Genetic and Phenotypic Analyses of a Papaver somniferum T-DNA Insertional Mutant with Altered Alkaloid Composition
Abstract
:1. Introduction
2. Results and Discussion
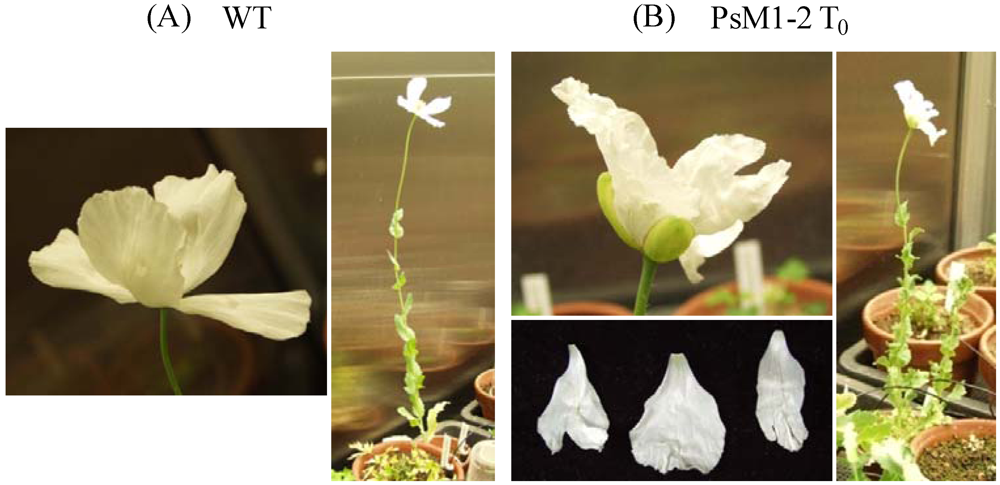
2.1. Morphological Characteristics of the PsM1-2 Mutants
| Progenies | Lines | Number of Plants | Days to Flowering (Mean ± SD) (days) | Number of Petals: Percentage (%) | Split on Petal Boundary (%) | Plant Height (Aerial Part) (Mean ± SD) (cm) |
|---|---|---|---|---|---|---|
| T0 | WT | 1 | 47 *1 | 4: 100 | 0 | 60.0 |
| T0 | 1 | 71 *1 | 4: 100 | 100 | 38.0 | |
| T1 | WT | 6 | 53.5 ± 4.8 | 4: 100 | 0 | 42.4 ± 5.8 |
| T1 | 60 | 100.6 ± 14.6 #### | 3: 1.7, 4: 41.7, 5: 35.0, 6: 16.7, 7: 3.3, 8: 1.7 | 45.0 | 52.1 ± 8.5 ## | |
| T2 | WT | 12 | 53.3 ± 4.0 | 3: 25.0, 4: 75.0 | 8.3 | 36.0 ± 7.6 |
| #1-27(HT) | 15 | 90.8 ± 12.6 #### | 5: 60.0, 6: 33.3, 10: 6.7 | 60.0 | 44.7 ± 5.4 ## | |
| #2-17(HT) | 6 | 79.8 ± 2.5 #### | 5: 50.0, 6: 33.3, 8: 16.7 | 83.3 | 45.1 ± 3.4 # | |
| #2-1(LT) | 12 | 83.3 ± 6.8 #### | 5: 66.7, 6: 16.7, 7: 8.3, 8: 8.3 | 33.3 | 35.6 ± 7.8 | |
| #2-6(LT) | 10 | 76.4 ± 3.6 #### | 5: 10.0, 6: 40.0,7: 30.0, 8: 10.0,12: 10.0 | 80.0 | 39.4 ± 3.1 | |
| T3 | WT | 6 | 109.4 ± 0.9 *2 | 4: 100 | 0 | 80.3 ± 5.8 |
| #1-27(HT)L#2 | 10 | 129.2 ± 11.9 *3, ### | 4: 40.0, 5: 50.0, 6: 10.0 | 10.0 | 45.8 ± 7.9 #### | |
| #2-17(HT)#2-1 | 12 | 131.1 ± 7.3 *4, #### | 3: 8.3, 4: 75.0, 5: 16.7 | 8.3 | 47.0 ± 13.4 #### |
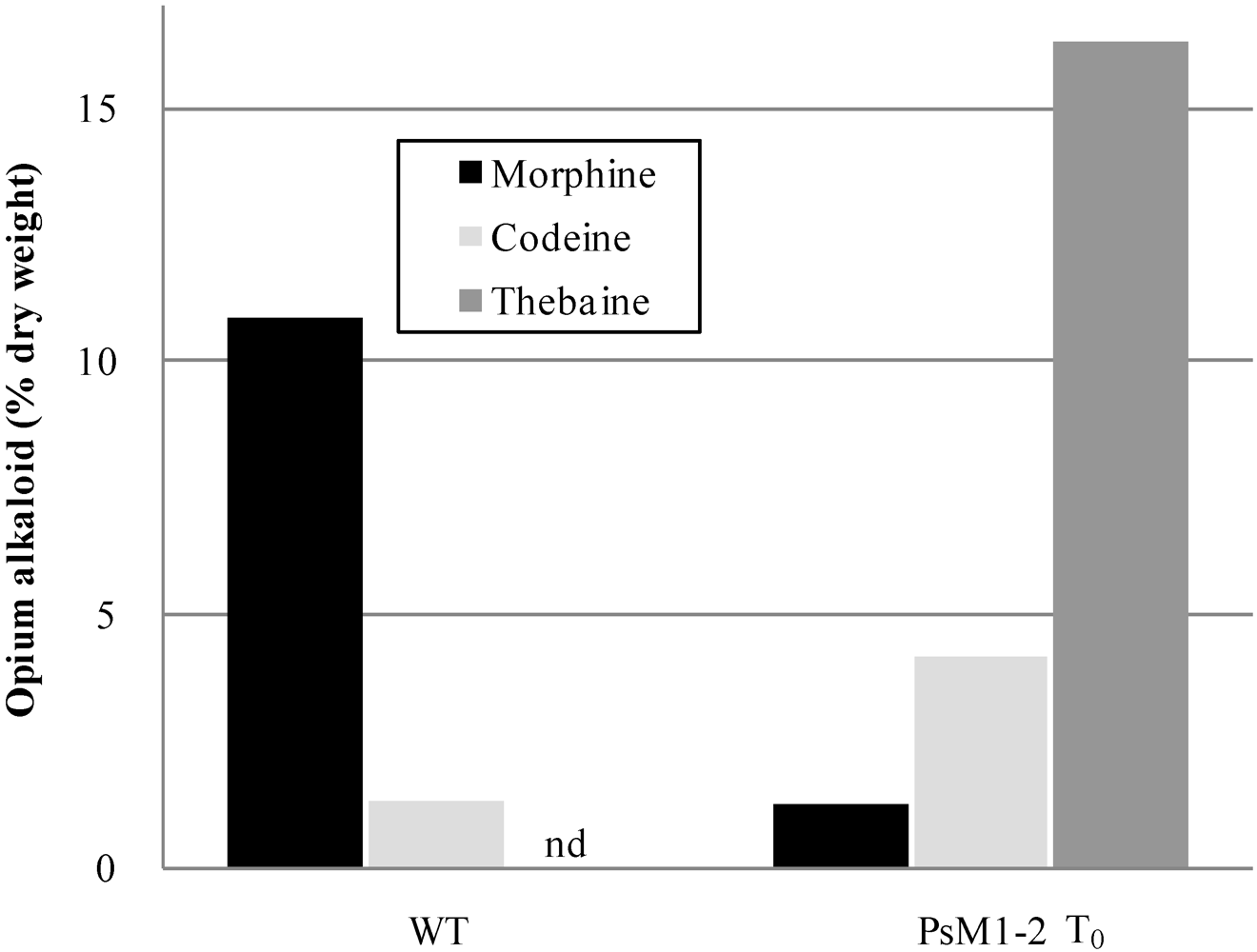
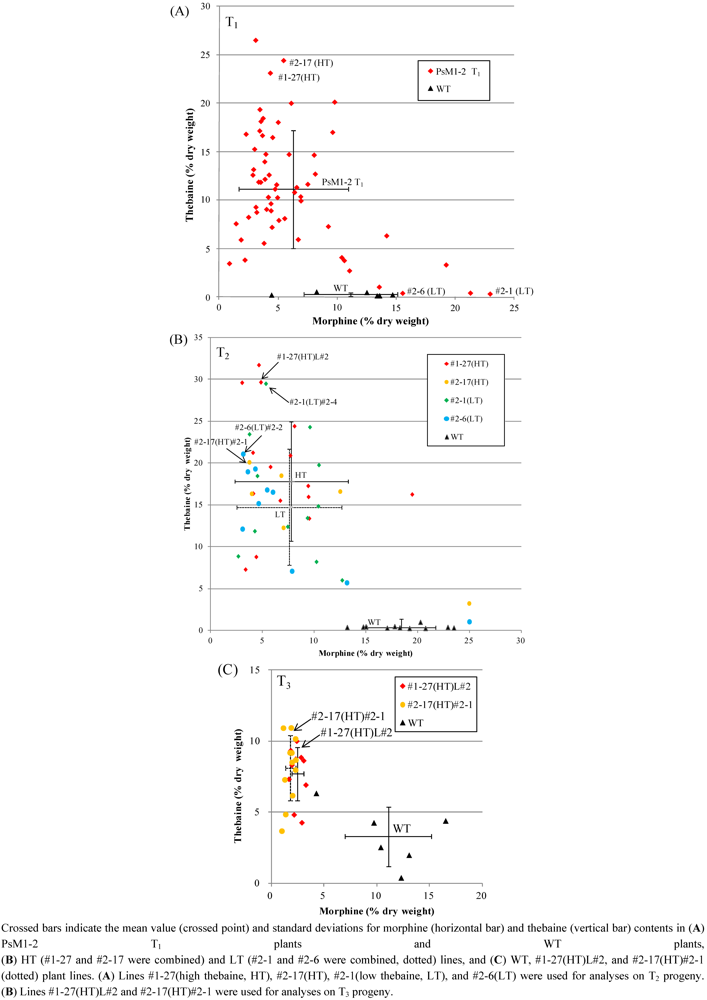
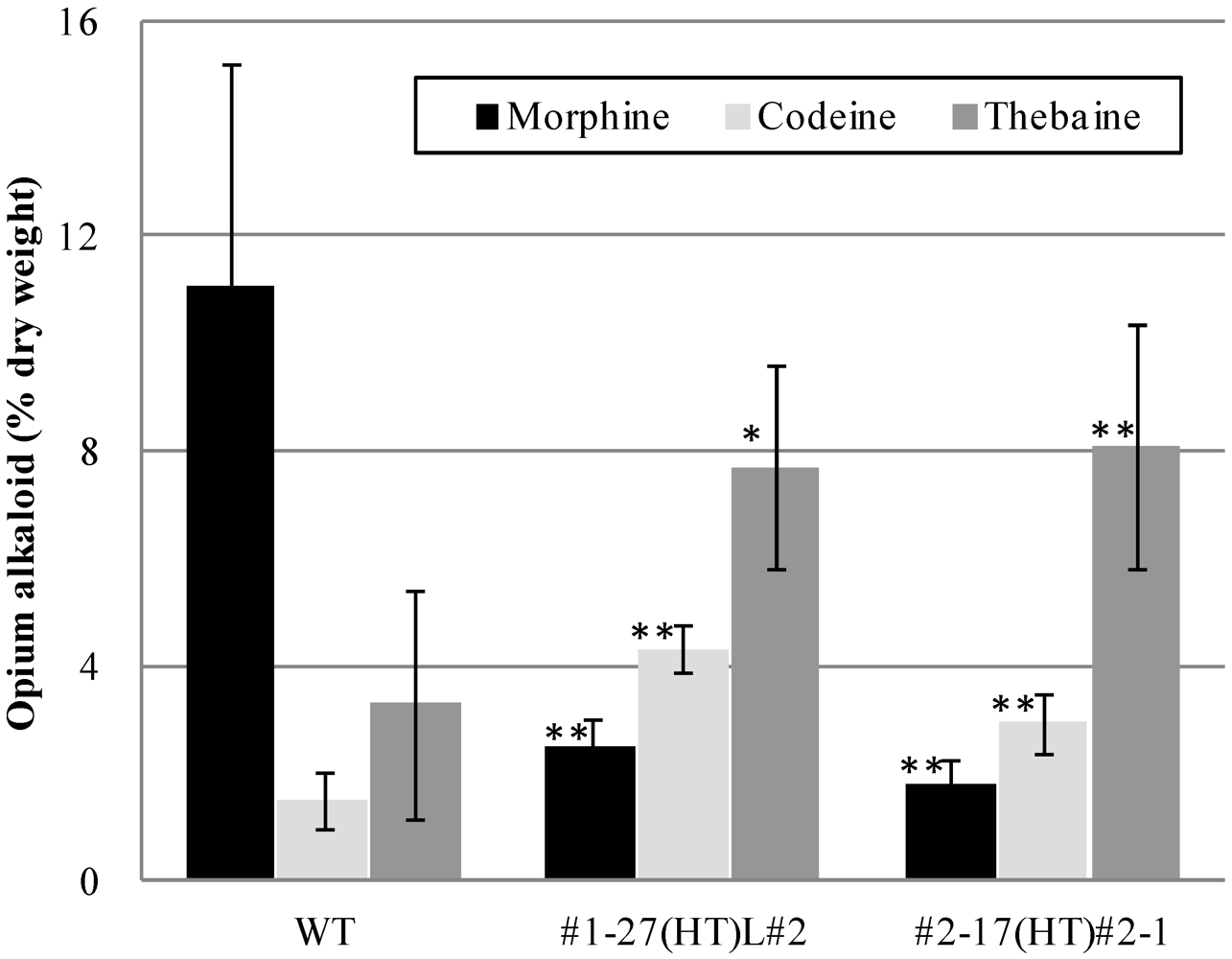
2.2. Alkaloid Composition in the PsM1-2 Mutants
| Progenies | Lines | Number of plants | Morphine | Codeine | Thebaine | Papaverine | Noscapine |
|---|---|---|---|---|---|---|---|
| T0 | WT | 1 | 10.9 | 1.3 | nd * | 2.0 | 9.2 |
| T0 | 1 | 1.3 | 4.2 | 16.3 | 2.3 | 10.2 | |
| T1 | WT | 6 | 11.2 ± 4.0 | 2.6 ± 2.1 | 0.3 ± 0.2 | 2.4 ± 0.7 | 11.6 ± 4.3 |
| T1 | 60 | 6.3 ± 4.6 # | 3.8 ± 1.5 | 11.1 ± 6.1 ### | 1.6 ± 0.5 | 7.9 ± 2.1 ### | |
| Selected lines (T1) | #1-27(HT) | - | 4.3 | 5.1 | 23.1 | 2.3 | 7.2 |
| #2-17(HT) | - | 5.5 | 3.7 | 24.4 | 1.9 | 6.8 | |
| #2-1(LT) | - | 23.0 | 1.3 | 0.3 | 1.8 | 8.4 | |
| #2-6(LT) | - | 13.6 | 2.1 | 1.0 | 1.5 | 6.9 | |
| T2 | WT | 11 | 18.4 ± 3.3 | 1.5 ± 0.9 | 0.4 ± 0.2 | 2.7 ± 1.0 | 18.4 ± 4.3 |
| #1-27(HT) | 15 | 7.0 ± 4.1 ### | 5.8 ± 1.6 ### | 19.1 ± 7.3 ### | 2.2 ± 0.4 | 9.6 ± 2.4 ### | |
| #2-17(HT) | 6 | 9.8 ± 8.1 # | 6.0 ± 0.5 ### | 14.5 ± 6.1 ### | 3.0 ± 0.4 | 9.2 ± 1.7 ### | |
| #2-1(LT) | 12 | 7.6 ± 3.3 ### | 5.8 ± 1.1 ### | 15.9 ± 7.2 ### | 2.8 ± 0.8 | 8.0 ± 2.0 ### | |
| #2-6(LT) | 10 | 7.6 ± 6.8 ### | 4.6 ± 1.3 ### | 13.4 ± 6.7 ### | 2.7 ± 0.5 | 11.7 ± 2.7 ### | |
| Selectedlines (T2) | #1-27(HT)L#2 | - | 4.9 | 6.1 | 29.6 | 2.4 | 9.4 |
| #2-17(HT)#2-1 | - | 3.7 | 6.5 | 20.0 | 3.1 | 10.4 | |
| #2-1(LT)#2-4 | - | 5.3 | 4.3 | 29.4 | 3.1 | 9.1 | |
| #2-6(LT)#2-2 | - | 3.1 | 4.9 | 21.1 | 2.7 | 13.2 | |
| T3 | WT | 6 | 11.1 ± 4.1 | 1.5 ± 0.5 | 3.3 ± 2.1 | 1.9 ± 0.5 | 4.2 ± 1.6 |
| #1-27(HT)L#2 | 10 | 2.5 ± 0.6 ### | 4.3 ± 0.4 ### | 7.7 ± 1.9 ## | 1.2 ± 0.1 ### | 5.1 ± 0.7 | |
| #2-17(HT)#2-1 | 12 | 1.8 ± 0.5 ### | 2.9 ± 0.5 ### | 8.1 ± 2.3 ### | 1.1 ± 0.2 ### | 4.1 ± 0.8 |
2.3. T-DNA Insertion Loci Analysis by IPCR and AL-PCR
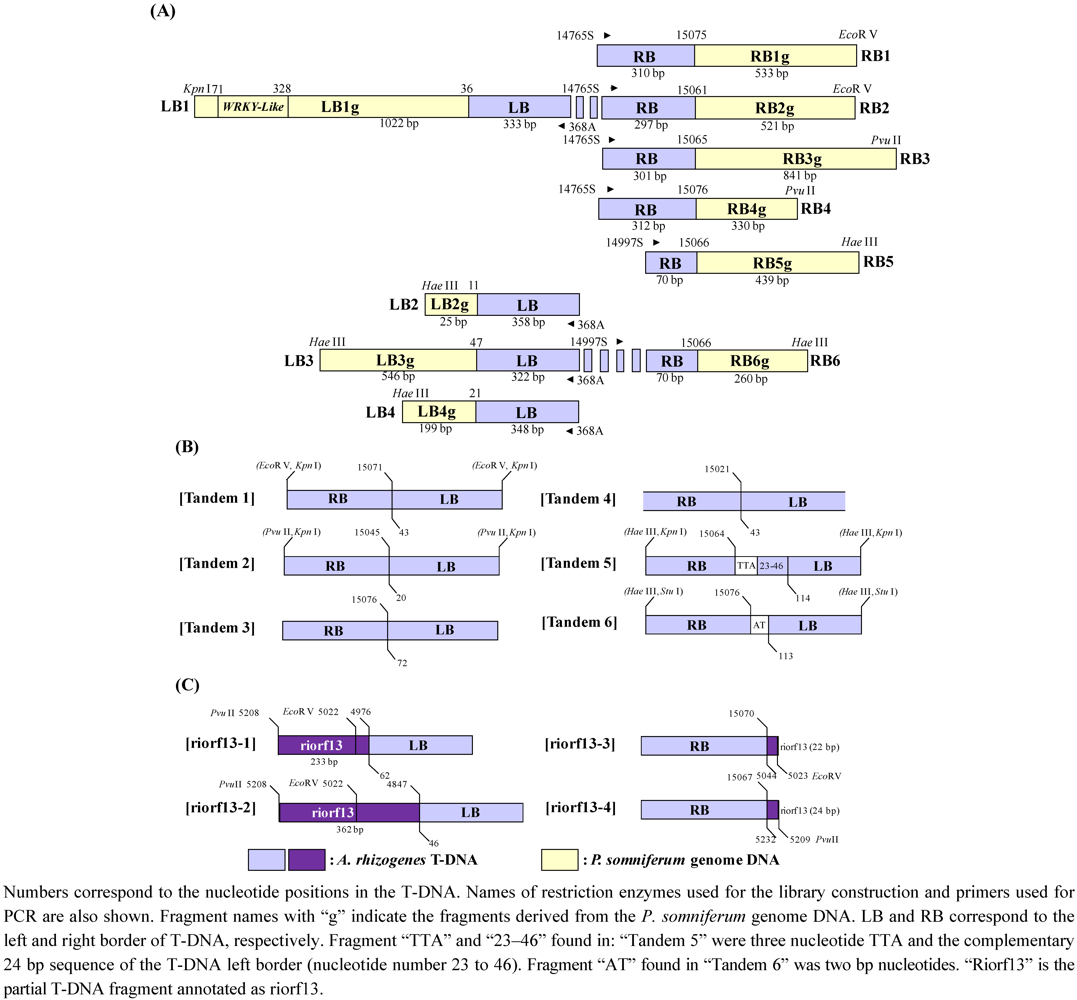
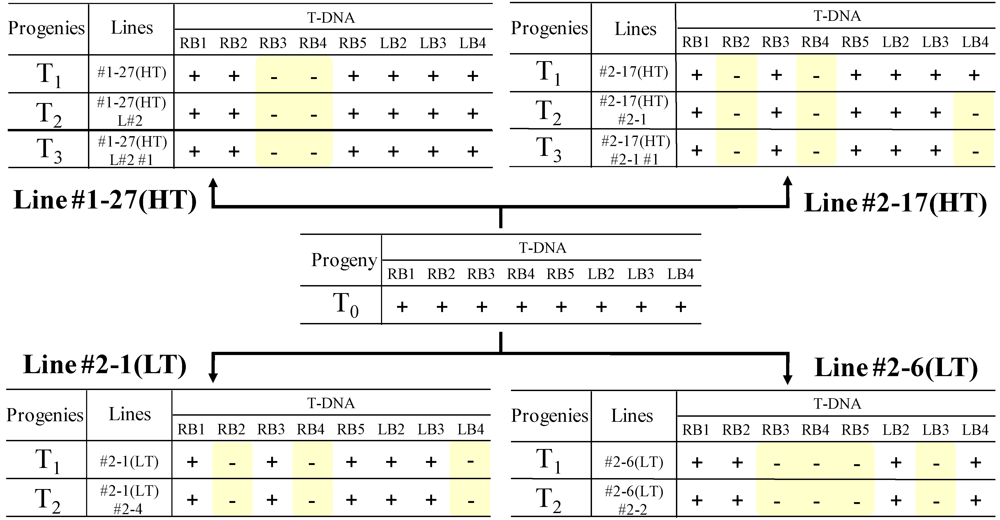
2.4. Analysis of the Heredity Manner of T-DNA Inserted Loci
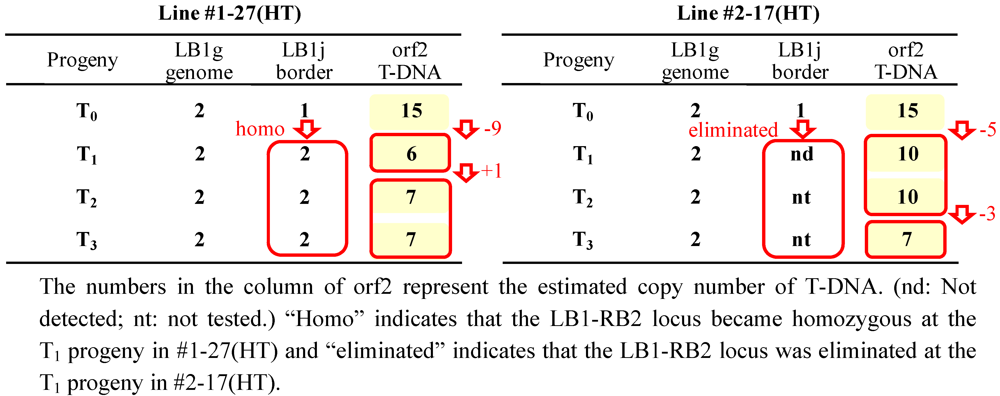
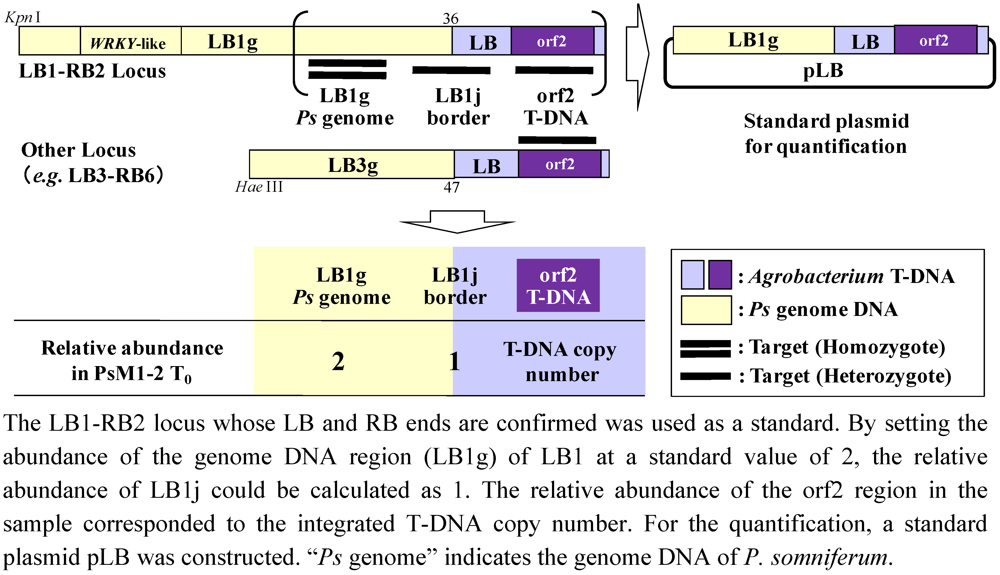
2.5. T-DNA Copy Number Analysis by Real-Time PCR
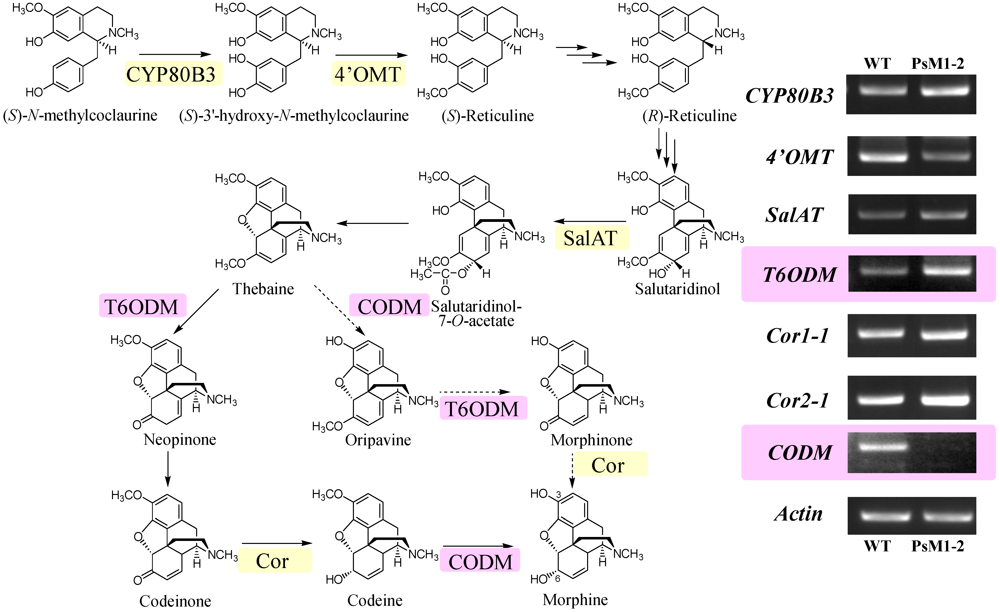
2.6. Expression Analyses on Morphine Biosynthetic Genes by RT-PCR
2.7. Discussion
3. Experimental Section
3.1. Plant Materials
3.2. Maintenance and Cultivation of Plant Materials
3.3. Phenotypic Observation of the PsM1-2 Mutants
3.4. HPLC Analysis of Alkaloid Content in the Latex
3.5. Genomic DNA Preparation from P. somniferum
3.6. Analysis of T-DNA Insertion Loci by IPCR and AL-PCR
3.7. Genomic Library Construction for IPCR
3.8. Genomic Library Construction for AL-PCR
3.9. IPCR and AL-PCR
3.10. Direct Amplification of T-DNA Borders Connected in Tandem
3.11. Analyses of T-DNA Insertion Loci and Heredity Manner by PCR
3.12. T-DNA Copy Number Analysis by Real-time PCR
3.13. Actin Gene Amplification from P. somniferum
3.14. Expression Analysis of the Morphine Biosynthetic Genes
3.15. Statistical Analysis
4. Conclusions
Acknowledgments
Conflict of Interest
References
- Frick, S.; Chitty, J.A.; Kramell, R.; Schmidt, J.; Allen, R.S.; Larkin, P.J.; Kutchan, T.M. Transformation of opium poppy (Papaver somniferum L.) with antisense berberine bridge enzyme gene (anti-bbe) via somatic embryogenesis results in an altered ratio of alkaloids in latex but not in roots. Transgenic Res. 2004, 13, 607–613. [Google Scholar] [CrossRef]
- Allen, R.S.; Millgate, A.G.; Chitty, J.A.; Thisleton, J.; Miller, J.A.C.; Fist, A.J.; Gerlach, W.L.; Larkin, P.J. RNAi-mediated replacement of morphine with the nonnarcotic alkaloid reticuline in opium poppy. Nat. Biotechnol. 2004, 22, 1559–1566. [Google Scholar] [CrossRef]
- Larkin, P.J.; Miller, J.A.; Allen, R.S.; Chitty, J.A.; Gerlach, W.L.; Frick, S.; Kutchan, T.M.; Fist, A.J. Increasing morphinan alkaloid production by over-expressing codeinone reductase in transgenic Papaver somniferum. Plant Biotechnol. J. 2007, 5, 26–37. [Google Scholar] [CrossRef]
- Frick, S.; Kramell, R.; Kutchan, T.M. Metabolic engineering with a morphine biosynthetic P450 in opium poppy surpasses breeding. Metab. Eng. 2007, 9, 169–176. [Google Scholar] [CrossRef]
- Allen, R.S.; Miller, J.A.; Chitty, J.A.; Fist, A.J.; Gerlach, W.L.; Larkin, P.J. Metabolic engineering of morphinan alkaloids by over-expression and RNAi suppression of salutaridinol 7-O-acetyltransferase in opium poppy. Plant Biotechnol. J. 2008, 6, 22–30. [Google Scholar]
- Kempe, K.; Higashi, Y.; Frick, S.; Sabarna, K.; Kutchan, T.M. RNAi suppression of the morphine biosynthetic gene salAT and evidence of association of pathway enzymes. Phytochemistry 2009, 70, 579–589. [Google Scholar] [CrossRef]
- Millgate, A.G.; Pogson, B.J.; Wilson, I.W.; Kutchan, T.M.; Zenk, M.H.; Gerlach, W.L.; Fist, A.J.; Larkin, P.J. Analgesia: Morphine-pathway block in top1 poppies. Nature 2004, 431, 413–414. [Google Scholar]
- Yoshimatsu, K.; Shimomura, K. Transformation of opium poppy (Papaver somniferum L.) with Agrobacterium rhizogenes MAFF 03-01724. Plant Cell Rep. 1992, 11, 132–136. [Google Scholar]
- Yoshimatsu, K.; Tono, K.; Shimomura, K. Cryopreservation of Medicinal Plant Resources—Retention of Biosynthetic Capabilities in Transformed Cultures. In Proceedings of the Japan International Research Center for Agricultural Sciences (JIRCAS)/International Plant Genetic Resources Institute (IPGRI) Joint International Workshop, Tsukuba, Japan, 20–23 October 1998; pp. S1–S6.
- Hagel, J.M.; Facchini, P.J. Dioxygenases catalyze the O-demethylation steps of morphine biosynthesis in opium poppy. Nat. Chem. Biol. 2010, 6, 273–275. [Google Scholar] [CrossRef]
- Nielsen, B.; Röe, J.; Brochmann-Hanssen, E. Oripavine—A new opium alkaloid. Planta Med. 1983, 48, 205–206. [Google Scholar] [CrossRef]
- Brochmann-Hanssen, E. A second pathway for the terminal steps in the biosynthesis of morphine. Planta Med. 1984, 50, 343–345. [Google Scholar] [CrossRef]
- Kato, N.; Dubouzet, E.; Kokabu, Y.; Yoshida, S.; Taniguchi, Y.; Dubouzet, J.G.; Yazaki, K.; Sato, F. Identification of a WRKY protein as a transcriptional regulator of benzylisoquinoline alkaloid biosynthesis in Coptis japonica. Plant Cell Physiol. 2007, 48, 8–18. [Google Scholar]
- Frick, S.; Kramell, R.; Schmidt, J.; Fist, A.J.; Kutchan, T.M. Comparative qualitative and quantitative determination of alkaloids in narcotic and condiment Papaver somniferum cultivars. J. Nat. Prod. 2005, 68, 666–673. [Google Scholar] [CrossRef]
- Facchini, P.J.; Park, S.-U. Developmental and inducible accumulation of gene transcripts involved in alkaloid biosynthesis in opium poppy. Phytochemistry 2003, 64, 177–186. [Google Scholar] [CrossRef]
- Yoshikawa, T.; Furuya, T. Morphinan alkaloid production by tissues differentiated from cultured cells of Papaver somniferum (1). Planta Med. 1985, 51, 110–113. [Google Scholar] [CrossRef]
- Weid, M.; Ziegler, J.; Kutchan, T.M. The roles of latex and the vascular bundle in morphine biosynthesis in the opium poppy, Papaver somniferum. Proc. Natl. Acad. Sci. USA 2004, 101, 13957–13962. [Google Scholar]
- Murashige, T.; Skoog, F. A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol. Plant 1962, 15, 473–497. [Google Scholar] [CrossRef]
- Yoshimatsu, K.; Yamaguchi, H.; Shimomura, K. Traits of Panax ginseng hairy roots after cold storage and cryopreservation. Plant Cell Rep. 1996, 15, 555–560. [Google Scholar] [CrossRef]
- Yoshimatsu, K.; Shimomura, K. Cryopreservation of Panax (Ginseng). In Biotechnology in Agriculture and Forestry 50,Cryopreservation of Plant Germplasm II; Towill, L.E., Bajaj, Y.P.S., Eds.; Springer-Verlag: Berlin/Heidelberg, Germany, 2002; pp. 164–179. [Google Scholar]
- Ochman, H.; Gerber, A.S.; Hartl, D.L. Genetic applications of an inverse polymerase chain reaction. Genetics 1988, 120, 621–623. [Google Scholar]
- Fladung, M. Gene stability in transgenic aspen (Populus). I. Flanking DNA sequences and T-DNA structure. Mol. Gen. Genet. 1999, 260, 574–581. [Google Scholar] [CrossRef]
- Arnold, C.; Hodgson, I.J. Vectorette PCR: A novel approach to genomic walking. PCR Methods Appl. 1991, 1, 39–42. [Google Scholar]
- Spertini, D.; Beliveau, C.; Bellemare, G. Screening of transgenic plants by amplification of unknown genomic DNA flanking T-DNA. Biotechniques 1999, 27, 308–314. [Google Scholar]
- Zheng, S.J.; Henken, B.; Sofiari, E.; Jacobsen, E.; Krens, F.A.; Kik, C. Molecular characterization of transgenic shallots (Allium cepa L.) by adaptor ligation PCR (AL-PCR) and sequencing of genomic DNA flanking T-DNA borders. Transgenic Res. 2001, 10, 237–245. [Google Scholar] [CrossRef]
- Bubner, B.; Baldwin, I.T. Use of real-time PCR for determining copy number and zygosity in transgenic plants. Plant Cell Rep. 2004, 23, 263–271. [Google Scholar] [CrossRef]
- Yang, L.; Ding, J.; Zhang, C.; Jia, J.; Weng, H.; Liu, W.; Zhang, D. Estimating the copy number of transgenes in transformed rice by real-time quantitative PCR. Plant Cell Rep. 2005, 23, 759–763. [Google Scholar] [CrossRef]
- Levy, A.; Milo, J. Genetics and breeding of Papaver somniferum. In Poppy: The Genus Papaver; Bernáth, J., Ed.; Harwood Academic Publishers: Chur, Switzerland, 1998; pp. 93–103. [Google Scholar]
- Huang, F.-C.; Kutchan, T.M. Distribution of morphinan and benzo[c]phenanthridine alkaloid gene transcript accumulation in Papaver somninferum. Phytochemistry 2000, 53, 555–564. [Google Scholar] [CrossRef]
- Grothe, T.; Lenz, R.; Kutchan, T.M. Molecular characterization of the salutaridinol 7-O-acetyltransferase involved in morphine biosynthesis in opium poppy Papaver somniferum. J. Biol. Chem. 2001, 276, 30717–30723. [Google Scholar] [CrossRef]
- Unterlinner, B.; Lenz, R.; Kutchan, T.M. Molecular cloning and functional expression of codeinone reductase: The penultimate enzyme in morphine biosynthesis in the opium poppy Papaver somniferum. Plant J. 1999, 18, 465–475. [Google Scholar] [CrossRef]
- Frohman, M.A.; Dush, M.K.; Martin, G.R. Rapid production of full-length cDNAs from rare transcripts: Amplification using a single gene-specific oligonucleotide primer. Proc. Natl. Acad. Sci. USA 1988, 85, 8998–9002. [Google Scholar]
- The R Project for Statistical Computing. Available online: http://www.r-project.org/ (accessed on 15 January 2012).
Supplementary Files
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Kawano, N.; Kiuchi, F.; Kawahara, N.; Yoshimatsu, K. Genetic and Phenotypic Analyses of a Papaver somniferum T-DNA Insertional Mutant with Altered Alkaloid Composition. Pharmaceuticals 2012, 5, 133-154. https://doi.org/10.3390/ph5020133
Kawano N, Kiuchi F, Kawahara N, Yoshimatsu K. Genetic and Phenotypic Analyses of a Papaver somniferum T-DNA Insertional Mutant with Altered Alkaloid Composition. Pharmaceuticals. 2012; 5(2):133-154. https://doi.org/10.3390/ph5020133
Chicago/Turabian StyleKawano, Noriaki, Fumiyuki Kiuchi, Nobuo Kawahara, and Kayo Yoshimatsu. 2012. "Genetic and Phenotypic Analyses of a Papaver somniferum T-DNA Insertional Mutant with Altered Alkaloid Composition" Pharmaceuticals 5, no. 2: 133-154. https://doi.org/10.3390/ph5020133
APA StyleKawano, N., Kiuchi, F., Kawahara, N., & Yoshimatsu, K. (2012). Genetic and Phenotypic Analyses of a Papaver somniferum T-DNA Insertional Mutant with Altered Alkaloid Composition. Pharmaceuticals, 5(2), 133-154. https://doi.org/10.3390/ph5020133




