Microbiology with Diseases by Taxonomy, 2nd ed; Pearson: San Francisco, CA, USA, 2007; p. 532. [Google Scholar]
- Moncrief, J.A.; Teplitz, C. Changing concepts in burn sepsis. J. Trauma 1964, 4, 233–245. [Google Scholar] [CrossRef]
- Bowser-Wallace, B.H.; Graves, D.B.; Caldwell, F.T. An epidemiological profile and trend analysis of wound flora in burned children: 7 Years’ experience. Burns Incl. Therm. Inj. 1984, 11, 16–25. [Google Scholar] [CrossRef]
- Reig, A.; Tejerina, C.; Codina, J.; Mirabet, V. Infections in burn patients. Ann. MBC 1992, 5. No. 2. [Google Scholar]
- Breen, J.D.; Karchmer, A.W. Staphylococcus aureus infections in diabetic patients. Infect. Dis. Clin. N. Am. 1995, 9, 11–24. [Google Scholar]
- Joshi, N.; Caputo, G.; Weitekamp, M.; Karchmer, A.W. Infections in patients with diabetes mellitus. N. Engl. J. Med. 1999, 341, 1906–1912. [Google Scholar] [CrossRef]
- Pantosti, A.; Venditti, M. What is MRSA? Eur. Respir. J. 2009, 34, 1190–1196. [Google Scholar] [CrossRef]
- Ghaznavi-Rad, E.; Nor Shamsudin, M.; Sekawi, Z.; Khoon, L.Y.; Aziz, M.N.; Hamat, R.A.; Othman, N.; Chong, P.P.; van Belkum, A.; Ghasemzadeh-Moghaddam, H.; et al. Predominance and emergence of clones of hospital-acquired methicillin-resistant Staphylococcus aureus in Malaysia. J. Clin. Microbiol. 2010, 48, 867–872. [Google Scholar] [CrossRef]
- Alexander, I.I.; Greenberger, P.A. Vancomycin-induced Stevens-Johnson syndrome. Allergy Asthma Proc. 1996, 17, 75–78. [Google Scholar] [CrossRef]
- Suwanna, T.; Somwang, D.; Yong, R.; Chertsak, D.; Wattanachai, S.; Teruyo, I.; Keiichi, H. First report of methicillin-resistant Staphylococcus aureus with reduced susceptibility to vancomycin in Thailand. J. Microbiol. 2001, 2, 591–595. [Google Scholar]
- McCollum, M.; Sorensen, S.V.; Liu, L.Z. A comparison of costs and hospital length stay associated with intravenous/oral linezolid or intravenous vancomycin treatment of complicated skin and soft-tissue infections caused by suspected or confirmed methicillin-resistant Staphylococcus aureus in elderly U.S. patients. Clin. Ther. 2007, 29, 469–477. [Google Scholar] [CrossRef]
- Pei, R.S.; Zhou, F.; Ji, B.P.; Xu, J. Evaluation of combined antibacterial effects of eugenol, cinnamaldehye, thymol, carvacrol against E. coli with an improved method. J. Food Sci. 2009, 74, M379–M383. [Google Scholar] [CrossRef]
- Glazebrook, J.; Ausubel, F.M. Isolation of phytoalexin-deficient mutants of Arabidopsis thaliana and characterization of their interactions with bacterial pathogens. Proc. Natl. Acad. Sci. USA 1994, 91, 8955–8959. [Google Scholar] [CrossRef]
- Hemaiswarya, S.; Kruthiventi, A.K.; Doble, M. Synergism between natural products and antibiotics against infectious diseases. Phytomedicine 2008, 15, 639–652. [Google Scholar] [CrossRef]
- Xiao, K.; Zhang, H.J.; Xuan, L.J.; Zhang, J.; Xu, Y.M.; Bai, D.L. Stilbenoid: Chemistry and bioactivities. Stud. Nat. Prod. Chem. 2008, 34, 453–646. [Google Scholar] [CrossRef]
- Atun, S.; Achmad, S.A.; Ghisalberti, E.L.; Hakim, E.H.; Makmur, L.; Shah, Y.M. Oligostilbenoids from Vatica umbonata (Dipterocarpaceae). Biochem. Syst. Ecol. 2004, 32, 1051–1053. [Google Scholar] [CrossRef]
- Kitanaka, S.; Ikezawa, T.; Yasukawa, K.; Yamanouchi, S.; Takido, M.; Sung, H.K.; Kim, I.H. (+)-Alpha-viniferin, an anti-inflammatory compound from Caragana chamlagu root. Chem. Pharm. Bull. (Tokyo) 1990, 38, 432–435. [Google Scholar] [CrossRef]
- Dai, J.R.; Hallock, Y.F.; John, H.; Cardellina, I.I.; Boyd, M.R. HIV-Inhibitory and cytotoxic oligostilbenoids isolated from the leaves of Hopea malibalo. J. Nat. Prod. 1988, 61, 351–353. [Google Scholar]
- Sotheeswaran, S.; Pasupathy, V. Distribution of resveratrol oligomers in plants. Phytochemistry 1993, 32, 1083–1092. [Google Scholar] [CrossRef]
- Bokel, M.; Diyasena, M.N.C.; Gunatilaka, A.A.L.; Kraus, W.; Shotheswaran, S. Canaliculatol, an antifungal resveratrol trimer from Stemonoporous canaliculatus. Phytochemistry 1988, 27, 377–380. [Google Scholar]
- Chemical Compound Database 2011. Available online: http://www.convertunits.com/compounds/ (accessed on 3 September 2012).
- McCafferty, D.G.; Cudic, P.; Yu, M.K.; Behenna, D.C.; Kruger, R. Synergy and duality in peptide antibiotic mechanisms. Curr. Opin. Chem. Biol. 1999, 3, 672–680. [Google Scholar] [CrossRef]
- Clinical and Laboratory Standards Institute, Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria that Grow Aerobically; Approved Standard, 8th edCLSI: Wayne, PA, USA, 2009.
- Yim, N.; Ha, D.T.; Trung, T.N.; Kim, J.P.; Lee, S.; Na, M.; Jung, H.; Kim, H.S.; Kim, Y.H.; Bae, K. The antimicrobial activity of compounds from the leaf and stem of Vitis amurensis against two oral pathogens. Bioorg. Med. Chem. Lett. 2010, 20, 1165–1168. [Google Scholar]
- Zain, W.Z.W.M.; Ahmat, N.; Norizan, H.H.; Nazri, N.A.A.M. The evaluation of antioxidant antibacterial and structural identification activity of trimer resveratrol from Malaysia’s Dipterocarpaceae. Aust. J. Basic Appl. Sci. 2011, 5, 926–929. [Google Scholar]
- Amalfitano, C.; Agrelli, D.; Arrigo, A.; Mugnai, L.; Surico, G.; Evidente, A. Stilbene polyphenols in the brown red wood of Vitis vinifera cv. Sangiovese affected by “esca proper”. Phytopathol. Mediterr 2011, 50, S224–S235. [Google Scholar]
- Kundakovic, T.; Stanojkovic, T.; Milenkovic, M.; Grubin, J.; Juranic, Z.; Stevanovic, B.; Kovacevic, N. Cytotoxic, antioxidant, and antimicrobial activities of Ampelopsis brevipedunculata and Parthenocessus tricuspidata (Vitaceae). Arch. Biol. Sci. Belgrade 2008, 60, 641–647. [Google Scholar] [CrossRef]
- Zain, W.Z.W.M.; Ahmat, N.; Daud, S.; Latip, J.; Syah, Y.M. Cytotoxic and antibacterial activity of laevifonol from the stem bark of Vatica odorata. Planta Med. 2009, 75, PD68. [Google Scholar]
- Ge, H.; Yang, W.; Shen, Y.; Jiang, N.; Guo, Z.; Luo, Q.; Xu, Q.; Ma, J.; Tan, R. Immunosuppressive resveratrol aneuploids from Hopea chinensis. Chem. Eur. J. 2010, 16, 6338–6345. [Google Scholar] [CrossRef]
- Atun, S.; Aznam, N.; Arianingrum, R.; Takaya, Y.; Masatake, N. Resveratrol derivatives from stem bark of Hopea and their biological activity test. J. Phys. Sci. 2008, 19, 7–21. [Google Scholar]
- Belur, P.D.; Mugeraya, G. Microbial production of tannase: State of the art. Res. J. Microbiol. 2011, 6, 25–40. [Google Scholar] [CrossRef]
- Kothari, V.; Naraniwal, M.; Gupta, A. Effect of certain phytochemicals on Aeromonas hydrophila. Res. Biotechnol. 2011, 2, 20–25. [Google Scholar]
- Fellenberg, M.A.; Espinoza, A.; Pena, I.; Alarcon, J. Antioxidant and bacteriostatic effects of the addition of extract of Quillay Polyphenols (Quillaja saponaria) in the marinade of broiler chicken. Braz. J. Poultry Sci. 2011, 13, 71–79. [Google Scholar]
- Jayaraman, P. Activity and interactions of antibiotic and phytochemical combinations against Pseudomonas aeruginosa in vitro. Int. J. Biol. Sci. 2010, 6, 556–568. [Google Scholar] [CrossRef]
- Wibowo, A.; Ahmat, N.; Hamzah, A.S.; Sufian, A.S.; Ismail, N.H.; Ahmad, R.; Jaafar, F.M.; Takayama, H.; Malaysianol, A. A new trimer resveratrol oligomer from the stem bark of Dryobalanops aromatica. Fitoterapia 2011, 82, 676–681. [Google Scholar] [CrossRef]
- Basri, D.F.; Tan, L.S.; Shafiei, Z.; Zin, N.M. In vitro antibacterial activity of galls of Quercus infectoria Olivier against oral pathogens. Evid. Based Complement. Alternat. Med. 2012, 632796. [Google Scholar]
- Zhao, W.H.; Hu, Z.Q.; Okubo, S.; Hara, Y.; Shimamura, T. Mechanism of synergy between epigallochatechin gallate and β-lactams against methicillin resistant Staphylococcus aureus. Antimicrob. Agents Chemother. 2001, 45, 1737–1742. [Google Scholar] [CrossRef]
- Reynolds, P.E. Structure, biochemistry and mechanism of action of glycopeptide antibiotics. Eur. J. Clin. Microbiol. Infect. Dis. 1989, 8, 943–950. [Google Scholar] [CrossRef]
- Aiyegoro, O.A.; Afolayan, A.J.; koh, A.I. Synergistic interaction of Helichrysum pedunculatum leaf extracts with antibiotics against wound infection associated bacteria. Biol. Res. 2009, 42, 327–338. [Google Scholar]
- Aiyegoro, O.A.; Okoh, A.L. Use of bioactive plant products in combination with standard antibiotics: Implications in antimicrobial chemotherapy. J. Med. Plant. Res. 2009, 3, 1147–1152. [Google Scholar]
- White, R.L.; Burgess, D.S.; Manduru, M.; Bosso, J.A. Comparison of three different in vitro methods of detecting synergy: Time-kill, checkerboard and E-test. Antimicrob. Agents Chemother. 1996, 40, 1914–1918. [Google Scholar]
- Moody, J.A.; Gerding, D.N.; Peterson, L.R. Evaluation of ciprofloxacin’s synergy with other agents by multiple in vitro methods. Am. J. Med. 1987, 82, 44–54. [Google Scholar] [CrossRef]
- Takahata, M.; Mitsuyama, J.; Yamashiro, Y.; Yonezawa, M.; Araki, H.; Todo, Y.; Minami, S.; Watanabe, Y.; Narita, H. In vitro and in vivo antimicrobial activities of T-381ME, a novel Des-F(6)-Quinolone. Antimicrob. Agents Chemother. 1999, 43, 1077–1084. [Google Scholar]
- Pillai, S.K.; Moellering, R.C.; Eliopoulos, G.M. Antimicrobial Combinations in Antibiotics in Laboratory Medicine, 5th ed; Lippincott Williams Wilkins: Philadelphia, PA, USA, 2005; p. 365. [Google Scholar]
- Bharadwaj, R.; Vidya, A.; Dewan, B.; Pal, A. An in vitro study to evaluate the synergistic activity of norfloxacin and metronidazole. Indian J. Pharm. 2003, 35, 220–226. [Google Scholar]
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Basri, D.F.; Luoi, C.K.; Azmi, A.M.; Latip, J. Evaluation of the Combined Effects of Stilbenoid from Shorea gibbosa and Vancomycin against Methicillin-Resistant Staphylococcus aureus (MRSA). Pharmaceuticals 2012, 5, 1032-1043. https://doi.org/10.3390/ph5091032
Basri DF, Luoi CK, Azmi AM, Latip J. Evaluation of the Combined Effects of Stilbenoid from Shorea gibbosa and Vancomycin against Methicillin-Resistant Staphylococcus aureus (MRSA). Pharmaceuticals. 2012; 5(9):1032-1043. https://doi.org/10.3390/ph5091032
Chicago/Turabian StyleBasri, Dayang Fredalina, Chan Kin Luoi, Abdul Muin Azmi, and Jalifah Latip. 2012. "Evaluation of the Combined Effects of Stilbenoid from Shorea gibbosa and Vancomycin against Methicillin-Resistant Staphylococcus aureus (MRSA)" Pharmaceuticals 5, no. 9: 1032-1043. https://doi.org/10.3390/ph5091032
APA StyleBasri, D. F., Luoi, C. K., Azmi, A. M., & Latip, J. (2012). Evaluation of the Combined Effects of Stilbenoid from Shorea gibbosa and Vancomycin against Methicillin-Resistant Staphylococcus aureus (MRSA). Pharmaceuticals, 5(9), 1032-1043. https://doi.org/10.3390/ph5091032




